Abstract
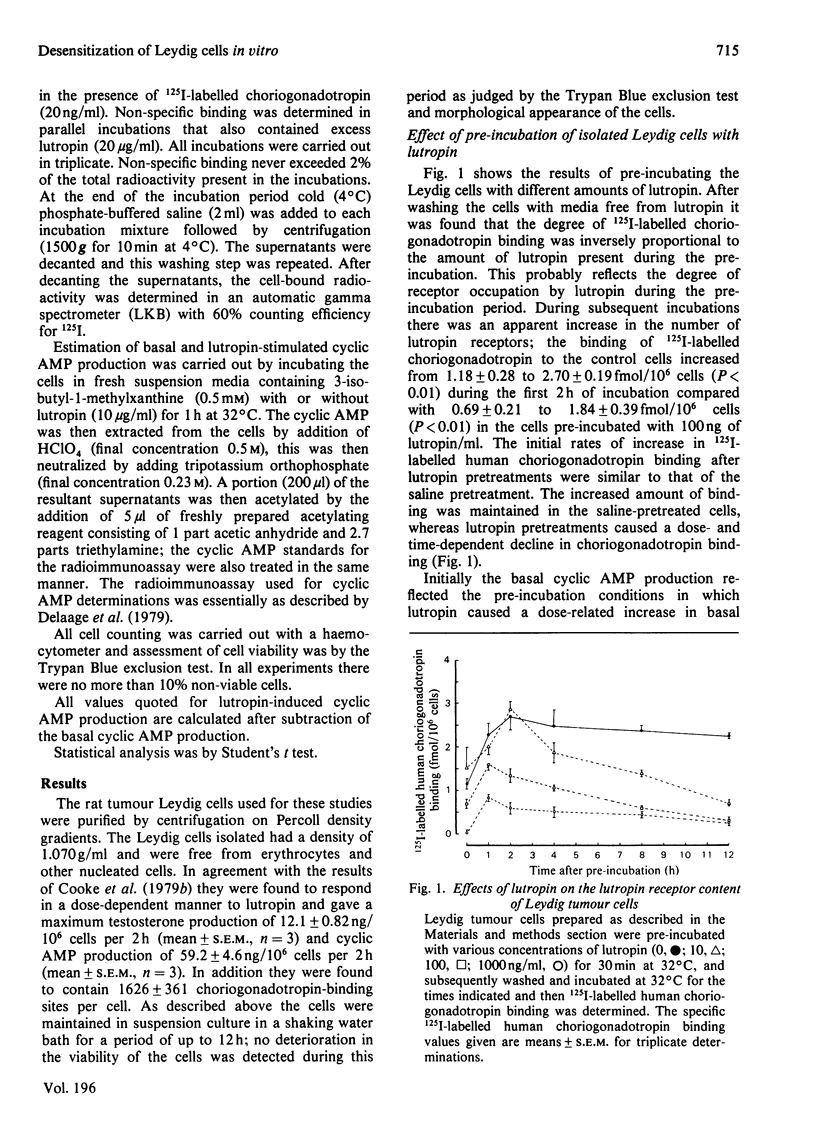
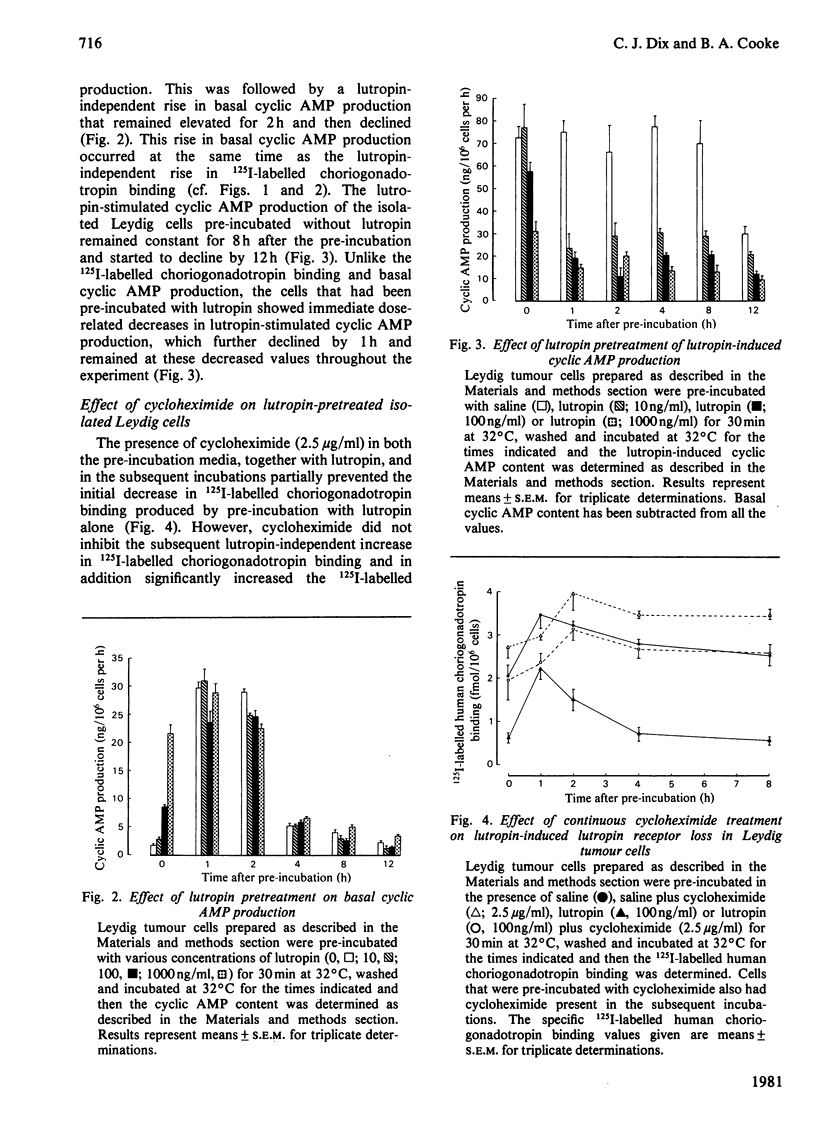
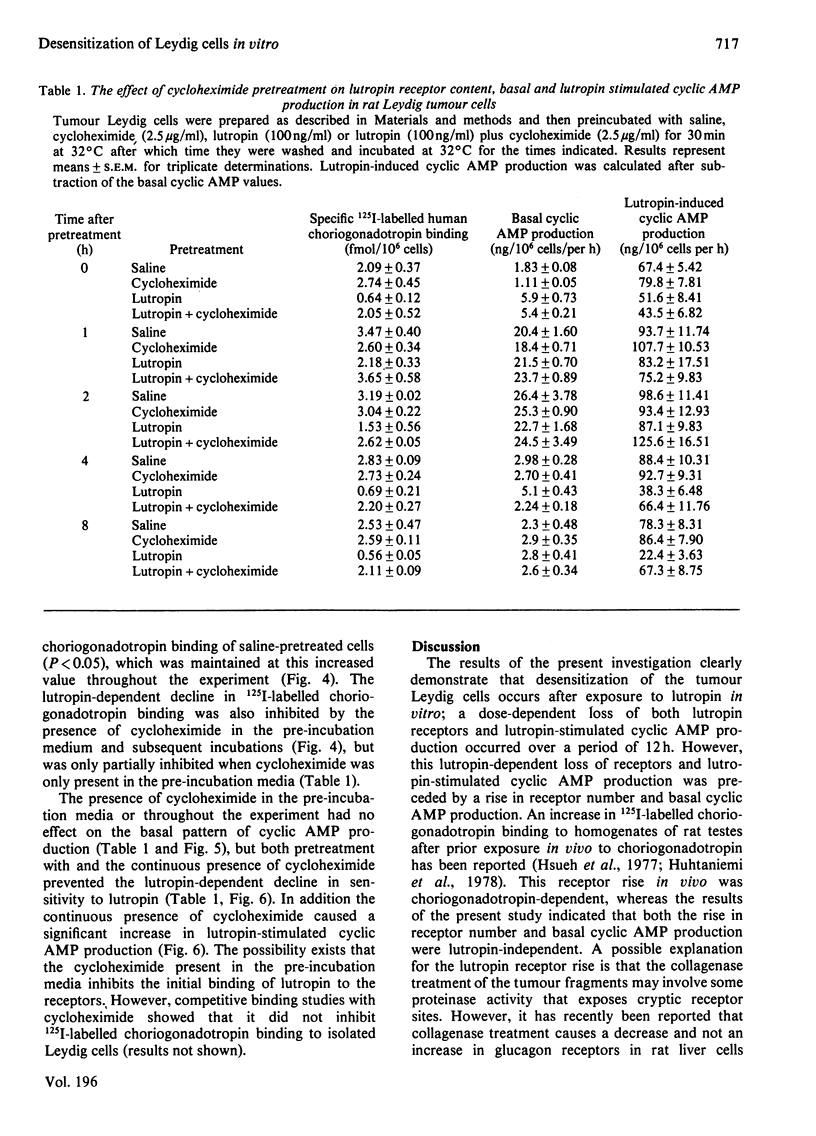
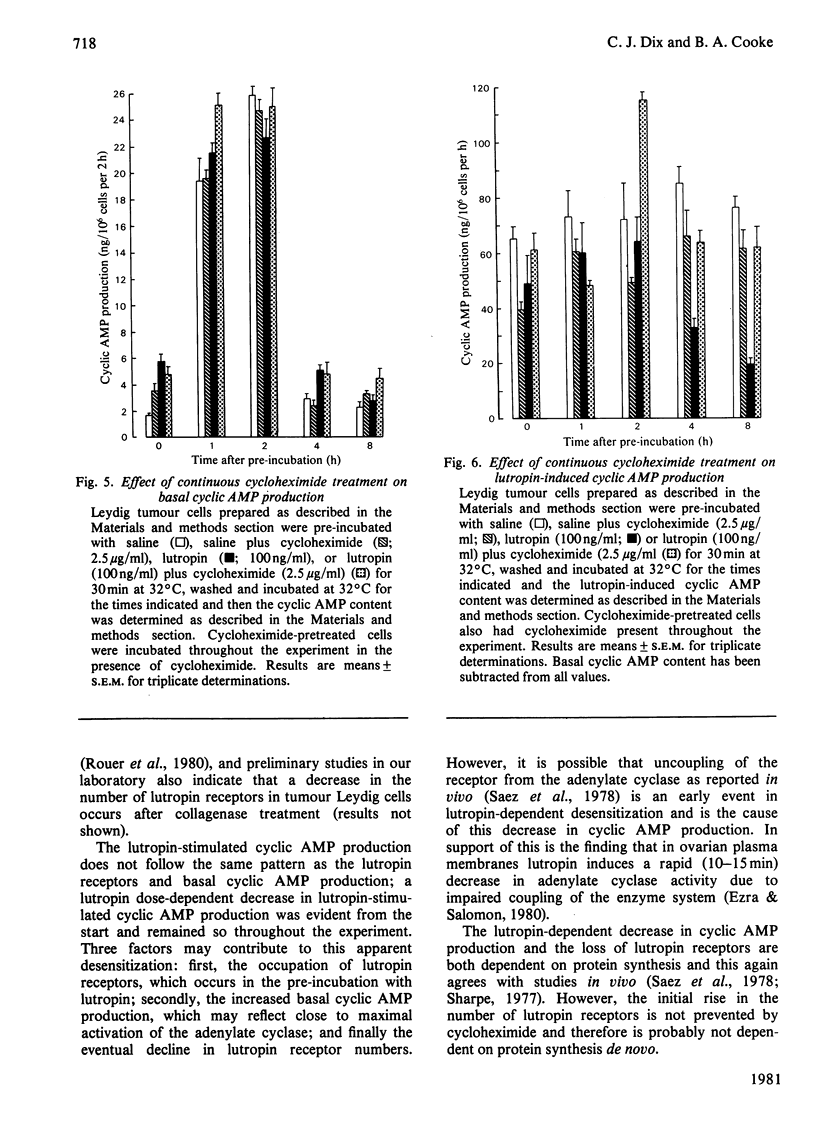
A system to study lutropin-induced desensitization of tumour Leydig cells in vitro has been investigated. Tumour Leydig cells were purified on a Percoll gradient and then incubated for 30 min with lutropin (0-1000ng/ml). The cells were then washed and incubated in suspension media at 32 degrees C. 125I-labelled human choriogonadotropin binding and basal and lutropin-stimulated cyclic AMP production were determined at various times. Initially the cells showed a dose-dependent decrease in human choriogonadotropin binding (1.18 and 0.13fmol/10(6) cells respectively) followed by an increase at 1 h (2.32 and 0.87fmol/10(6) cells respectively). Human choriogonadotropin binding remained elevated in the cells pre-incubated without lutropin, whereas the cells pre-incubated with lutropin showed a dose-dependent decrease over the next 10 h (2.20-0.18fmol/10(6) cells respectively). Basal production of cyclic AMP initially reflected the pre-incubation conditions (1.17-21.19ng/10(6) cells per h for 0-1000ng of lutropin/ml respectively). However, by 1 h there was a marked rise in basal cyclic AMP production which returned to the initial lower values by 4 h. At all time intervals studied, lutropin-induced cyclic AMP production showed a decrease that was proportional to lutropin concentration in the pre-incubated media. The decreases in human choriogonadotropin binding produced by pre-incubations with lutropin (100ng/ml) was partially inhibited by the presence of cycloheximide in the pre-incubation media and totally prevented by the continuous presence of cycloheximide. These results demonstrate that desensitization of tumour Leydig cells occurs after exposure to lutropin in vitro. This desensitization involves both a loss of plasma membrane receptors for lutropin and lutropin-stimulated adenylate cyclase. These events can be prevented by cycloheximide and are therefore probably dependent on protein synthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cailla H., Delaage M. Succinyl derivatives of adenosine 3',5'-cyclic monophosphate: synthesis and purification. Anal Biochem. 1972 Jul;48(1):62–72. doi: 10.1016/0003-2697(72)90170-4. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Harwood J. P., Aguilera G., Dufau M. L. Hormonal regulation of peptide receptors and target cell responses. Nature. 1979 Jul 12;280(5718):109–116. doi: 10.1038/280109a0. [DOI] [PubMed] [Google Scholar]
- Cooke B. A., Janszen F. H., van Driel M. J., van der Molen H. J. Evidence for the involvement of lutropin-independent RNA synthesis in Leydig cell steroidogenesis. Mol Cell Endocrinol. 1979 Jun;14(3):181–189. doi: 10.1016/0303-7207(79)90043-1. [DOI] [PubMed] [Google Scholar]
- Cooke B. A., Lindh L. M., Janszen F. H. Role of cyclic AM in steroidogenesis in Leydig cells: discrepancies' between effects of luteinizing hormone and cholera toxin. FEBS Lett. 1977 Jan 15;73(1):67–71. [PubMed] [Google Scholar]
- Cooke B. A., Lindh L. M., Janszen F. H., van Driel M. J., Bakker C. P., van der Plank M. P., van der Molen H. J. A Leydig cell tumour: a model for the study of lutropin action. Biochim Biophys Acta. 1979 Mar 22;583(3):320–331. doi: 10.1016/0304-4165(79)90456-2. [DOI] [PubMed] [Google Scholar]
- Cooke B. A., Lindh M. L., Janszen F. H. Correlation of protein kinase activation and testosterone production after stimulation of Leydig cells with luteinizing hormone. Biochem J. 1976 Dec 15;160(3):439–446. doi: 10.1042/bj1600439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezra E., Salomon Y. Mechanism of desensitization of adenylate cyclase in lutropin. GTP-dependent uncoupling of the receptor. J Biol Chem. 1980 Jan 25;255(2):653–658. [PubMed] [Google Scholar]
- Hsueh A. J., Dufau M. L., Catt K. J. Gonadotropin-induced regulation of luteinizing hormone receptors and desensitization of testicular 3':5'-cyclic AMP and testosterone responses. Proc Natl Acad Sci U S A. 1977 Feb;74(2):592–595. doi: 10.1073/pnas.74.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtaniemi I., Martikainen H., Tikkala L. HCG-induced changes in the number of rat testis LH/HCG receptors. Mol Cell Endocrinol. 1978 Jun;11(1):43–50. doi: 10.1016/0303-7207(78)90031-x. [DOI] [PubMed] [Google Scholar]
- Rouer E., Desbuquois B., Postel-Vinay M. C. Interactions of glucagon with isolated rat-liver cells. Fate and subcellular localization of cell-associated hormone. Mol Cell Endocrinol. 1980 Aug;19(2):143–164. doi: 10.1016/0303-7207(80)90018-0. [DOI] [PubMed] [Google Scholar]
- Saez J. M., Haour F., Cathiard A. M. Early hCG-induced desensitization in Leydig cells. Biochem Biophys Res Commun. 1978 Mar 30;81(2):552–558. doi: 10.1016/0006-291x(78)91570-x. [DOI] [PubMed] [Google Scholar]
- Schumacher M., Schäfer G., Holstein A. F., Hilz H. Rapid isolation of mouse Leydig cells by centrifugation in Percoll density gradients with complete retention of morphological and biochemical integrity. FEBS Lett. 1978 Jul 15;91(2):333–338. doi: 10.1016/0014-5793(78)81204-6. [DOI] [PubMed] [Google Scholar]
- Sharpe R. M. Relationship between testosterone, fluid content and luteinizing hormone receptors in the rat testis. Biochem Biophys Res Commun. 1977 Apr 11;75(3):711–717. doi: 10.1016/0006-291x(77)91530-3. [DOI] [PubMed] [Google Scholar]
- Thorell J. I., Johansson B. G. Enzymatic iodination of polypeptides with 125I to high specific activity. Biochim Biophys Acta. 1971 Dec 28;251(3):363–369. doi: 10.1016/0005-2795(71)90123-1. [DOI] [PubMed] [Google Scholar]
- Tsuruhara T., Dufau M. L., Cigorraga S., Catt K. J. Hormonal regulation of testicular luteinizing hormone receptors. Effects on cyclic AMP and testosterone responses in isolated Leydig cells. J Biol Chem. 1977 Dec 25;252(24):9002–9009. [PubMed] [Google Scholar]


