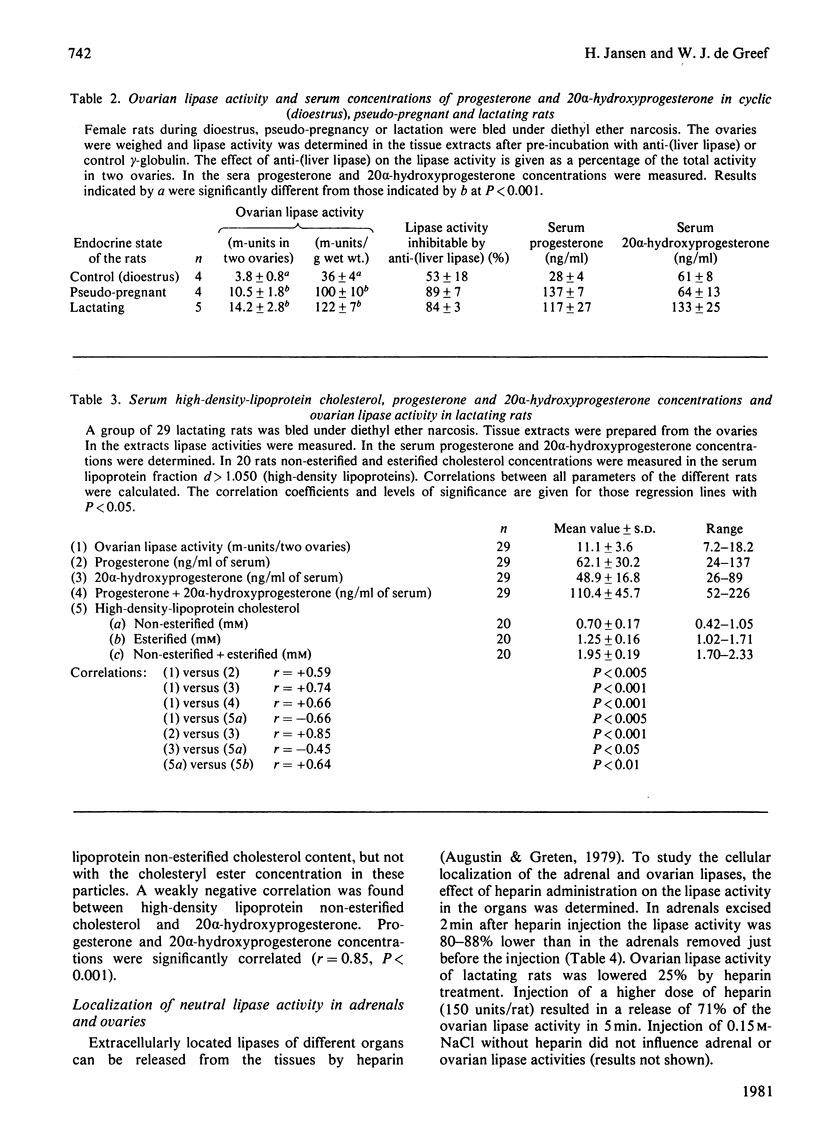
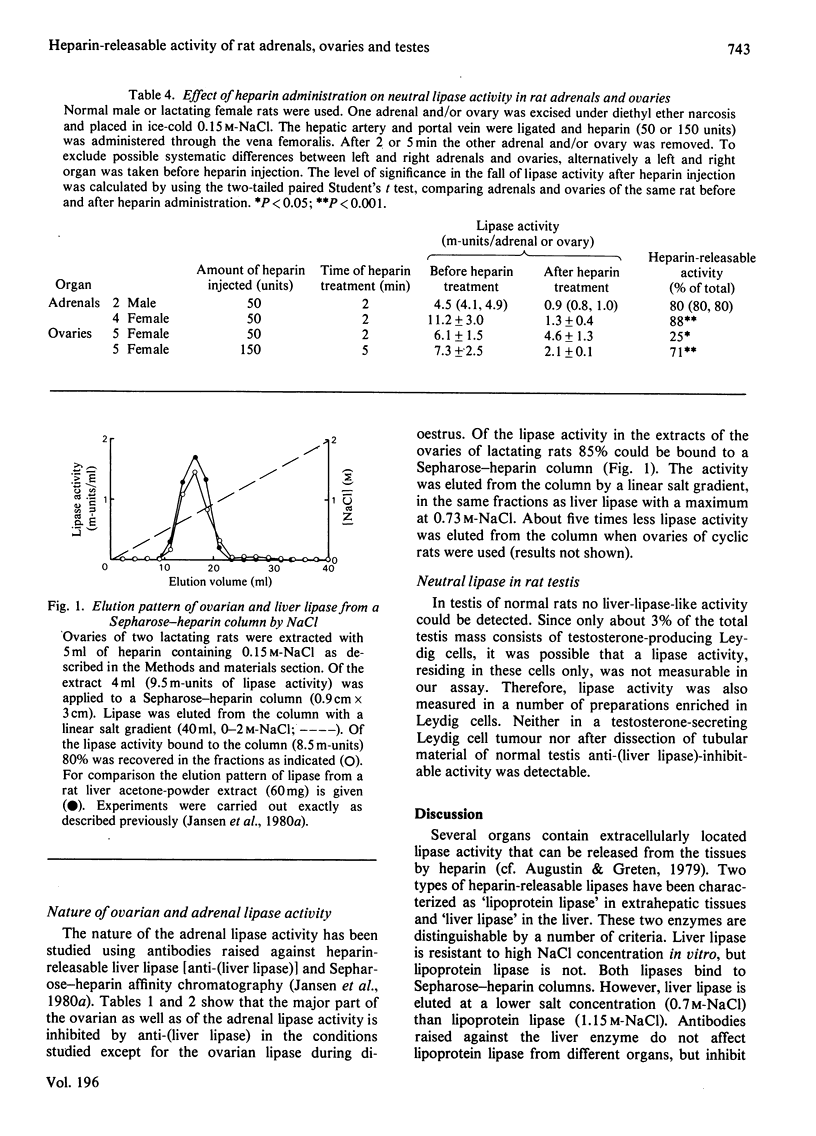
Abstract
The presence of NaCl-resistant, neutral triacylglycerol hydrolase (lipase) activity in rat adrenal gland, ovary and testis was studied. Both adrenals and ovaries but not testes were found to contain such a lipase. The activity of the enzyme in the adrenal gland was lowered during cortisol treatment and hypothyroidism. An elevated adrenal lipase activity was found during hyperthyroidism. Pseudo-pregnant and lactating rats had higher ovarian lipase activities than cyclic rats. Ovarian lipase activity in lactating rats was positively correlated with the serum concentrations of progesterone and of 20 alpha-hydroxyprogesterone and negatively correlated with the high-density-lipoprotein non-esterified cholesterol concentration. The lipase activity of adrenals and of ovaries was largely releasable from these organs by heparin and could be inhibited by an antibody against heparin-releasable liver lipase. This indicated that the lipase is extracellularly located and is similar to 'liver' lipase. A possible role of this lipase in adrenals and ovaries is discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J. M., Dietschy J. M. Relative importance of high and low density lipoproteins in the regulation of cholesterol synthesis in the adrenal gland, ovary, and testis of the rat. J Biol Chem. 1978 Dec 25;253(24):9024–9032. [PubMed] [Google Scholar]
- Balasubramaniam S., Goldstein J. L., Faust J. R., Brunschede G. Y., Brown M. S. Lipoprotein-mediated regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and cholesteryl ester metabolism in the adrenal gland of the rat. J Biol Chem. 1977 Mar 10;252(5):1771–1779. [PubMed] [Google Scholar]
- Bronzert T. J., Brewer H. B., Jr New micromethod for measuring cholesterol in plasma lipoprotein fractions. Clin Chem. 1977 Nov;23(11):2089–2098. [PubMed] [Google Scholar]
- Brown M. S., Kovanen P. T., Goldstein J. L. Receptor-mediated uptake of lipoprotein-cholesterol and its utilization for steroid synthesis in the adrenal cortex. Recent Prog Horm Res. 1979;35:215–257. doi: 10.1016/b978-0-12-571135-7.50009-6. [DOI] [PubMed] [Google Scholar]
- FEKETE G., GOEROEG P. THE INHIBITORY ACTION OF NATURAL AND SYNTHETIC GLUCOCORTICOIDS ON ADRENAL STEROIDOGENESIS AT THE ADRENAL LEVEL. J Endocrinol. 1963 Oct;27:123–126. doi: 10.1677/joe.0.0270123. [DOI] [PubMed] [Google Scholar]
- Glomset J. A. Lecithin: cholesterol acyltransferase. An exercise in comparative biology. Prog Biochem Pharmacol. 1979;15:41–66. [PubMed] [Google Scholar]
- Gwynne J. T., Hess B. Binding and degradation of human 125I-HDL by rat adrenocortical cells. Metabolism. 1978 Nov;27(11):1593–1600. doi: 10.1016/0026-0495(78)90281-0. [DOI] [PubMed] [Google Scholar]
- Gwynne J. T., Mahaffee D., Brewer H. B., Jr, Ney R. L. Adrenal cholesterol uptake from plasma lipoproteins: regulation by corticotropin. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4329–4333. doi: 10.1073/pnas.73.12.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen J. K., Ehnholm C., Kinnunen P. K., Nikkilä E. A. An immunochemical method for the selective measurement of two triglyceride lipases in human postheparin plasma. Clin Chim Acta. 1975 Sep 16;63(3):335–347. doi: 10.1016/0009-8981(75)90055-8. [DOI] [PubMed] [Google Scholar]
- Hülsmann W. C., Oerlemans M. C., Geelhoed-Mieras M. M. Effect of hypothyroidism, diabetes and polyunsaturated fatty acids on heparin-releasable rat liver lipase. Biochem Biophys Res Commun. 1977 Dec 7;79(3):784–788. doi: 10.1016/0006-291x(77)91180-9. [DOI] [PubMed] [Google Scholar]
- Jansen H., Kalkman C., Birkenhäger J. C., Hülsmann W. C. Demonstration of a heparin-releasable liver-lipase-like activity in rat adrenals. FEBS Lett. 1980 Mar 24;112(1):30–34. doi: 10.1016/0014-5793(80)80119-0. [DOI] [PubMed] [Google Scholar]
- Jansen H., van Berkel T. J., Hülsmann W. C. Binding of liver lipase to parenchymal and non-parenchymal rat liver cells. Biochem Biophys Res Commun. 1978 Nov 14;85(1):148–152. doi: 10.1016/s0006-291x(78)80022-9. [DOI] [PubMed] [Google Scholar]
- Jansen H., van Tol A., Hülsmann W. C. On the metabolic function of heparin-releasable liver lipase. Biochem Biophys Res Commun. 1980 Jan 15;92(1):53–59. doi: 10.1016/0006-291x(80)91518-1. [DOI] [PubMed] [Google Scholar]
- Kovanen P. T., Goldstein J. L., Chappell D. A., Brown M. S. Regulation of low density lipoprotein receptors by adrenocorticotropin in the adrenal gland of mice and rats in vivo. J Biol Chem. 1980 Jun 25;255(12):5591–5598. [PubMed] [Google Scholar]
- Kuusi T., Kinnunen P. K., Nikkilä E. A. Hepatic endothelial lipase antiserum influences rat plasma low and high density lipoproteins in vivo. FEBS Lett. 1979 Aug 15;104(2):384–388. doi: 10.1016/0014-5793(79)80858-3. [DOI] [PubMed] [Google Scholar]
- Labrie F., Pelletier G., Labrie R., Ho-Kim M. A., Delgado A., MacIntosh B., Fortier C. Liaison transcortine-corticostérone et contrôle de l'activité hypophyso-surrénalienne chez le rat. Interactions hypophyse-thyroïde-surrénales-gonades. Ann Endocrinol (Paris) 1968 Jan-Feb;29(1):29–43. [PubMed] [Google Scholar]
- Meijs-Roelofs H. M., de Greef W. J., Uilenbroek J. T. Plasma progesterone and its relationship to serum gonadotrophins in immature female rats. J Endocrinol. 1975 Feb;64(2):329–336. doi: 10.1677/joe.0.0640329. [DOI] [PubMed] [Google Scholar]
- PERON F. G., MONCLOA F., DORFMAN R. I. Studies on the possible inhibitory effect of corticosterone on corticosteroidogenesis at the adrenal level in the rat. Endocrinology. 1960 Sep;67:379–388. doi: 10.1210/endo-67-3-379. [DOI] [PubMed] [Google Scholar]
- PETERSON R. E. The influence of the thyroid on adrenal cortical function. J Clin Invest. 1958 May;37(5):736–743. doi: 10.1172/JCI103659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepe G. J., Rothchild I. Metabolic clearance rate of progesterone: comparison between ovariectomized, pregnant, pseudopregnant and deciduoma-bearing pseudopregnant rats. Endocrinology. 1973 Nov;93(5):1200–1205. doi: 10.1210/endo-93-5-1200. [DOI] [PubMed] [Google Scholar]
- Visser T. J., van den Hout-Goemaat N. L., Docter R., Hennemann G. Radio-immunoassay of thyroxine in unextracted serum. Neth J Med. 1975;18(3):111–115. [PubMed] [Google Scholar]
- Welschen R., Osman P., Dullaart J., de Greef W. J., Uilenbroek J. T., de Jong F. H. Levels of follicle-stimulating hormone, luteinizing hormone, oestradiol-17 beta and progesterone, and follicular growth in the pseudopregnant rat. J Endocrinol. 1975 Jan;64(1):37–47. doi: 10.1677/joe.0.0640037. [DOI] [PubMed] [Google Scholar]
- de Greef W. J., Dullaart J., Zeilmaker G. H. Serum luteinizing hormone, follicle-stimulating hormone, prolactin and progesterone concentrations and follicular development in the pseudopregnant rat after unilateral ovariectomy. J Endocrinol. 1975 Aug;66(2):249–256. doi: 10.1677/joe.0.0660249. [DOI] [PubMed] [Google Scholar]
- de Greef W. J., Zeilmaker G. H. Blood pregesterone levels in pseudopregnant rats: effects of partial removal of luteal tissue. Endocrinology. 1974 Aug;95(2):565–571. doi: 10.1210/endo-95-2-565. [DOI] [PubMed] [Google Scholar]
- de Jong F. H., Baird D. T., van der Molen H. J. Ovarian secretion rates of oestrogens, androgens and progesterone in normal women and in women with persistent ovarian follicles. Acta Endocrinol (Copenh) 1974 Nov;77(3):575–587. doi: 10.1530/acta.0.0770575. [DOI] [PubMed] [Google Scholar]


