Abstract
Cardiovascular diseases and dermatological conditions are prevalent health issues worldwide. Previous studies have suggested that risk factors for cardiovascular diseases may be associated with the development of dermatological conditions. However, the causal association between these factors remain unclear. This study utilized data from genome-wide association studies and applied Mendelian randomization (MR) to explore the potential causal association between cardiovascular risk factors and common dermatological conditions. Genetic variants significantly associated with low-density lipoprotein cholesterol (LDL-C), serum uric acid, blood glucose, and hypertension were selected as instrumental variables. We employed inverse variance weighted, MR Egger, and weighted mode methods for analysis. Sensitivity analyses, including Cochran Q test, MR-Egger intercept test, MR-PRESSO global test, and leave-one-out analysis, were conducted to ensure the robustness of the results. The MR analysis indicated a positive association between LDL-C levels and the risk of psoriasis (odds ratio [OR] = 1.23, 95% confidence interval [CI]: 1.03–1.47, P = .02). Additionally, hypertension and serum uric acid levels were positively associated with the risk of dermatitis eczema (hypertension: OR = 2.77, 95% CI: 1.23–6.24, P = .01; serum uric acid: OR = 1.09, 95% CI: 1.01–1.06, P = .01). This study provides evidence of a potential causal association between LDL-C levels and psoriasis, as well as between hypertension and serum uric acid levels and dermatitis eczema. These findings highlight the potential importance of cardiovascular health management in the prevention and treatment of common dermatological conditions. Further research is needed to validate these results and explore the underlying biological mechanisms.
Keywords: dermatitis eczema, hypertension, LDL cholesterol, psoriasis, uric acid
1. Introduction
Cardiovascular diseases (CVD) and dermatological conditions are prevalent and significant global health issues, placing a substantial burden on patients’ quality of life and healthcare resources.[1–4]
Previous research has suggested that some risk factors for cardiovascular diseases, such as low-density lipoprotein cholesterol (LDL-C), serum uric acid, blood glucose, and hypertension, may be associated with the development of skin conditions. These risk factors may affect skin health through various mechanisms.[5,6] Elevated LDL-C levels can lead to atherosclerosis and vascular inflammation, and psoriasis patients often have a higher risk of cardiovascular diseases; however, whether these 2 conditions influence each other remains unclear.[7,8] Hyperuricemia is closely related to gout, and elevated uric acid levels may affect the risk of skin-related diseases through inflammatory responses and oxidative stress.[9–11] However, the causality of these association has not been fully verified, and traditional observational studies are often affected by confounding factors, making it difficult to establish causal links.
Mendelian randomization (MR) uses genetic variants as instrumental variables (IVs) to effectively avoid the influence of confounding factors, providing a robust method for exploring potential causal association.[12,13] This study aims to utilize MR to investigate the potential causal association between cardiovascular disease risk factors and common skin conditions. By providing new perspectives and evidence on the potential causal links between cardiovascular risk factors and skin diseases, this research contributes to a better understanding that can inform clinical prevention and treatment strategies.
2. Materials and methods
2.1. Study design
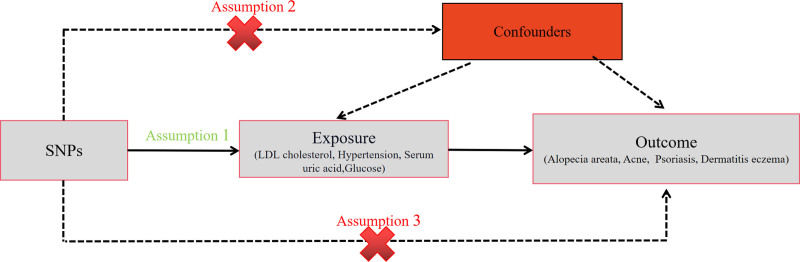
This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology Using MR guidelines (STROBE-MR, S1 Checklist).[14,15] This study examines LDL-C, serum uric acid, blood glucose, and hypertension as exposure factors to explore their potential causal association with 4 outcome variables: acne, alopecia areata, psoriasis, and dermatitis eczema. The MR design follows 3 assumptions: first, single nucleotide polymorphisms (SNPs) must be strongly associated with the exposure; second, SNPs should not be related to the outcome through confounding factors; third, SNPs should not directly influence the outcome (Fig. 1). This method has been detailed in previous studies and relies on published research and publicly available summary data, thus not requiring further ethical approval or participant consent.
Figure 1.
This study follows the 3 assumptions of MR study.
2.2. Date sources
To avoid population overlap between exposure factors and outcome variables, all datasets for the exposure factors in this study were sourced from the UK Biobank (https://gwas.mrcieu.ac.uk/) and the European Bioinformatics Institute (https://gwas.mrcieu.ac.uk/). The datasets related to the outcome variables were obtained from FinnGen (https://www.finngen.fi/fi). All datasets were derived from European populations and included both male and female participants. Detailed information on the datasets included in this study is presented in Table 1.
Table 1.
Source of the GWAS datas.
| Exposure/outcome | Database | Year | Author | Participants | Number of SNPs | Web source if publicly |
|---|---|---|---|---|---|---|
| LDL cholesterol (ieu-b-110) (PMID: 32203549) |
UK Biobank | 2020 | Richardson, Tom | 440,546 individuals of European |
12,321,875 |
https://gwas.mrcieu.ac.uk/datasets/ieu-b-110/ (Access time: July, 2024) |
| Hypertension (ukb-b-12493) |
UK Biobank | 2018 | Ben Elsworth | 463,010 individuals (54,358 use cases and 408,652 controls) of European |
9,851,867 |
https://gwas.mrcieu.ac.uk/datasets/ukb-b-12493/ (Access time: July, 2024) |
| Serum uric acid levels (ebi-a-GCST90018977) (PMID: 34594039) |
EBI | 2021 | Sakaue S | 343,836 individuals of European |
19,041,286 |
https://gwas.mrcieu.ac.uk/datasets/ebi-a-GCST90018977/ (Access time: July, 2024) |
| Glucose levels (ebi-a-GCST90018955) (PMID: 34594039) |
EBI | 2021 | Sakaue S | 314,916 individuals (60,620 cases and 970,216 controls) of European |
19,046,716 |
https://gwas.mrcieu.ac.uk/datasets/ebi-a-GCST90018955/ (Access time: July, 2024) |
| Alopecia areata (finn-b-L12_ALOPECAREATA) |
Finngen | 2021 | NA | 211,428 individuals (289 cases and 211,139 controls) of European |
16,380,450 |
https://gwas.mrcieu.ac.uk/datasets/finn-b-L12_ALOPECAREATA/ (Access time: July, 2024) |
| Acne (finn-b-L12_ACNE) |
Finngen | 2021 | NA | 212,438 individuals (1299 cases and 211,139 controls) of European ancestry |
16,380,454 |
https://gwas.mrcieu.ac.uk/datasets/finn-b-L12_ACNE/ (Access time: July, 2024) |
| Zoster (finn-b-AB1_ZOSTER) |
Finngen | 2021 | NA | 213,936 individuals (2080 cases and 211,856 controls) of European |
16,380,433 |
https://gwas.mrcieu.ac.uk/datasets/finn-b-AB1_ZOSTER/ (Access time: July, 2024) |
| Dermatitis and eczema (finn-b-L12_DERMATITISECZEMA) |
Finngen | 2021 | NA | 218,792 individuals (20,052 cases and 198,740 controls) of European |
16,380,466 |
https://gwas.mrcieu.ac.uk/datasets/finn-b-L12_DERMATITISECZEMA/ (Access time: July, 2024) |
| Psoriasis (finn-b-L12_PSORIASIS) |
Finngen | 2021 | NA | 216,752 individuals (4510 cases and 212,242 controls) of European |
16,380,464 |
https://gwas.mrcieu.ac.uk/datasets/finn-b-L12_PSORIASIS/ (Access time: July, 2024) |
EBI = European Bioinformatics Institute, GWAS = genome wide association study, LDL = low-density lipoprotein.
2.3. Genetic instrumental variables
To avoid analysis bias caused by strong linkage disequilibrium among SNPs, the genetic instrumental variables screening criteria were: (1) P < 5 × 10‐8; (2) physical distance M > 10,000 kb between every 2 genes; (3) r2 threshold of LD between genes < 0.001. In addition, this study searched the table-scanning database for secondary phenotypes of each SNP and excluded SNPs associated with outcome confounders to avoid potential multiple effects. R2 is the proportion of variance in the exposure variable explained by the instrumental variable in the regression model. The R2 was calculated using the formula: R2=β2(1 − EAF) × 2EAF. EAF is the frequency of mutated genes. SNPs with F statistics > 10 was defined as reliable and valid IVs.[16] The F-statistic is calculated as: F = R2(N − K − 1)/[K(1 − R2)], K is the number of SNP-exposure association, and N is the sample size for the SNP-exposure association.[17]
2.4. Mendelian randomization analysis
This study adopted 3 analytical methods of inverse variance weighting (IVW), MR Egger regression and weighted mode for analysis. The IVW is the most commonly used analytical method, which is mainly used to calculate the weighted average of the effect values of all IVs, and its estimation results and accuracy are similar to the two-stage least squares method, so the main reference is the results of the IVW analysis. The results presented as odds ratio (OR) and 95% confidence interval (CI) to estimate the causal effect of exposure on the outcome.
2.5. Bonferroni correction
This study conducted a multivariate MR analysis and therefore Bonferroni calibration was chosen to validate the results. Bonferroni calibration is a multiple comparisons calibration method which helps the researcher to control the overall error rate when conducting multiple hypothesis tests, thus making the results more reliable.
2.6. Sensitivity analysis
This study was conducted with Cochran Q statistic to test for heterogeneity. If the heterogeneity was not statistically significant (P > .05), MR analysis was performed using the fixed-effects model of IVW. Otherwise, the random effects model was used. The MR Egger intercept test and the MR residuals and MR-PRESSO test were used to detect multidimensionality and remove outliers to correct for horizontal multidimensionality. Leave-one-out analyses were used to assess whether MR results were altered by specific SNPs.
2.7. Statistical methods
All data analyses were performed using R software (version 4.3.1) and the R packages “TwosampleMR.” Differences were considered statistically significant only when the P-value < .05.[18]
3. Result
3.1. Genetic instrument variables
According to the SNP selection criteria, 158 SNPs were obtained from the LDL-C dataset, 70 SNPs from the hypertension dataset, 242 SNPs from the serum uric acid dataset, and 104 SNPs from the blood glucose dataset (Tables S1–S4, Supplemental Digital Content, http://links.lww.com/MD/O124).
3.2. Causal association between LDL cholesterol and 4 skin diseases
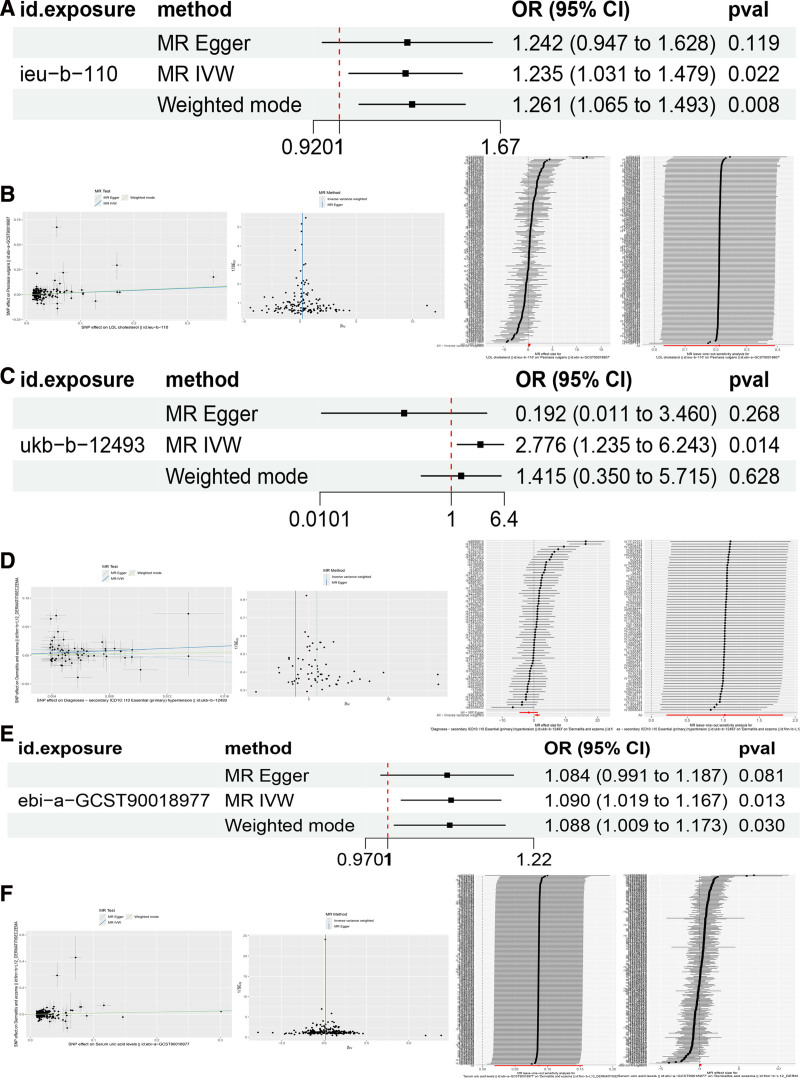
IVW analysis indicated a potential positive association between LDL-C and the risk of psoriasis (OR = 1.23, 95% CI: 1.03–1.47, P = .02). However, after Bonferroni correction, the adjusted P-value was .08 (Fig. 2A). The IVW analysis results showed no potential causal association between LDL-C and alopecia areata, dermatitis eczema, or acne (Table 2).
Figure 2.
(A) Causal association between LDL cholesterol and psoriasis. (B) Scatter plot, funnel plot, forest plot, and leave-one-out sensitivity analysis in MR analysis of LDL and psoriasis. (C) Causal association between hypertension and dermatitis eczema. (D) Scatter plot, funnel plot, forest plot, and leave-one-out sensitivity analysis in MR analysis of hypertension and dermatitis eczema. (E) Causal association between serum uric acid and dermatitis eczema. (F) Scatter plot, funnel plot, forest plot, and leave-one-out sensitivity analysis in MR analysis of serum uric acid and dermatitis eczema. LDL = low-density lipoprotein, MR = Mendelian randomization.
Table 2.
Results of Mendelian randomization analysis.
| Outcome | Exposure | SNPs | P-value | Padjust-value | or | or -lci95 | or-uci95 |
|---|---|---|---|---|---|---|---|
| LDL cholesterol | Alopecia areata | 148 | 0.36 | 1 | 1.24 | 0.77 | 1.99 |
| LDL cholesterol | Acne | 148 | 0.60 | 1 | 0.92 | 0.69 | 1.23 |
| LDL cholesterol | Dermatitis eczema | 148 | 0.92 | 1 | 0.99 | 0.92 | 1.06 |
| LDL cholesterol | Psoriasis | 148 | 0.02 | 0.08 | 1.23 | 1.03 | 1.47 |
| Hypertension | Alopecia areata | 65 | 0.37 | 1 | 8.49 | 0.07 | 914.67 |
| Hypertension | Acne | 65 | 0.23 | 0.92 | 0.26 | 0.02 | 2.40 |
| Hypertension | Dermatitis eczema | 65 | 0.01 | 0.04 | 2.77 | 1.23 | 6.24 |
| Hypertension | Psoriasis | 66 | 0.09 | 0.36 | 3.36 | 0.82 | 13.72 |
| Glucose levels | Alopecia areata | 97 | 0.80 | 1 | 1.08 | 0.56 | 2.08 |
| Glucose levels | Acne | 97 | 0.53 | 1 | 0.89 | 0.63 | 1.25 |
| Glucose levels | Dermatitis eczema | 97 | 0.10 | 0.04 | 0.92 | 0.84 | 1.01 |
| Glucose levels | Psoriasis | 102 | 0.42 | 1 | 1.08 | 0.88 | 1.33 |
| Serum uric acid levels | Alopecia areata | 228 | 0.43 | 1 | 1.19 | 0.76 | 1.85 |
| Serum uric acid levels | Acne | 228 | 0.82 | 1 | 0.97 | 0.79 | 1.21 |
| Serum uric acid levels | Dermatitis eczema | 228 | 0.01 | 0.04 | 1.09 | 1.01 | 1.16 |
| Serum uric acid levels | Psoriasis | 234 | 0.36 | 1 | 0.91 | 0.75 | 1.11 |
LDL = low-density lipoprotein.
Heterogeneity analysis for LDL-C and psoriasis showed no evidence of heterogeneity (P = .07), and no evidence of horizontal pleiotropy was detected (Egger intercept = 0.002, se = 0.005, P = .956). Both the funnel plot and leave-one-out sensitivity analysis indicated that the results are robust (Fig. 2B).
3.3. Causal association between hypertension and 4 skin diseases
VW analysis results showed that hypertension may be positively associated with the risk of dermatitis eczema (OR = 2.77, 95% CI: 1.23–6.24, P = .007). After Bonferroni correction, the adjusted P-value was .04 (Fig. 2C). Meanwhile, IVW analysis indicated no potential causal association between hypertension and alopecia areata, acne, or psoriasis (Table 2).
Heterogeneity analysis for hypertension and dermatitis eczema suggested the presence of heterogeneity (P < .05), so a random-effects model was used. However, no evidence of horizontal pleiotropy was detected (Egger_intercept = 0.015, SE = 0.008, P = .064). Both the funnel plot and leave-one-out sensitivity analysis indicated that the results are robust (Fig. 2D).
3.4. Causal association between glucose levels and 4 skin diseases
IVW analysis results showed that glucose levels are not potentially causally related to the risk of any of the 4 skin diseases (Table 2). Additionally, heterogeneity analysis for glucose levels and the 4 skin diseases indicated no presence of heterogeneity (P all > .05).
3.5. Causal association between serum uric acid levels and 4 skin diseases
IVW analysis results showed that serum uric acid levels may be positively associated with the risk of dermatitis eczema (OR = 1.09, 95% CI: 1.01–1.16, P = .007). After Bonferroni correction, the adjusted P-value was .04 (Fig. 2E). Meanwhile, IVW analysis indicated no potential causal association between serum uric acid levels and alopecia areata, acne, or psoriasis (Table 2).
Heterogeneity analysis for serum uric acid levels and dermatitis eczema showed no evidence of heterogeneity (P = .13). Additionally, no evidence of horizontal pleiotropy was detected (Egger intercept = 0.001, SE = 0.001, P = .859). Both the funnel plot and leave-one-out sensitivity analysis indicated that the results are robust (Fig. 2F).
4. Discussion
This study utilized MR study to explore potential causal association between common cardiovascular disease risk factors: LDL-C, serum uric acid, blood glucose, and hypertension, and 4 common dermatological conditions. The study found that LDL-C may be positively associated with the risk of psoriasis, although this association did not reach significance after Bonferroni correction (adjusted P-value = .08). This suggests that LDL-C levels may have an impact on psoriasis risk, but further research is needed for validation. Hypertension and serum uric acid levels were significantly associated with the risk of dermatitis eczema, with the corrected P-values remaining significant, indicating that elevated uric acid levels may increase the risk of dermatitis eczema. Meanwhile, no significant causal association were found between blood glucose levels and the 4 dermatological conditions.
Psoriasis is a chronic inflammatory skin disease, and previous studies have shown that patients with psoriasis have a higher risk of cardiovascular diseases.[19,20] This study’s finding that LDL-C levels may be associated with psoriasis risk suggests that inflammatory responses could be a common mechanism linking the 2 conditions.[21] Elevated LDL-C can lead to atherosclerosis by promoting endothelial cell activation and the release of inflammatory factors, which may exacerbate psoriasis inflammation.[22] Additionally, psoriasis is often accompanied by metabolic syndrome, including hyperlipidemia, which further supports the study’s findings.
Hypertension may increase the risk of dermatitis eczema through several mechanisms. Firstly, hypertension can induce systemic inflammation, elevating levels of inflammatory markers such as C-reactive protein and interleukin-6, which may affect skin health through various pathways.[23–25] Secondly, antihypertensive medications, such as β-blockers, have also been reported to be associated with skin conditions.[26] These findings are consistent with this study’s results, further supporting the causal association between hypertension and dermatitis eczema.
Elevated uric acid levels may contribute to dermatitis eczema through inflammation and oxidative stress.[27,28] Uric acid is a potent oxidant that can activate the NLRP3 inflammasome, leading to the release of inflammatory cytokines such as IL-1β and IL-18.[29,30] These cytokines are elevated in the skin of dermatitis eczema patients and may increase the risk of the condition by exacerbating skin barrier dysfunction and local inflammation.
However, this study has some limitations. First, the data were exclusively from European populations, which may limit the generalizability of the findings to other ethnic groups. Future studies should include diverse populations to enhance the applicability of the results. Second, while the data came from large biobanks, sample sizes in certain specific analyses may still be insufficient to detect small effects, particularly when significance was lost after Bonferroni correction.
This study underscores the potential importance of cardiovascular health management in the prevention and treatment of dermatological conditions. Clinicians should be aware of cardiovascular disease risk factors, such as LDL-C, hypertension, and elevated uric acid levels. Effective management of these factors may not only reduce cardiovascular disease risk but also help prevent and alleviate certain skin conditions. Additionally, this finding suggests that cardiovascular health factors should be considered in the comprehensive management of skin disease patients to provide a more holistic treatment approach.
5. Conclusion
This MR study explored the causal association between CVD risk factors: LDL-C, serum uric acid, blood glucose, and hypertension, and common skin diseases like psoriasis and dermatitis eczema. The findings suggest LDL-C may increase psoriasis risk, while hypertension and serum uric acid levels significantly raise the risk of dermatitis eczema. These results underscore the importance of managing CVD risk factors not only to prevent heart disease but also to potentially reduce skin disease risks. Further research is needed to confirm these findings and understand the underlying mechanisms. The study highlights the interconnectedness of cardiovascular and skin health, advocating for integrated clinical management approaches.
Author contributions
Conceptualization: Shaoyi Peng, Kaiyuan Li, Lingyu Han, Peng Liu.
Data curation: Shaoyi Peng, Kaiyuan Li, Lingyu Han, Peng Liu.
Formal analysis: Shaoyi Peng, Peng Liu.
Investigation: Shaoyi Peng.
Methodology: Shaoyi Peng, Lingyu Han.
Project administration: Peng Liu.
Software: Shaoyi Peng.
Validation: Shaoyi Peng.
Writing – original draft: Shaoyi Peng, Kaiyuan Li, Lingyu Han.
Writing – review & editing: Shaoyi Peng, Kaiyuan Li, Peng Liu.
Supplementary Material
Abbreviations:
- CI
- confidence interval
- CVD
- cardiovascular diseases
- IVs
- instrumental variables
- IVW
- inverse variance weighted
- LDL-C
- low-density lipoprotein cholesterol
- MR
- Mendelian randomization
- OR
- odds ratio
- SNPs
- single nucleotide polymorphisms
Our analysis used publicly available genome-wide association study summary statistics. No new data were collected, and no new ethical approval was required.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
Supplemental Digital Content is available for this article.
How to cite this article: Peng S, Li K, Han L, Liu P. Causal association between cardiovascular risk factors and common skin diseases: A multivariate Mendelian randomization study. Medicine 2024;103:49(e40631).
SP, KL, and LH contributed equally to this work.
Contributor Information
Shaoyi Peng, Email: 1239852850@qq.com.
Kaiyuan Li, Email: 986970919@qq.com.
Lingyu Han, Email: 2238368188@qq.com.
References
- [1].Bolling MC, Jonkman MF. Skin and heart: une liaison dangereuse. Exp Dermatol. 2009;18:658–68. [DOI] [PubMed] [Google Scholar]
- [2].Zundell MP, Woodbury MJ, Lee K, et al. Report of the Skin Research Workgroups From the IDEOM Breakout at the GRAPPA 2022 Annual Meeting. J Rheumatol. 2023;50(Suppl 2):47–50. [DOI] [PubMed] [Google Scholar]
- [3].Kraus WE, Powell KE, Haskell WL, et al. Physical activity, all-cause and cardiovascular mortality, and cardiovascular disease. Med Sci Sports Exerc. 2019;51:1270–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lim HW, Collins SAB, Resneck JS, Jr., et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958–72.e2. [DOI] [PubMed] [Google Scholar]
- [5].Zhang ZY, Jian ZY, Tang Y, Li W. The relationship between blood lipid and risk of psoriasis: univariable and multivariable Mendelian randomization analysis. Front Immunol. 2023;14:1174998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ben Abdallah H, Vestergaard C. Atopic dermatitis, hypertension and cardiovascular disease. Br J Dermatol. 2022;186:203–4. [DOI] [PubMed] [Google Scholar]
- [7].Verma S, Mazer CD, Connelly KA. Inflammation and cholesterol at the crossroads of vascular risk. Cell Metab. 2023;35:1095–8. [DOI] [PubMed] [Google Scholar]
- [8].Masson W, Lobo M, Molinero G. Psoriasis and cardiovascular risk: a comprehensive review. Adv Ther. 2020;37:2017–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kanellis J, Kang DH. Uric acid as a mediator of endothelial dysfunction, inflammation, and vascular disease. Semin Nephrol. 2005;25:39–42. [DOI] [PubMed] [Google Scholar]
- [10].Jin M, Yang F, Yang I, et al. Uric acid, hyperuricemia and vascular diseases. Front Biosci (Landmark Ed). 2012;17:656–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gherghina ME, Peride I, Tiglis M, Neagu TP, Niculae A, Checherita IA. Uric acid and oxidative stress-relationship with cardiovascular, metabolic, and renal impairment. Int J Mol Sci . 2022;23:3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Larsson SC, Butterworth AS, Burgess S. Mendelian randomization for cardiovascular diseases: principles and applications. Eur Heart J. 2023;44:4913–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sekula P, Del Greco MF, Pattaro C, Kottgen A. Mendelian randomization as an approach to assess causality using observational data. J Am Soc Nephrol. 2016;27:3253–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomization: the STROBE-MR Statement. JAMA. 2021;326:1614–21. [DOI] [PubMed] [Google Scholar]
- [15].Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the reporting of observational studies in epidemiology using Mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ. 2021;375:n2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. 2016;45:1961–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Burgess S, Thompson SG, Collaboration CCG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. 2011;40:755–64. [DOI] [PubMed] [Google Scholar]
- [18].Chen L, Yang H, Li H, He C, Yang L, Lv G. Insights into modifiable risk factors of cholelithiasis: a Mendelian randomization study. Hepatology. 2022;75:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang L, Wang Y, Qiu L, Wu J. Psoriasis and cardiovascular disease risk in European and East Asian populations: evidence from meta-analysis and Mendelian randomization analysis. BMC Med. 2022;20:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Weber B, Merola JF, Husni ME, Di Carli M, Berger JS, Garshick MS. Psoriasis and cardiovascular disease: novel mechanisms and evolving therapeutics. Curr Atheroscler Rep. 2021;23:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Matwiejuk M, Mysliwiec H, Jakubowicz-Zalewska O, Chabowski A, Flisiak I. Effects of hypolipidemic drugs on psoriasis. Metabolites. 2023;13:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Pietrzak A, Chabros P, Grywalska E, et al. Serum concentration of interleukin 6 is related to inflammation and dyslipidemia in patients with psoriasis. Postepy Dermatol Alergol. 2020;37:41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang Z, Zhao L, Zhou X, Meng X, Zhou X. Role of inflammation, immunity, and oxidative stress in hypertension: new insights and potential therapeutic targets. Front Immunol. 2022;13:1098725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Guzik TJ, Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. 2017;70:660–7. [DOI] [PubMed] [Google Scholar]
- [25].Xiao L, Harrison DG. Inflammation in hypertension. Can J Cardiol. 2020;36:635–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ye M, Chan LN, Douglas I, Margolis DJ, Langan SM, Abuabara K. Antihypertensive medications and eczematous dermatitis in older adults. JAMA Dermatol. 2024;160:710–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Strazzullo P, Puig JG. Uric acid and oxidative stress: relative impact on cardiovascular risk? Nutr Metab Cardiovasc Dis. 2007;17:409–14. [DOI] [PubMed] [Google Scholar]
- [28].Narang RK, Dalbeth N. Pathophysiology of Gout. Semin Nephrol. 2020;40:550–63. [DOI] [PubMed] [Google Scholar]
- [29].Shen S, He F, Cheng C, Xu B, Sheng J. Uric acid aggravates myocardial ischemia-reperfusion injury via ROS/NLRP3 pyroptosis pathway. Biomed Pharmacother. 2021;133:110990. [DOI] [PubMed] [Google Scholar]
- [30].Wu M, Hu X, Lu T, Liu C, Lu H. Uric acid is independently associated with interleukin-1beta levels in tear fluid of hyperuricemia and gout patients. Immun Inflamm Dis. 2023;11:e805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.