Abstract
Systemic inflammatory indices, originally developed to predict the prognosis of cancer patients, have found increasing application in various medical areas, including cardiovascular research. This study aimed to investigate the relationship between ascending aortic dilatation in bicuspid aortic valve patients and systemic inflammatory indices. This retrospective cross-sectional study included 122 patients with bicuspid aortic valves. These patients were divided into 2 groups based on the presence or absence of dilatation according to ascending aorta z-scores. Complete blood counts were analyzed, focusing on leukocyte, neutrophil, lymphocyte, monocyte, and platelet counts. Additionally, systemic inflammatory indices including neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio (PLR), Systemic Immune-Inflammation Index (SII), Systemic Inflammatory Response Index (SIRI), and monocyte-to-lymphocyte ratio (MLR) and pan-immune-inflammation value (PIV) were calculated from these parameters. MLR, SIRI, and PIV demonstrated acceptable diagnostic power in detecting ascending aortic dilatation in bicuspid aortic valve patients, with area under the curve (AUC) values of 0.709, 0.741, and 0.779, respectively. PLR and SII exhibited fair diagnostic power, with AUC values of 0.673 and 0.688, respectively. According to the receiver operating characteristic analysis, PIV had the highest AUC value of 0.779 (95% confidence interval [CI] = 0.69–0.86), with a sensitivity of 70.9% and specificity of 70.8% at a cutoff value of 224.93. A relationship exists between PLR, MLR, SII, SIRI, PIV, and ascending aorta dilatation in pediatric patients with bicuspid aortic valves. These findings suggest that inflammation may play a role in the dilatation of the ascending aorta in bicuspid aortic valve patients.
Keywords: ascending aortic dilation, bicuspid aortic valve, pediatric cardiology, PIV, SII, SIRI, systemic inflammatory indices
1. Introduction
Bicuspid aortic valve (BAV) is one of the most common congenital heart diseases, affecting 0.5% to 2% of the general population.[1–3] Both the ascending aorta and the aortic valve develop from neural crest cells, but they have a common embryonic origin. Consequently, BAV not only causes problems at the valve level but also leads to dilatation, particularly of the ascending aorta, as well as aortopathy, affecting other areas, such as coarctation in the aortic arch.[4–8]
Dilatation of the ascending aorta is observed in more than one-third of patients with BAV.[9] If left unchecked, progressive dilatation may lead to aneurysm formation and potentially life-threatening dissection.[10,11] While the pathogenesis of BAV is not fully understood, ascending aortic dilatation occurs as a result of various histopathological changes. These changes include medial degeneration in the aortic wall, increased matrix metalloproteinase activity, decreased fibrillin-1 levels, genetic reasons, and hemodynamic stress.[12,13] The morphology of the bicuspid valve and the degree of stenosis and insufficiency also contribute to increased hemodynamic stress on the aortic root and ascending aorta dilatation.[14–16] Notably, even with a functionally normal valve, BAV patients can exhibit larger ascending aortic dilatations compared to control groups.[10]
Patients with a dilated ascending aorta and BAV undergo periodic follow-up using imaging tools such as echocardiography, computed tomography, and magnetic resonance imaging. Although surgical operations for isolated ascending aortic aneurysms secondary to BAV are uncommon in pediatric populations, the condition causes significant concern for families and increases physician visits.[17] The medical community continually seeks biomarkers or indices to predict prognosis in these patient groups.
Recent studies have revealed a relationship between systemic inflammatory indices and vascular remodeling, suggesting that inflammation may play a role in ascending aortic dilatation.[18–22] The main parameters investigated in the literature include the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), Systemic Immune-Inflammation Index (SII), Systemic Inflammatory Response Index (SIRI), and monocyte-to-lymphocyte ratio (MLR). In addition to these, the pan-immune-inflammation value (PIV) has gained attention in both cardiovascular and other fields. These parameters are valuable due to their accessibility, ease of calculation, and cost-effectiveness, as they can be derived from a single complete blood count (CBC) sample.[18–22]
To date, no published study has investigated the relationship between ascending aortic dilatation in BAV patients and the new systemic inflammation indices SII, SIRI, and PIV. Accordingly, in this study, we aimed to investigate the relationship between these indices and ascending aortic dilatation in patients with BAV.
2. Materials and methods
2.1. Patient population
This study was conducted using a descriptive cross-sectional method with a retrospective design at the Pediatric Cardiology Clinic of Giresun Women’s and Children’s Health Education and Research Hospital between June 2022 and August 2024. All patients with BAV whose radiological, clinical, and demographic data were available were included in the study.
The exclusion criteria are as follows:
Patients with a history of acute infection within the last week,
Complex cardiac disease,
Moderate or higher aortic stenosis,
A history of chronic inflammatory disease,
Suspicious findings on genetic tests or physical exams and a family history suggesting a connective tissue disease, autoimmune diseases,
A history of hematological/oncological, renal and liver disease, those undergoing corticosteroid treatment, and
Older than 18 years old,
Those whose data were not available were excluded from the study.
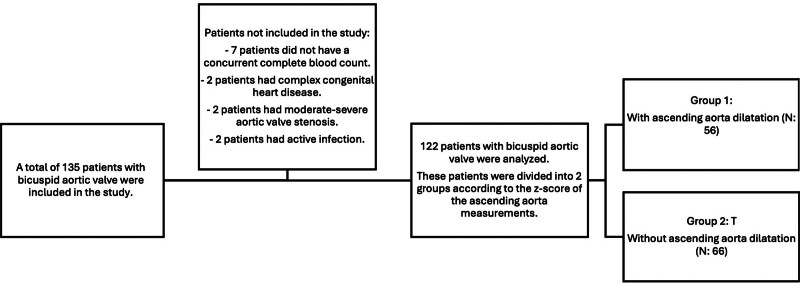
A total of 135 pediatric patients under 18 years of age with bicuspid aortic valve were included in the study, considering the previously mentioned exclusion criteria. However, 13 patients were excluded due to the absence of simultaneous CBC results (7 patients), the presence of complex heart disease (2 patients), elevated acute phase reactants (2 patients), and moderate or higher aortic stenosis (2 patients). Consequently, data from 122 patients were analyzed (Fig. 1). Ascending aorta measurements with z-scores of 2 were classified as indicating dilatation. Based on these criteria, patients with BAV were divided into 2 groups: those with ascending aorta dilatation (Group 1, n: 56) and those without (Group 2, n: 66) (Fig. 1).
Figure 1.
Flowchart of included and excluded patients in the study.
The study was approved by the Giresun Education and Research Hospital Ethics Committee (decision number 06, dated October 09, 2023). Written informed consent was obtained from all patients participating in the study.
2.2. Echocardiography
Echocardiographic examinations were performed using a Philips Affiniti 50 (Philips Healthcare, Best, The Netherlands) echocardiography device. Measurements were taken from parasternal long-axis views to assess the diameters of the aortic root, sinuses of Valsalva, sinotubular junction, and ascending aorta. All measurements were obtained at their maximum dimension during peak flow in the mid-systolic phase using the inner edge to inner edge method.[23] To ensure accuracy, each measurement was repeated 3 times, and the average of these measurements was used for analysis. The echocardiographic data were the normalized for z-score calculations, adjusting for the patients’ height and body surface area.
In addition to these measurements, left ventricular end-diastolic diameter, left ventricular end-systolic diameter, and shortening fraction (%) were evaluated. Left ventricular end-diastolic diameter and left ventricular end-systolic diameter were measured in millimeters (mm) during diastole and systole, respectively, while shortening fraction was calculated as a percentage to assess left ventricular systolic function. All parameters were based on “Guidelines for Performing a Comprehensive Pediatric Transthoracic Echocardiogram: Recommendations From the American Society of Echocardiography.”[23]
The classification of BAV was performed using the Sievers classification system, which categorizes BAV based on the number of raphes observed: type 0 (no raphe), type 1 (1 raphe), and type 2 (2 raphes).[24]
2.3. Biochemical analysis
Blood samples were collected in K2-EDTA (ethylenediaminetetraacetic acid) tubes, and CBC parameters were analyzed using the XN1000 (Sysmex Co., Kobe, Japan) device. The following equations were used to calculate inflammatory indices: NLR = (neutrophil count/lymphocyte count), MLR = (monocyte count/lymphocyte count), PLR = (platelet count/lymphocyte count), SII = (platelet count * neutrophil count/lymphocyte count), SIRI = (monocyte count * neutrophil count/lymphocyte count), PIV = (platelet count * monocyte count * neutrophil count)/lymphocyte count).
2.4. Statistical analysis
All statistical analyses were performed using SPSS version 26.0 (IBM Corp, Armonk, NY). The Kolmogorov–Smirnov test was used to assess data normality. Continuous data with a normal distribution (parametric) are reported as mean (standard deviation), while non-normally distributed data (nonparametric) are reported as median (25th–75th percentile). Categorical variables are presented as frequency and percentage values. The Chi-square test was used for discrete data. To compare demographic, echocardiographic, and biochemical data of patients with and without ascending aorta dilatation in the bicuspid aorta, the Mann–Whitney U test was used for nonparametric variables and Student t test for parametric variables. Receiver operating characteristic (ROC) analysis was used to evaluate the diagnostic performance of the tests, providing area under the curve (AUC), sensitivity and specificity values. An AUC > 0.9 was interpreted as outstanding, 0.8 to 0.9 as excellent, 0.7 to 0.8 as acceptable, 0.6 to 0.7 as moderate, and 0.5 to 0.6 as poor. Statistical significance was set at P < .05.
3. Results
The median ages (25th–75th percentile) of patients in Group 1 and Group 2 were 9 years (5–15) and 8 years (4–12), respectively. There was no statistically significant difference between the demographic data of the 2 groups (Table 1).
Table 1.
Comparison of demographic data of the participants.
| Group 1 | Group 2 | P-value* | ||
|---|---|---|---|---|
| Age (year) | 9 (5–15) | 8 (4–12) | .14 | |
| Gender n (%) | Female | 24 (42.8%) | 29 (43.9%) | .09 |
| Male | 32 (57.2%) | 37 (56.1%) | ||
| Weight (kg) | 33 (17.40–53.25) | 31 (15.75–50.50) | .43 | |
| Height (m) | 140.5 (110–160) | 135 (98.5–159) | .26 | |
| Body mass index (kg/m2) | 17.26 (15.16–21.30) | 17.75 (15.51–21.48) | .67 | |
P < .05 statistical significance. The data are presented as a number (percentage %) or median (25th–75th percentile). The Mann–Whitney U test was utilized for continuous variables, while the Chi-square test was used for discrete variables.
The distribution of patients according to bicuspid valve types and other echocardiographic data is presented in Table 2. Among the echocardiographic parameters, only the ascending aorta z-score was significantly higher in Group 1 than in Group 2 (P = .006). No statistically significant differences were found between the groups in other echocardiographic parameters. The most common BAV morphology in Groups 1 and 2 was type 1 R/L (53.5% and 59.1%, respectively).
Table 2.
Comparison of echocardiography data of groups.
| Group 1 | Group 2 | P-value | ||
|---|---|---|---|---|
| Bicuspid aortic types n (%) | Type 0 | 7 (12.5%) | 15 (22.7%) | .12 |
| Type 1, R/L | 30 (53.5%) | 39 (59.1%) | ||
| Type 1, R/N | 13 (23.2%) | 10 (15.2%) | ||
| Type 1, L/N | 1 (1.8%) | 1 (1.5%) | ||
| Type 2 | 2 (3.6%) | 1 (1.5%) | ||
| Unclassified types | 3 (5.4%) | 0 | ||
| Aortic anulus z-score | 1.30 (0.55–1.73) | 1.31 (0.10–14.32) | .26 | |
| SVS z-score | 0.79 (0.20–1.36) | 1.03 (0.25–20.15) | .14 | |
| STJ z-score | 1.05 (0.60–1.63) | 0.47 (0.10–16.75) | .98 | |
| Ascending aorta z-score | 2.80 (2.22–3.34) | 1.08 (0.42–1.57) | .006* | |
| LVEDD (mm) | 41 (34.5–48.1) | 39.5 (34.5–45.75) | .15 | |
| LVEDS (mm) | 25 (21.5–30.5) | 24.5 (19.25–27.5) | .16 | |
| SF (%) | 38 (36–40) | 38 (35–39.75) | .51 | |
LVEDD = left ventricular end-diastolic diameter, LVESD = left ventricular end-systolic diameter, SF = shortening fraction, STJ = sinotubular junction, SVS = sinüs valsalva, type 1, L/N = raphe between the left and noncoronary sinuses, type 1, R/L = raphe between the right and left coronary sinuses, type 1, R/N = raphe between the right and non-coronary sinuses, type 2 = valve with 2 raphes.
P < .05 statistical significance. The data are given as a number (percentage %) or as the median (25th–75th percentile). The Chi-square test was utilized for discrete variables, and the Mann–Whitney U test was used for continuous variables.
Table 3 presents the comparison of laboratory data between the groups. Group 1 showed significantly higher levels of white blood cell count, monocyte count, platelet count, PLR, MLR, SII, SIRI, and PIV compared to Group 2. Conversely, lymphocyte count was significantly lower in Group 1 (P = .03). No statistically significant differences between the 2 groups in terms of neutrophil count, MPV, CRP, and NLR.
Table 3.
Comparison of laboratory data of the groups.
| Group 1 | Group 2 | P-value | |
|---|---|---|---|
| White blood cell count (109/L) | 7.99 (1.44) | 7.18 (1.66) | .02 * |
| Neutrophil count (109/L) | 4.01 (1.15) | 3.82 (1.21) | .07* |
| Lymphocyte count (109/L) | 2.49 (0.89) | 2.90 (1.13) | .03 * |
| Monocyte count (109/L) | 0.65 (0.2) | 0.46 (0.18) | <.001 * |
| Platelet count (109/L) | 338.81 (84.4) | 263.35 (69.18) | <.001 * |
| MPV (fL) | 8.90 (0.86) | 8.62 (0.85) | .08* |
| CRP (mg/L) | 0.30 (0.20–0.50) | 0.40 (0.20–1.20) | .38† |
| NLR | 1.41 (1.17–1.88) | 1.38 (1.13–1.57) | .06† |
| PLR | 143.7 (117.6–183.7) | 94.26 (78.06–119.1) | .006 † |
| MLR | 0.25 (0.20–0.34) | 0.15 (0.12–0.22) | <.001 † |
| SII | 537 (383–676) | 381 (295–505) | <.001 † |
| SIRI | 0.95 (0.70–1.24) | 0.61 (0.47–0.90) | <.001 † |
| PIV | 289.9 (216.3–409.4) | 163.6 (117.8–239.1) | <.001 † |
MLR = monocyte/lymphocyte ratio, MPV = mean platelet volüme, NLR = neutrophil/lymphocyte ratio, PIV = pan-immune inflammation value, PLR = platelet/lymphocyte ratio, SII = systemic immune inflammatory index, SIRI = systemic inflammatory response index.
Parametric data are given as mean (SD). Nonparametric data are given as median (25-75th percentile). Bold numbers are statistical significance.
Student’s t test.
Mann–Whitney U test (P < .05 significance).
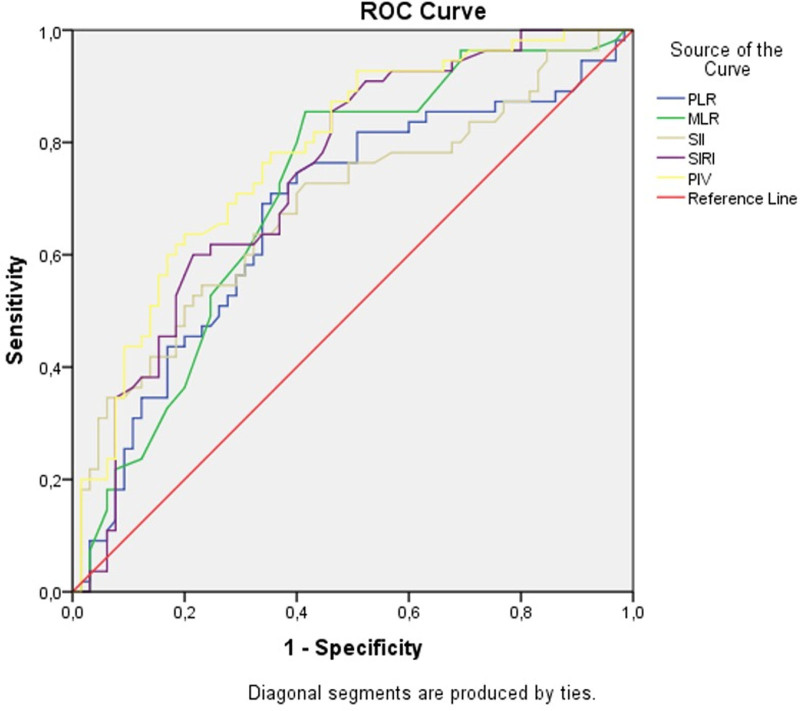
The ROC curve and diagnostic accuracy of the new inflammatory parameters in predicting ascending aortic dilatation in patients with BAV are presented in Table 4 and Figure 2. MLR, SIRI, and PIV demonstrated acceptable diagnostic power in detecting ascending aortic dilatation in patients with BAV, with AUC values of 0.709, 0.741, and 0.779, respectively. PLR and SII showed fair diagnostic power, with AUC values of 0.673 and 0.688, respectively. ROC analysis revealed that PIV exhibited the highest diagnostic accuracy, with an AUC value of 0.779 (95% confidence interval [CI] = 0.69–0.86). At a cutoff value of 224.93, PIV demonstrated a sensitivity of 70.9% and specificity of 70.8% (Table 4).
Table 4.
Diagnostic accuracy of new inflammatory parameters to predicting ascending aortic dilatation in patients with bicuspid aorta.
| Parameters | AUC | Cutoff | Sensitivity % | Specificity % | %95 CI | P-value |
|---|---|---|---|---|---|---|
| PLR | 0.673 | >117.08 | 69.1 | 66.2 | (0.57–0.77) | <.001 |
| MLR | 0.709 | >0.19 | 72.7 | 69.2 | (0.61–0.80) | <.001 |
| SII | 0.688 | >435.5 | 67.3 | 63.1 | (0.59–0.78) | <.001 |
| SIRI | 0.741 | >0.715 | 74.5 | 61.5 | (0.65–0.82) | <.001 |
| PIV | 0.779 | >224.93 | 70.9 | 70.8 | (0.69–0.86) | <.001 |
AUC = area under curve, CI = confidence interval; see Table 3.
Figure 2.
The ROC curve of new inflammatory parameters for predicting ascending aortic dilatation in patients with bicuspid aorta. See Table 3. ROC = receiver operating characteristic.
4. Discussion
In our study, we found that MLR, SIRI, and PIV were the most significant systemic inflammatory indices in predicting ascending aortic dilatation in patients with BAV. BAV is one of the most common congenital heart diseases, affecting 0.5% to 2% of the population.[1–3,25,26] It not only causes problems at the valve level but also impacts other parts of the aorta, particularly the ascending aorta. Approximately 50% of patients with a bicuspid aorta experience dilatation (z-score > 2 SD).[17] The pathogenesis of ascending aorta dilatation in BAV patients is not fully understood. Factors such as genetic conditions (Marfan syndrome and Loeys–Dietz syndrome) and hemodynamic stress on the aortic wall due to blood flow through the bicuspid valve are known to contribute to the development of ascending aortic dilatation.[27] Additionally, some studies suggest that systemic and localized inflammation may play a role in the pathophysiology of ascending aortic dilatation in patients with BAV.[18–22,28]
Systemic inflammatory indices, calculated using the neutrophil, lymphocyte, monocyte, and platelet counts from routine CBCs, provide valuable information about inflammation. Initially used to predict survival and recurrence in cancer patients, these indices are now applied to a wide range of diseases, including cardiovascular diseases.[29] For example, high SII and SIRI values have been associated with increased mortality in patients with osteoarthritis,[29] a higher risk of stroke,[30] as well as cardiovascular and all-cause mortality.[30–35] Our study also suggests a close relationship between these inflammatory indices and ascending aorta dilation in BAV patients.
To the best of our knowledge, our study is the first to investigate the effects of PLR, SII, SIRI, and PIV on ascending aorta dilation in BAV patients, based on an extensive literature review. According to ROC analysis, PIV demonstrated the highest AUC value of 0.779 (95% CI = 0.69–0.86), with a sensitivity of 70.9% and specificity of 70.8% at a cutoff value of 224.93. Thus, PIV was identified as the parameter with the highest AUC value for predicting ascending aorta dilation in patients with BAV.
In the study conducted by Aslan et al, white blood cell count and NLR were found to be significantly higher in the group with ascending aorta dilation, consistent with our findings. However, while they found a significantly higher neutrophil count in the group with dilatation, we did not observe a significant difference between the groups.[22] In contrast, another study found no significant differences was found between groups in terms of leukocyte, lymphocyte, neutrophil counts, and NLR.[19]
Another study reported a significant difference in CRP and monocyte counts related to dilatation of the ascending aorta in BAV patients, whereas our study revealed a similar significant difference in monocyte counts but not in CRP values.[20] We believe that the lack of a significant difference in CRP values in our study may be due to the exclusion of patients with acute infections.
In the study by Chen et al, which examined the impact of inflammatory biomarkers on the development of poststenotic ascending aortic dilatation in patients with aortic stenosis, a significant increase in monocytes was observed in the dilation group, similar to our study. The authors also found a significantly low lymphocyte/monocyte ratio.[21] Since we formulated this parameter in the opposite way (MLR, monocyte/lymphocyte ratio), we found it to be significantly higher in the group with ascending aorta dilatation. Additionally, their study found no significant differences between the groups in terms of leukocyte, neutrophil, and CRP values.[21]
5. Study limitations
Our study was conducted in a single center with a small population, which limits the generalizability of the results. Another limitation is that the echocardiography values were measured by a single physician due to being in a peripheral health center. While we examined the relationship between ascending aortic dilatation in patients with BAV and systemic inflammatory indices, the cause–effect relationship was not investigated. In addition, the fact that there were no patients who underwent aortic surgery due to ascending aortic dilatation indicates that our follow-up period was short, and our center was not an advanced center. Future research should include studies on patients with ascending aortic dilatation and tricuspid aortic valve to better understand the relationship between systemic inflammatory indices and aortopathy.
6. Conclusion
In conclusion, we identified a positive relationship between systemic inflammatory indices: MLR, PLR, SII, SIRI, and PIV, and ascending aortic dilation in patients with BAV. To the best of our knowledge, this is the first study to explore this relationship using the SII, SIRI, and PIV indices. We found a positive relationship between patients with ascending aortic dilatation and BAV and high SII, SIRI, and PIV and high z-scores. Although these indices do not establish a cause-and-effect relationship, they offer a practical and cost-effective means of predicting ascending aortic dilatation in patients with BAV. Consequently, they may contribute to a better understanding of the pathophysiology of this condition, which remains unclear. These indices could also aid in the effective monitoring of at-risk populations and offer new avenues for early intervention strategies. However, larger-scale studies are necessary to generalize these findings across the entire patient population.
Acknowledgments
We would like to thank to Professor Dr Murat Usta, Chief of the Department of Biochemistry at Giresun Training and Research Hospital, for his supervision of the statistical analysis. We also thanks to Mustafa Altun for his significant contributions to data collection. Consent has been obtained from the individuals involved for the inclusion of their names in this manuscript, acknowledging their respective contributions to the study.
Author contributions
Conceptualization: Bekir Yükcü.
Data curation: Bekir Yükcü, Hilmi Furkan Arslan.
Formal analysis: Bekir Yükcü, Hilmi Furkan Arslan.
Investigation: Bekir Yükcü.
Methodology: Bekir Yükcü, Hilmi Furkan Arslan.
Project administration: Bekir Yükcü.
Resources: Bekir Yükcü, Hilmi Furkan Arslan.
Supervision: Bekir Yükcü.
Validation: Bekir Yükcü.
Visualization: Bekir Yükcü.
Writing – original draft: Bekir Yükcü.
Writing – review & editing: Bekir Yükcü, Hilmi Furkan Arslan.
Abbreviations:
- AUC
- area under the curve
- BAV
- bicuspid aortic valve
- CBC
- complete blood count
- MLR
- monocyte-to-lymphocyte ratio
- NLR
- neutrophil-to-lymphocyte ratio
- PIV
- pan-immune-inflammation value
- PLR
- platelet-to-lymphocyte ratio
- ROC
- receiver operating characteristic
- SII
- Systemic Immune-Inflammation Index
- SIRI
- Systemic Inflammatory Response Index
The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Institutional Ethics Committee. During data collection and processing, the protocols were approved by the Giresun University Ethical Committee (decision number 06, dated October 09, 2023).
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Yükcü B, Arslan HF. New systemic inflammatory indices as predictors of ascending aortic dilation in children with bicuspid aortic valve: A retrospective cross-sectional study. Medicine 2024;103:49(e40904).
References
- [1].Roberts WC. The congenitally bicuspid aortic valve: a study of 85 autopsy cases. Am J Cardiol. 1970;26:72–83. [DOI] [PubMed] [Google Scholar]
- [2].Steinberger J, Moller JH, Berry JM, Sinaiko AR. Echocardiographic diagnosis of heart disease in apparently healthy adolescents. Pediatrics. 2000;105:815–8. [DOI] [PubMed] [Google Scholar]
- [3].Basso C, Boschello M, Perrone C, et al. An echocardiographic survey of primary school children for bicuspid aortic valve. Am J Cardiol. 2004;93:661–3. [DOI] [PubMed] [Google Scholar]
- [4].Kappetein A, Gittenberger-de Groot A, Zwinderman A, Rohmer J, Poelmann R, Huysmans H. The neural crest as a possible pathogenetic factor in coarctation of the aorta and bicuspid aortic valve. J Thorac Cardiovasc Surg. 1991;102:830–6. [PubMed] [Google Scholar]
- [5].Kirby ML, Waldo KL. Role of neural crest in congenital heart disease. Circulation. 1990;82:332–40. [DOI] [PubMed] [Google Scholar]
- [6].Ausoni S, Sartore S. Cell lineages and tissue boundaries in cardiac arterial and venous poles: developmental patterns, animal models, and implications for congenital vascular diseases. Arterioscler Thromb Vasc Biol. 2001;21:312–20. [DOI] [PubMed] [Google Scholar]
- [7].Morrison-Graham K, Schatteman GC, Bork T, Bowen-Pope DF, Weston JA. A PDGF receptor mutation in the mouse (Patch) perturbs the development of a non-neuronal subset of neural crest-derived cells. Development. 1992;115:133–42. [DOI] [PubMed] [Google Scholar]
- [8].Becker AE, Becker MJ, Edwards JE. Anomalies associated with coarctation of aorta particular reference to infancy. Circulation. 1970;41:1067–75. [DOI] [PubMed] [Google Scholar]
- [9].Michelena HI, Desjardins VA, Avierinos J-F, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation. 2008;117:2776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Beroukhim RS, Kruzick TL, Taylor AL, Gao D, Yetman AT. Progression of aortic dilation in children with a functionally normal bicuspid aortic valve. Am J Cardiol. 2006;98:828–30. [DOI] [PubMed] [Google Scholar]
- [11].Warren AE, Boyd ML, O’Connell C, Dodds L. Dilatation of the ascending aorta in paediatric patients with bicuspid aortic valve: frequency, rate of progression and risk factors. Heart. 2006;92:1496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nataatmadja M, West M, West J, et al. Abnormal extracellular matrix protein transport associated with increased apoptosis of vascular smooth muscle cells in Marfan syndrome and bicuspid aortic valve thoracic aortic aneurysm. Circulation. 2003;108(10_suppl_1):II-329–34. [DOI] [PubMed] [Google Scholar]
- [13].Tadros TM, Klein MD, Shapira OM. Ascending aortic dilatation associated with bicuspid aortic valve: pathophysiology, molecular biology, and clinical implications. Circulation. 2009;119:880–90. [DOI] [PubMed] [Google Scholar]
- [14].Keane MG, Wiegers SE, Plappert T, Pochettino A, Bavaria JE, Sutton MGSJ. Bicuspid aortic valves are associated with aortic dilatation out of proportion to coexistent valvular lesions. Circulation. 2000;102(suppl_3):III–35. [DOI] [PubMed] [Google Scholar]
- [15].Novaro GM, Tiong IY, Pearce GL, Grimm RA, Smedira N, Griffin BP. Features and predictors of ascending aortic dilatation in association with a congenital bicuspid aortic valve. Am J Cardiol. 2003;92:99–101. [DOI] [PubMed] [Google Scholar]
- [16].Bauer M, Siniawski H, Pasic M, Schaumann B, Hetzer R. Different hemodynamic stress of the ascending aorta wall in patients with bicuspid and tricuspid aortic valve. J Card Surg. 2006;21:218–20. [DOI] [PubMed] [Google Scholar]
- [17].Morris SA, Flyer JN, Yetman AT, et al. Cardiovascular management of aortopathy in children: a scientific statement from the American Heart Association. Circulation. 2024;150:228–54. [DOI] [PubMed] [Google Scholar]
- [18].Grewal N, Franken R, Mulder BJ, et al. Histopathology of aortic complications in bicuspid aortic valve versus Marfan syndrome: relevance for therapy? Heart Vessels. 2016;31:795–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kasapkara HA, Aslan AN, Ayhan H, et al. Higher neutrophil to lymphocyte ratio is related to a lower ejection fraction in bicuspid aortic valve patients. Turk J Med Sci. 2016;46:1144–50. [DOI] [PubMed] [Google Scholar]
- [20].Acar B, Yayla C, Gul M, et al. Monocyte-to-HDL-cholesterol ratio is associated with ascending aorta dilatation in patients with bicuspid aortic valve. Afr Health Sci. 2021;21:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen SH, Wu ZZ, Yun Y, et al. Lymphocyte-to-monocyte ratio associated with severe post-stenotic aortic dilation in a case–control study. BMC Cardiovasc Disord. 2022;22:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Aslan AN, Baştuğ S, Çelik MC, et al. Relation of red cell distribution width with ascending aortic diameter in bicuspid aortic valve patients. Sakarya Med J. 2018;8:327–35. [Google Scholar]
- [23].Lopez L, Saurers DL, Barker PC, et al. Guidelines for performing a comprehensive pediatric transthoracic echocardiogram: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2024;37:119–70. [DOI] [PubMed] [Google Scholar]
- [24].Sievers H-H, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007;133:1226–33. [DOI] [PubMed] [Google Scholar]
- [25].Beckman JA, Members WC. 2022 ACC/AHA guideline for the diagnosis and management of aortic disease: a report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022;146:e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Erbel R, Aboyans V, Boileau C, et al. ESC guidelines on the diagnosis and treatment of aortic diseases. Kardiol Pol (Pol Heart J). 2014. 2014;72:1169–252. [DOI] [PubMed] [Google Scholar]
- [27].Fedak PW, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation. 2002;106:900–4. [DOI] [PubMed] [Google Scholar]
- [28].Artemiou P, Charokopos N, Rouska E, et al. C-reactive protein/interleukin-6 ratio as marker of the size of the uncomplicated thoracic aortic aneurysms. Interact Cardiovasc Thorac Surg. 2012;15:871–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhou E, Wu J, Zhou X, Yin Y. Systemic inflammatory biomarkers are novel predictors of all-cause and cardiovascular mortality in individuals with osteoarthritis: a prospective cohort study using data from the NHANES. BMC Public Health. 2024;24:1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Zhang YH, Xing ZK, Zhou KC, Jiang SH. The predictive role of systemic inflammation response index (SIRI) in the prognosis of stroke patients. Clin Interv Aging. 2021;Volume 16:1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cao LX, Liu XY, Sun TT, et al. Predictive and diagnostic values of systemic inflammatory indices in bronchopulmonary dysplasia. Children-Basel. 2024;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sen F, Kurtul A, Bekler O. Pan-immune-inflammation value is independently correlated to impaired coronary flow after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2024;211:153–9. [DOI] [PubMed] [Google Scholar]
- [33].Murat B, Murat S, Altinbas ME, et al. Association of pan immune-inflammation value with long term outcomes of acute decompensated heart failure. Arq Bras Cardiol. 2024;121:e20230817. [DOI] [PubMed] [Google Scholar]
- [34].Menyhart O, Fekete JT, Győrffy B. Inflammation and colorectal cancer: a meta-analysis of the prognostic significance of the systemic immune–inflammation index (SII) and the systemic inflammation response index (SIRI). Int J Mol Sci . 2024;25:8441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Xia YY, Xia CL, Wu LD, Li Z, Li H, Zhang JX. Systemic immune inflammation index (SII), system inflammation response index (SIRI) and risk of all-cause mortality and cardiovascular mortality: a 20-year follow-up cohort study of 42,875 US adults. J Clin Med. 2023;12:1128. [DOI] [PMC free article] [PubMed] [Google Scholar]