Abstract
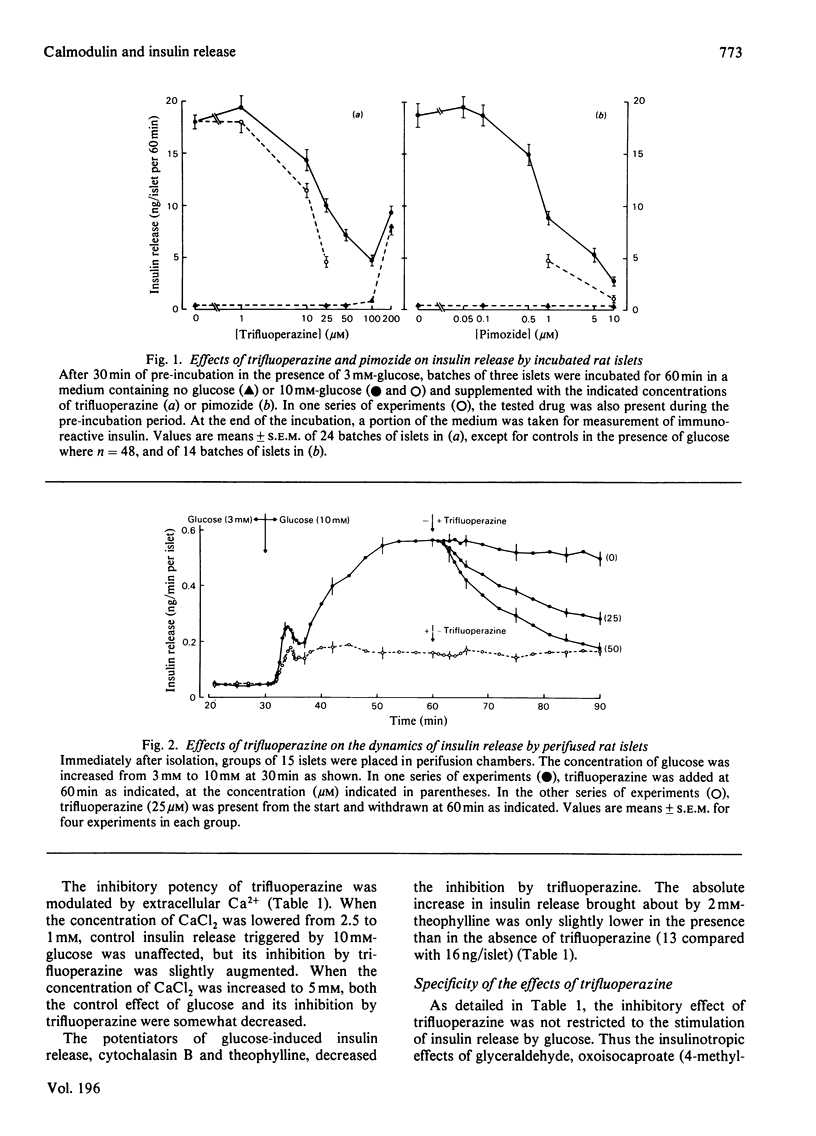
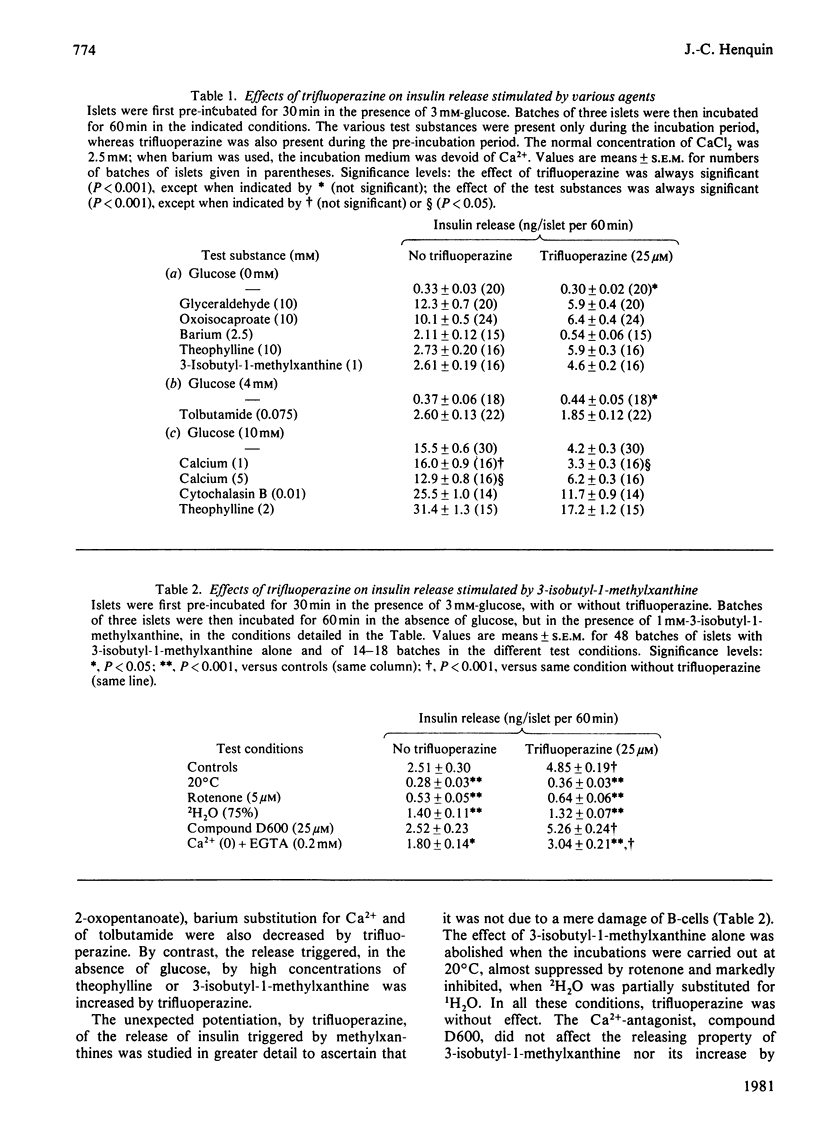
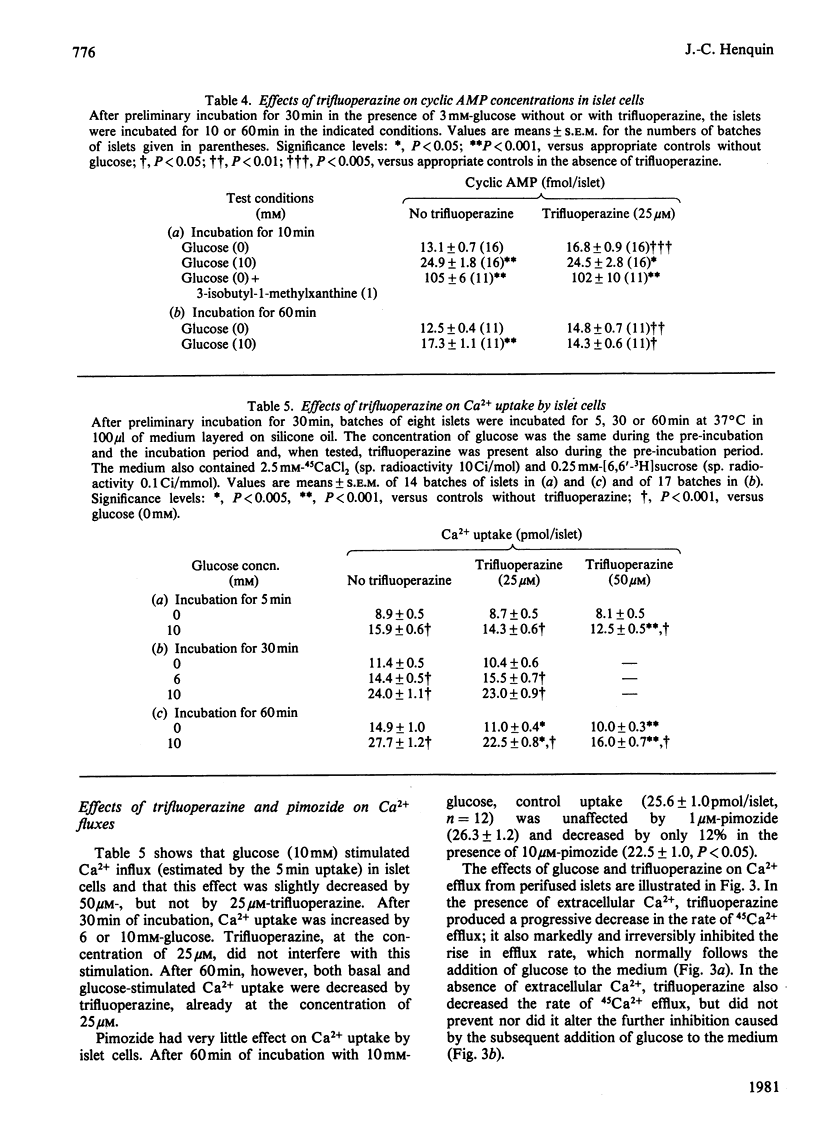
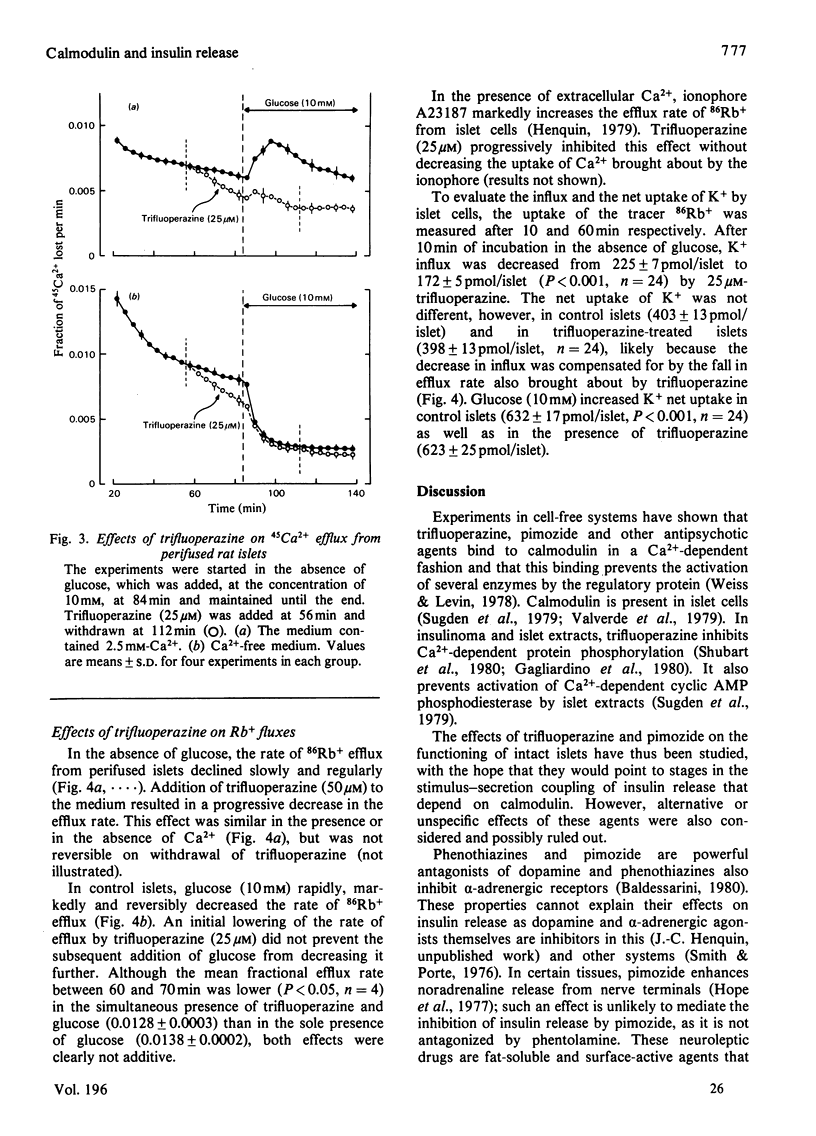
The possible involvement of calmodulin in insulin release was evaluated by studying the effects on intact islets of trifluoperazine and pimozide, two antipsychotic agents known to bind strongly to calmodulin in cell-free systems. Trifluoperazine (10–100μm) produced a dose- and time-dependent inhibition of the two phases of glucose-stimulated insulin release. The effect was not reversible by simple washing of the drug, but could be prevented by cytochalasin B or theophylline. Trifluoperazine also inhibited the release induced by glyceraldehyde, oxoisocaproate, tolbutamide or barium, but not that stimulated by 10mm-theophylline or 1mm-3-isobutyl-1-methylxanthine. Pimozide (0.5–10μm) also produced a dose-dependent inhibition of insulin release triggered by glucose, leucine or barium, but did not affect the release induced by methylxanthines. Glucose utilization by islet cells was not modified by trifluoperazine (25μm), which slightly increased cyclic AMP concentration in islets incubated without glucose. The drug did not prevent the increase in cyclic AMP concentration observed after 10min of glucose stimulation, but suppressed it after 60min. Basal or glucose-stimulated Ca2+ influx (5min) was unaffected by 25μm-trifluoperazine, whereas Ca2+net uptake (60min) was inhibited by 20%. Glucose-stimulated Ca2+ uptake was almost unaffected by pimozide. In a Ca2+-free medium, trifluoperazine decreased Ca2+ efflux from the islets and did not prevent the further decrease by glucose; in the presence of Ca2+, the drug again decreased Ca2+ efflux and inhibited the stimulation normally produced by glucose. In the absence of glucose, trifluoperazine lowered the rate of Rb+ efflux from the islets, decreased Rb+ influx (10min), but did not affect Rb+ net uptake (60min). It did not interfere with the ability of glucose to decrease Rb+ efflux rate further and to increase Rb+ net uptake. The results show thus that trifluoperazine does not alter the initial key events of the stimulus–secretion coupling. Its inhibition of insulin release suggests a role of calmodulin at late stages of the secretory process.
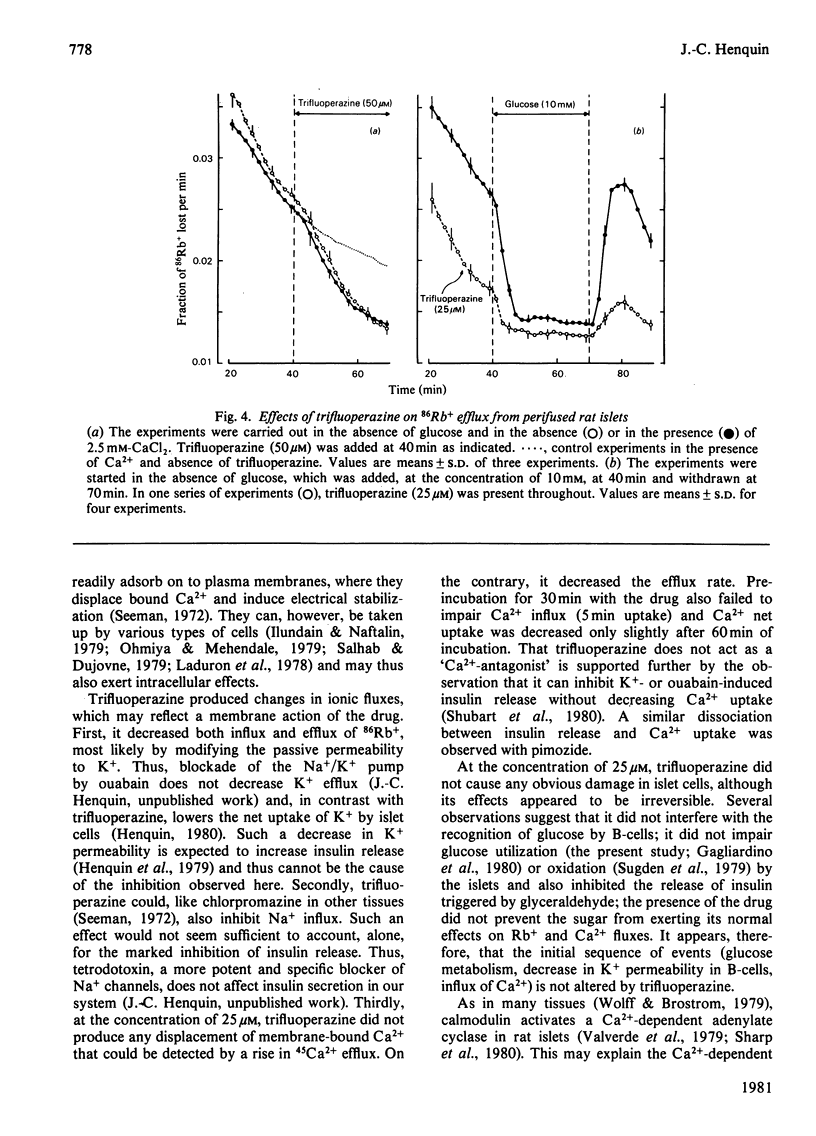
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Weerasinghe L. C., Bassett J. M., Randle P. J. The pentose cycle and insulin release in mouse pancreatic islets. Biochem J. 1972 Feb;126(3):525–532. doi: 10.1042/bj1260525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Knight D. E. Gaining access to the site of exocytosis in bovine adrenal medullary cells. J Physiol (Paris) 1980 Sep;76(5):497–504. [PubMed] [Google Scholar]
- Berridge M. J. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv Cyclic Nucleotide Res. 1975;6:1–98. [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R. J., Freedman S. D., Yohe W. B., Maurer S. C. Stimulation of Ca2+-dependent neurotransmitter release and presynaptic nerve terminal protein phosphorylation by calmodulin and a calmodulin-like protein isolated from synaptic vesicles. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1838–1842. doi: 10.1073/pnas.76.4.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliardino J. J., Harrison D. E., Christie M. R., Gagliardino E. E., Ashcroft S. J. Evidence for the participation of calmodulin in stimulus-secretion coupling in the pancreatic beta-cell. Biochem J. 1980 Dec 15;192(3):919–927. doi: 10.1042/bj1920919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C. D-glucose inhibits potassium efflux from pancreatic islet cells. Nature. 1978 Jan 19;271(5642):271–273. doi: 10.1038/271271a0. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Lambert A. E. Bicarbonate modulation of glucose-9nduced biphasic insulin release by rat islets. Am J Physiol. 1976 Sep;231(3):713–721. doi: 10.1152/ajplegacy.1976.231.3.713. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P., Preissler M. 9-Aminoacridine- and tetraethylammonium-induced reduction of the potassium permeability in pancreatic B-cells. Effects on insulin release and electrical properties. Biochim Biophys Acta. 1979 Nov 1;587(4):579–592. doi: 10.1016/0304-4165(79)90010-2. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Metabolic control of potassium permeability in pancreatic islet cells. Biochem J. 1980 Feb 15;186(2):541–550. doi: 10.1042/bj1860541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C. Opposite effects of intracellular Ca2+ and glucose on K+ permeability of pancreatic islet cells. Nature. 1979 Jul 5;280(5717):66–68. doi: 10.1038/280066a0. [DOI] [PubMed] [Google Scholar]
- Hope W., McCulloch M. W., Story D. F., Rand M. J. Effects of pimozide on noradrenergic transmission in rabbit isolated ear arteries. Eur J Pharmacol. 1977 Nov 15;46(2):101–111. doi: 10.1016/0014-2999(77)90245-x. [DOI] [PubMed] [Google Scholar]
- Ilundain A., Naftalin R. J. Role of Ca(2+)-dependent regulator protein in intestinal secretion. Nature. 1979 May 31;279(5712):446–448. doi: 10.1038/279446a0. [DOI] [PubMed] [Google Scholar]
- Krausz Y., Wollheim C. B., Siegel E., Sharp G. W. Possible role for calmodulin in insulin release. Studies with trifluoperazine in rat pancreatic islets. J Clin Invest. 1980 Sep;66(3):603–607. doi: 10.1172/JCI109893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laduron P. M., Janssen P. F., Leysen J. E. Spiperone: a ligand of choice for neuroleptic receptors. 3. Subcellular distribution of neuroleptic drugs and their receptors in various rat brain areas. Biochem Pharmacol. 1978 Feb 1;27(3):323–328. doi: 10.1016/0006-2952(78)90235-6. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Binding of trifluoperazine to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. Mol Pharmacol. 1977 Jul;13(4):690–697. [PubMed] [Google Scholar]
- Means A. R., Dedman J. R. Calmodulin--an intracellular calcium receptor. Nature. 1980 May 8;285(5760):73–77. doi: 10.1038/285073a0. [DOI] [PubMed] [Google Scholar]
- Ohmiya Y., Mehendale H. M. Uptake and accumulation of chlorpromazine in the isolated perfused rabbit lung. Drug Metab Dispos. 1979 Nov-Dec;7(6):442–443. [PubMed] [Google Scholar]
- Pershadsingh H. A., McDaniel M. L., Landt M., Bry C. G., Lacy P. E., McDonald J. M. Ca2+-activated ATPase and ATP-dependent calmodulin-stimulated Ca2+ transport in islet cell plasma membrane. Nature. 1980 Dec 4;288(5790):492–495. doi: 10.1038/288492a0. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B. Relationships between calcium and cyclic nucleotides in cell activation. Physiol Rev. 1977 Jul;57(3):421–509. doi: 10.1152/physrev.1977.57.3.421. [DOI] [PubMed] [Google Scholar]
- Salhab A. S., Dujovne C. A. Chlorpromazine uptake by isolated rat hepatocytes. Proc Soc Exp Biol Med. 1979 Jul;161(3):270–274. doi: 10.3181/00379727-161-40534. [DOI] [PubMed] [Google Scholar]
- Schubart U. K., Erlichman J., Fleischer N. The role of calmodulin in the regulation of protein phosphorylation and insulin release in hamster insulinoma cells. J Biol Chem. 1980 May 10;255(9):4120–4124. [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Sehilin J. Calcium uptake by subcellular fractions of pancreatic islets. Effects of nucleotides and theophylline. Biochem J. 1976 Apr 15;156(1):63–69. doi: 10.1042/bj1560063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. W. The adenylate cyclase-cyclic AMP system in islets of Langerhans and its role in the control of insulin release. Diabetologia. 1979 May;16(5):287–296. doi: 10.1007/BF01223617. [DOI] [PubMed] [Google Scholar]
- Sharp G. W., Wiedenkeller D. E., Kaelin D., Siegel E. G., Wollheim C. B. Stimulation of adenylate cyclase by Ca2+ and calmodulin in rat islets of langerhans: explanation for the glucose-induced increase in cyclic AMP levels. Diabetes. 1980 Jan;29(1):74–77. doi: 10.2337/diab.29.1.74. [DOI] [PubMed] [Google Scholar]
- Smith P. H., Porte D., Jr Neuropharmacology of the pancreatic islets. Annu Rev Pharmacol Toxicol. 1976;16:269–285. doi: 10.1146/annurev.pa.16.040176.001413. [DOI] [PubMed] [Google Scholar]
- Somers G., Devis G., van Obberghen E., Malaisse W. J. Calcium-antagonists and islet function. VI. Effects of barium. Pflugers Arch. 1976 Sep 3;365(1):21–28. doi: 10.1007/BF00583624. [DOI] [PubMed] [Google Scholar]
- Sugden M. C., Ashcroft S. J. Effects of phosphoenolpyruvate, other glycolytic intermediates and methylxanthines on calcium uptake by a mitochondrial fraction from rat pancreatic islets. Diabetologia. 1978 Sep;15(3):173–180. doi: 10.1007/BF00421235. [DOI] [PubMed] [Google Scholar]
- Sugden M. C., Christie M. R., Ashcroft S. J. Presence and possible role of calcium-dependent regulator (calmodulin) in rat islets of Langerhans. FEBS Lett. 1979 Sep 1;105(1):95–100. doi: 10.1016/0014-5793(79)80894-7. [DOI] [PubMed] [Google Scholar]
- Valverde I., Vandermeers A., Anjaneyulu R., Malaisse W. J. Calmodulin activation of adenylate cyclase in pancreatic islets. Science. 1979 Oct 12;206(4415):225–227. doi: 10.1126/science.225798. [DOI] [PubMed] [Google Scholar]
- Vincenzi F. F., Larsen F. L. The plasma membrane calcium pump: regulation by a soluble Ca2+ binding protein. Fed Proc. 1980 May 15;39(7):2427–2431. [PubMed] [Google Scholar]
- Weiss B., Levin R. M. Mechanism for selectively inhibiting the activation of cyclic nucleotide phosphodiesterase and adenylate cyclase by antipsychotic agents. Adv Cyclic Nucleotide Res. 1978;9:285–303. [PubMed] [Google Scholar]
- Wolff D. J., Brostrom C. O. Properties and functions of the calcium-dependent regulator protein. Adv Cyclic Nucleotide Res. 1979;11:27–88. [PubMed] [Google Scholar]



