Abstract
Weight-adjusted-waist index (WWI) is an anthropometric indicator of central obesity, which is calculated by dividing the waist circumference (WC) by the squared weight. The purpose of this study was to investigate the association between WWI and psoriasis in adults. Multivariate logistic regression and smoothing curve fitting were used to investigate the relationship between WWI and psoriasis based on data from the National Health and Nutrition Examination Survey (NHANES) 2009 to 2014. Subgroup analysis and interaction tests were employed to examine the population-level stability of this connection. There was a positive association between WWI and psoriasis in 15,932 participants > 20 years of age. In the fully adjusted model, each 1-unit increase in WWI was associated with a 14% increase in the risk of developing psoriasis [1.14 (1.01, 1.32)]. Participants in the highest quartile of WWI had a 38% higher risk of developing psoriasis than those in the lowest quartile [1.38 (1.01, 1.94)]. This positive association was more pronounced in males. WWI is positively associated with psoriasis in US adults. Our findings imply that WWI has the potential to improve psoriasis prevention in the general population.
Keywords: metabolism, NHANES, obese, psoriasis, weight-adjusted-waist index
1. Introduction
Psoriasis is a common inflammatory skin disease that can influence the skin and/or joints, serious disease is linked to significant impairment in physical and mental health.[1] It has recently been determined that psoriasis is an inflammatory disease affecting multiple organ systems that can lead to metabolic syndrome, inflammatory bowel disease, diabetes, and cardiovascular disease.[2,3] It has also been observed that the prevalence of disease is rising.[4] As a result, it is imperative to identify causes that may be controlled or prevented to reduce the prevalence of psoriasis.
The number of people affected by obesity is rising, and its prevalence has reached epidemic proportions worldwide.[5–7] Obesity has been associated with the onset of various diseases, including cardiovascular, diabetes, osteoarticular, immune system diseases and several malignancies.[8–12] Waist circumference (WC) and body mass index (BMI) are 2 commonly used measurements for evaluating obesity. Nevertheless, it is impossible to distinguish between muscle and fat mass using such signs.[13–15] According to recent research, body composition and fat distribution can be utilized to more precisely identify metabolic diseases.[16] Weight-adjusted-waist index (WWI) is an anthropometric indicator of central obesity, which is calculated by dividing the WC by the squared weight.[17] Even within distinct BMI categories, it could reflect components of both muscle and fat mass.[18,19] WWI as a new type of obesity index, standardizes WC with weight, incorporating the strengths of WC while attenuating the relationship with BMI.[17] The WWI accounts for central obesity concerns unrelated to weight in addition to differentiating between fat and muscle mass.[18] According to previous studies, it outperforms BMI, body shape index (ABSI), and waist-to-height ratio (WHtR) as a notable predictor of cardiovascular morbidity and mortality.[20] Furthermore, WWI is a greater predictor of incident hypertension compared to BMI and WC.[21]
Scientific evidence suggests that the relationship between obesity and psoriasis may be complex, with dietary practices, lifestyle choices, genetic predispositions, and the microbiome all being important in the development of both diseases due to their associations with long-term pro-inflammatory states.[22] The association between WWI and psoriasis has not been investigated before despite the possibility that WWI is a sign of central obesity. It is important to explore the association of obesity evaluated by WWI and psoriasis to gain more awareness about the negative effects of obesity on psoriasis. Therefore, we aimed to investigate the association between WWI and psoriasis among the US population using data from National Health and Nutrition Examination Survey 2009 to 2014 (NHANES).
2. Methods
2.1. Study population
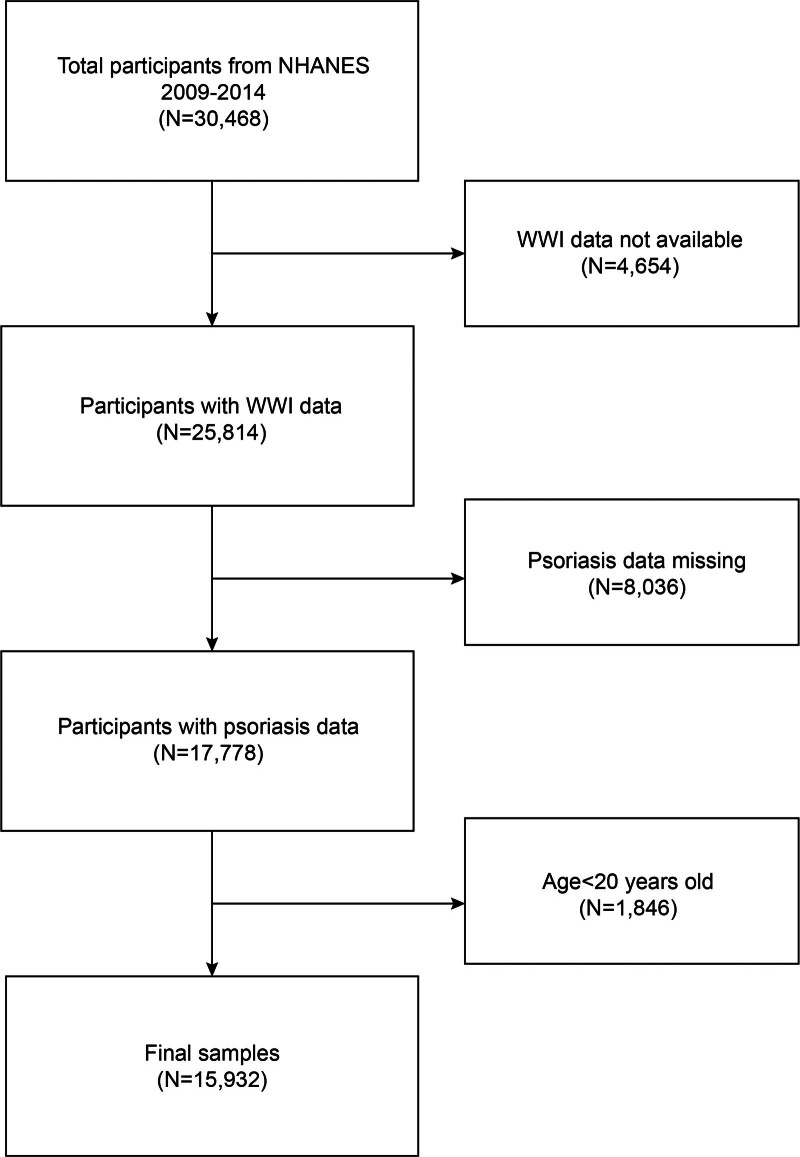
The NHANES is a nationally representative survey conducted by the Centers for Disease Control and Prevention.[23,24] The study procedure received approval from the Research Ethics Review Board of the National Center for Health Statistics (NCHS). Every participant gave written consent at the time of recruiting.[25,26] The NHANES study designs and data are all available to the public at www.cdc.gov/nchs/nhanes/. Psoriasis data were only available to individuals aged 16 to 80 in the NHANES 2009 to 2014 cycles. We excluded 4654 participants without available WWI data, 8036 participants with missing psoriasis data, and 1846 participants under 20 years old. The study eventually included 15,932 participants (Fig. 1).
Figure 1.
Flow chart of participants selection. NHANES = National Health and Nutrition Examination Survey.
2.2. Weight-adjusted-waist index
WWI is an anthropometric index based on WC and weight to measure central obesity. In NHANES, weight and WC measurements were made by trained health technicians. Each participant’s WWI was determined by dividing their weight in kilograms squared by their WC in centimeters and rounding to 2 decimal places. (WWI = WC/Weight2, WC in centimeters, Weight in kilograms). Higher WWI was associated with higher levels of obesity. WWI was 1 of the exposure variables in our study.
2.3. Diagnosis of psoriasis
In response to the question, “Have you ever been told by a health care provider that you had psoriasis?” psoriasis was self-reported.
2.4. Covariables
Covariates included age, race, gender, smoking status, alcohol drinking status, coronary heart disease, high blood pressure, diabetes, low-density lipoprotein cholesterol (LDL-C), triglyceride, total cholesterol, income-to-poverty ratio (PIR), education level and BMI.
2.5. Statistical analysis
The participant’s demographics were evaluated by WWI quartile using the chi-square test and t-test. The linear associations between WWI and psoriasis were examined using weighted multivariate linear and logistic regression analyses. After WWI was transformed from a continuous variable to a categorical variable (quartile), a trend test was used to examine the linear association between WWI and psoriasis. The “P for trend” represents the level of significance in testing the null hypothesis. Specifically, it examines whether there is a systematic change in the outcome variable (psoriasis) as the levels of an ordered categorical variable increase (WWI). Subgroup analysis was used to investigate the association between WWI and psoriasis in people of different gender, age, and diabetes status, and interaction tests were used to investigate whether the relationships were consistent across subgroups. Smoothing curve fitting was used to explore the positive association between WWI and psoriasis.[27] R (version 4.2) or Empowerstats (version 5.0) were used for all analyses. The definition of statistical significance is 2-sided P < .05.
2.6. Ethical statement
The portions of this study involving human participants, human materials, or human data were conducted by the Declaration of Helsinki and were approved by the NCHS Ethics Review Board. The patients/participants provided written informed consent to participate in this study.
3. Results
3.1. Baseline characteristics
Of a total of 15,932 participants older than 20 years, the mean (SD) age was 48.69 (17.49) years, with 51.20% female and 42.81% non-Hispanic White. The WWI ranges for tertiles 1 to 4 were 8.37 to 10.46, 10.47 to 11.03, 11.04 to 11.61, and 11.62 to 14.80, respectively. There was an overall prevalence of 2.75% (weighed proportion) of psoriasis and it also increased as the WWI tertile increased (tertile 1: 2.03%; tertile 2: 2.71%; tertile 3: 2.74%; tertile 4: 3.51%; P < .001). We observed statistically significant differences by gender, race, education, BMI, smoking, diabetes, hypertension, coronary heart disease, high blood pressure, drinking, family PIR, triglycerides, LDL-C, asthma, arthritis, thyroid problems, chronic bronchitis, and total cholesterol (all P < .001) among WWI tertiles (Table 1).
Table 1.
Basic characteristics of participants by weight-adjusted-waist index among U.S. adults.
| Characteristics | Weight-adjusted waist index | P value | |||
|---|---|---|---|---|---|
| Q1 (n = 3983) | Q2 (n = 3983) | Q3 (n = 3983) | Q4 (n = 3983) | ||
| Age (yr) | 37.34 ± 14.11 | 46.20 ± 15.63 | 52.71 ± 16.17 | 58.51 ± 16.47 | <.001 |
| Gender, (%) | |||||
| Male | 61.74 | 52.90 | 47.05 | 33.54 | <.001 |
| Female | 38.26 | 47.10 | 52.95 | 66.46 | |
| Race/ethnicity, (%) | |||||
| Mexican American | 7.53 | 13.56 | 16.92 | 18.73 | <.001 |
| Other Hispanic | 7.58 | 9.09 | 11.78 | 11.00 | |
| Non-Hispanic White | 42.93 | 41.88 | 41.02 | 45.44 | |
| Non-Hispanic Black | 27.74 | 20.86 | 19.61 | 16.09 | |
| Other races | 14.21 | 14.61 | 10.67 | 8.74 | |
| Education level, (%) | |||||
| <high school | 14.26 | 20.97 | 27.60 | 34.22 | <.001 |
| High school | 20.54 | 21.24 | 22.72 | 23.88 | |
| >high school | 65.20 | 57.79 | 49.68 | 41.90 | |
| Smoked at least 100 cigarettes in life, (%) | |||||
| Yes | 41.01 | 43.56 | 45.49 | 46.82 | <.001 |
| No | 58.99 | 56.44 | 54.51 | 53.18 | |
| Diabetes, (%) | |||||
| Yes | 2.61 | 6.38 | 14.01 | 24.38 | <.001 |
| No | 97.39 | 93.62 | 85.99 | 75.62 | |
| Coronary heart disease, (%) | |||||
| Yes | 0.85 | 2.66 | 4.47 | 6.90 | <.001 |
| No | 99.15 | 97.34 | 95.53 | 93.10 | |
| High blood pressure, (%) | |||||
| Yes | 15.06 | 29.73 | 41.28 | 54.96 | <.001 |
| No | 84.94 | 70.27 | 58.72 | 45.04 | |
| Family PIR | 2.65 ± 1.70 | 2.63 ± 1.68 | 2.47 ± 1.64 | 2.12 ± 1.50 | <.001 |
| BMI | 24.75 ± 4.58 | 27.72 ± 5.41 | 30.04 ± 6.18 | 33.21 ± 7.54 | <.001 |
| Triglyceride (mg/dL) | 101.97 ± 85.91 | 118.36 ± 89.17 | 139.27 ± 156.51 | 148.95 ± 100.57 | <.001 |
| LDL-C (mg/dL) | 107.89 ± 32.28 | 116.61 ± 35.06 | 116.64 ± 36.60 | 114.61 ± 35.85 | <.001 |
| Total cholesterol (mg/dL) | 184.21 ± 37.90 | 195.62 ± 40.68 | 196.23 ± 42.04 | 195.68 ± 44.58 | <.001 |
| Had at least 12 alcohol drinks/1 yr (%) | |||||
| Yes | 80.81 | 76.31 | 71.88 | 63.96 | <.001 |
| No | 19.19 | 23.69 | 28.12 | 36.04 | |
| Psoriasis, (%) | |||||
| Yes | 2.03 | 2.71 | 2.74 | 3.51 | <.001 |
| No | 97.97 | 97.29 | 97.26 | 96.49 | |
| Asthma, (%) | |||||
| Yes | 13.73 | 12.98 | 13.91 | 17.55 | <.001 |
| No | 86.27 | 87.02 | 86.09 | 82.45 | |
| Arthritis, (%) | |||||
| Yes | 10.42 | 18.80 | 30.36 | 41.78 | <.001 |
| No | 89.58 | 81.20 | 69.64 | 58.22 | |
| Thyroid problems, (%) | |||||
| Yes | 4.87 | 7.68 | 10.92 | 15.49 | <.001 |
| No | 95.13 | 92.32 | 89.08 | 84.51 | |
| Chronic bronchitis, (%) | |||||
| Yes | 2.74 | 4.14 | 5.02 | 9.26 | <.001 |
| No | 97.26 | 95.86 | 94.98 | 90.74 | |
Mean ± SD for continuous variables: the P value was calculated by the weighted linear regression model; (%) for categorical variables: the P value was calculated by the weighted chi-square test.
Abbreviations: BMI = body mass index, LDL-C = low-density lipoprotein, PIR = the ratio of income-to-poverty, Q = quartile.
3.2. Higher WWI in associated with psoriasis
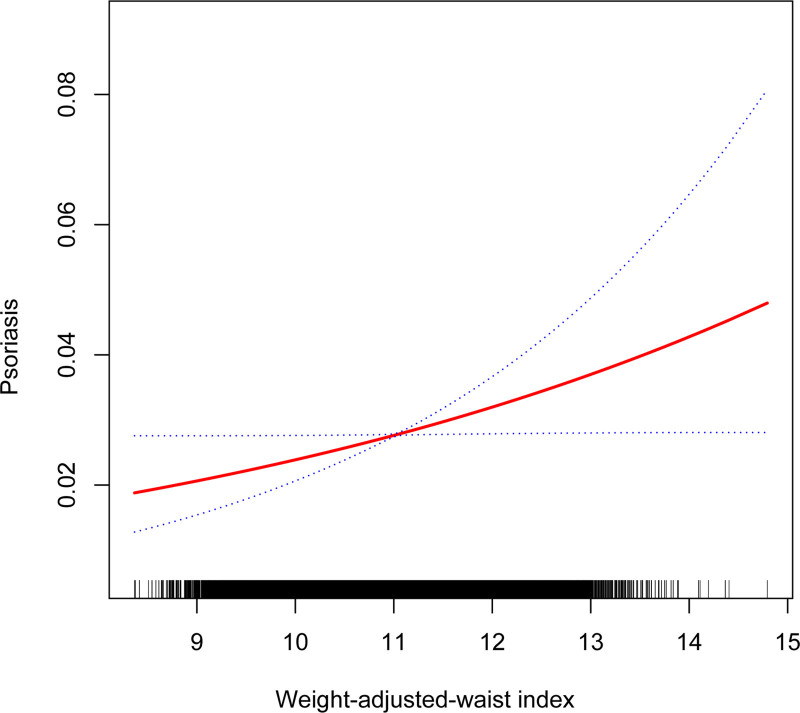
Table 2 shows the association between WWI and psoriasis. We found higher WWI was correlated with psoriasis both in the crude model [1.28 (1.15, 1.44)] and adjusted model [1.23 (1.07, 1.40)]. After full adjustment, each unit of higher WWI score was found to be associated with a 21% increased risk of developing psoriasis (OR = 1.21, 95% CI 1.05–1.45). After WWI was classified as quartiles, the above correlation remained statistically significant (all P for trend < .05). In the fully adjusted model, compared with the lowest WWI tertile (quartile 1), participants in the highest WWI tertile exhibited a significantly 0.83-fold increased likelihood. (OR = 1.83, 95% CI 1.01–2.34; P for trend = .0214), respectively. In addition, the smoothed curve fitting results further validated the positive association between WII with psoriasis (Fig. 2).
Table 2.
Association between weight-adjusted waist index and psoriasis.
| WWI | Psoriasis | P for tend |
|---|---|---|
| OR (95% CI) | ||
| Crude model (model 1) | ||
| Continuous | 1.28 (1.15, 1.44) | |
| Categories | ||
| Quartile 1 | 0 (ref) | <.0001 |
| Quartile 2 | 1.34 (1.00, 1.80) | |
| Quartile 3 | 1.36 (1.01, 1.81) | |
| Quartile 4 | 1.75 (1.33, 2.32) | |
| Minimally adjusted model (model 2) | ||
| Continuous | 1.23 (1.07, 1.40) | |
| Categories | ||
| Quartile 1 | 0 (ref) | .0123 |
| Quartile 2 | 1.27 (0.94, 1.71) | |
| Quartile 3 | 1.25 (0.91, 1.71) | |
| Quartile 4 | 1.54 (1.12, 2.12) | |
| Fully adjusted model (model 3) | ||
| Continuous | 1.21 (1.05, 1.45) | |
| Categories | ||
| Quartile 1 | 0 (ref) | .0214 |
| Quartile 2 | 1.21 (0.87, 1.92) | |
| Quartile 3 | 1.76 (0.91, 1.94) | |
| Quartile 4 | 1.83 (1.01, 2.34) | |
Model 1: no covariates were adjusted.
Model 2: age, gender, and race were adjusted.
Model 3: age, gender, race, education level, PIR, BMI, drinking alcohol, smoking, diabetes, coronary heart disease, high blood pressure, LDL-C, asthma, arthritis, thyroid problems, chronic bronchitis, and triglycerides were adjusted.
Abbreviations: BMI = body mass index, LDL-C = low-density lipoprotein, PIR = the ratio of income-to-poverty, Q = quartile, WWI = weight-adjusted waist index.
Figure 2.
The nonlinear associations between WWI and psoriasis. The solid red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit. WWI = weight-adjusted-waist index.
3.3. Subgroup analyses
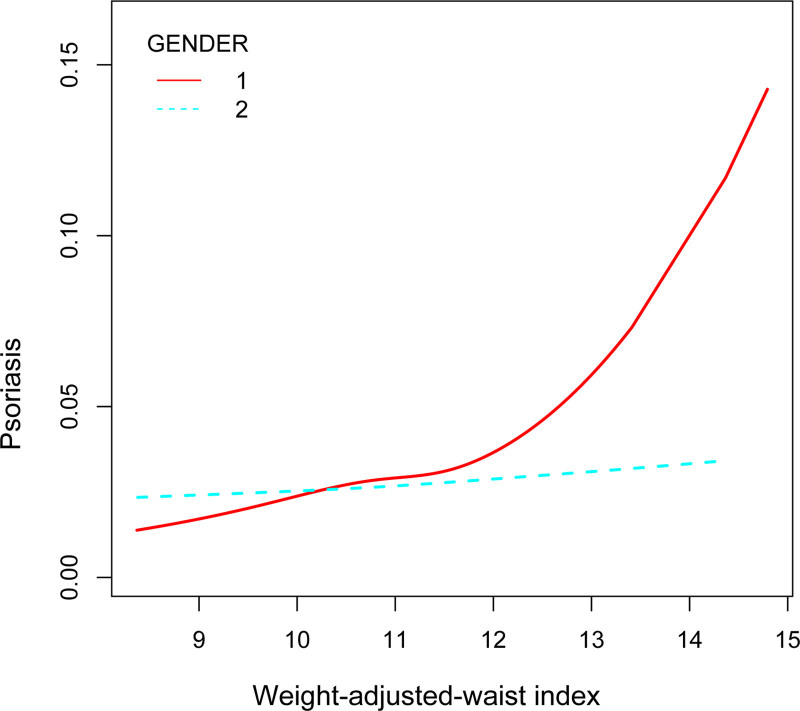
To evaluate whether the association between WWI and psoriasis was consistent in the overall population and for the potentially different population settings, we conducted subgroup analysis and interaction tests stratified by age, gender, and diabetes (Table 3). Our results showed that the associations were inconsistent. As is shown in Figure 3, we detected significant interaction for sex (P for interaction < .05), while there was no statistical significance for age and BMI. WWI with psoriasis remained positively associated in male, older adults greater than or equal to 60 years. Taken together, our results demonstrated the association of WWI and psoriasis showed dependence on sex, it may be appropriate for male.
Table 3.
Subgroup analysis of the association between WWI and psoriasis.
| Subgroup | Psoriasis [OR (95%CI)] | P for interaction |
|---|---|---|
| Sex | ||
| Male | 1.49 (1.26, 1.77) | .0331 |
| Female | 1.16 (0.99, 1.36) | |
| Age | ||
| <60 yr | 1.21 (1.04, 1.40) | .2759 |
| ≥60 yr | 1.40 (1.12, 1.73) | |
| Diabetes, (%) | ||
| Yes | 1.66 (1.00, 2.78) | .8841 |
| No | 1.47 (1.20, 1.81) | |
Age, gender, race, education level, PIR, BMI, drinking alcohol, diabetes, coronary heart disease, high blood pressure, LDL-C, asthma, arthritis, thyroid problems, chronic bronchitis, and triglycerides were adjusted.
Figure 3.
Association between WWI and psoriasis stratified by gender. WWI = weight-adjusted-waist index.
4. Discussion
In the cross-sectional research that enrolled 15,932 representative participants, we observed the positive associations between WWI and psoriasis, and there was significant dependence of gender on this association, indicating that higher levels of WWI may lead to an increased risk of developing psoriasis, especially in the males. Our results suggest that WWI may have potential clinical value in the diagnosis of psoriasis risk and disease severity.
To our knowledge, this is the first study to assess the relationship between WWI and psoriasis, which emphasizes the positive association of WWI level and higher psoriasis risks. Previous studies have found that obesity plays a fundamental role in psoriasis.[28–30] Owing to the widespread occurrence and significant negative effects of obesity, an increasing number of indicators are being employed to assess obesity, with a particular focus on the identified detrimental intra-abdominal fat mass. BMI, as a widely used obesity index, was found to possess a “U-shaped pattern” with all-cause mortality, with the highest risk in the most overweight and underweight participant groups.[13,31] However, numerous studies proposed the “obesity paradox” phenomenon of BMI, which refers to the relatively obese participants embracing a better prognosis than those with a normal range of BMI.[17,20,32] A comparable occurrence was also observed with WC when it was used in a study that focused on heart failure.[15] The unanticipated paradox might be brought on by BMI and WC’s incapacity to differentiate between fat and muscle mass. WWI could be a more complete and reliable indicator of obesity.
As obesity increasingly becomes a serious global public health concern and its close link with many diseases is revealed.[33] Ko et al[30] evaluated ten randomized controlled trials among 1,163 participants and interventions lasting at least 12 weeks, highlighted the potential connection between psoriasis and obesity. A recent large-scale population-based Norwegian study including over 35,000 participants has found a link between metabolic syndrome and a higher chance of developing psoriasis. The examination of metabolic variables revealed that obesity plays an important part in this correlation.[34] A study by Setty et al[35] involved 78,626 women (of whom 892 reported having psoriasis) indicating that adiposity and weight gain were potential risk factors for the development of psoriasis. According to a recent prospective study, obesity and excessive abdominal fat mass quadrupled the incidence of psoriasis.[28] According to a meta-analysis of 7 prospective studies with 17,636 participants, higher BMI, WC, WHtR, and weight gain were linked to an increased risk of psoriasis, with a 2 to 4-fold increase in the risk of psoriasis among those at the high end of each adiposity measures.[36] Besides that, the association between obesity and psoriasis has been demonstrated in animal studies. It was demonstrated using an obese mouse model with imiquimod-induced psoriasiform dermatitis that obesity may acutely worsen the severity of the condition in mice.[37] In our analysis, it showed a positive correlation between WWI and psoriasis in both the crude and adjusted models. This positive association was more pronounced in males. Accordingly, when faced with nutritional problems, males are more prone than females to acquire obesity, insulin resistance, and hyperglycemia when taking into account practically all animal models.[38] The protective effects of endogenous estrogens are demonstrated by clinical and experimental studies, mostly due to the activation of estrogen receptor α in a variety of organs, such as the brain, liver, skeletal muscle, adipose tissue, and pancreatic beta cells. A deeper examination of the underlying mechanisms, particularly the function of sex chromosomes, fetal/neonatal programming, and epigenetic alterations, is necessary in addition to sex steroids.[39]
The psoriasis and obesity etiology involve alterations to the microbiota, a feature that is also shared by other chronic inflammatory diseases.[40] They have a strong connection to autoimmune illnesses,[41,42] which are characterized by an imbalance in lymphocyte production and an increase in IL-17 production. Furthermore, obesity modifies the inflammatory cells’ biological makeup and activity in the skin. Nakamizo et al[43] reported an accumulation of γδ T cells that produce IL-17A in psoriatic skin lesions of high-fat diet (HFD)-induced obese mice, which exacerbates psoriatic dermatitis. Additionally, genetically engineered diabetic (db/db) mice demonstrated heightened psoriatic skin inflammation with enhanced levels of IL-17A and IL-22 [37]. Another study demonstrated that a prolonged HFD lasting 9 months stimulated the skin’s accumulation of specific CD11c + macrophages, in a way that was dependent on the epidermal fatty acid binding protein (E-FABP).[44]
The association between obesity and psoriasis has been previously well-documented in the literature, and the use of WWI in this study provides further evidence in this area. However, since NHANES is a cross-sectional study, we are unable to provide risk stratification based on WWI levels and psoriasis, as it does not follow individuals over time. One of our study’s strengths is the intricate multi-stage probability sampling design we used, which improved the study’s representativeness and dependability. Our research has several limitations. First, we were unable to determine a causal association between WWI and psoriasis because of the design of the cross-sectional analysis. Even after adjusting for some confounding variables, the results might have been affected by additional confounding variables, such as the use of steroids and diuretics. We were limited in our ability to include these covariables in our analysis since information on them was not gathered in NHANES and was not available in the original dataset. Besides that, although the diagnosis of psoriasis comes from a medical professional or health care practitioner, reliance on self-reported psoriasis conditions can lead to reporting bias and lack of clinical validation. The specific types of psoriasis were not delineated in detail, which could potentially affect the results of the study. In addition, NHANES was a US-based study, thus we could only evaluate the association between WWI and psoriasis in US adults.
5. Conclusion
In conclusion, our study found that WWI and psoriasis were positively correlated, and this association was more significant in males. The WWI may be of potential value in clinical practice for identifying psoriasis severity.
Acknowledgments
We would like to thank all participants in this study.
Author contributions
Conceptualization: Yanan Tuo.
Data curation: Yanan Tuo.
Investigation: Yanan Tuo.
Methodology: Yanan Tuo.
Supervision: Junchen He.
Validation: Junchen He.
Writing – original draft: Tao Guo.
Writing – review & editing: Tao Guo.
Abbreviations:
- ABSI
- body shape index
- BMI
- body mass index
- LDL-C
- low-density lipoprotein cholesterol
- NCHS
- National Center for Health Statistics
- NHANES
- National Health and Nutrition Examination Survey
- PIR
- income-to-poverty ratio
- WC
- waist circumference
- WHtR
- waist-to-height ratio
- WWI
- weight-adjusted-waist index
The authors have no funding and conflicts of interest to disclose.
Consent is not applicable to this study.
The datasets generated during and/or analyzed during the current study are publicly available.
How to cite this article: Tuo Y, He J, Guo T. The association between weight-adjusted-waist index and psoriasis: A cross-sectional study based on NHANES 2009 to 2014. Medicine 2024;103:49(e40808).
Contributor Information
Yanan Tuo, Email: 623733360@qq.com.
Junchen He, Email: 420359745@qq.com.
References
- [1].Iskandar IY, Ashcroft DM, Warren RB, et al. Demographics and disease characteristics of patients with psoriasis enrolled in the British Association of Dermatologists Biologic Interventions Register. Br J Dermatol. 2015;173:510–8. [DOI] [PubMed] [Google Scholar]
- [2].Fu Y, Lee CH, Chi CC. Association of psoriasis with inflammatory bowel disease: a systematic review and meta-analysis. JAMA Dermatol. 2018;154:1417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dauden E, Blasco AJ, Bonanad C, et al. Position statement for the management of comorbidities in psoriasis. J Eur Acad Dermatol Venereol. 2018;32:2058–73. [DOI] [PubMed] [Google Scholar]
- [4].van Eeden AE, van Hoeken D, Hoek HW. Incidence, prevalence and mortality of anorexia nervosa and bulimia nervosa. Curr Opin Psychiatry. 2021;34:515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yanovski JA. Obesity: trends in underweight and obesity: scale of the problem. Nat Rev Endocrinol. 2018;14:5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].NCD Risk Factor Collaboration. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kanda N, Hoashi T, Saeki H. Nutrition and psoriasis. Int J Mol Sci . 2020;21:5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu W, Liu J, Shao S, et al. Obesity at a young age is associated with development of diabetes mellitus: a prospective cohort study in rural China. Postgrad Med. 2020;132:709–13. [DOI] [PubMed] [Google Scholar]
- [10].Timmins KA, Leech RD, Batt ME, Edwards KL. Running and knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2017;45:1447–57. [DOI] [PubMed] [Google Scholar]
- [11].Ying W, Fu W, Lee YS, Olefsky JM. The role of macrophages in obesity-associated islet inflammation and β-cell abnormalities. Nat Rev Endocrinol. 2020;16:81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–98. [DOI] [PubMed] [Google Scholar]
- [13].Antonopoulos AS, Oikonomou EK, Antoniades C, Tousoulis D. From the BMI paradox to the obesity paradox: the obesity-mortality association in coronary heart disease. Obes Rev. 2016;17:989–1000. [DOI] [PubMed] [Google Scholar]
- [14].Hainer V, Aldhoon-Hainerová I. Obesity paradox does exist. Diabetes Care. 2013;36(Suppl 2):S276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Clark AL, Fonarow GC, Horwich TB. Waist circumference, body mass index, and survival in systolic heart failure: the obesity paradox revisited. J Card Fail. 2011;17:374–80. [DOI] [PubMed] [Google Scholar]
- [16].Shieh A, Karlamangla AS, Karvonen-Guttierez CA, Greendale GA. Menopause-related changes in body composition are associated with subsequent bone mineral density and fractures: study of women’s health across the nation. J Bone Miner Res. 2023;38:395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Park Y, Kim NH, Kwon TY, Kim SG. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci Rep. 1675;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kim NH, Park Y, Kim NH, Kim SG. Weight-adjusted waist index reflects fat and muscle mass in the opposite direction in older adults. Age Ageing. 2021;50:780–6. [DOI] [PubMed] [Google Scholar]
- [19].Kim JY, Choi J, Vella CA, Criqui MH, Allison MA, Kim NH. Associations between weight-adjusted waist index and abdominal fat and muscle mass: multi-ethnic study of atherosclerosis. Diabetes Metab J. 2022;46:747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ding C, Shi Y, Li J, et al. Association of weight-adjusted-waist index with all-cause and cardiovascular mortality in China: a prospective cohort study. Nutr Metab Cardiovasc Dis. 2022;32:1210–7. [DOI] [PubMed] [Google Scholar]
- [21].Li Q, Qie R, Qin P, et al. Association of weight-adjusted-waist index with incident hypertension: the rural Chinese cohort study. Nutr Metab Cardiovasc Dis. 2020;30:1732–41. [DOI] [PubMed] [Google Scholar]
- [22].Barros G, Duran P, Vera I, Bermúdez V. Exploring the links between obesity and psoriasis: a comprehensive review. Int J Mol Sci . 2022;23:7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang Y, Xie R, Ou J. A U-shaped association between serum albumin with total triiodothyronine in adults. J Clin Lab Anal. 2022;36:e24473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Xie R, Zhang Y. Is assessing the degree of hepatic steatosis and fibrosis based on index calculations the best choice for epidemiological studies? Environ Pollut. 1207;317:120783. [DOI] [PubMed] [Google Scholar]
- [25].Xie R, Zhang Y. Association between 19 dietary fatty acids intake and rheumatoid arthritis: results of a nationwide survey. Prostaglandins Leukot Essent Fatty Acids. 2022;188:102530. [DOI] [PubMed] [Google Scholar]
- [26].Xie R, Liu Y, Wang J, et al. Race and gender differences in the associations between cadmium exposure and bone mineral density in US adults. Biol Trace Elem Res. 2022;201:4254–61. [DOI] [PubMed] [Google Scholar]
- [27].Xie R, Zhang Y. Index-based calculation or transient elastography to assess the degree of hepatic steatosis and fibrosis. J Nutr. 2023;153:909. [DOI] [PubMed] [Google Scholar]
- [28].Snekvik I, Smith CH, Nilsen TIL, et al. Obesity, waist circumference, weight change, and risk of incident psoriasis: prospective data from the HUNT study. J Invest Dermatol. 2017;137:2484–90. [DOI] [PubMed] [Google Scholar]
- [29].Kumar S, Han J, Li T, Qureshi AA. Obesity, waist circumference, weight change and the risk of psoriasis in US women. J Eur Acad Dermatol Venereol. 2013;27:1293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ko SH, Chi CC, Yeh ML, Wang SH, Tsai YS, Hsu MY. Lifestyle changes for treating psoriasis. Cochrane Database Syst Rev. 2019;7:CD011972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Elagizi A, Kachur S, Lavie CJ, et al. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2018;61:142–50. [DOI] [PubMed] [Google Scholar]
- [33].Stefan N, Häring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1:152–62. [DOI] [PubMed] [Google Scholar]
- [34].Snekvik I, Nilsen TIL, Romundstad PR, Saunes M. Metabolic syndrome and risk of incident psoriasis: prospective data from the HUNT Study, Norway. Br J Dermatol. 2019;180:94–9. [DOI] [PubMed] [Google Scholar]
- [35].Setty AR, Curhan G, Choi HK. Obesity, waist circumference, weight change, and the risk of psoriasis in women: nurses’ health study II. Arch Intern Med 2007;167:1670–5. [DOI] [PubMed] [Google Scholar]
- [36].Aune D, Snekvik I, Schlesinger S, Norat T, Riboli E, Vatten LJ. Body mass index, abdominal fatness, weight gain and the risk of psoriasis: a systematic review and dose-response meta-analysis of prospective studies. Eur J Epidemiol. 2018;33:1163–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kanemaru K, Matsuyuki A, Nakamura Y, Fukami K. Obesity exacerbates imiquimod-induced psoriasis-like epidermal hyperplasia and interleukin-17 and interleukin-22 production in mice. Exp Dermatol. 2015;24:436–42. [DOI] [PubMed] [Google Scholar]
- [38].Tramunt B, Smati S, Grandgeorge N, et al. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia. 2020;63:453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Dearden L, Bouret SG, Ozanne SE. Sex and gender differences in developmental programming of metabolism. Mol Metab. 2018;15:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chen L, Li J, Zhu W, et al. Skin and gut microbiome in psoriasis: gaining insight into the pathophysiology of it and finding novel therapeutic strategies. Front Microbiol. 5897;11:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hedin CRH, Sonkoly E, Eberhardson M, Ståhle M. Inflammatory bowel disease and psoriasis: modernizing the multidisciplinary approach. J Intern Med. 2021;290:257–78. [DOI] [PubMed] [Google Scholar]
- [42].Todberg T, Egeberg A, Zachariae C, Sørensen N, Pedersen O, Skov L. Patients with psoriasis have a dysbiotic taxonomic and functional gut microbiota. Br J Dermatol. 2022;187:89–98. [DOI] [PubMed] [Google Scholar]
- [43].Nakamizo S, Honda T, Adachi A, et al. High fat diet exacerbates murine psoriatic dermatitis by increasing the number of IL-17-producing γδ T cells. Sci Rep. 1407;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhang Y, Li Q, Rao E, et al. Epidermal fatty acid binding protein promotes skin inflammation induced by high-fat diet. Immunity. 2015;42:953–64. [DOI] [PMC free article] [PubMed] [Google Scholar]