Abstract
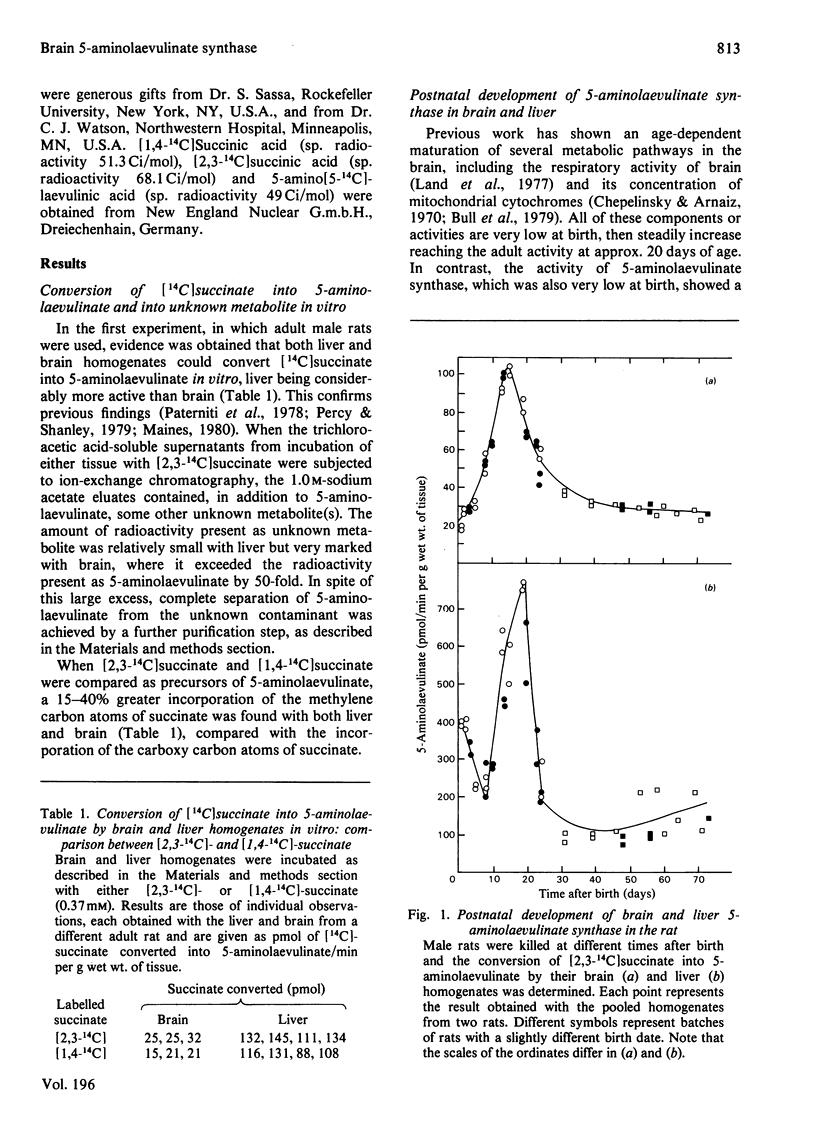
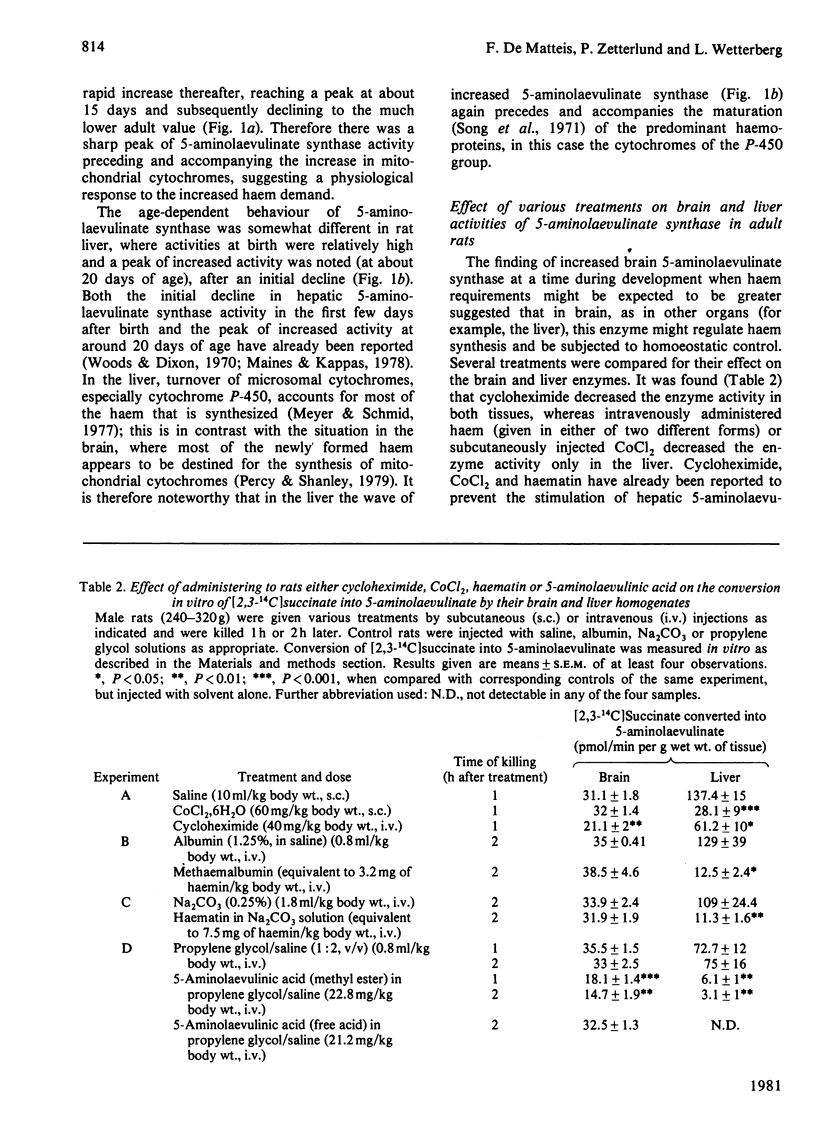
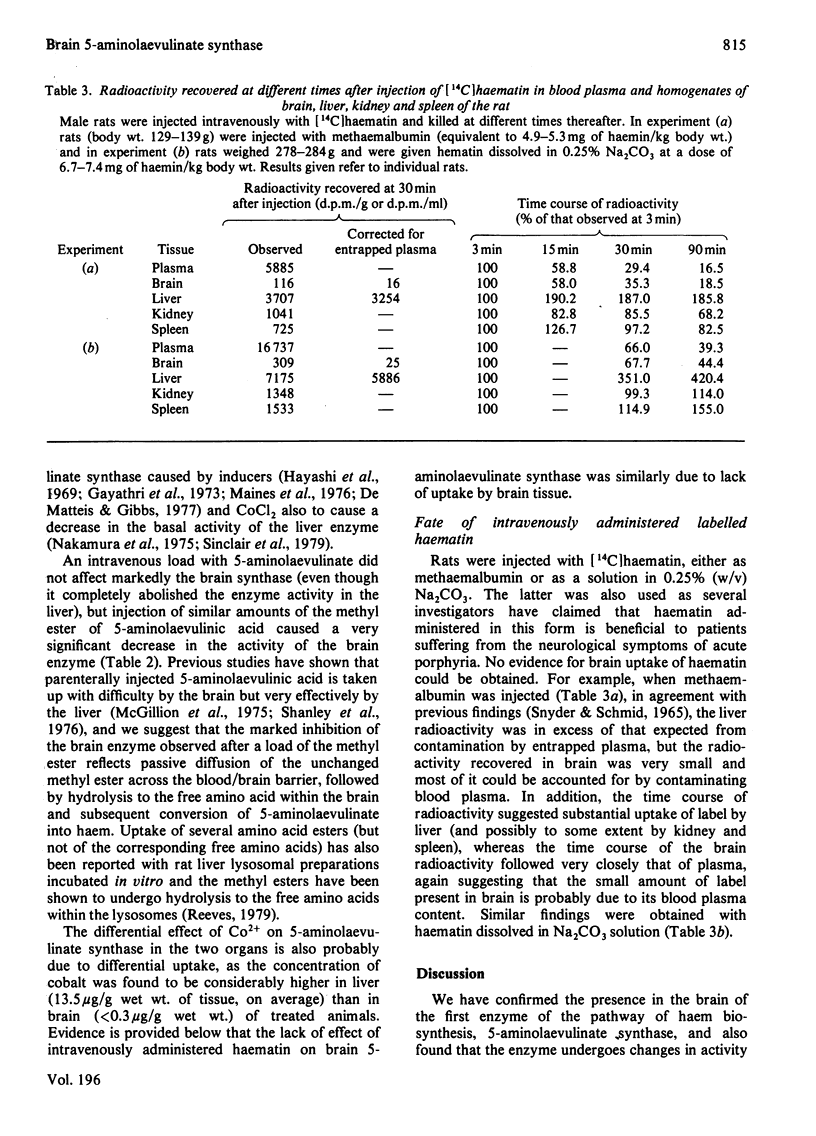
1. Brain 5-aminolaevulinate synthase showed a peak of increased activity in the first few weeks of life, which preceded and accompanied the development of brain cytochromes. 2. In the brain of the adult rat the activity of the enzyme was only 20% of that in the liver (on a per g wet wt. basis), but it was still probably sufficient to maintain the turnover of brain cytochromes. 3. The brain synthase activity could be decreased by treatment of rats with cycloheximide or with large doses of 5-aminolaevulinate, especially when this precursor was given as the methyl ester. 4. Injected haematin and CoCl2 markedly inhibited the synthase activity in the liver but failed to affect the brain enzyme; neither was taken up by the brain in vivo. 5. It is concluded that the brain can itself produce the haem required for the synthesis and turnover of its own haemoproteins and that 5-aminolaevulinate synthase may regulate the pathway in brain as in other tissues. 6. The relevance of the present findings to the pathogenesis of the neurological symptoms of acute porphyria and to the beneficial effect of exogenous haematin in porphyric patients is briefly considered.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aschenbrenner B., Druyan R., Albin R., Rabinowitz M. Haem a, cytochrome c and total protein turnover in mitochondria from rat heart and liver. Biochem J. 1970 Sep;119(2):157–160. doi: 10.1042/bj1190157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell D. M., Liem H. H., Muller-Eberhard U. Secretion of haem by hepatic parenchymal cells. Biochem J. 1979 Dec 15;184(3):689–694. doi: 10.1042/bj1840689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowsky H. L., Tschudy D. P., Collins A., Doherty J., Bossenmaier I., Cardinal R., Watson C. J. Repression of the overproduction of porphyrin precursors in acute intermittent porphyria by intravenous infusions of hematin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2725–2729. doi: 10.1073/pnas.68.11.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull R. J., Lutkenhoff S. D., McCarty G. E., Miller R. G. Delays in the postnatal increase of cerebral cytochrome concentrations in lead-exposed rats. Neuropharmacology. 1979 Jan;18(1):83–92. doi: 10.1016/0028-3908(79)90013-3. [DOI] [PubMed] [Google Scholar]
- Chepelinsky A. B., Rodríguez de Lores Arn Levels of cytochromes in rat-brain mitochondria during post-natal development. Biochim Biophys Acta. 1970 Mar 3;197(2):321–323. doi: 10.1016/0005-2728(70)90044-7. [DOI] [PubMed] [Google Scholar]
- Chiueh C. C., Sun C. L., Kopin I. J., Fredericks W. R., Rapoport S. I. Entry of [3H]norepinephrine, [125I]albumin and Evans blue from blood into brain following unilateral osmotic opening of the blood-brain barrier. Brain Res. 1978 Apr 28;145(2):291–301. doi: 10.1016/0006-8993(78)90863-6. [DOI] [PubMed] [Google Scholar]
- Condie L. W., Tephly T. R. delta-Aminolevulinic acid synthetase-sensitive methods in liver for hemoprotein biosynthesis. Methods Enzymol. 1978;52:350–354. doi: 10.1016/s0076-6879(78)52038-7. [DOI] [PubMed] [Google Scholar]
- Cunningham V. J., De Matteis F., Stonard M. D. The effect of cobalt on the uptake of plasma iron by the liver. Biochem J. 1976 Jul 15;158(1):105–108. doi: 10.1042/bj1580105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H. Inhibition of haem synthesis caused by cobalt in rat liver. Evidence for two different sites of action. Biochem J. 1977 Jan 15;162(1):213–216. doi: 10.1042/bj1620213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar G. J., Bossenmaier I., Petryka Z. J., Cardinal R., Watson C. J. Effects of hematin in hepatic porphyria. Further studies. Ann Intern Med. 1975 Jul;83(1):20–30. doi: 10.7326/0003-4819-83-1-20. [DOI] [PubMed] [Google Scholar]
- GRANICK S., URATA G. Increase in activity of alpha-aminolevulinic acid synthetase in liver mitochondria induced by feeding of 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biol Chem. 1963 Feb;238:821–827. [PubMed] [Google Scholar]
- Gayathri A. K., Rao M. R., Padmanaban G. Studies on the induction of -aminolevulinic acid synthetase iun mouse liver. Arch Biochem Biophys. 1973 Apr;155(2):299–306. doi: 10.1016/0003-9861(73)90118-5. [DOI] [PubMed] [Google Scholar]
- Hayashi N., Yoda B., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of delta-aminolevulinate synthetase in liver mitochondria. IV. Accumulation of the enzyme in the soluble fraction of rat liver. Arch Biochem Biophys. 1969 Apr;131(1):83–91. doi: 10.1016/0003-9861(69)90107-6. [DOI] [PubMed] [Google Scholar]
- Land J. M., Booth R. F., Berger R., Clark J. B. Development of mitochondrial energy metabolism in rat brain. Biochem J. 1977 May 15;164(2):339–348. doi: 10.1042/bj1640339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Maines M. D., Janousek V., Tomio J. M., Kappas A. Cobalt inhibition of synthesis and induction of delta-aminolevulinate synthase in liver. Proc Natl Acad Sci U S A. 1976 May;73(5):1499–1503. doi: 10.1073/pnas.73.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Prematurely evoked synthesis and induction of delta-aminolevulinate synthetase in neonatal liver. Evidence for metal ion repression of enzyme formation. J Biol Chem. 1978 Apr 10;253(7):2321–2326. [PubMed] [Google Scholar]
- Maines M. D. Regional distribution of the enzymes of haem biosynthesis and the inhibition of 5-aminolaevulinate synthase by manganese in the rat brain. Biochem J. 1980 Aug 15;190(2):315–321. doi: 10.1042/bj1900315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillion F. B., Thompson G. G., Goldberg A. Tissue uptake of o-aminolaevulinic acid. Biochem Pharmacol. 1975 Jan 15;24(2):99–301. doi: 10.1016/0006-2952(75)90295-6. [DOI] [PubMed] [Google Scholar]
- Meyer U. A., Schmid R. Intermittent acute porphyria: the enzymatic defect. Res Publ Assoc Res Nerv Ment Dis. 1974;53:211–224. [PubMed] [Google Scholar]
- Nakamura M., Yasukochi Y., Minakami S. Effect of cobalt on heme biosynthesis in rat liver and spleen. J Biochem. 1975 Aug;78(2):373–380. doi: 10.1093/oxfordjournals.jbchem.a130917. [DOI] [PubMed] [Google Scholar]
- Paterniti J. R., Jr, Simone J. J., Beattie D. S. Detection and regulation of delta-aminolevulinic acid synthetase activity in the rat brain. Arch Biochem Biophys. 1978 Jul;189(1):86–91. doi: 10.1016/0003-9861(78)90117-0. [DOI] [PubMed] [Google Scholar]
- Percy V. A., Shanley B. C. Studies on haem biosynthesis in rat brain. J Neurochem. 1979 Dec;33(6):1267–1274. doi: 10.1111/j.1471-4159.1979.tb05273.x. [DOI] [PubMed] [Google Scholar]
- Peterson A., Bossenmaier I., Cardinal R., Watson C. J. Hematin treatment of acute porphyria. Early remission of an almost fatal relapse. JAMA. 1976 Feb 2;235(5):520–522. [PubMed] [Google Scholar]
- Reeves J. P. Accumulation of amino acids by lysosomes incubated with amino acid methyl esters. J Biol Chem. 1979 Sep 25;254(18):8914–8921. [PubMed] [Google Scholar]
- SNYDER A. L., SCHMID R. THE CONVERSION OF HEMATIN TO BILE PIGMENT IN THE RAT. J Lab Clin Med. 1965 May;65:817–824. [PubMed] [Google Scholar]
- Sasame H. A., Ames M. M., Nelson S. D. Cytochrome P-450 and NADPH cytochrome c reductase in rat brain: formation of catechols and reactive catechol metabolites. Biochem Biophys Res Commun. 1977 Oct 10;78(3):919–926. doi: 10.1016/0006-291x(77)90510-1. [DOI] [PubMed] [Google Scholar]
- Shanley B. C., Percy V. A., Neethling A. C. Pathogenesis of neural manifestations in acute porphyria. S Afr Med J. 1977 Apr 2;51(14):458–460. [PubMed] [Google Scholar]
- Sinclair P., Gibbs A. H., Sinclair J. F., de Matteis F. Formation of cobalt protoporphyrin in the liver of rats. A mechanism for the inhibition of liver haem biosynthesis by inorganic cobalt. Biochem J. 1979 Mar 15;178(3):529–538. doi: 10.1042/bj1780529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C. S., Moses H. L., Rosenthal A. S., Gelb N. A., Kappas A. The influence of postnatal development on drug-induced hepatic porphyria and the synthesis of cytochrome P-450. A biochemical and morphological study. J Exp Med. 1971 Nov 1;134(5):1349–1371. doi: 10.1084/jem.134.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- URATA G., GRANICK S. Biosynthesis of alpha-aminoketones and the metabolism of aminoacetone. J Biol Chem. 1963 Feb;238:811–820. [PubMed] [Google Scholar]
- Watson C. J., Dhar G. J., Bossenmaier I., Cardinal R., Petryka Z. J. Effect of hematin in acute porphyric relapse. Ann Intern Med. 1973 Jul;79(1):80–83. doi: 10.7326/0003-4819-79-1-80. [DOI] [PubMed] [Google Scholar]
- Watson C. J. Editorial: Hematin and porphyria. N Engl J Med. 1975 Sep 18;293(12):605–607. doi: 10.1056/NEJM197509182931210. [DOI] [PubMed] [Google Scholar]
- Woods J. S., Dixon F. L. Perinatal differences in delta-aminolevulinic acid synthetase activity. Life Sci II. 1970 Jun 22;9(12):711–719. doi: 10.1016/0024-3205(70)90279-1. [DOI] [PubMed] [Google Scholar]


