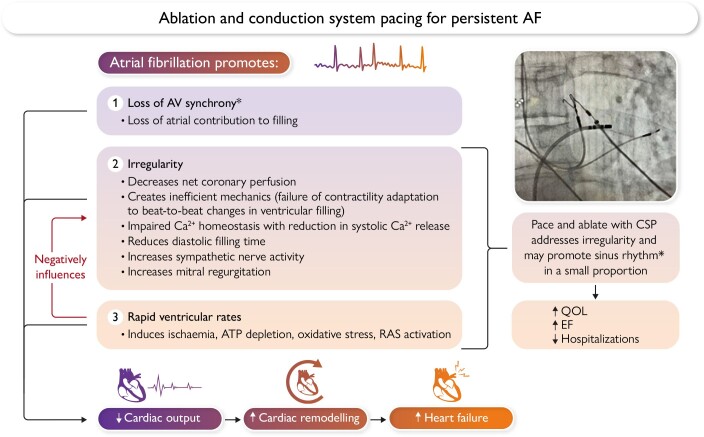
Graphical Abstract
Graphical Abstract.
The mechanisms through which the pace-and-ablate strategy influences the hemodynamic effects of atrial fibrillation.
Keywords: Atrial fibrillation, Catheter ablation, Conduction system pacing, Atrioventricular node ablation
Abstract
Despite key advances in catheter-based treatments, the management of persistent atrial fibrillation (AF) remains a therapeutic challenge in a significant subset of patients. While success rates have improved with repeat AF ablation procedures and the concurrent use of antiarrhythmic drugs, the likelihood of maintaining sinus rhythm during long-term follow-up is still limited. Atrioventricular node ablation (AVNA) has returned as a valuable treatment option given the recent developments in cardiac pacing. With the advent of conduction system pacing, AVNA has seen a revival where pacing-induced cardiomyopathy after AVNA is felt to be overcome. This review will discuss the role of permanent pacemaker implantation and AVNA for AF management in this new era of conduction system pacing. Specifically, this review will discuss the haemodynamic consequences of AF and the mechanisms through which ‘pace-and-ablate therapy’ enhances outcomes, analyse historical and more recent literature across various pacing methods, and work to identify patient groups that may benefit from earlier implementation of this approach.
Introduction
Atrial fibrillation (AF) is a common cardiac arrhythmia, resulting in substantial impairments in morbidity and mortality.1 While the management of paroxysmal AF has become increasingly straightforward, persistent AF continues to present a clinical dilemma, having undergone several substantial philosophical shifts in management over the last decade. Historically, the management of persistent AF was centred on pharmacological rate control strategies, with permanent pacemaker implantation and atrioventricular node ablation (AVNA) being considered a reasonable second-line strategy when drug therapy was ineffective or poorly tolerated. In these patients, AVNA was demonstrated to be highly effective in improving quality of life (QOL)2,3 while decreasing healthcare costs through a reduction in hospitalizations, outpatient visits, and antiarrhythmic drug use (AAD).4 However, concern regarding an increased risk of heart failure (HF) secondary to chronic right ventricular apical pacing-induced ventricular dyssynchrony limits the widespread use of this strategy. With the advent of conduction system pacing (CSP) targeting the His bundle or left bundle branch area, AVNA has seen a revival where ventricular dyssynchrony may be less of an issue.
This review will discuss the role of permanent pacemaker implantation and AVNA (‘pace-and-ablate’) for AF management in this new era of CSP. The haemodynamic consequences of AF and mechanisms by which the pace-and-ablate approach proposes to improve outcomes will be identified. Finally, a thorough analysis of the historical and more current literature on pace-and-ablate therapy across various pacing methods and the critical areas for research necessary to move this field forward will be considered.
Persistent atrial fibrillation as a clinical problem
Atrial fibrillation ablation is an established rhythm control strategy to prevent recurrence. It has shown to be superior to AAD therapy in maintaining sinus rhythm and for symptomatic improvement when performed as either an initial (‘first-line’) or ‘second-line’ therapy (i.e. when antiarrhythmic drugs have been ineffective, are contraindicated, or produce intolerable adverse effects).1,5 However, left atrial catheter ablation is not universally curative. Several factors predictive of AF recurrence have been identified, including increasing age, female sex, increased left atrial dimensions, renal dysfunction, longer duration of AF, and the presence of coronary artery disease.6 In those with persistent AF, there is an average 40%–45% chance of maintaining sinus rhythm at two years following a single ablation procedure without the use of AADs.7 While success is improved with repeat ablation procedures and the concurrent use of AADs, the likelihood of maintaining sinus rhythm at 5 years is only 50% in those with persistent AF and decreases to 40% in those with long-standing persistent AF.8 The CABANA study has shown lower efficacy of catheter ablation in the elderly.9 Importantly, trials investigating patients with persistent AF ablation have been constrained by limited follow-up durations, with many patients still necessitating AAD therapy despite intervention. This potentially leaves many patients with symptomatic persistent AF without adequate relief where it may be reasonable to consider an earlier rate control approach with a pace-and-ablate strategy.10
Haemodynamic consequences of atrial fibrillation: irregulopathy
Through excessive heart rates, beat-to-beat irregularity, absence of atrial contraction, and reduced coronary flow reserve, AF causes harmful effects upon left ventricular (LV) mechanical stretch and sympathetic nerve activity, leading to unfavourable haemodynamic consequences (Graphical Abstract). The cycle ensues with increased filling pressures and neurohormonal changes, resulting in atrial fibrosis and altered calcium handling, leading to further adverse cardiac remodelling, referred to as arrhythmia-induced cardiomyopathy.11
Early pioneering work attempting to elucidate the effect of ‘irregularity’ on intracardiac pressures and cardiac output (CO) began in 1983 when Naito et al.12 studied the haemodynamic effects of atrioventricular (AV) block in canine hearts. After open surgical AVNA was performed in 20 mongrels, ventricular pacing with a regular and irregular (five extra-stimuli introduced at varying coupling intervals) pattern was performed with each sequence from the left atrium and the LV apex: AV sequential pacing at 100 ms; ventricular-only pacing during sinus rhythm (with AV dissociation); AF with ventricular pacing; and AV sequential pacing but with an AV interval of −100 ms. They found that a normal AV sequence was of ultimate importance as AV sequential pacing resulted in optimal haemodynamics during both regular and irregular pacing. The most deleterious haemodynamic effects (reduced CO, increased left atrial pressure, and reduced LV pressure) were observed when atrial systole was superimposed on a ventricular contraction resulting in active retrograde atrial emptying, and although AV dissociation resulted in overall lower CO than during sequential AV pacing, only irregularly pacing the ventricle during AF resulted in a further drop in CO. The CO during this particularly deleterious sequence was indistinguishable from AF with irregular ventricular pacing. The loss of the atrial kick appeared to be the causal factor for the initial 22% reduction in CO, but pacing irregularly resulted in a further 9% CO drop. In other words, regular paced rhythms were still better than irregularly paced rhythms. Mitral valve regurgitation during angiography was observed in this study only in the presence of irregularity.
Further exploration into ‘irregulopathy’ was pursued by haemodynamic evaluation at the time of AVNA.13,14 Both regular and irregular ventricular pacing protocols at faster and slower heart rates were tested in patients. An irregular ventricular rhythm, independent of rate, was found to decrease the CO by 12%–15% when compared with a regular ventricular-paced rhythm.13,14 Irregular pacing also increased pulmonary capillary wedge pressure and right atrial pressure.14 Failure of cardiac contractility adaptation during beat-to-beat changes in ventricular filling may explain part of the mechanism for haemodynamic deterioration during irregular rhythms. The reduction in stroke volume that occurs with short RR intervals may not be entirely compensated for by the increase in stroke volume accompanying long RR intervals. Combined computational modelling of beat-to-beat speckle tracking on echocardiographic images confirms these findings, where beat-to-beat changes in preload explain the differences in LV systolic function, and that a reduced diastolic filling time can explain the variability of LV function in patients with AF.15 Geelen et al.16 further demonstrated consistent haemodynamic improvements over time post-AVNA,16 accompanied by improvements in cardiac index, and reductions in LV end-diastolic pressures and dimensions at 6 months.
Other underlying mechanisms for the reduced output attributed to irregularity invoke neurohormonal and vasomotor factors. A greater increase in atrial natriuretic peptide (ANP) is measured during irregular vs. regular ventricular pacing causing arterial and venous dilation with vagally mediated inhibition of cardiac sympathetic input. Plasma ANP and brain natriuretic peptide levels have been shown to progressively decrease after rate regularization post-AVNA.17
Irregularity may also reduce CO through a reduction in myocardial perfusion. Atrial fibrillation causes an increase in coronary flow that is insufficient to meet the increased myocardial oxygen demand. Using Doppler guide wires positioned in the left coronary system, coronary flow measurements were taken during sinus rhythm, induced AF, and right atrial pacing.18 The increase in coronary flow following AF induction is independent of the changes in heart rate and blood pressure, suggesting this increase is not proportional to that required by an augmented myocardial oxygen demand. Importantly, the loss of atrial contraction during AF induction did not affect the coronary vascular resistance, suggesting that the irregularity itself is solely responsible for the coronary vasoconstriction that acts in opposition to dilation during AF, thus impeding coronary flow and reducing coronary flow reserve.
An irregular rhythm also causes distinct functional and molecular LV remodelling in the absence of tachycardia. Irregularity is associated with alterations in ventricular cardiomyocyte Ca2+ haemostasis with a reduction in systolic Ca2+ release.19 A diminished Ca2+ load within the sarcoplasmic reticulum (SR) together with increased SR Ca2+ leak via hyperphosphorylation of the ryanodine receptor has been shown to lower the systolic Ca2+ amplitude. This reduction is a key participant in contractile dysfunction present in patients with HF.20 An increase in cytosolic Na+ causing action potential prolongation and oxidative stress within the LV myocardium further contributes to the Ca2+ handling changes in the AF ventricle.19,21
Increased sympathetic nerve activity is observed after AF induction which is in part attributable to the irregular ventricular response.22 Efferent post-ganglionic muscle sympathetic nerve activity was recorded from the left peroneal nerve in eight patients undergoing electrophysiology study. Patients underwent induction of AF, and in those who converted to sinus rhythm afterwards, irregular and regular RA pacing was performed. During AF, an increase in sympathetic activity was noted when compared with sinus rhythm. A further increase was noted during irregular pacing when compared with regular pacing without a significant change in blood pressure or central venous pressure. Given that increased sympathetic activity is known to be detrimental, particularly in patients with LV dysfunction, removing irregularity by restoration of sinus rhythm or through AV node ablation should help patients.
Impacts of pace-and-ablate therapy on atrial fibrillation management
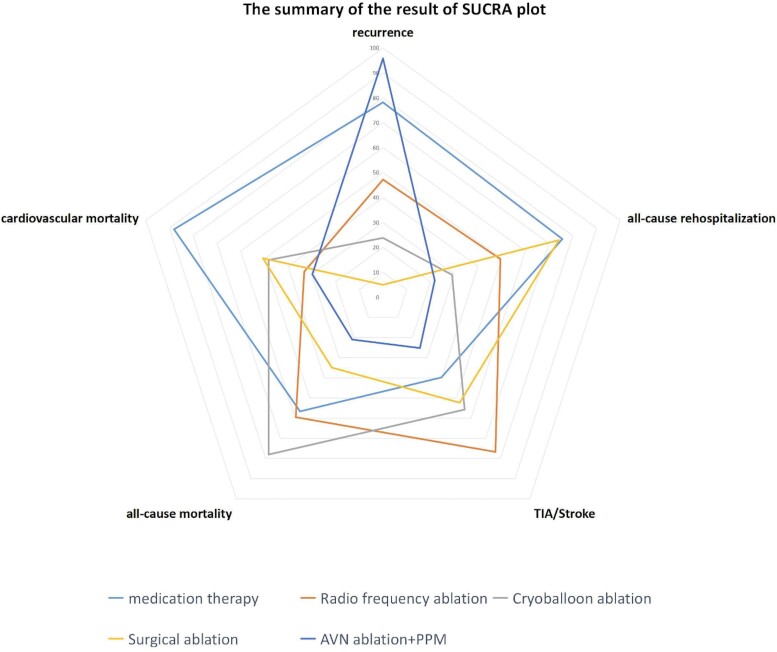
A recent network meta-analysis aimed to compare various AF therapies, ranking the efficacy and safety among drug therapy, AF ablation strategies (RF, cryoballoon, and surgical ablation), and pace-and-ablate therapy. In a pairwise comparison, it was shown that although AF ablation strategies performed best in terms of reducing AF recurrence, a pace-and-ablate approach was consistently better than any other treatment in reducing cardiovascular and all-cause mortality, re-hospitalization, and stroke as shown in Figure 1.23
Figure 1.
Radar plot indicating the overall risk of efficacy and safety endpoints from the different atrial fibrillation management strategies. It illustrates that with the exception of recurrences, pace-and-ablate strategy for atrial fibrillation has a good efficacy and safety profile. See permission letter from Professor Zeyi Cheng for Figure 1.23
Interestingly, a recent prospective observational study of patients with persistent AF demonstrated spontaneous return to sinus rhythm in a small subset of patients after pace-and-ablate therapy with CSP.24 We speculate that the treatment of the irregulopathy favours normalization of atrial pressures and volumes, with a reduction in atrial stretch, which may lead to reverse atrial remodelling responsible for spontaneous restoration of sinus rhythm. Alternatively, the return to sinus rhythm may also simply reflect the routine chance of spontaneous conversion to sinus rhythm.
Pace-and-ablate therapy in heart failure
A decision on rate or rhythm control with drugs or invasive treatment is complex and highlights the importance of an electrophysiologist in the multidisciplinary team setting to improve the decision-making process on arrhythmia management in patients with HF. There is mounting evidence that AF ablation improves clinical outcomes in paroxysmal and patients with persistent AF with HF.25,26 The patients included in these trials were relatively young without multiple comorbidities not necessarily reflecting the make-up of a typical HF clinic.25 The PABA-CHF trial showed superiority of pulmonary vein isolation (PVI) when compared with AVNA with biventricular cardiac resynchronization therapy (BIV-CRT) in patients with low ejection fraction (EF) in terms of improved EF, QOL, and 6-min walk scores, underscoring the importance of sinus rhythm when attainable.27 The average age of these patients was 60 years with left atrial diameters of 4.7 cm reflecting a population more likely to maintain sinus rhythm. Conversely, the RAFT-AF trial failed to demonstrate superiority of ablation-based rhythm management over rate control for the primary endpoint of HF hospitalization and mortality, although the trial was underpowered having stopped early due to recruitment concerns.28 Both studies had limited follow-up and the longer-term costs of re-hospitalization and repeat procedures were not considered.
The APAF-CRT trial highlighted the potential re-emergence of pace-and-ablate therapy in patients with HF.29 Atrioventricular node ablation and BiV-CRT implantation in patients with HF with permanent AF and at least one recent hospitalization were shown to reduce mortality compared with medical rate control over a follow-up of 4 years. It is worth noting that many patients included in this study had good rate control with medical therapy. The clinical benefit was hypothesized to result from the combination of rate lowering and rate regularization by AVNA in combination with BiV pacing to avoid ventricular dyssynchrony.
Pacing strategy with atrioventricular node ablation
Atrioventricular node ablation and right ventricular pacing
For decades, the right ventricle (RV) has been the preferred location for pacing given its reliability, stability of lead parameters over time, and easy accessibility. Early studies evaluating the role of AVNA and RV pacing (RVP) therapy when compared with medical therapy have been summarized30 and are re-adapted in Table 1. In a total of 21 studies consisting of 1181 patients including two randomized controlled trials,2,38 pace-and-ablate therapy with RV apical pacing significantly reduced cardiac symptoms and healthcare use while improving exercise tolerance, QOL, and left ventricular ejection fraction (LVEF).30 The success of this strategy stems directly from rhythm regularization, as it is well known that introducing iatrogenic pacing-induced ventricular dyssynchrony in patients with a baseline narrow QRS may simultaneously be harmful, particularly for those with a reduced LVEF. This dyssynchrony may manifest as a reduction in LVEF causing pacing-induced cardiomyopathy (PICM), the prevalence of which is ∼12% increasing to 15%–20% after 5 years in those with normal baseline ventricular function who have >20% pacing.43–45 Atrioventricular node ablation and RVP studies therefore may not necessarily account for the development of PICM given the insufficient follow-up duration, the lack of follow-up imaging, and the absence of a widely acknowledged definition of PICM. Nevertheless, AVNA with permanent RVP is currently recommended as a reasonable strategy to control the heart rate in AF irrespective of QRS duration in patients with preserved EF (Class IIa).46
Table 1.
Studies comparing atrial fibrillation node ablation with right ventricle pacing vs. medical therapy (limited to studies including 20 or more patients)
| Author, year | No. of pts | Trial design | PAF/permanent AF %/% | EF | Comparison groups | Post-AVNA pacing rate | F/up (m) | Sudden death or early VT/VF, n (%) |
Total Mort (%) |
Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|
| Geelen16 1997 | 235 | Retr + Pros | 0/100 | 58 ± 16 | Analysis of 100 AVNA pts with pacing set at ≤70 vs. prosp. eval of 135 post-AVNA pace set at 90 b.p.m. for 1–3 m (then down to 70 b.p.m. thereafter) with an initial 48 h of in-hospital monitoring | 20 | 6 (6) in post-pacing at ≤70 vs. 0 (0) in 90 b.p.m. group |
|
||
| Morady31 1993 |
40 | pros RCT |
45/55 | 54 ± 14 | Direct current shocks vs. radiofrequency ablation for AVNA | 70–120 b.p.m. | 12 | 0 (0) | 0 (0) |
|
| Olgin32 1997 |
54 | Retr | - | 51 ± 13 | RF energy vs. direct current energy for AVNA | 24 | 2 (4) | 4 (7) |
|
|
| Brignole33 1994 |
23 | Pros RCT | 0/23 | 46 ± 11 | AVNA vs. medical therapy | VVIR 70–130 b.p.m. | 3 | 0 (0) | 1 (4) |
|
| Jensen4 1995 |
50 | Retr | 46/54 | Retro review of all AVNA | 80 b.p.m. | 17 (4–36) | 2 (4) | 6 (12) |
|
|
| Edner34 1995 | 29 | Pros | 41/59 | 45 | LVEF and early filling deceleration times (Edec) after 1–2 h of v-pacing at 80 b.p.m. -Pts divided into baseline EF <50% and ≥50% |
VVI/VVIR 80 b.p.m. × 1 week and for 1–2 h prior to each echo | 7 | 1 (3) |
|
|
| Fitzpatrick35 1996 |
107 | Retr | 46/54 | 51 ± 10 | Retro review of all AVNA | 2.3 y | 3 (3) | 17 (16) |
|
|
| Bubien36 1996 |
44 | Pros | 229 | ? | QOL at 6 mo vs. QOL pre-AVNA | 6 | 2 (4) |
|
||
| Darpo37 1997 |
220 | Retr | 48/52 | 50 ± 13 | Retro review of all AVNA | DDDR or VVIR 70–80 b.p.m. first 1–3 weeks | 31 | 11 (5) | 31 (14) |
|
| Brignole38 1997 |
43 | RCT | 100/0 | 58 ± 11 | DDDR + AVNA vs. drugs | DDDR 70–130 | 6 | 0 (0) | 0 (0) |
|
| Buys39 1997 |
25 | Pros | 0/100 | Exercise capacity pre- and post-AVNA | VVIR | 7 | 0 (0) | 0 (0) |
|
|
| Twidale40 1998 |
22 | Pros | 0/100 | 33 ± 9 | HF pts with digoxin and ACEi + diuretic and EF < 45%. AVNA vs. AVN mod. | 14 | 2 (9) | 2 (23) |
|
|
| Lee2 1998 |
30 | RCT | 50/50 | 51 ± 6 | AVNA vs. AV node modification without pacemaker | VVIR 70–120 | 6 | 0 (0) | 0 (0) |
|
| Ablate and Pace Trial Kay3 1998 |
156 | Pros | 55/45 | 50 | AVNA on QOL, survival, exercise capacity and vent function | Operator choice |
12 | 5 (3) | 23 (15) |
|
| Brignole38 1997 |
66 | RCT | 100/0 | VVIR + AVNA vs. meds | VVIR 70–130 b.p.m. | 6 | 0 (0) | 0 (0) |
|
|
| Marshall41 1999 |
56 | RCT | 100/0 | Fractional shortening 30% | DDDR/MS + AVNA vs. medical therapy | lower rate 70 b.p.m. | 4 | 0 (0) | 0 (0) |
|
| AIRCRAFT Weerasooriya42 2003 |
99 | RCT | 0/100 | 54% AVNA 61% med | AVNA vs. medical therapy | VVIR 80–90 b.p.m. × 1 month then as per treating physician | 12 | 3 (3) 2 in AVNA arm, both with low EF; 1 in med arm |
3 (3) in total |
|
SD, sudden death; Sx, symptoms; QOL, quality of life; S, significant; NS, non-significant; HF, heart Failure; HFH, heart failure hospitalization; AVNA, atrioventricular nodal ablation; AF, atrial fibrillation; BiV, biventricular pacing; RV, right ventricular pacing; EF, ejection fraction; 6MWD, 6-min walk distance; NYHA, New York Heart Association; Resp, respectively; h, hours; m, months; Retro, retrospective; Pros, prospective; RCT, randomized controlled trial; RF, radiofrequency.
Atrial fibrillation node ablation and biventricular pacing
Biventricular pacing (BiVP) is considered a superior alternative for patients undergoing AVNA because it effectively addresses the dyssynchrony caused by RVP. Supported by extensive data (Table 2), BiVP corrects inter- and intraventricular mechanical dyssynchrony, reduces mitral regurgitation, and contributes to long-term beneficial effects on myocardial remodelling.55 The evidence supporting BiVP primarily comes from studies in patients with AV block and reduced LVEF;56–58 however, there have been no separate analyses of outcomes for patients with wide vs. narrow baseline QRS. Biventricular pacing in patients with narrow QRS will always result in QRS prolongation that invariably introduces a degree of electrical and likely mechanical dyssynchrony. Harmful effects have been demonstrated when BiVP is performed in patients with a baseline narrow QRS with pre-existing LV dysfunction59–62 because slow myocyte-to-myocyte conduction from an LV pacing site will not reproduce normal ventricular activation. Consequently, the 2021 ESC guidelines gives BiVP a Class IIb indication for those undergoing AVNA, irrespective of QRS duration, where the LVEF is preserved, a Class IIa for those with mildly reduced LVEF, and a Class I for patients with reduced LVEF.46
Table 2.
Studies comparing biventricular vs. right ventricular pacing for atrioventricular node ablation
| Author, year | No. of pts | Trial Design | EF | Pt Population | Experimental group |
Control group | 1° endpt and pacing rate post | F/up (m) | Conclusions |
|---|---|---|---|---|---|---|---|---|---|
| Leon47 2002 |
20 | Prosp | 21 ± 7% | pts with previous AVNA for permanent AF | Upgrade from RV pacing to BiV pacing | none | NYHA, hospitalizations, QOL ECG and echo |
17 |
|
| MUSTIC Linde48 2002 |
43 | RCT | 26% | EF < 35% NYHA III, persistent AF AVNA | 3-month crossover btw BiV vs. RVp | 6MWD | n/a |
|
|
| OPSITE Puggioni49 2004 |
44 | RCT | 36.6% | Permanent AF | LVp + AVNA | RVp + AVNA | EF |
|
|
| Simantirakis50 2004 | 12 | Prosp | 44% | Permanent AF | LV pacing | RVp + AVNA | Haemodynamics | 24 h |
|
| PAVE Doshi51 2005 |
184 | RCT | Permanent AF undergoing AVNA | BiV + AVNA | RVp + AVNA | 6MWD | 6 |
|
|
| OPSITE Brignole52 2011 |
56 | Crossover RCT | 38 ± 14 | Permanent AF undergoing AVNA with or without HF | 3-month crossover btw (i) RV and LV pacing (ii) RV and BiV |
QOL Exercise capacity |
n/a |
|
|
| AVAIL Orlov53 |
153 | RCT 4:1 CRT: RV |
BiV: 56% RVp 57% |
Persistent or permanent AF with AVNA NYHA II–III | BiV pacing | RVp | Echo | 6 |
|
| APAF Brignole54 2018 |
186 | RCT | 38% | Permanent AF undergoing AVNA with or without HF | BiV pacing | RVp | Composite of Death, HF or HFH | 20 |
|
| APAF-CRT Mortality Trial Brignole29 2021 |
133 | RCT | 41% | Permanent AF, narrow QRS, HFH, severe symptoms | BiV pacing | Meds | 1° endpt all-cause mortality 2° endpt combined mortality or HFH |
29 |
|
S, Significant; NS, non-significant; HF, heart failure; HFH, heart failure hospitalization; AVNA, atrioventricular nodal ablation; AF, atrial fibrillation; BiV, biventricular pacing; RV, right ventricular pacing; EF, ejection fraction; 6MWD, 6-min walk distance; NYHA, New York Heart Association; resp, respectively; h, hours; m, months; endpt, endpoint; RVp, right ventricular pacing.
Atrioventricular node ablation and conduction system pacing
Pacing the proximal conduction system has become a blossoming area of interest (Figure 2, Table 3). His bundle pacing (HBP) has shown to be a safe and efficacious pacing strategy where direct capture of the His bundle maintains perfect biventricular activation with a resulting narrow QRS. After the initial achievement of HBP in 12 patients with refractory AF undergoing AVNA by Deshmukh et al.64 HBP has demonstrated in several small randomized trials and larger non-randomized studies to be superior to RVP and even at least as good as BiVP post-AVNA.78 Small but significant improvements in LVEF were noted in 38 patients with HBP when compared with BiVP in those with LVEF ≤40% and narrow QRS undergoing a pace-and-ablate strategy in the ALTERNATIVE-AF trial.73 Its documented feasibility alongside AVNA however must be considered in the light of the unique challenges posed by the close proximity of the ablation site to the pacing electrode and the general concern for low sensing values with possible atrial oversensing and late rises in capture thresholds frequently necessitating an RV backup lead.79–81 The HBP implant remains challenging,82 compounded by the limited availability of advanced implantation tools and a decreasing number of specialized operators. Nevertheless, once in experienced hands, success rates for HBP in patients with narrow QRS with AV block are similar to success rates in left bundle branch area pacing (LBBAP).83
Figure 2.
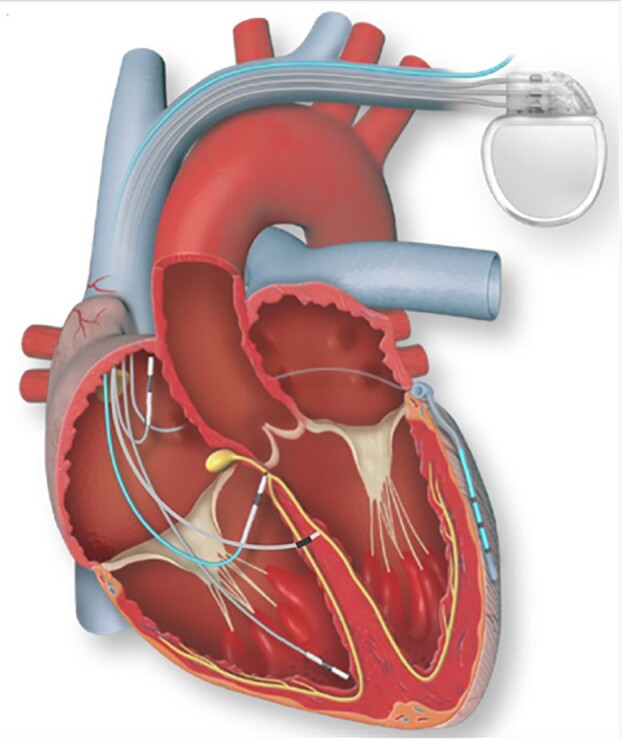
Pacing modalities in patients undergoing atrioventricular node ablation: right ventiricular pacing, his bundle pacing, left bundle branch pacing, and biventricular pacing. See permission letter from Professor Glikson Figure 2.63
Table 3.
Studies to date comparing conduction system pacing with any alternative for atrioventricular node ablation
| Author, year | n | Trial design | Base EF |
Pt population | Exp. group |
Cont. group | 1° endpt | F/ up (m) |
Conclusions |
|---|---|---|---|---|---|---|---|---|---|
| Deshmukh64 2000 | 14 | Prosp | 20% | Permanent AF, dilated CM, narrow QRS | HBP + AVNA | n/a | Echo response | 23 |
|
| Deshmukh65 2004 | 54 | Prosp | 23% | Persistent AF, CM, narrow QRS | HBP + AVNA | n/a | Echo response, cardio- pulmonary response | 42 |
|
| Occhetta66 2006 | 18 | RCT | 52% | AF refractory to medical therapy | 6-month crossover between HBP vs. RVp and AVNA | Safety Feasibility 6MWD QOL NYHA |
12 |
|
|
| Occhetta67 2007 | 68 | Retro | 51% | AF refractory to medical therapy; narrow QRS | HBP + AVNA | n/a | NYHA, QOL, echo response | 21 |
|
| Vijayaraman68 2017 | 42 | Retro | 45% | AF refractory to medical therapy | HBP + AVNA | n/a | Success Echo response NYHA |
19 |
|
| Huang69 2017 | 42 | Prosp | 45% | Symptomatic HF with persistent AF | HBP | none | Echo, NYHA, mortality, hosp, HF meds | 20 |
|
| Wang70 2019 | 86 | Retro case-control | 35% | Persistent AF with HF and ICD indication | HBP + ICD + AVNA | ICD | Safety Echo response, NYHA |
30 |
|
| Su71 2020 | 81 | Prosp | 45% ± 14.9 | Long-standing persistent AF with HF and narrow QRS | HBP | none | Echo NYHA mortality |
Mean 36 |
|
| 2021 Senes72 | 24 | Prosp propensity match | 35% BiV | CRT indication for HF and AVNA | HBP/HBP + LV lead | BiV | Comp of death, HFH, HF | 9.6 |
|
| ALTERNATIVE-AF trial Huang73 2022 |
50 | RCT | 32.8 ± 8.9 | Persistent AF and LVEF ≤40%, NYHA II–IV, QRS < 120 ms or RBBB | HBP on for 9 mo. | BiV On for 9 mo. |
LVEF change NYHA QOL LVEDd BNP |
18 |
|
| Ivanovski74 2022 | 50 | Retro | 39% | Sympt and refractory AF EF < 50% NYHA II–IV QRS < 120 ms |
CSP: HBP or LBBP |
BiV | QRS Echo NYHA |
2–6 |
|
| Vijayaraman75 2022 | 223 | Retro | 43 ± 15 | Rates refractory to meds, > 6 mo follow-up | CSP | RV | 27 ± 19 |
|
|
| Qi76 2023 | 31 | Prosp | 60% | Persistent AF refractory to 2 ablations; symptomatic | CSP 22 HBP 9 LBBP |
none | Symptoms Echo BNP |
6 |
|
| Palmisano77 2023 | 373 | Prosp multicentre |
41%–42% | CSP: LBBaP (42) or HBP 9 (68) | CRT | incidence of device-related complications Secondary: HF hospitalization, pacing performance, AVNA outcome |
12 |
|
|
S, significant; NS, non-significant; HF, heart failure; HFH, heart failure hospitalization; AVNA, atrioventricular nodal ablation; AF, atrial fibrillation; BiV, biventricular pacing; RV, right ventricular pacing; EF, ejection fraction; 6MWD, 6-min walk distance; NYHA, New York Heart Association; resp, respectively; h, hours; m, months; CM, cardiomyopathy; Prosp, prospective; Retro, retrospective; RCT, randomized controlled trial; HBP, His bundle pacing; LBBP, left bundle branch pacing.
Left bundle branch area pacing was introduced after showing it was possible to penetrate the interventricular septum to target the left side of the septum,84 and capture the left bundle.85 Stimulating the left bundle maintains near-normal LV electrical activation.86,87 In the large multicentre MELOS registry, the initial experience from 14 European sites performing LBBAP demonstrated feasibility as a primary technique for both patients with bradycardia and CRT indications where only 1.8% of patients experienced a threshold rise to an absolute value >2.0 V over 18 months of observation.88 Vijayaraman et al.75 demonstrated similar electrical and procedural outcomes of CSP (HBP or LBBAP) vs. conventional pacing (RVP or BiVP) in a retrospective cohort of 223 patients undergoing AVNA. Similar findings were observed in a prospective evaluation where at 12-month follow-up, LBBAP maintained the lowest capture thresholds and longest estimated residual battery longevity with a similar risk of device-related complications and HF hospitalizations.77
Biventricular pacing vs. His bundle pacing vs. left bundle branch area pacing
Despite the level of complexity that BiVP may require pre-AVNA (need for implant expertise and precise tools, suitable coronary sinus branches, and the presence of a complex device with an additional lead), its main default is that it does not avoid the dyssynchrony created in patients with a baseline narrow QRS. While there is a strong rationale for superiority when compared with conventional RVP, when a narrow QRS is obtained, the same cannot be assumed in cases where a non-physiological wide QRS persists post-implant. Furthermore, the baseline QRS in BiV pacing studies was wide (>120 ms), where benefit may have been observed from resynchronization itself. The typical candidate for BiVP is a patient with wide LBBB and HF with reduced EF (HFrEF). In these patients, BiVP is proved to be effective in reducing HF and mortality where electrical uncoupling, septal myocardial scar, or functional conduction block will create lateral activation delays that will never respond to CSP in isolation. In theory, when considering AVNA, BiVP should primarily be used in those with a baseline wide QRS >120 ms and the presence of HF as opposed to any QRS duration as suggested in the guidelines and in the absence of data for CSP at the time of writing. Certainly, the reality is more nuanced as CSP does not always achieve a consistently narrow QRS complex particularly in patients with HF with a wide baseline QRS complex. While there are established criteria for successful implantation, particularly for LBBAP, left bundle capture cannot always be obtained.89 When suboptimal electrical resynchronization is obtained with CSP due to additional distal conduction delay, septal myocardial scar, or lack of conduction system capture for other reasons, the addition of a lead within the coronary sinus for HBP-optimized CRT (HOT-CRT) or LBBAP-optimized CRT (LOT-CRT) may be necessary.90,91 His bundle pacing-optimized CRT resulted in a significant narrowing of the QRS (183 ms at baseline to 162 ms with BiVP, to 151 ms during HBP, to 120 ms with HOT-CRT) with improved LVEF over 14 months of follow-up in patients initially referred for CRT therapy.70 Greater resynchronization was similarly seen with LOT-CRT with similar QRS narrowing (182 ms at baseline to 170 ms with BiV and 144 ms with LOT-CRT),90 emphasizing the need for further decision aids to attain a more personalized approach to CRT. Further advancements in tools, rigorous training, and implementation of quality control measures will be crucial to ensure that these procedures (BiVP and CSP) are both performed effectively. Finally, until an RCT will prove superiority (or at least non-inferiority) of CSP vs. BiVP, CSP cannot be proposed as a first-line alternative with the intention of CRT for HFrEF either in patients with sinus rhythm or in those with AF.
There also remains ongoing debate about the merits of HBP vs. LBBAP. His bundle pacing results in much higher rates of confirmed conduction system capture and offers pure physiologic activation of both the RV and LV with selective capture in patients with narrow QRS. While left bundle branch pacing (LBBP) ensures physiological activation of the left ventricle, it creates a right bundle activation pattern leading to concern about the lack of physiological activation of the RV. However, recent echocardiographic observations have seen improvements in RV systolic function with LBBAP as evaluated by RV free wall strain.92 Propensity score matching of a pace-and-ablate strategy of 99 LBBAP with 86 HBP patients demonstrated similar improvements in echocardiographic and HF outcomes, whereas higher implant success rates, better pacing parameters, and fewer late lead-related complications were present in the LBBAP group.93 Importantly, late increased thresholds were noted in 9.3% of the HBP group requiring re-programming to RVP via the previously placed backup lead. Albeit it seems that LBBAP might be more suitable as a first-line CSP strategy for patients undergoing AVNA.94,95
Risks of pace-and-ablate therapy
The pace-and-ablate strategy is often perceived as a harmful therapy as a result of pacemaker dependency, to be avoided at all costs. Apart from the potential for PICM, complications include those related to the device itself: pocket haematoma and infection, pneumothorax, perforation, tricuspid regurgitation, and the need for generator change all in a dependent patient, and AVNA: vascular access complication, lead dislodgement, and rarely sudden cardiac death (SCD). However, the overall complication is low, at 0.8% in the NASPE registry96 and 1.8% in the MERFS survey97—where these represent historical studies where the incidence of complications over time has likely decreased.
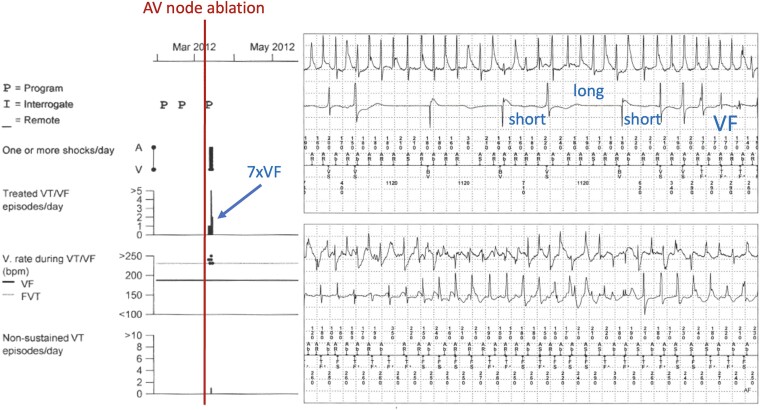
Reports of ventricular arrhythmia and SCD are known, albeit rare, complications of pace-and-ablate therapy (an example of such a case is presented in Figure 3). The underlying mechanism most likely contributing to early SCD is that of dispersion of refractoriness due to an abrupt heart rate decrease post-AVNA with abnormally prolonged QT intervals, but underlying cardiac disease or aggravated repolarization abnormalities are certainly contributing causes.98 In a large retrospective study evaluating 334 patients with AF who underwent AVNA and pacemaker implantation during the 1990s, nine patients had SCD post-ablation.99 Of them, seven were determined to have suffered SCD as a result of the procedure: two within 48 h as out-of-hospital arrests (1.2%) and five in-hospital where two survivors had documented polymorphic ventricular tachycardia (VT) or ventricular fibrillation (VF). In 1997, prevention of post-AVNA malignant arrhythmias was demonstrated by programming devices to a lower rate of 90 b.p.m. for the initial 1–3 months post-AVNA.16
Figure 3.
An illustration of a patient who developed polymorphic ventricular fibrillation 1 day after atrioventricular node ablation when the cardiac resynchronization therapy-D device was programmed to a lower rate of 55 b.p.m. post-atrioventricular node ablation. The patient suffered seven ICD shocks in the nights following atrioventricular node ablation
Knowledge gaps and future directions
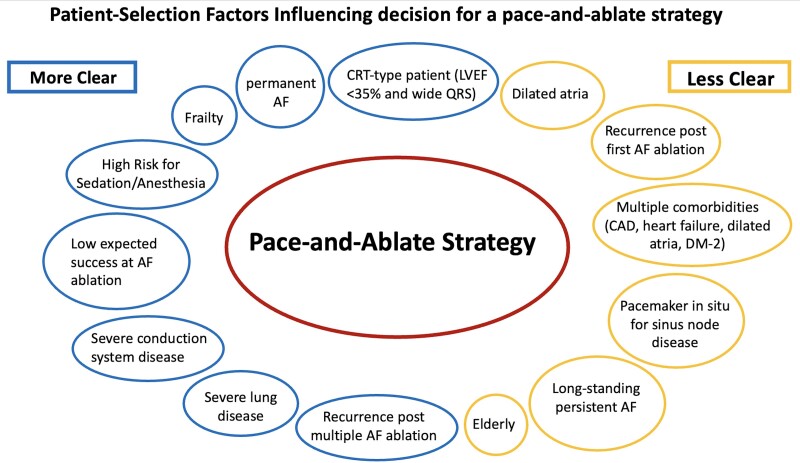
The current approach to determine eligibility for pace-and-ablate therapy remains grounded on the chance of AF ablation failure or heightened risk profile as determined by physician experience and opinion, as well as any given centre’s expertise where multiple redo ablation procedures may be part of the normal culture. No formal criteria exist beyond ‘AF ablation non-eligible’. We refer to prior studies (Tables 1–3) that consider the safety of the pace-and-ablate approach in addition to the clear benefits of QOL and symptom management. We can similarly consider risk models predicting AF ablation success despite one or more ablations, although their absolute value remains limited to date. When considering a first-line pace-and-ablate approach, it is crucial to account for various patient-specific characteristics (Figure 4). Future research on pace-and-ablate therapy should focus on patient selection, timing of the intervention, and long-term follow-up of patient outcomes including stroke and HF hospitalizations, particularly those with less clear indications to proceed with pace-and-ablate therapy.
Figure 4.
Patient selection factors influencing decision for a pace-and-ablate strategy
In patients with HF with reduced EF (HFrEF), CASTLE25 and CASTLE-HTX26 showed improved clinical outcome after PVI when compared with medical therapy. However, the RAFT-AF28 trial failed to observe this benefit. A careful evaluation of these trials reveals different patient profiles and AF types which could explain the differences in outcome. A pace-and-ablate strategy may provide a very reasonable option in select patients with HFrEF.
A number of trials evaluating AVNA + CSP are ongoing. The PACE-FIB trial (Clinicaltrials.gov; NCT05029570) is randomizing 366 patients with HFpEF/HFmrEF and permanent AF to pharmacological rate control or LBBAP with AVNA. The primary outcome measure will be the composite of all-cause mortality, HF hospitalization, and worsening HF at three years.100 The ABACUS trial (Clinicaltrials.gov: NCT06207383) will randomize PVI (with additional lesions if deemed necessary) and AVNA + CSP in 220 patients aged >60 years with persistent AF (with at most one previous ablation) and symptomatic HF. The two primary endpoints are superiority of AVNA + CSP for mortality and cardiovascular hospitalizations (including redo procedures) or non-inferiority for mortality and HF hospitalization.
An issue in clinical practice is upgrade to CSP in patients who require AVNA and who are already implanted with a DF-4 implantable cardioverter defibrillator (ICD). These patients may have a device that still has several years of longevity but require a new generator due to the inability to connect the new pacing lead to the existing device. The older DF-1 standard offers this possibility and should be maintained, also for other reasons which are outlined elsewhere.101 Direct delivery of LBBAP by ICD leads would avoid the requirement for upgrade. A pilot case series reported successful temporary LBBAP implantation in three of five patients. A lumenless 4.7 F ICD lead is currently being evaluated for RV septal or apical placement.102 Due to its resemblance with the 4.2F lumenless 4.2F 3830 lead (Medtronic, MN, USA), this lead may be suitable for LBBAP. However, due to its integrated bipolar design, the entire coil needs to be positioned in the RV to avoid atrial oversensing (which is not an issue with true bipolar ICD leads) and needs to be formally tested for this indication.
Technology is constantly evolving, both in the field of AF ablation and in CSP. The question is to what extent sinus rhythm can be maintained in sick and scarred atria (e.g. atrial cardiomyopathy) and for how long, even with the best of technologies and the most skilled operators. Evolution in lead design and ancillary tools will facilitate CSP implantation (as it did with BiVP) and thereby offer a simple, safe, and pragmatic solution to a growing problem.
Supplementary data
Supplementary data are not available at European Heart Journal online.
Contributor Information
Jacqueline Joza, Department of Medicine, McGill University Health Center, Montreal, Quebec, Canada.
Haran Burri, Cardiology Department, University Hospital of Geneva, Geneva, Switzerland.
Jason G Andrade, Department of Medicine, Vancouver General Hospital, Vancouver, British Columbia, Canada.
Dominik Linz, Department of Cardiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Center, Maastricht, The Netherlands.
Kenneth A Ellenbogen, Virginia Commonwealth University Medical Center, Richmond, VA, USA.
Kevin Vernooy, Department of Cardiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Center, Maastricht, The Netherlands.
Declarations
Disclosure of Interest
J.J. reports an investigator-initiated external research programme grant from Medtronic Inc and honoraria from Boston Scientific and Medtronic. H.B. reports honoraria from Abbott, Biotronik, Medtronic, and Microport, paid to the institution, and participation on a data safety monitoring board for Medtronic and Boston Scientific, paid to the institution. J.G.A. reports honoraria from Boston Scientific, Medtronic, Abbott, and Biosense Webster. D.L. has nothing to disclose. K.A.E. reports royalties from textbooks and CME for ACC journals and participation on several data safety monitoring boards. K.V. reports grants from Medtronic, Boston Scientific, Biosense Webster, Abbott, and NWO-ZonMW and consulting and honoraria from Medtronic, Boston Scientific, Biosense Webster, and Abbott all paid to the institution.
Data Availability
No data were generated or analysed for or in support of this paper.
Funding
J.J. is supported by a Clinical Research Scholar Award from the Fonds de Recherche du Québec - Santé (FRQS).
References
- 1. Cheung CC, Nattel S, Macle L, Andrade JG. Management of atrial fibrillation in 2021: an updated comparison of the current CCS/CHRS, ESC, and AHA/ACC/HRS guidelines. Can J Cardiol 2021;37:1607–18. 10.1016/j.cjca.2021.06.011 [DOI] [PubMed] [Google Scholar]
- 2. Lee SH, Chen SA, Tai CT, Chiang CE, Wen ZC, Cheng JJ, et al. Comparisons of quality of life and cardiac performance after complete atrioventricular junction ablation and atrioventricular junction modification in patients with medically refractory atrial fibrillation. J Am Coll Cardiol 1998;31:637–44. 10.1016/S0735-1097(97)00530-5 [DOI] [PubMed] [Google Scholar]
- 3. Kay GN, Ellenbogen KA, Giudici M, Redfield MM, Jenkins LS, Mianulli M, et al. The ablate and pace trial: a prospective study of catheter ablation of the AV conduction system and permanent pacemaker implantation for treatment of atrial fibrillation. APT investigators. J Interv Card Electrophysiol 1998;2:121–35. 10.1023/A:1009795330454 [DOI] [PubMed] [Google Scholar]
- 4. Jensen SM, Bergfeldt L, Rosenqvist M. Long-term follow-up of patients treated by radiofrequency ablation of the atrioventricular junction. Pacing Clin Electrophysiol 1995;18:1609–14. 10.1111/j.1540-8159.1995.tb06982.x [DOI] [PubMed] [Google Scholar]
- 5. Andrade JG, Wazni OM, Kuniss M, Hawkins NM, Deyell MW, Chierchia GB, et al. Cryoballoon ablation as initial treatment for atrial fibrillation: JACC state-of-the-art review. J Am Coll Cardiol 2021;78:914–30. 10.1016/j.jacc.2021.06.038 [DOI] [PubMed] [Google Scholar]
- 6. Winkle RA, Jarman JW, Mead RH, Engel G, Kong MH, Fleming W, et al. Predicting atrial fibrillation ablation outcome: the CAAP-AF score. Heart Rhythm 2016;13:2119–25. 10.1016/j.hrthm.2016.07.018 [DOI] [PubMed] [Google Scholar]
- 7. Clarnette JA, Brooks AG, Mahajan R, Elliott AD, Twomey DJ, Pathak RK, et al. Outcomes of persistent and long-standing persistent atrial fibrillation ablation: a systematic review and meta-analysis. Europace 2018;20:f366–f76. 10.1093/europace/eux297 [DOI] [PubMed] [Google Scholar]
- 8. Teunissen C, Kassenberg W, van der Heijden JF, Hassink RJ, van Driel VJ, Zuithoff NP, et al. Five-year efficacy of pulmonary vein antrum isolation as a primary ablation strategy for atrial fibrillation: a single-centre cohort study. Europace 2016;18:1335–42. 10.1093/europace/euv439 [DOI] [PubMed] [Google Scholar]
- 9. Poole JE, Bahnson TD, Monahan KH, Johnson G, Rostami H, Silverstein AP, et al. Recurrence of atrial fibrillation after catheter ablation or antiarrhythmic drug therapy in the CABANA trial. J Am Coll Cardiol 2020;75:3105–18. 10.1016/j.jacc.2020.04.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schnabel RB, Marinelli EA, Arbelo E, Boriani G, Boveda S, Buckley CM, et al. Early diagnosis and better rhythm management to improve outcomes in patients with atrial fibrillation: the 8th AFNET/EHRA consensus conference. Europace 2023;25:6–27. 10.1093/europace/euac062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Prinzen FW, Auricchio A, Mullens W, Linde C, Huizar JF. Electrical management of heart failure: from pathophysiology to treatment. Eur Heart J 2022;43:1917–27. 10.1093/eurheartj/ehac088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naito M, David D, Michelson EL, Schaffenburg M, Dreifus LS. The hemodynamic consequences of cardiac arrhythmias: evaluation of the relative roles of abnormal atrioventricular sequencing, irregularity of ventricular rhythm and atrial fibrillation in a canine model. Am Heart J 1983;106:284–91. 10.1016/0002-8703(83)90194-1 [DOI] [PubMed] [Google Scholar]
- 13. Daoud EG, Weiss R, Bahu M, Knight BP, Bogun F, Goyal R, et al. Effect of an irregular ventricular rhythm on cardiac output. Am J Cardiol 1996;78:1433–6. 10.1016/S0002-9149(97)89297-1 [DOI] [PubMed] [Google Scholar]
- 14. Clark DM, Plumb VJ, Epstein AE, Kay GN. Hemodynamic effects of an irregular sequence of ventricular cycle lengths during atrial fibrillation. J Am Coll Cardiol 1997;30:1039–45. 10.1016/S0735-1097(97)00254-4 [DOI] [PubMed] [Google Scholar]
- 15. Lyon A, van Mourik M, Cruts L, Heijman J, Bekkers SCAM, Schotten U, et al. Both beat-to-beat changes in RR-interval and left ventricular filling time determine ventricular function during atrial fibrillation. Europace 2021;23:i21–8. 10.1093/europace/euaa387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Geelen P, Goethals M, de Bruyne B, Brugada P. A prospective hemodynamic evaluation of patients with chronic atrial fibrillation undergoing radiofrequency catheter ablation of the atrioventricular junction. Am J Cardiol 1997;80:1606–9. 10.1016/S0002-9149(97)00779-0 [DOI] [PubMed] [Google Scholar]
- 17. Takahashi Y, Yoshito I, Takahashi A, Harada T, Mitsuhashi T, Shirota K, et al. AV nodal ablation and pacemaker implantation improves hemodynamic function in atrial fibrillation. Pacing Clin Electrophysiol 2003;26:1212–7. 10.1046/j.1460-9592.2003.t01-1-00171.x [DOI] [PubMed] [Google Scholar]
- 18. Kochiadakis GE, Skalidis EI, Kalebubas MD, Igoumenidis NE, Chrysostomakis SI, Kanoupakis EM, et al. Effect of acute atrial fibrillation on phasic coronary blood flow pattern and flow reserve in humans. Eur Heart J 2002;23:734–41. 10.1053/euhj.2001.2894 [DOI] [PubMed] [Google Scholar]
- 19. Pabel S, Knierim M, Stehle T, Alebrand F, Paulus M, Sieme M, et al. Effects of atrial fibrillation on the human ventricle. Circ Res 2022;130:994–1010. 10.1161/CIRCRESAHA.121.319718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sossalla S, Fluschnik N, Schotola H, Ort KR, Neef S, Schulte T, et al. Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ Res 2010;107:1150–61. 10.1161/CIRCRESAHA.110.220418 [DOI] [PubMed] [Google Scholar]
- 21. Ling LH, Khammy O, Byrne M, Amirahmadi F, Foster A, Li G, et al. Irregular rhythm adversely influences calcium handling in ventricular myocardium: implications for the interaction between heart failure and atrial fibrillation. Circ Heart Fail 2012;5:786–93. 10.1161/CIRCHEARTFAILURE.112.968321 [DOI] [PubMed] [Google Scholar]
- 22. Wasmund SL, Li JM, Page RL, Joglar JA, Kowal RC, Smith ML, et al. Effect of atrial fibrillation and an irregular ventricular response on sympathetic nerve activity in human subjects. Circulation 2003;107:2011–5. 10.1161/01.CIR.0000064900.76674.CC [DOI] [PubMed] [Google Scholar]
- 23. Wang T, Fang T, Cheng Z. Comparison of the efficacy and safety endpoints of five therapies for atrial fibrillation: a network meta-analysis. Front Cardiovasc Med 2022;9:853149. 10.3389/fcvm.2022.853149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palmisano P, Parlavecchio A, Vetta G, Crea P, Carerj S, Della Rocca DG, et al. Spontaneous sinus rhythm restoration in patients with refractory, permanent atrial fibrillation who underwent conduction system pacing and atrioventricular junction ablation. Am J Cardiol 2023;209:76–84. 10.1016/j.amjcard.2023.09.093 [DOI] [PubMed] [Google Scholar]
- 25. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. 10.1056/NEJMoa1707855 [DOI] [PubMed] [Google Scholar]
- 26. Sohns C, Fox H, Marrouche NF, Crijns HJGM, Costard-Jaeckle A, Bergau L, et al. Catheter ablation in end-stage heart failure with atrial fibrillation. N Engl J Med 2023;389:1380–9. 10.1056/NEJMoa2306037 [DOI] [PubMed] [Google Scholar]
- 27. Khan MN, Jaïs P, Cummings J, Di Biase L, Sanders P, Martin DO, et al. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med 2008;359:1778–85. 10.1056/NEJMoa0708234 [DOI] [PubMed] [Google Scholar]
- 28. Parkash R, Wells GA, Rouleau J, Talajic M, Essebag V, Skanes A, et al. Randomized ablation-based rhythm-control versus rate-control trial in patients with heart failure and atrial fibrillation: results from the RAFT-AF trial. Circulation 2022;145:1693–704. 10.1161/CIRCULATIONAHA.121.057095 [DOI] [PubMed] [Google Scholar]
- 29. Brignole M, Pentimalli F, Palmisano P, Landolina M, Quartieri F, Occhetta E, et al. AV junction ablation and cardiac resynchronization for patients with permanent atrial fibrillation and narrow QRS: the APAF-CRT mortality trial. Eur Heart J 2021;42:4731–9. 10.1093/eurheartj/ehab569 [DOI] [PubMed] [Google Scholar]
- 30. Wood MA, Brown-Mahoney C, Kay GN, Ellenbogen KA. Clinical outcomes after ablation and pacing therapy for atrial fibrillation: a meta-analysis. Circulation 2000;101:1138–44. 10.1161/01.CIR.101.10.1138 [DOI] [PubMed] [Google Scholar]
- 31. Morady F, Calkins H, Langberg JJ, Armstrong WF, de Buitleir M, el-Atassi R, et al. A prospective randomized comparison of direct current and radiofrequency ablation of the atrioventricular junction. J Am Coll Cardiol 1993;21:102–9. 10.1016/0735-1097(93)90723-E [DOI] [PubMed] [Google Scholar]
- 32. Olgin JE, Scheinman MM. Comparison of high energy direct current and radiofrequency catheter ablation of the atrioventricular junction. J Am Coll Cardiol 1993;21:557–64. 10.1016/0735-1097(93)90084-E [DOI] [PubMed] [Google Scholar]
- 33. Brignole M, Gianfranchi L, Menozzi C, Bottoni N, Bollini R, Lolli G, et al. Influence of atrioventricular junction radiofrequency ablation in patients with chronic atrial fibrillation and flutter on quality of life and cardiac performance. Am J Cardiol 1994;74:242–6. 10.1016/0002-9149(94)90364-6 [DOI] [PubMed] [Google Scholar]
- 34. Edner M, Caidahl K, Bergfeldt L, Darpö B, Edvardsson N, Rosenqvist M. Prospective study of left ventricular function after radiofrequency ablation of atrioventricular junction in patients with atrial fibrillation. Br Heart J 1995;74:261–7. 10.1136/hrt.74.3.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fitzpatrick AP, Kourouyan HD, Siu A, Lee RJ, Lesh MD, Epstein LM, et al. Quality of life and outcomes after radiofrequency His-bundle catheter ablation and permanent pacemaker implantation: impact of treatment in paroxysmal and established atrial fibrillation. Am Heart J 1996;131:499–507. 10.1016/S0002-8703(96)90528-1 [DOI] [PubMed] [Google Scholar]
- 36. Bubien RS, Knotts-Dolson SM, Plumb VJ, Kay GN. Effect of radiofrequency catheter ablation on health-related quality of life and activities of daily living in patients with recurrent arrhythmias. Circulation 1996;94:1585–91. 10.1161/01.CIR.94.7.1585 [DOI] [PubMed] [Google Scholar]
- 37. Darpö B, Walfridsson H, Aunes M, Bergfeldt L, Edvardsson N, Linde C, et al. Incidence of sudden death after radiofrequency ablation of the atrioventricular junction for atrial fibrillation. Am J Cardiol 1997;80:1174–7. 10.1016/S0002-9149(97)00635-8 [DOI] [PubMed] [Google Scholar]
- 38. Brignole M, Gianfranchi L, Menozzi C, Alboni P, Musso G, Bongiorni MG, et al. Assessment of atrioventricular junction ablation and DDDR mode-switching pacemaker versus pharmacological treatment in patients with severely symptomatic paroxysmal atrial fibrillation: a randomized controlled study. Circulation 1997;96:2617–24. 10.1161/01.CIR.96.8.2617 [DOI] [PubMed] [Google Scholar]
- 39. Buys EM, van Hemel NM, Kelder JC, Ascoop CA, van Dessel PF, Bakema L, et al. Exercise capacity after His bundle ablation and rate response ventricular pacing for drug refractory chronic atrial fibrillation. Heart 1997;77:238–41. 10.1136/hrt.77.3.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Twidale N, McDonald T, Nave K, Seal A. Comparison of the effects of AV nodal ablation versus AV nodal modification in patients with congestive heart failure and uncontrolled atrial fibrillation. Pacing Clin Electrophysiol 1998;21:641–51. 10.1111/j.1540-8159.1998.tb00119.x [DOI] [PubMed] [Google Scholar]
- 41. Marshall HJ, Harris ZI, Griffith MJ, Gammage MD. Prospective, randomized study of atrioventricular node ablation and mode-switching, dual chamber pacemaker implantation using two different algorithms in patients with paroxysmal atrial fibrillation. Europace 1999;1:20–1. 10.1053/eupc.1998.0003 [DOI] [PubMed] [Google Scholar]
- 42. Weerasooriya R, Davis M, Powell A, Szili-Torok T, Shah C, Whalley D, et al. The Australian intervention randomized control of rate in atrial fibrillation trial (AIRCRAFT). J Am Coll Cardiol 2003;41:1697–702. 10.1016/S0735-1097(03)00338-3 [DOI] [PubMed] [Google Scholar]
- 43. Kiehl EL, Makki T, Kumar R, Gumber D, Kwon DH, Rickard JW, et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm 2016;13:2272–8. 10.1016/j.hrthm.2016.09.027 [DOI] [PubMed] [Google Scholar]
- 44. Khurshid S, Epstein AE, Verdino RJ, Lin D, Goldberg LR, Marchlinski FE, et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy. Heart Rhythm 2014;11:1619–25. 10.1016/j.hrthm.2014.05.040 [DOI] [PubMed] [Google Scholar]
- 45. Somma V, Ha FJ, Palmer S, Mohamed U, Agarwal S. Pacing-induced cardiomyopathy: a systematic review and meta-analysis of definition, prevalence, risk factors, and management. Heart Rhythm 2023;20:282–90. 10.1016/j.hrthm.2022.09.019 [DOI] [PubMed] [Google Scholar]
- 46. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, et al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2021;42:3427–520. 10.1093/eurheartj/ehab364 [DOI] [PubMed] [Google Scholar]
- 47. Leon AR, Greenberg JM, Kanuru N, Baker CM, Mera FV, Smith AL, et al. Cardiac resynchronization in patients with congestive heart failure and chronic atrial fibrillation: effect of upgrading to biventricular pacing after chronic right ventricular pacing. J Am Coll Cardiol 2002;39:1258–63. 10.1016/S0735-1097(02)01779-5 [DOI] [PubMed] [Google Scholar]
- 48. Linde C, Leclercq C, Rex S, Garrigue S, Lavergne T, Cazeau S, et al. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol 2002;40:111–8. 10.1016/S0735-1097(02)01932-0 [DOI] [PubMed] [Google Scholar]
- 49. Puggioni E, Brignole M, Gammage M, Soldati E, Bongiorni MG, Simantirakis EN, et al. Acute comparative effect of right and left ventricular pacing in patients with permanent atrial fibrillation. J Am Coll Cardiol 2004;43:234–8. 10.1016/j.jacc.2003.09.027 [DOI] [PubMed] [Google Scholar]
- 50. Simantirakis EN, Vardakis KE, Kochiadakis GE, Manios EG, Igoumenidis NE, Brignole M, et al. Left ventricular mechanics during right ventricular apical or left ventricular-based pacing in patients with chronic atrial fibrillation after atrioventricular junction ablation. J Am Coll Cardiol 2004;43:1013–8. 10.1016/j.jacc.2003.10.038 [DOI] [PubMed] [Google Scholar]
- 51. Doshi RN, Daoud EG, Fellows C, Turk K, Duran A, Hamdan MH, et al. Left ventricular-based cardiac stimulation post AV nodal ablation evaluation (the PAVE study). J Cardiovasc Electrophysiol 2005;16:1160–5. 10.1111/j.1540-8167.2005.50062.x [DOI] [PubMed] [Google Scholar]
- 52. Brignole M, Gammage M, Puggioni E, Alboni P, Raviele A, Sutton R, et al. Comparative assessment of right, left, and biventricular pacing in patients with permanent atrial fibrillation. Eur Heart J 2005;26:712–22. 10.1093/eurheartj/ehi069 [DOI] [PubMed] [Google Scholar]
- 53. Orlov MV, Gardin JM, Slawsky M, Bess RL, Cohen G, Bailey W, et al. Biventricular pacing improves cardiac function and prevents further left atrial remodeling in patients with symptomatic atrial fibrillation after atrioventricular node ablation. Am Heart J 2010;159:264–70. 10.1016/j.ahj.2009.11.012 [DOI] [PubMed] [Google Scholar]
- 54. Brignole M, Botto G, Mont L, Iacopino S, De Marchi G, Oddone D, et al. Cardiac resynchronization therapy in patients undergoing atrioventricular junction ablation for permanent atrial fibrillation: a randomized trial. Eur Heart J 2011;32:2420–9. 10.1093/eurheartj/ehr162 [DOI] [PubMed] [Google Scholar]
- 55. Cleland JG, Ghio S. The determinants of clinical outcome and clinical response to CRT are not the same. Heart Fail Rev 2012;17:755–66. 10.1007/s10741-011-9268-9 [DOI] [PubMed] [Google Scholar]
- 56. Curtis AB, Worley SJ, Adamson PB, Chung ES, Niazi I, Sherfesee L, et al. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med 2013;368:1585–93. 10.1056/NEJMoa1210356 [DOI] [PubMed] [Google Scholar]
- 57. Martinelli Filho M, de Siqueira SF, Costa R, Greco OT, Moreira LF, D'avila A, et al. Conventional versus biventricular pacing in heart failure and bradyarrhythmia: the COMBAT study. J Card Fail 2010;16:293–300. 10.1016/j.cardfail.2009.12.008 [DOI] [PubMed] [Google Scholar]
- 58. Kindermann M, Hennen B, Jung J, Geisel J, Böhm M, Fröhlig G. Biventricular versus conventional right ventricular stimulation for patients with standard pacing indication and left ventricular dysfunction: the Homburg Biventricular Pacing Evaluation (HOBIPACE). J Am Coll Cardiol 2006;47:1927–37. 10.1016/j.jacc.2005.12.056 [DOI] [PubMed] [Google Scholar]
- 59. Thibault B, Harel F, Ducharme A, White M, Ellenbogen KA, Frasure-Smith N, et al. Cardiac resynchronization therapy in patients with heart failure and a QRS complex <120 milliseconds: the evaluation of resynchronization therapy for heart failure (LESSER-EARTH) trial. Circulation 2013;127:873–81. 10.1161/CIRCULATIONAHA.112.001239. [DOI] [PubMed] [Google Scholar]
- 60. Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J, et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med 2013;369:1395–405. 10.1056/NEJMoa1306687 [DOI] [PubMed] [Google Scholar]
- 61. Beshai JF, Grimm RA, Nagueh SF, Baker JH 2nd, Beau SL, Greenberg SM, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med 2007;357:2461–71. 10.1056/NEJMoa0706695 [DOI] [PubMed] [Google Scholar]
- 62. Shah RM, Patel D, Molnar J, Ellenbogen KA, Koneru JN. Cardiac-resynchronization therapy in patients with systolic heart failure and QRS interval ≤130 ms: insights from a meta-analysis. Europace 2015;17:267–73. 10.1093/europace/euu214. [DOI] [PubMed] [Google Scholar]
- 63. Glikson M, Jastrzebski M, Gold MR, Ellenbogen K, Burri H. Conventional biventricular pacing is still preferred to conduction system pacing for atrioventricular block in patients with reduced ejection fraction and narrow QRS. Europace 2023;26:euad337. 10.1093/europace/euad337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Deshmukh P, Casavant DA, Romanyshyn M, Anderson K. Permanent, direct His-bundle pacing: a novel approach to cardiac pacing in patients with normal His-Purkinje activation. Circulation 2000;101:869–77. 10.1161/01.CIR.101.8.869 [DOI] [PubMed] [Google Scholar]
- 65. Deshmukh PM, Romanyshyn M. Direct His-bundle pacing: present and future. Pacing Clin Electrophysiol 2004;27:862–70. 10.1111/j.1540-8159.2004.00548.x [DOI] [PubMed] [Google Scholar]
- 66. Occhetta E, Bortnik M, Magnani A, Francalacci G, Piccinino C, Plebani L, et al. Prevention of ventricular desynchronization by permanent para-Hisian pacing after atrioventricular node ablation in chronic atrial fibrillation: a crossover, blinded, randomized study versus apical right ventricular pacing. J Am Coll Cardiol 2006;47:1938–45. 10.1016/j.jacc.2006.01.056 [DOI] [PubMed] [Google Scholar]
- 67. Occhetta E, Bortnik M, Marino P. Permanent parahisian pacing. Indian Pacing Electrophysiol J 2007;7:110–25. PMID: 17538702 PMC1877829 [PMC free article] [PubMed] [Google Scholar]
- 68. Vijayaraman P, Subzposh FA, Naperkowski A. Atrioventricular node ablation and His bundle pacing. Europace 2017;19:iv10–6. 10.1093/europace/eux263 [DOI] [PubMed] [Google Scholar]
- 69. Huang W, Su L, Wu S, Xu L, Xiao F, Zhou X, et al. Benefits of permanent His bundle pacing combined with atrioventricular node ablation in atrial fibrillation patients with heart failure with both preserved and reduced left ventricular ejection fraction. J Am Heart Assoc 2017;6:e005309. 10.1161/JAHA.116.005309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang S, Wu S, Xu L, Xiao F, Whinnett ZI, Vijayaraman P, et al. Feasibility and efficacy of His bundle pacing or left bundle pacing combined with atrioventricular node ablation in patients with persistent atrial fibrillation and implantable cardioverter-defibrillator therapy. J Am Heart Assoc 2019;8:e014253. 10.1161/JAHA.119.014253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Su L, Cai M, Wu S, Wang S, Xu T, Vijayaraman P, et al. Long-term performance and risk factors analysis after permanent His-bundle pacing and atrioventricular node ablation in patients with atrial fibrillation and heart failure. Europace 2020;22:ii19–26. 10.1093/europace/euaa306 [DOI] [PubMed] [Google Scholar]
- 72. Senes J, Mascia G, Bottoni N, Oddone D, Donateo P, Grimaldi T, et al. Is His-optimized superior to conventional cardiac resynchronization therapy in improving heart failure? Results from a propensity-matched study. Pacing Clin Electrophysiol 2021;44:1532–9. 10.1111/pace.14336 [DOI] [PubMed] [Google Scholar]
- 73. Huang W, Wang S, Su L, Fu G, Su Y, Chen K, et al. His-bundle pacing vs biventricular pacing following atrioventricular nodal ablation in patients with atrial fibrillation and reduced ejection fraction: a multicenter, randomized, crossover study-the ALTERNATIVE-AF trial. Heart Rhythm 2022;19:1948–55. 10.1016/j.hrthm.2022.07.009 [DOI] [PubMed] [Google Scholar]
- 74. Ivanovski M, Mrak M, Mežnar AZ, Žižek D. Biventricular versus conduction system pacing after atrioventricular node ablation in heart failure patients with atrial fibrillation. J Cardiovasc Dev Dis 2022;9:209. 10.3390/jcdd9070209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Vijayaraman P, Mathew AJ, Naperkowski A, Young W, Pokharel P, Batul SA, et al. Conduction system pacing versus conventional pacing in patients undergoing atrioventricular node ablation: nonrandomized, on-treatment comparison. Heart Rhythm O2 2022;3:368–76. 10.1016/j.hroo.2022.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Qi P, An H, Lv Y, Geng Y, Chen S, Li S, et al. His-Purkinje conduction system pacing and atrioventricular node ablation in treatment of persistent atrial fibrillation refractory to multiple ablation procedures: a case report. SAGE Open Med Case Rep 2023;11:2050313X231172873. 10.1177/2050313X231172873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Palmisano P, Ziacchi M, Dell'Era G, Donateo P, Ammendola E, Aspromonte V, et al. Ablate and pace: comparison of outcomes between conduction system pacing and biventricular pacing. Pacing Clin Electrophysiol 2023;46:1258–68. 10.1111/pace.14813 [DOI] [PubMed] [Google Scholar]
- 78. Salden FCWM, Luermans JGLM, Westra SW, Weijs B, Engels EB, Heckman LIB, et al. Short-term hemodynamic and electrophysiological effects of cardiac resynchronization by left ventricular septal pacing. J Am Coll Cardiol 2020;75:347–59. 10.1016/j.jacc.2019.11.040 [DOI] [PubMed] [Google Scholar]
- 79. Vijayaraman P, Dandamudi G. Permanent his-bundle pacing: case studies. Pacing Clin Electrophysiol 2016;39:1305–12. 10.1111/pace.12910 [DOI] [PubMed] [Google Scholar]
- 80. Zweerink A, Bakelants E, Stettler C, Burri H. Cryoablation vs. radiofrequency ablation of the atrioventricular node in patients with his-bundle pacing. Europace 2021;23:421–30. 10.1093/europace/euaa344 [DOI] [PubMed] [Google Scholar]
- 81. Herbert J, Kovacsovics A, Brito R, Masson N, Burri H. Mid-term performance of His-bundle pacing and usefulness of backup leads. Europace 2024; 26:euae168. 10.1093/europace/euae168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Burri H, Jastrzebski M, Cano Ó, Čurila K, de Pooter J, Huang W, et al. EHRA clinical consensus statement on conduction system pacing implantation: executive summary. Endorsed by the Asia-Pacific Heart Rhythm Society (APHRS), Canadian Heart Rhythm Society (CHRS) and Latin-American Heart Rhythm Society (LAHRS). Europace 2023;25:1237–48. 10.1093/europace/euad044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Vijayaraman P, Naperkowski A, Ellenbogen KA, Dandamudi G. Electrophysiologic insights into site of atrioventricular block: lessons from permanent His bundle pacing. JACC Clin Electrophysiol 2015;1:571–81. 10.1016/j.jacep.2015.09.012 [DOI] [PubMed] [Google Scholar]
- 84. Mafi-Rad M, Luermans JG, Blaauw Y, Janssen M, Crijns HJ, Prinzen FW, et al. Feasibility and acute hemodynamic effect of left ventricular septal pacing by transvenous approach through the interventricular septum. Circ Arrhythm Electrophysiol 2016;9:e003344. 10.1161/CIRCEP.115.003344 [DOI] [PubMed] [Google Scholar]
- 85. Huang W, Su L, Wu S, Xu L, Xiao F, Zhou X, et al. A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol 2017;33:1736.e1–e3. 10.1016/j.cjca.2017.09.013 [DOI] [PubMed] [Google Scholar]
- 86. Heckman LIB, Luermans JGLM, Curila K, Van Stipdonk AMW, Westra S, Smisek R, et al. Comparing ventricular synchrony in left bundle branch and left ventricular septal pacing in pacemaker patients. J Clin Med 2021;10:822. 10.3390/jcm10040822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Curila K, Jurak P, Jastrzebski M, Prinzen F, Waldauf P, Halamek J, et al. Left bundle branch pacing compared to left ventricular septal myocardial pacing increases interventricular dyssynchrony but accelerates left ventricular lateral wall depolarization. Heart Rhythm 2021;18:1281–9. 10.1016/j.hrthm.2021.04.025 [DOI] [PubMed] [Google Scholar]
- 88. Jastrzebski M, Kielbasa G, Cano O, Curila K, Heckman L, De Pooter J, et al. Left bundle branch area pacing outcomes: the multicentre European MELOS study. Eur Heart J 2022;43:4161–73. 10.1093/eurheartj/ehac445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Cano Ó, Jover P, Ayala HD, Navarrete-Navarro J, Osca J, Izquierdo M, et al. Left bundle branch pacing versus left ventricular septal pacing as a primary procedural endpoint during left bundle branch area pacing: evaluation of two different implant strategies. J Cardiovasc Electrophysiol 2024;35:120–9. 10.1111/jce.16128 [DOI] [PubMed] [Google Scholar]
- 90. Jastrzębski M, Moskal P, Huybrechts W, Curila K, Sreekumar P, Rademakers LM, et al. Left bundle branch-optimized cardiac resynchronization therapy (LOT-CRT): results from an international LBBAP collaborative study group. Heart Rhythm 2022;19:13–21. 10.1016/j.hrthm.2021.07.057 [DOI] [PubMed] [Google Scholar]
- 91. Vijayaraman P, Herweg B, Ellenbogen KA, Gajek J. His-optimized cardiac resynchronization therapy to maximize electrical resynchronization: a feasibility study. Circ Arrhythm Electrophysiol 2019;12:e006934. 10.1161/CIRCEP.118.006934 [DOI] [PubMed] [Google Scholar]
- 92. Bednarek A, Kiełbasa G, Moskal P, Ostrowska A, Bednarski A, Sondej T, et al. Left bundle branch area pacing improves right ventricular function and synchrony. Heart Rhythm 2024:S1547-5271(24)02561-X. 10.1016/j.hrthm.2024.05.019 [DOI] [PubMed] [Google Scholar]
- 93. Cai M, Wu S, Wang S, Zheng R, Jiang L, Lian L, et al. Left bundle branch pacing postatrioventricular junction ablation for atrial fibrillation: propensity score matching with His bundle pacing. Circ Arrhythm Electrophysiol 2022;15:e010926. 10.1161/CIRCEP.122.010926 [DOI] [PubMed] [Google Scholar]
- 94. Keene D, Anselme F, Burri H, Pérez ÓC, Čurila K, Derndorfer M, et al. Conduction system pacing, a European survey: insights from clinical practice. Europace 2023;25: euad019. 10.1093/europace/euad019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kircanski B, Boveda S, Prinzen F, Sorgente A, Anic A, Conte G, et al. Conduction system pacing in everyday clinical practice: EHRA physician survey. Europace 2023;25:682–7. 10.1093/europace/euac201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Scheinman MM, Huang S. The 1998 NASPE prospective catheter ablation registry. Pacing Clin Electrophysiol 2000;23:1020–8. 10.1111/j.1540-8159.2000.tb00891.x [DOI] [PubMed] [Google Scholar]
- 97. Hindricks G. Incidence of complete atrioventricular block following attempted radiofrequency catheter modification of the atrioventricular node in 880 patients. Results of the Multicenter European Radiofrequency Survey (MERFS) the working group on arrhythmias of the European Society of Cardiology. Eur Heart J 1996;17:82–8. 10.1093/oxfordjournals.eurheartj.a014696 [DOI] [PubMed] [Google Scholar]
- 98. Moss AJ. Long QT syndromes. Curr Treat Options Cardiovasc Med 2000;2:317–22. 10.1007/s11936-996-0005-y [DOI] [PubMed] [Google Scholar]
- 99. Ozcan C, Jahangir A, Friedman PA, Hayes DL, Munger TM, Rea RF, et al. Sudden death after radiofrequency ablation of the atrioventricular node in patients with atrial fibrillation. J Am Coll Cardiol 2002;40:105–10. 10.1016/S0735-1097(02)01927-7 [DOI] [PubMed] [Google Scholar]
- 100. Rodríguez Muñoz D, Crespo-Leiro MG, Fernández Lozano I, Zamorano Gómez JL, Peinado Peinado R, Manzano Espinosa L, et al. Conduction system pacing and atrioventricular node ablation in heart failure: the PACE-FIB study design. ESC Heart Fail 2023;10:3700–9. 10.1002/ehf2.14488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sticherling C, Ellenbogen KA, Burri H. Stepping back for good reasons: a reappraisal of the DF-1 connector for defibrillator leads. Europace 2024;26:euae057. 10.1093/europace/euae057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Crossley GH, Sanders P, De Filippo P, Tarakji KG, Hansky B, Shah M, et al. Rationale and design of the lead evaluation for defibrillation and reliability study: safety and efficacy of a novel ICD lead design. J Cardiovasc Electrophysiol 2023;34:257–67. 10.1111/jce.15747 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were generated or analysed for or in support of this paper.