Abstract
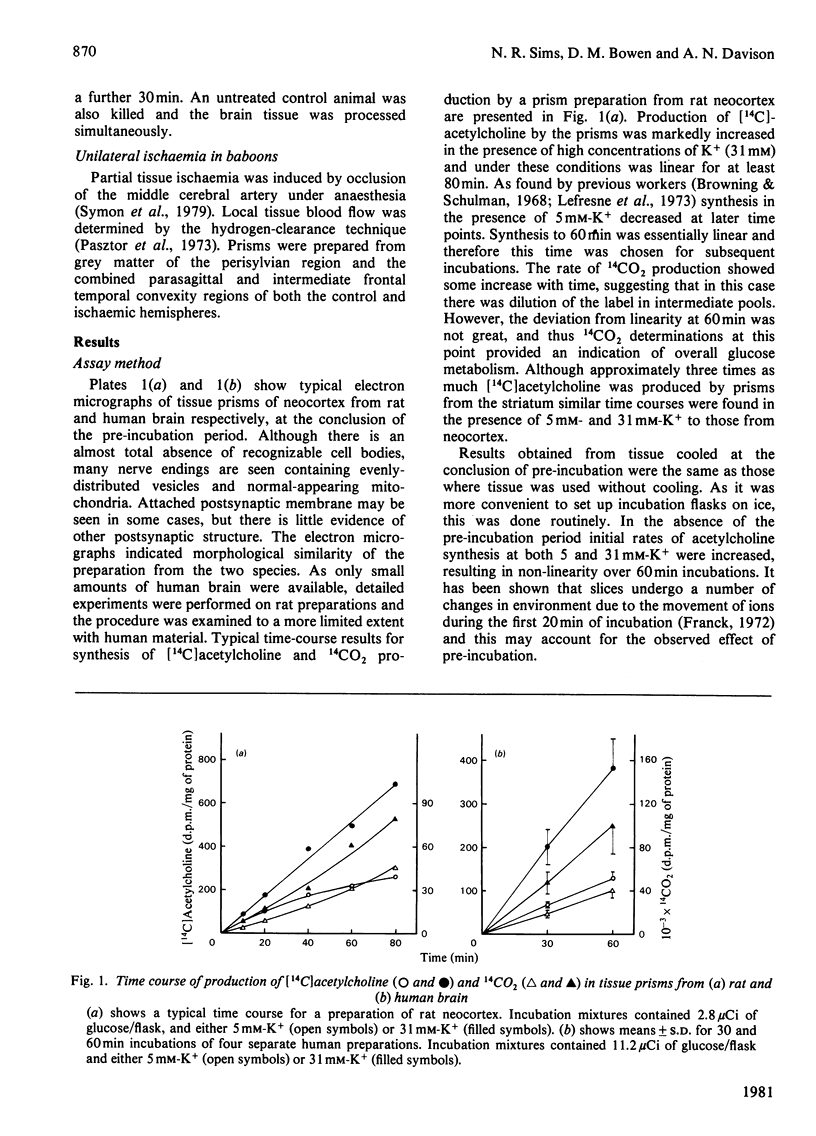
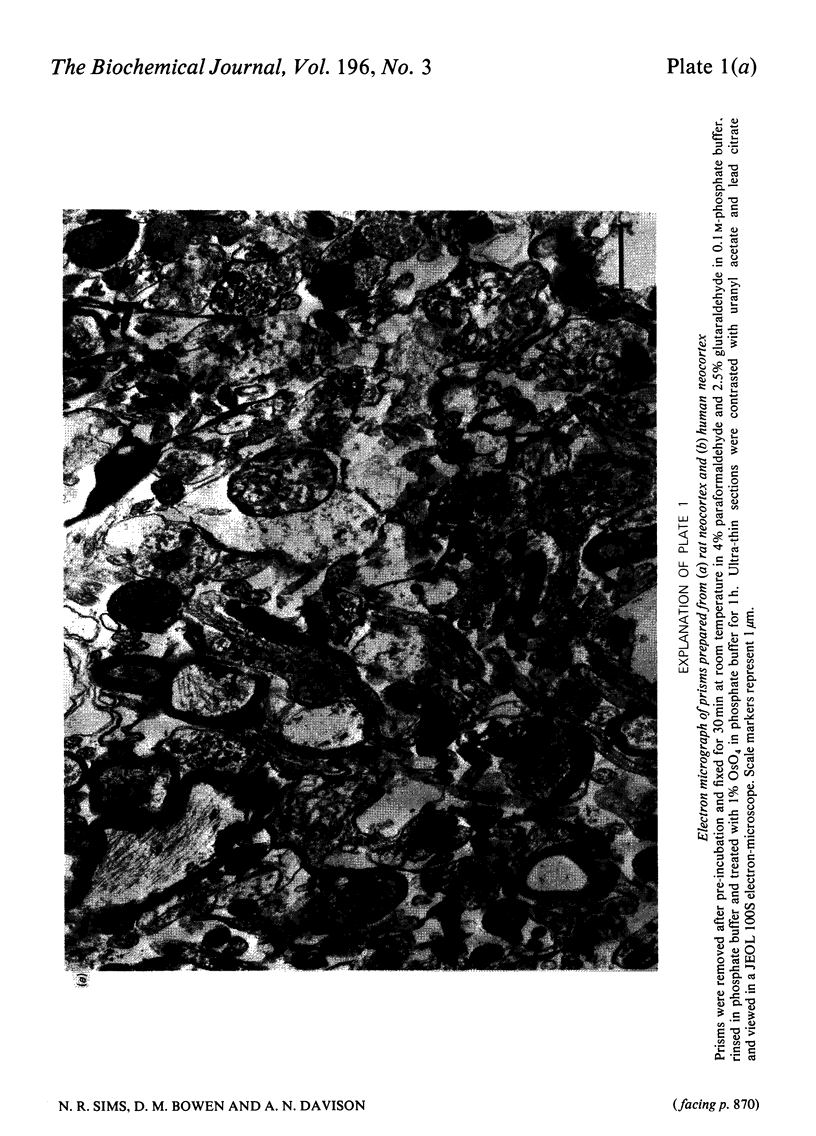
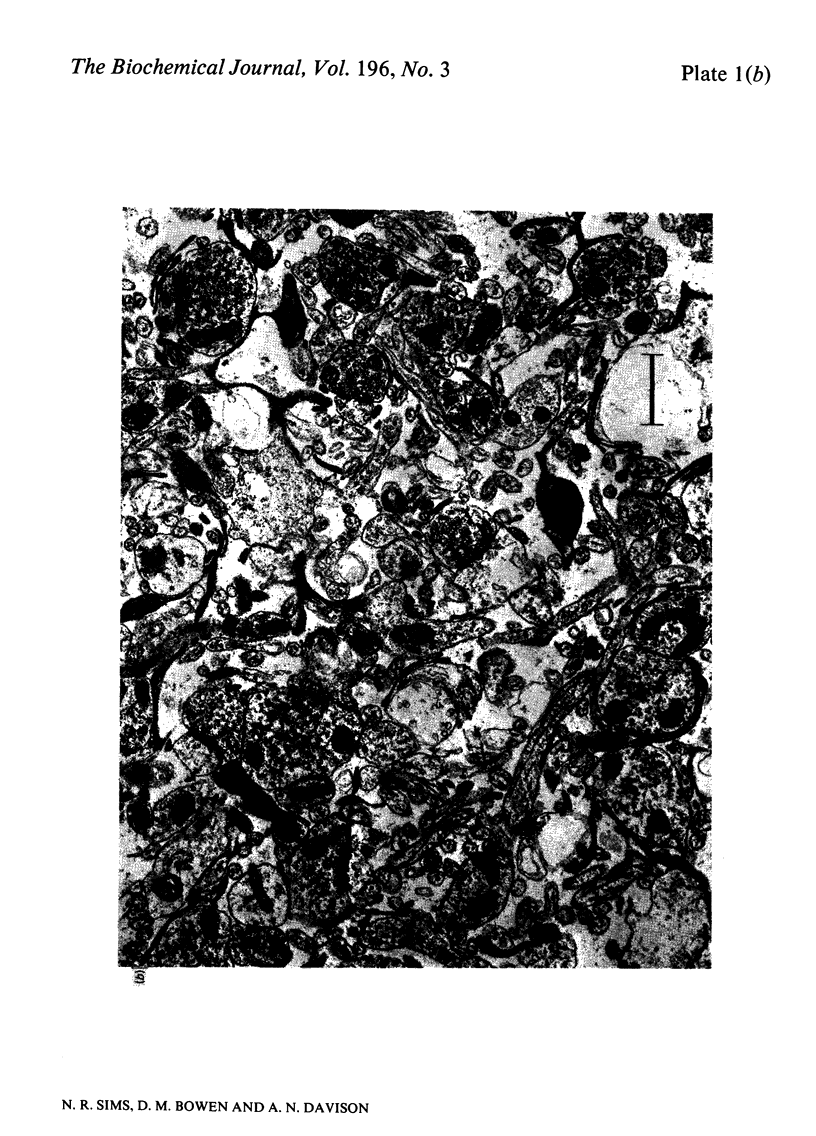
1. [14C]Acetylcholine synthesis and 14CO2 production from [U-14C]glucose has been measured in tissue prism preparations from human neocortex. 2. Electron micrographs of prisms from human and rat neocortex show that both contain intact synaptic endings with evenly-distributed vesicles and normal-appearing mitochondria, but only poorly preserved cell body structure. 3. Synthesis of [14C]acetylcholine in prisms from rat neocortex is similar to estimates for turnover in vivo. Synthesis in prisms from human neocortex is 18% of that in rat tissue and 64% of that in tissue from baboon neocortex for incubations performed in 31 mM-K+. 4. Investigations of prisms prepared from rat brains stored at 37 degrees C after death revealed that synthesis of [14C]acetylcholine in the presence of 31 mM-K+ was greatly decreased within 30 min of post-mortem incubation, whereas synthesis at 5 mM-K+ and production of 14CO2 at both K+ concentrations were only significantly affected after longer periods. Changes were similar in neocortex and striatum. Thus human autopsy material is unlikely to be suitable for use with this system. 5. Investigations using animal models suggest that [14C]acetylcholine synthesis and 14CO2 production are not affected by surgical or anaesthetic procedures. 6. Neither [14C]acetylcholine synthesis nor 14CO2 production in human prisms was significantly changed with age between 15 and 68 years. 7. Samples from patients with the dementing condition Alzheimer's disease showed a significant decrease in [14C]acetylcholine synthesis to 47% of normal samples and a significant increase of 39% in production of 14CO2.
Full text
PDF


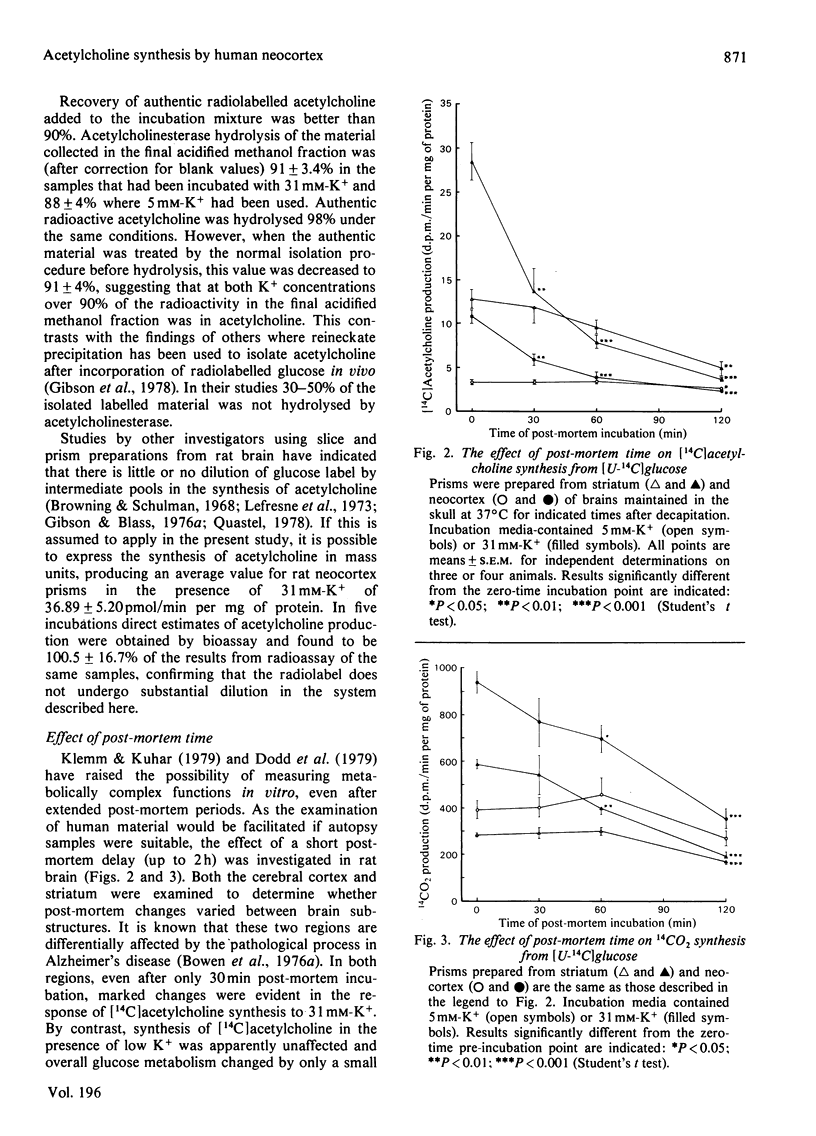




Images in this article
Selected References
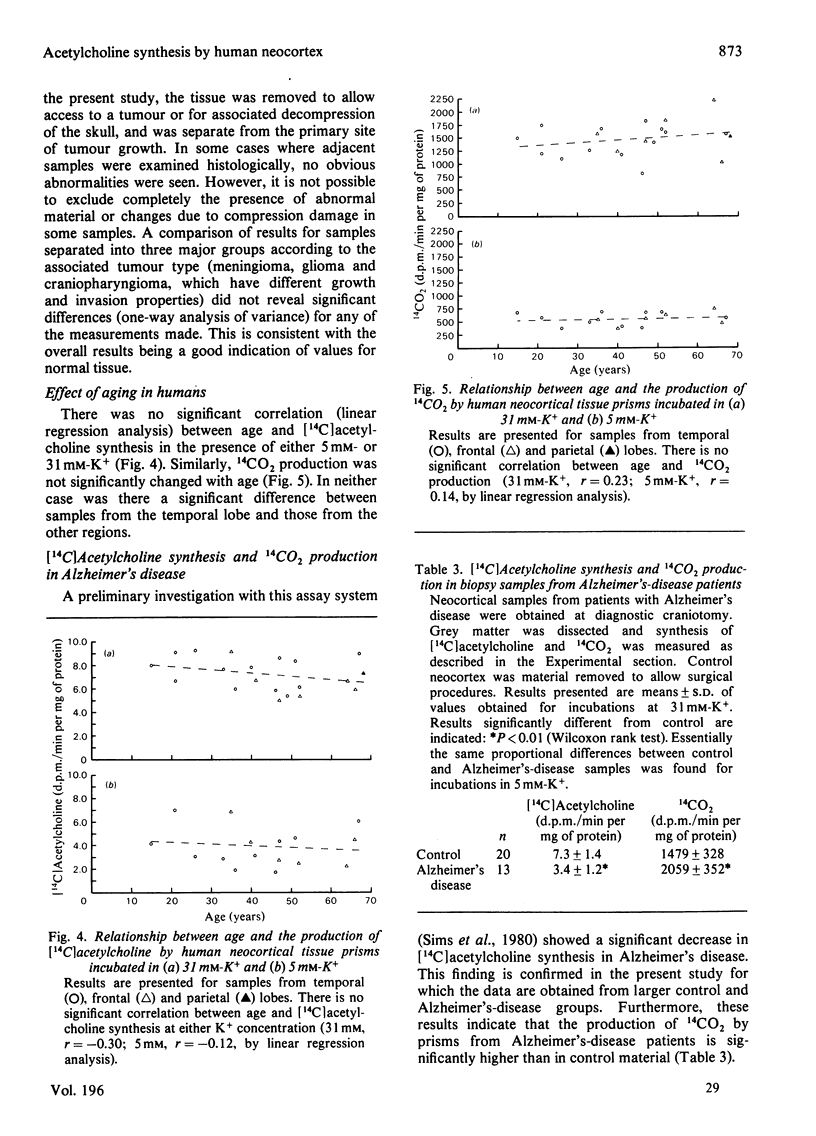
These references are in PubMed. This may not be the complete list of references from this article.
- BOURA A., MONGAR J. L., SCHILD H. O. Improved automatic apparatus for pharmacological assays on isolated preparations. Br J Pharmacol Chemother. 1954 Mar;9(1):24–30. doi: 10.1111/j.1476-5381.1954.tb00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayoumi R. A., Smith W. R. Regional distribution of glutamic acid decarboxylase in the developing brain of the pyridoxine-deficient rat. J Neurochem. 1973 Sep;21(3):603–613. doi: 10.1111/j.1471-4159.1973.tb06005.x. [DOI] [PubMed] [Google Scholar]
- Bowen D. M., Goodhardt M. J., Strong A. J., Smith C. B., White P., Branston N. M., Symon L., Davison A. N. Biochemical indices of brain structure, function and "hypoxia" in cortex from baboons with middle cerebral artery occlusion. Brain Res. 1976 Dec 3;117(3):503–507. doi: 10.1016/0006-8993(76)90757-5. [DOI] [PubMed] [Google Scholar]
- Bowen D. M., Smith C. B., White P., Davison A. N. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain. 1976 Sep;99(3):459–496. doi: 10.1093/brain/99.3.459. [DOI] [PubMed] [Google Scholar]
- Bowen D. M., White P., Spillane J. A., Goodhardt M. J., Curzon G., Iwangoff P., Meier-Ruge W., Davison A. N. Accelerated ageing or selective neuronal loss as an important cause of dementia? Lancet. 1979 Jan 6;1(8106):11–14. doi: 10.1016/s0140-6736(79)90454-9. [DOI] [PubMed] [Google Scholar]
- Brizzee K. R., Ordy J. M. Age pigments, cell loss and hippocampal function. Mech Ageing Dev. 1979 Jan;9(1-2):143–162. doi: 10.1016/0047-6374(79)90126-x. [DOI] [PubMed] [Google Scholar]
- Browning E. T., Schulman M. P. (14C) acetylcholine synthesis by cortex slices of rat brain. J Neurochem. 1968 Dec;15(12):1391–1405. doi: 10.1111/j.1471-4159.1968.tb05921.x. [DOI] [PubMed] [Google Scholar]
- Chalmers A., McGeer E. G., Wickson V., McGeer P. L. Distribution of glutamic acid decarboxylase in the brains of various mammalian species. Comp Gen Pharmacol. 1970 Dec;1(4):385–390. doi: 10.1016/0010-4035(70)90062-5. [DOI] [PubMed] [Google Scholar]
- Cragg B. G. The density of synapses and neurons in normal, mentally defective ageing human brains. Brain. 1975 Mar;98(1):81–90. doi: 10.1093/brain/98.1.81. [DOI] [PubMed] [Google Scholar]
- Davies P. Neurotransmitter-related enzymes in senile dementia of the Alzheimer type. Brain Res. 1979 Aug 3;171(2):319–327. doi: 10.1016/0006-8993(79)90336-6. [DOI] [PubMed] [Google Scholar]
- Dodd P. R., Hardy J. A., Bradford H. F., Bennett G. W., Edwardson J. A., Harding B. N. Metabolic and secretory processes in nerve-endings isolated from post-mortem brain. Neurosci Lett. 1979 Jan;11(1):87–92. doi: 10.1016/0304-3940(79)90061-2. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Garthwaite J., Woodhams P. L., Collins M. J., Balazs R. On the preparation of brain slices: morphology and cyclic nucleotides. Brain Res. 1979 Sep 14;173(2):373–377. doi: 10.1016/0006-8993(79)90641-3. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Woodhams P. L., Collins M. J., Balázs R. A morphological study of incubated slices of rat cerebellum in relation to postnatal age. Dev Neurosci. 1980;3(2):90–99. doi: 10.1159/000112381. [DOI] [PubMed] [Google Scholar]
- Gibson G. E., Blass J. P. Impaired synthesis of acetylcholine in brain accompanying mild hypoxia and hypoglycemia. J Neurochem. 1976 Jul;27(1):37–42. doi: 10.1111/j.1471-4159.1976.tb01540.x. [DOI] [PubMed] [Google Scholar]
- Gibson G. E., Blass J. P. Inhibition of acetylcholine synthesis and of carbohydrate utilization by maple-syrup-urine disease metabolites. J Neurochem. 1976 Jun;26(6):1073–1078. doi: 10.1111/j.1471-4159.1976.tb06988.x. [DOI] [PubMed] [Google Scholar]
- Gibson G. E., Blass J. P., Jenden D. J. Measurement of acetylcholine turnover with glucose used as precursor: evidence for compartmentation of glucose metabolism in brain. J Neurochem. 1978 Jan;30(1):71–76. doi: 10.1111/j.1471-4159.1978.tb07036.x. [DOI] [PubMed] [Google Scholar]
- Gibson G. E., Jope R., Blass J. P. Decreased synthesis of acetylcholine accompanying impaired oxidation of pyruvic acid in rat brain minces. Biochem J. 1975 Apr;148(1):17–23. doi: 10.1042/bj1480017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewaal D. S., Quastel J. H. Control of synthesis and release of radioactive acetylcholine in brain slices from the rat. Effects of neurotropic drugs. Biochem J. 1973 Jan;132(1):1–14. doi: 10.1042/bj1320001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher P. R. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979 Mar 16;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Klemm N., Kuhar M. J. Post-mortem changes in high affinity choline uptake. J Neurochem. 1979 May;32(5):1487–1494. doi: 10.1111/j.1471-4159.1979.tb11089.x. [DOI] [PubMed] [Google Scholar]
- Lefresne P., Guyenet P., Glowinski J. Acetylcholine synthesis from (2- 14 C)pyruvate in rat striatal slices. J Neurochem. 1973 Apr;20(4):1083–1097. doi: 10.1111/j.1471-4159.1973.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Malthe-Sorenssen D., Wood P. L., Cheney D. L., Costa E. Modulation of the turnover rate of acetylcholine in rat brain by intraventricular injections of thyrotropin-releasing hormone, somatostatin, neurotensin and angiotensin II. J Neurochem. 1978 Sep;31(3):685–691. doi: 10.1111/j.1471-4159.1978.tb07841.x. [DOI] [PubMed] [Google Scholar]
- Marchbanks R. M., Israël M. Aspects of acetylcholine metabolism in the electric organ of Torpedo marmorata. J Neurochem. 1971 Mar;18(3):439–448. doi: 10.1111/j.1471-4159.1971.tb11971.x. [DOI] [PubMed] [Google Scholar]
- Marchbanks R. M., Wonnacott S. Relationship of choline uptake to acetylcholine synthesis and release. Prog Brain Res. 1979;49:77–88. doi: 10.1016/S0079-6123(08)64623-3. [DOI] [PubMed] [Google Scholar]
- McGeer P. L., McGeer E. G. Enzymes associated with the metabolism of catecholamines, acetylcholine and gaba in human controls and patients with Parkinson's disease and Huntington's chorea. J Neurochem. 1976 Jan;26(1):65–76. [PubMed] [Google Scholar]
- Pasztor E., Symon L., Dorsch N. W., Branston N. M. The hydrogen clearance method in assessment of blood flow in cortex, white matter and deep nuclei of baboons. Stroke. 1973 Jul-Aug;4(4):556–567. doi: 10.1161/01.str.4.4.556. [DOI] [PubMed] [Google Scholar]
- Perry R. H., Tomlinson B. E., Taylor M. J., Perry E. K. Human brain temperature at necropsy: a guide in post-mortem biochemistry. Lancet. 1977 Jan 1;1(8001):38–38. doi: 10.1016/s0140-6736(77)91669-5. [DOI] [PubMed] [Google Scholar]
- Richter J. A., Perry E. K., Tomlinson B. E. Acetylcholine and choline levels in post-mortem human brain tissue: preliminary observations in Alzheimer's disease. Life Sci. 1980 May 19;26(20):1683–1689. doi: 10.1016/0024-3205(80)90176-9. [DOI] [PubMed] [Google Scholar]
- Roberts E., Kuriyama K. Biochemical-physiological correlations in studies of the gamma-aminobutyric acid system. Brain Res. 1968 Apr;8(1):1–35. doi: 10.1016/0006-8993(68)90170-4. [DOI] [PubMed] [Google Scholar]
- Sims N. R., Bowen D. M., Smith C. C., Flack R. H., Davison A. N., Snowden J. S., Neary D. Glucose metabolism and acetylcholine synthesis in relation to neuronal activity in Alzheimer's disease. Lancet. 1980 Feb 16;1(8164):333–336. doi: 10.1016/s0140-6736(80)90884-3. [DOI] [PubMed] [Google Scholar]
- Spillane J. A., White P., Goodhardt W. J., Flack R. H., Bowen D. M., Davison A. N. Selective vulnerability of neurones in organic dementia. Nature. 1977 Apr 7;266(5602):558–559. doi: 10.1038/266558a0. [DOI] [PubMed] [Google Scholar]
- Spokes E. G. An analysis of factors influencing measurements of dopamine, noradrenaline, glutamate decarboxylase and choline acetylase in human post-mortem brain tissue. Brain. 1979 Jun;102(2):333–346. doi: 10.1093/brain/102.2.333. [DOI] [PubMed] [Google Scholar]
- Symon L., Branston N. M., Chikovani O. Ischemic brain edema following middle cerebral artery occlusion in baboons: relationship between regional cerebral water content and blood flow at 1 to 2 hours. Stroke. 1979 Mar-Apr;10(2):184–191. doi: 10.1161/01.str.10.2.184. [DOI] [PubMed] [Google Scholar]
- TOWER D. B., ELLIOTT K. A. C. Activity of acetylcholine system in cerebral cortex of various unanesthetized mammals. Am J Physiol. 1952 Mar;168(3):747–759. doi: 10.1152/ajplegacy.1952.168.3.747. [DOI] [PubMed] [Google Scholar]
- Weiler M. H., Misgeld U., Bak I. J., Jenden D. J. Acetylcholine synthesis in rat neostriatal slices. Brain Res. 1979 Nov 2;176(2):401–406. doi: 10.1016/0006-8993(79)90997-1. [DOI] [PubMed] [Google Scholar]
- White P., Hiley C. R., Goodhardt M. J., Carrasco L. H., Keet J. P., Williams I. E., Bowen D. M. Neocortical cholinergic neurons in elderly people. Lancet. 1977 Mar 26;1(8013):668–671. doi: 10.1016/s0140-6736(77)92114-6. [DOI] [PubMed] [Google Scholar]
- Wood P. L., Cheney D. L., Costa E. A prolactin action on acetylcholine metabolism in striatum, hippocampus, and thalamus. J Neurochem. 1980 May;34(5):1053–1057. doi: 10.1111/j.1471-4159.1980.tb09938.x. [DOI] [PubMed] [Google Scholar]