ABSTRACT
We herein report a neonatal case showing specific findings of blood perfusion in the anastomosed esophagus of esophageal atresia (EA) and tracheoesophageal fistula (TEF) using indocyanine green (ICG) fluorescence during thoracoscopic surgery. The patient was a 3054 g, 0‐day neonatal boy diagnosed with EA‐TEF based on a coil‐up sign of the nasogastric tube. Thoracoscopic surgery was performed on Day 4 after birth. After TEF transection, esophageal anastomosis was performed using interrupted sutures. ICG was administered intravenously to confirm blood perfusion at the anastomotic site. Initially, the upper esophagus was visualized, and 5 s later, the lower esophagus was visualized. However, no fluorescence signal was detected at the anastomotic site. The postoperative course was uneventful without anastomotic leakage. After discharge, mild anastomotic stenosis was observed, which required balloon dilatation. The time lag of fluorescent findings was considered to reflect differences in the feeding artery.
Keywords: esophageal atresia, indocyanine green fluorescence, thoracoscopic surgery
1. Introduction
Indocyanine green (ICG) fluorescence imaging is useful for evaluating blood perfusion to organs and has recently been used in a variety of pediatric surgical procedures. Sufficient blood perfusion to the anastomosed esophagus is important to reduce the risk of complications, such as anastomotic leakage and anastomotic stenosis, in EA surgery. Its application has been reported in secondary thoracoscopic surgery for long‐gap EA [1], revision surgery for EA [2], and open surgery for EA [3]. However, there have been no reports on ICG fluorescence in EA‐TEF primary thoracoscopic surgery.
We revealed the specific findings of blood perfusion in the anastomosed esophagus of neonatal EA‐TEF using ICG fluorescence during thoracoscopic surgery.
2. Case Presentation
2.1. Patient
The patient was born via Caesarean section at 41 weeks' gestation. The birth weight was 3054 g, and the Apgar score was 3/9. After birth, EA‐TEF was suspected because of the difficulty in inserting a nasogastric tube. He was diagnosed with EA‐TEF (Gross type C) based on the coil‐up sign of the nasogastric tube and the presence of gastric bubbles on x‐ray (Figure 1). No complications, such as cardiac anomalies or anorectal malformation, were observed. Thoracoscopic repair for EA‐TEF was performed on Day 4 after birth.
FIGURE 1.
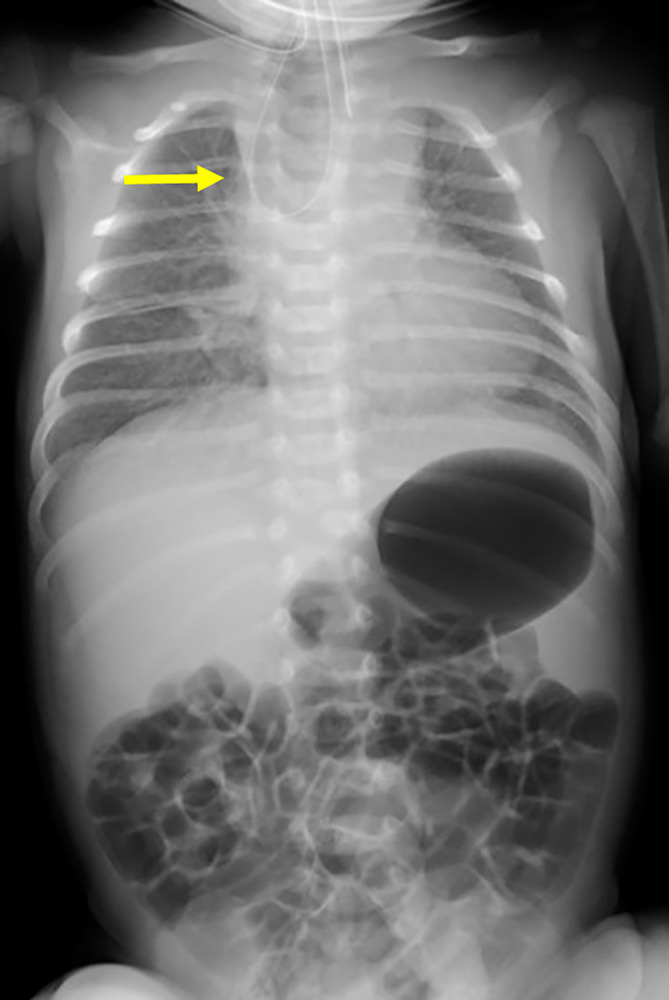
X‐ray findings at 0 days. A coil‐up sign in the nasogastric tube (yellow arrow) and gastric bubbles were detected.
2.2. Operative Findings and Procedure
Preoperatively, flexible bronchoscopy was performed under general anesthesia with tracheal intubation to confirm the location of the TEF under general anesthesia with tracheal intubation. The TEF was identified 5 mm proximal to the tracheal bifurcation. Following bronchoscopy, the patient's position was changed to a left three‐quarters prone position. A 5‐mm trocar was initially inserted in the sixth intercostal space (ICS) on the posterior axial line using an optical procedure. Artificial pneumothorax was established with 5 mmHg CO2 insufflation (1 L/min). Two additional trocars were then inserted under thoracoscopic inspection: a 3.5‐mm trocar was placed in the fourth ICS on the anterior axial line for the operator's right forceps, and a 3.5‐mm trocar was placed in the eighth ICS on the posterior axial line for the operator's left forceps. The azygos vein was coagulated with a vessel‐sealing system (Cool Seal, Bolder Surgical, Louisville, CO, USA) and divided. The TEF and lower esophagus were minimally dissected to secure blood supply while preserving the vagal nerve. TEF was initially ligated by Loeders' knot with 5‐0 monofilament absorbable suture (PDS II; Ethicon, Cincinnati, OH, USA), and then a transfixing suture with 5‐0 PDS II was also added. After double ligation, the TEF was transected close to the trachea.
An 8‐Fr catheter was inserted orally to identify the upper esophagus. The catheter was pushed by an anesthesiologist, and the upper esophagus was dissected bluntly and sharply. There was almost no gap between the upper and lower esophagus. After incision of the upper esophagus using cold scissors, esophageal anastomosis was successfully performed with eight interrupted sutures using 5‐0 PDS II (Figure 2a).
FIGURE 2.
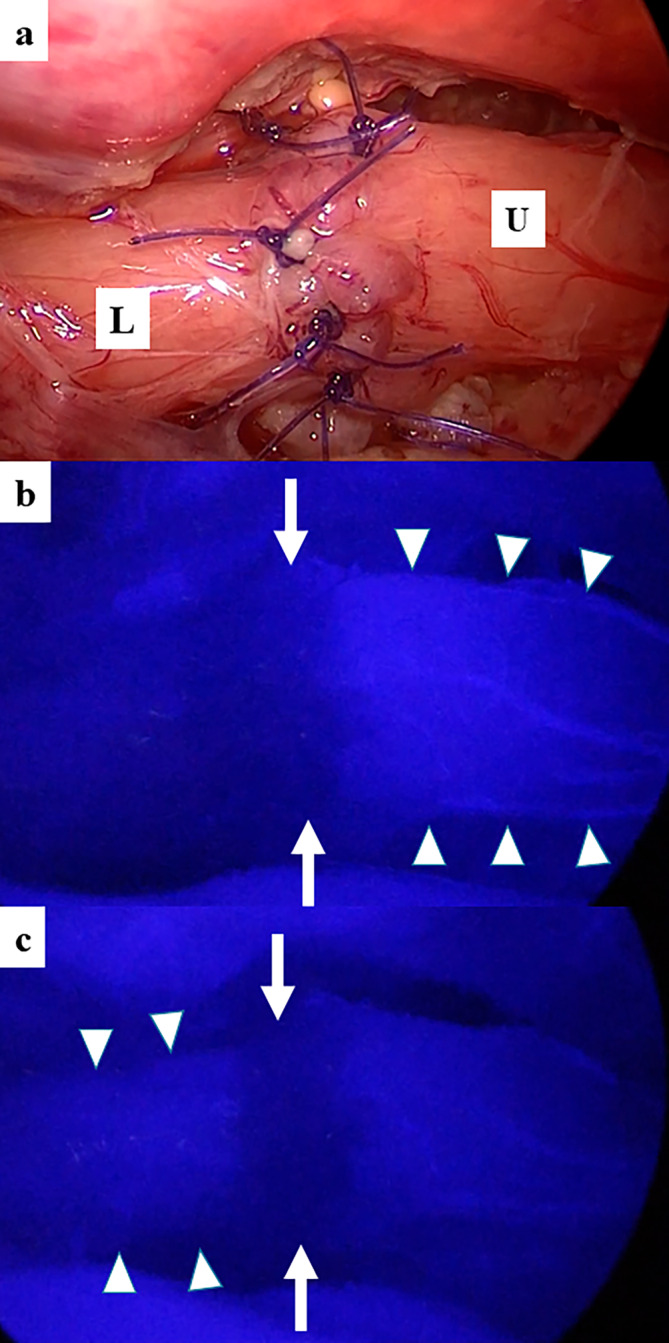
Intraoperative ICG fluorescence findings. (a) At the end of esophageal anastomosis. U, the upper esophagus; L, the lower esophagus. (b) At 25 s after ICG was injected intravenously. Arrow, the anastomotic site; Arrowhead, the upper esophagus. (c) At 30 s after ICG was injected intravenously. Arrow, the anastomotic site; Arrowhead, the lower esophagus.
ICG (0.5 mg/kg: DIAGNOGREEN, Daiichi Sankyo) was administered intravenously to confirm blood perfusion at the anastomotic site. Initially, the upper esophagus was visualized 25 s after administration (Figure 2b), and 5 s later, the lower esophagus was visualized (Figure 2c and Video S1). However, the fluorescence signal at the anastomotic site was not observed. After placing a 10‐Fr drain in the thoracic cavity, fibrin glue (BOLHEAL; KM Biologics Co. Ltd., Kumamoto, Japan) was sprayed on the anastomotic site. There were no intraoperative complications, and the operating time was 215 min.
A postoperative esophagogram 7 days after the operation showed no leakage at the anastomotic site. The patient was started on postoperative day 7 for milk feeding and discharged home on postoperative Day 24. Four months after discharge, mild anastomotic stenosis was observed on the esophagogram. He started eating baby food and occasionally choked on it. The patient underwent balloon dilatation 9 months postoperatively.
3. Discussion
ICG shows fluorescence when irradiated with a specific excitation light, and this characteristic has been used in various surgical procedures, such as gastrointestinal and hepatobiliary surgery, to evaluate blood perfusion of those organs. It is being used increasingly frequently in pediatric surgery as well [4, 5].
Anastomotic leakage is one of the most important complications of EA‐TEF surgery. The risk of anastomotic leakage includes excessive anastomotic tension when the gap between the upper and lower esophagus is large. Insufficient blood perfusion at the anastomotic site may also lead to anastomotic leakage.
ICG may be useful in the evaluation of blood perfusion at the anastomosis during repair of EA‐TEF [1, 2, 3]. There are probably two reasons for the time lag in fluorescent visualization between the upper and lower esophagus. The main reason may be the different sources of the feeding arteries of the upper and lower esophagus. The cervical and thoracic upper esophagus are mainly fed by the inferior thyroid artery and tracheobronchial artery close to the tracheal bifurcation. Caudal blood perfusion is also supplied by the native esophageal artery, which is a direct branch of the aorta, left gastric artery, and left inferior phrenic artery [6]. The second potential reason is that the blood supply to the lower esophagus is affected by the degree of dissection. In addition, no fluorescence signal was detected at the anastomotic site. Postoperatively, there was no anastomotic leakage; however, the patient developed mild anastomotic stenosis requiring balloon dilatation. From these findings, it is expected that the decreased blood perfusion at the anastomotic site did not cause anastomotic leakage but did cause anastomotic stenosis.
There is no report or discussion of the timing of blood flow assessment with ICG in surgery for EA [3]. As in the previous literature, we performed ICG fluorescence evaluation after anastomosis [3]. This is because the purpose of ICG evaluation, in this case, was to assess anastomotic outcomes such as anastomotic leakage and stenosis after surgery. However, by performing ICG fluorescence before esophageal anastomosis as well, it may be possible to determine whether the reason the fluorescent signal was not observed at the anastomotic site is an artifact of the anastomosis itself or is due to decreased blood flow. Further studies are needed to determine whether ICG fluorescence evaluation before esophageal anastomosis provides more useful information.
In conclusion, the ICG fluorescence technique may be useful for the visual confirmation of the blood supply at the esophageal anastomosis in primary thoracoscopic surgery for EA‐TEF. However, this technique is a qualitative evaluation, and a quantitative method is required for the objective evaluation of anastomosis.
Author Contributions
All authors contributed to the study design and concept. Patient data were collected from chart records by Yudai Tsuruno. The draft of this article was written by Yudai Tsuruno and other authors commented on the draft versions of this article. All the authors have proofread and approved the final version of the article.
Ethics Statement
The authors have nothing to report.
Consent
Written informed consent was obtained from the patient's parents for publication of this case report and accompanying images. A copy of the consent document is available for review by the editor‐in‐chief of the journal.
Conflicts of Interest
Dr. Satoshi Ieiri is an Editorial Board member of ASES Journal and a coauthor of this article. To minimize bias, they were excluded from all editorial decision‐making related to the acceptance of this article for publication.
Supporting information
Video S1. Operative findings and procedure of transection of the TEF, esophageal anastomosis, and ICG fluorescence.
Acknowledgments
We thank Mr. Brian Quinn for his comments and assistance with this manuscript.
Funding: The authors received no specific funding for this work.
Data Availability Statement
The data analyzed in this study are not publicly available because of privacy and ethical concerns.
References
- 1. Onishi S., Muto M., Yamada K., et al., “Feasibility of Delayed Anastomosis for Long Gap Esophageal Atresia in the Neonatal Period Using Internal Traction and Indocyanine Green‐Guided Near‐Infrared Fluorescence,” Asian Journal of Endoscopic Surgery 15, no. 4 (2022): 877–881, 10.1111/ases.13098. [DOI] [PubMed] [Google Scholar]
- 2. Zhang Y., Wang M., Li S., et al., “Indocyanine Green Fluorescence Imaging Localization‐Assisted Thoracoscopy Revision Surgery After Repair of Esophageal Atresia,” BMC Gastroenterology 22, no. 1 (2022): 373, 10.1186/s12876-022-02444-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meisner J. W., Kamran A., Staffa S. J., et al., “Qualitative Features of Esophageal Fluorescence Angiography and Anastomotic Outcomes in Children,” Journal of Pediatric Surgery 58, no. 7 (2023): 1359–1367, 10.1016/j.jpedsurg.2022.07.007. [DOI] [PubMed] [Google Scholar]
- 4. Yamamoto N., Kitagawa H., Orihashi K., Yokota K., Namikawa T., and Seo S., “Blood Flow Evaluation of Reconstructed Gastric Tube in Esophageal Surgery Using Near‐Infrared Imaging and Retrospective Time‐Intensity Curve Analysis,” Langenbeck's Archives of Surgery 409, no. 1 (2024): 90, 10.1007/s00423-024-03284-1. [DOI] [PubMed] [Google Scholar]
- 5. Sincavage J., Gulack B. C., and Zamora I. J., “Indocyanine Green (ICG) Fluorescence‐Enhanced Applications in Pediatric Surgery,” Seminars in Pediatric Surgery 33, no. 1 (2024): 151384, 10.1016/j.sempedsurg.2024.151384. [DOI] [PubMed] [Google Scholar]
- 6. Netter F. H., Atlas of Human Anatomy, 8th ed. (Philadelphia: Elsevier, 2023). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Operative findings and procedure of transection of the TEF, esophageal anastomosis, and ICG fluorescence.
Data Availability Statement
The data analyzed in this study are not publicly available because of privacy and ethical concerns.


