Abstract
Mesenchymal stem cells (MSCs) are multipotent cells with high self-renewal and multilineage differentiation abilities, playing an important role in tissue healing. Recent advancements in stem cell-based technologies have offered new and promising therapeutic options in regenerative medicine. Upon tissue damage, MSCs are immediately mobilized from the bone marrow and move to the injury site via blood circulation. Notably, allogenically transplanted MSCs can also home to the damaged tissue site. Therefore, MSCs hold great therapeutic potential for curing various diseases. However, one major obstacle to this approach is attracting MSCs specifically to the injury site following systemic administration. In this review, we describe the molecular pathways governing the homing mechanism of MSCs and various strategies for improving this process, including targeted stem cell administration, target tissue modification, in vitro priming, cell surface engineering, genetic modifications, and magnetic guidance. These strategies are crucial for directing MSCs precisely to the injury site and, consequently, enhancing their migration and local tissue repair properties. Specifically, our review provides a guide to improving the therapeutic efficacy of clinical applications of MSCs through optimized in vivo administration and homing capacities.
Keywords: mesenchymal stem cells, homing, improvement strategies, administration, migration, target tissue modification, priming
Graphical Abstract
Graphical Abstract.
Significance Statement.
Mesenchymal stem cells (MSCs) hold tremendous potential for regenerative medicine and the treatment of currently incurable diseases. These adult multipotent progenitor cells are essential for tissue regeneration and wound repair due to their high capacity to differentiate into various tissues. Through paracrine effects, MSCs can regulate immune responses, enhance neovascularization, and improve cell survival. Moreover, MSCs have the ability to preferentially home to damaged tissues and serve as a reservoir of growth and proregenerative factors. Therefore, understanding and enhancing MSC homing efficiency at injury sites is crucial to maximizing their therapeutic effects. In this review, we analyze a variety of strategies to optimize MSC homing and foster the potential of MSC-based cell therapies in regenerative medicine.
Introduction
Adult stem cells such as mesenchymal stem cells (MSCs), induced pluripotent stem cells (iPSCs), and embryonic stem cells (ESCs) are 3 main types of stem cells. MSCs are adult multipotent progenitor cells that can differentiate into various tissues, including adipose, bone, and cartilage.1 MSCs are characterized morphologically by a small cell body with a few cell processes that are long and thin. Importantly, those fibroblast-like cells were identified first by Friedenstein et al1 in the 1960s. The observations of Friedenstein have laid a milestone for the later discovery of what is now known as MSCs. In 1974, Friedenstein et al2 isolated MSCs from bone marrow for the first time. Since then, MSCs have been isolated from various other tissues, which include fetal tissue,3 perivascular tissue,4 muscle,5 dermis,5 adipose tissues,6 and dental pulp.7
Based on the criteria of the International Society for Cellular Therapy MSCs show the following characteristics8: (1) plastic adherence; (2) positive expression of CD90, CD105, and CD73 surface markers; (3) negative expression of stem cell lineage markers including CD34, present on hematopoietic and endothelial cells, CD79a or CD19 present on B cells, and CD45 present on pan-leukocyte; (4) negative expression of myeloid markers including CD14 or CD11b; and (5) tri-lineage differentiation potential into chondrocytes, osteocytes, and adipocytes.8 These minimal criteria must be met by MSCs isolated from any tissue. Furthermore, certain nonclassical differentiation potential of MSCs including neural,9 hepatocytic,10 and myoblastic4 lineages has been demonstrated, although the neural differentiation remains controversial.
In contrast, ESCs are pluripotent stem cells that may differentiate into any mature cell of the 3 germlines after being isolated from the inner cell mass of a mouse early preimplantation blastocyst.11 In general, stem cell-based research aims to improve therapies for currently untreatable diseases. Presently, tissues derived from stem cells, stem cell-based products, and biomaterials combined with stem cells offer a promising alternative in regenerative medicine.12 MSCs have demonstrated superior therapeutic effects because of their ability to regulate many types of immune cells of the adaptive and innate immune systems. In particular, MSCs promote neovascularization, enhance angiogenesis, inhibit cell death, increase cell proliferation and viability, and regulate immune responses by exosomes, cell-to-cell contacts, and paracrine effects.13,14
Although MSC treatments have made significant advances in recent decades, several challenges remain. High levels of heterogeneity, issues regarding immune compatibility, differentiation capacity, phenotype stability, and migratory capabilities are the key points.15 Upon tissue damage, MSCs are rapidly mobilized into the bloodstream,16 moving to the injury site, where they create a proregenerative microenvironment for proper wound healing.17,18 Importantly, allogenically transplanted MSCs can also home to the damaged tissue site and support the recovery process or act as activators for the regeneration of tissues. This concept underpins the therapeutic potential of the administration of MSCs for clinical purposes. Table 1 represents a summary of preclinical and clinical trials involving MSCs.17
Table 1.
Summary of preclinical trials and clinical trials involving mesenchymal stem cells (MSCs).
| Disease/condition | Clinical application | Status | References |
|---|---|---|---|
| Graft-versus-host disease (GVHD) | Immunomodulation | Clinical | Dominici et al8, Chinnadurai et al19 |
| Multiple sclerosis (MS) | Neuroregeneration | Clinical | Chinnadurai et al19 |
| Crohn’s disease (CD) | Tissue homeostasis | Clinical | Lotfy et al20 |
| Amyotrophic lateral sclerosis (ALS) | Regeneration | Clinical | Lotfy et al20, Rendra et al21 |
| Myocardial infarction (MI) | Cardiac repair | Clinical | Kahrizi et al22 |
| Acute respiratory distress syndrome (ARDS) | Anti-inflammatory effects | Clinical | Lotfy et al20 |
| Heart failure | Cardiac repair | Preclinical | Kahrizi et al22 |
| Lung injury | Anti-inflammatory effects | Preclinical | Galipeau23 |
| Liver disease | Tissue regeneration | Preclinical | Chinnadurai et al19 |
Once localized at the target site, MSCs release various factors, which have angiogenic, immunomodulatory, and antiapoptotic effects.24-26 Based on these characteristics, MSCs have been applied in clinical settings including regulation of immune response in autoimmune and inflammatory diseases, protection of tissue after injury, and regenerative medicine.27 Therefore, the improvement of MSCs’ homing efficiency is necessary since delivering MSCs to the injury site represents the key feature of their therapeutic efficacy. Hence, this review elaborates on different strategies for improving MSC homing efficiency at the molecular level.
MSCs homing mechanism
The ability of MSCs to home to damaged tissues is a key benefit and a requirement for a successful stem cell-based therapy. Therefore, it is important to first define the homing mechanism, including both systemic and nonsystemic homing.28 In nonsystemic homing, MSCs are implanted locally at the target site, and a chemokine gradient guides them to the injury.29 In contrast, in systemic homing, MSCs are administered into the bloodstream and migrate through a multistep process to the injury site after leaving circulation.30
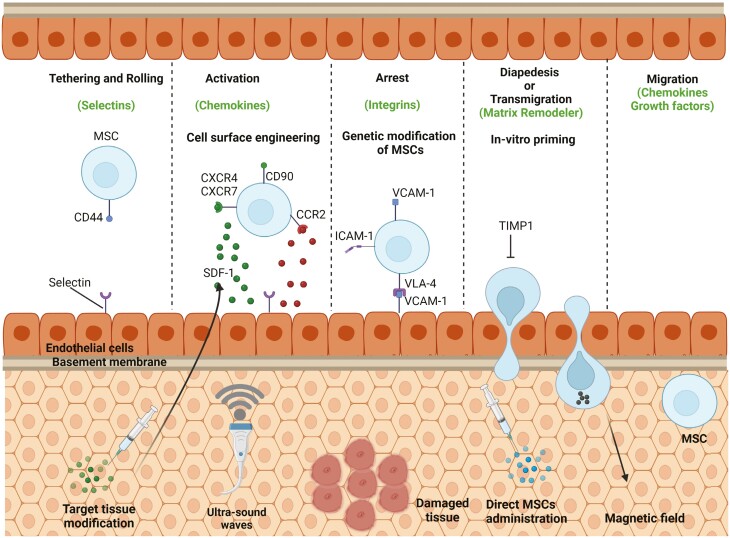
The systemic homing can be divided into 5 distinct steps (Figure 1):
Figure 1.
Overview of MSC homing mechanism. The systemic homing of MSCs comprises 5 distinct steps, including tethering and rolling, activation, arrest, diapedesis or transmigration, and migration. Tethering and rolling, which are the first steps of this mechanism, are facilitated by interactions of selectins and ligands. The second step activation is facilitated by G protein-coupled chemokine receptors, and the third step of cell arrest involves integrins. In the fourth step of diapedesis or transmigration, MSCs cross the endothelial cell layer and basement membrane by secreting matrix metalloproteinases (MMPs). In the final step, MSCs migrate to the injury site through the interstitium due to chemotactic signals released upon tissue damage. Modified from Ullah et al.31
tethering and rolling,
activation,
arrest,
diapedesis or transmigration, and
migration.32
Importantly, endothelial cells express selectins which facilitate initial tethering.33 On the other hand, CD44 is expressed by MSCs, which bind to endothelial selectins, and upon this, MSCs begin to roll on the vasculature. Table 2 depicts all important cell surface markers and integrins expressed on distinct types of MSCs.
Table 2.
Summary of surface markers and integrins relevant to homing mechanisms and expressed on various types of MSCs.
| Surface marker | Expression in MSCs | Function | MSC types | References |
|---|---|---|---|---|
| CD90 (Thy-1) | High expression | Associated with MSC identity and immunomodulation | Bone marrow-derived MSCs (BM-MSCs), adipose tissue-derived MSCs (AT-MSCs) | Dominici et al8 |
| CD73 | High expression | Involved in adenosine production and immunosuppression | BM-MSCs, AT-MSCs | Dominici et al8 |
| CD105 (endoglin) | High expression | Regulates angiogenesis and tissue repair | BM-MSCs, AT-MSCs | Dominici et al8 |
| CD44 | High expression | Cell adhesion and migration | BM-MSCs, AT-MSCs | Sackstein et al34 |
| CD273 (PD-L2) | Variable expression | Immunomodulatory role | BM-MSCs, AT-MSCs | Wu et al29 |
| CD146 | Variable expression | Associated with angiogenesis and tissue regeneration | BM-MSCs, AT-MSCs | Caplan et al,13 Fan et al14 |
| CD248 (endosialin) | Variable expression | Implicated in tissue remodeling and angiogenesis | BM-MSCs, AT-MSCs | Caplan et al,13 Fan et al14 |
| VLA-4 (α4β1 integrin) | Expressed by MSCs | Mediates binding to endothelial cells via its ligand VCAM-1 | BM-MSCs, AT-MSCs | Rüster et al,35 Segers et al,36 Steingen et al37 |
| VCAM-1 (vascular cell adhesion molecule-1) | Expressed by endothelial cells | Facilitates MSC binding to endothelial surfaces during inflammation | Rüster et al,35 Segers et al,36 Steingen et al37 |
It is known that P-selectin glycoprotein ligand-1 (PSGL-1) and the hematopoietic cell E-/L-selectin ligand (HCELL) bind to specific selectins expressed by endothelial cells, triggering initial tethering. However, in the case of MSCs, it is still not completely understood which selectins bind to MSCs, as they express neither PSGL-1 nor HCELL.34 Some in vitro models mimicking the homing of MSCs have been reported previously.35 Interestingly, Rüster et al35 reconstructed a model demonstrating a coordinated sequence of adhesion steps of human MSCs with human endothelium, initiated by tethering events in a parallel plate flow chamber. The authors confirmed that the binding of human MSCs to human endothelial cells could be suppressed by anti-P-selectin antibodies, while the rolling of MSCs increased when exposed to a P-selectin-containing plate in the chamber. Since MSCs do not express PSGL-1, a different ligand is used for binding with P-selectin. In one study, galectin-1 was identified as a possible ligand for P-selectin.38 Moreover, Bailey et al39 identified CD24 as another potential P-selectin ligand in stromal cells derived from human adipose tissue.
G protein-coupled chemokine receptors facilitate the second step, “activation,” typically in response to inflammatory signals. Animal experiments, where either the entire body or a local area was irradiated, showed higher numbers of MSCs in response to inflammation.40 The critical factor for this step is stromal cell-derived factor-1 (SDF-1) expression on endothelial cells,41 interacting with CXC chemokine receptors type 4 (CXCR4) expressed by MSCs.42-44 Homing to the bone marrow was reported to increase with overexpression of CXCR4 on MSCs.45 However, other receptors may be involved in this process, as some studies reported that MSCs do not express CXCR4.46
In this regard, another receptor, CXC chemokine receptor type 7 (CXCR7), was identified to be expressed on MSCs. Like CXCR4, CXCR7 also binds to SDF-1.47-49 Other receptors and chemokines also play crucial roles in the homing process. For example, monocyte chemoattractant protein-1 (MCP-1), an anti-inflammatory marker expressed in the myocardium of mice, enhances MSC homing by binding to its corresponding receptor, CC chemokine receptor type 2 (CCR2).50 Mice expressing MCP-1 recruited MSCs expressing the CCR2 receptor through this interaction.50 In another study, MSC homing was significantly increased by MCP-3 expression in the myocardium.51 MSCs also express several other receptors, including CCR1, CCR4, CCR5, CCR6, CCR7, CXCR9, and CXCR10.43,46 However, the roles of these receptors are not yet fully understood.
Furthermore, integrin affinity increases through conformational changes in their extracellular domains, a process also involved in the MSC activation step. Integrins are vital for cell adhesion to target tissues.52,53 For example, Talin and Kindlin signaling molecules interact with the cytoplasmic domain of very late antigen-4 (VLA-4) upon SDF-1 stimulation, changing VLA-4 from an inactive to an active form, promoting MSC migration and binding to receptors.54
Integrins facilitate the third step, cell adherence. High integrin expression significantly enhances MSC adherence. Chemokines like SDF-1 activate VLA-4, an integrin expressed in MSCs. Upon activation, vascular cell adhesion molecule 1 (VCAM-1), expressed by endothelial cells, binds to activated VLA-4 integrin.35-37 The homing of MSCs to bone marrow increases when VLA-4 integrin is overexpressed. VCAM-1 and other integrin ligands are also expressed by MSCs.55,56
In the final step, MSCs migrate to the injury site through the interstitium.57 Chemotactic signals which are released upon tissue damage guide this step. MSCs move toward those specific signals which include insulin-like growth factor (IGF)-1 and platelet-derived growth factor-AB (PDGF-AB). Further, MSCs can be also attracted by chemokines like SDF-1, macrophage-derived chemokine, as well as RANTES.57 MSCs migration toward chemokines can be increased by preincubating MSCs with tumor necrosis factor (TNF)-alpha, which upregulates their CCR2, CCR3, and CCR4 receptors.57 MSCs migration toward sites of injury may also be promoted by inflammatory chemokines such as interleukin (IL)-8.58,59 This chemokine also stimulates in MSCs the secretion of regenerative factors like vascular endothelial growth factor (VEGF).60 Thus, comprehensive knowledge of molecular events that are involved in MSC homing provides different strategies for the optimization of the MSC homing process for therapeutic purposes.
Currently, MSC homing efficiency remains one of the key challenges in MSC therapies. Multiple reports suggest that only a small percentage of MSCs reach the target tissue when administered intravenously.61-63 Several factors contribute to low MSC homing efficiency. One reason is that MSCs become trapped in the lung capillaries after intravenous administration. Anticoagulants like heparin and vasodilators have been shown to increase MSC homing to the liver and bone marrow by reducing lung trapping.64,65 Another reason for low homing efficiency may be the reduced expression of specific homing molecules like CXCR4 on MSCs,44,46 as homing molecule expression decreases with in vitro MSC expansion.43,66
Enhancing of MSCs homing efficiency
MSCs homing efficiency can be improved by a variety of approaches:
targeted administration,
target tissue modification,
in vitro priming,
cell surface engineering,
genetic modification,
magnetic guidance, and
radiotherapeutic techniques.
Targeted administration
Targeted administration represents a method, which can significantly improve MSCs’ homing. In this method, cells are at a target site or near to it (Figure 2) instead of introducing them by intravenous routes. Retention of MSCs may increase by targeted administration, for example, by intracerebral application for neurological diseases, intratracheal application for lung disease, or intramyocardial injection for heart disease. A lot of research has been performed in the development of new medical technologies required for targeted administration. In particular, transcatheter injections into the myocardium have been used in several clinical trials of MSCs therapies for ischemic cardiomyopathy.67,68
Figure 2.
Targeted administration of MSCs at or near the damaged tissue. This process is called nonsystemic homing. It is the chemokines which guide the MSCs toward damaged tissue when administered near the damaged tissue. Modified from Ullah et al.31
In a porcine model, Dick et al69. identified infarct borders by utilizing magnetic resonance fluoroscopy. The authors delivered MSCs to the infarcted region by safely navigating the catheter to the target site. The applied MSCs were visible and detectable even after administration near the damaged tissue using MRI. This study confirmed the successful migration of MSCs into the infarct area; however, no quantification of MSCs retained in the target tissue was reported.
Targeted administration of MSCs has been described in many studies, but very few compared standard intravenous injection with targeted administration. The optimal route of MSC administration can be determined by meta-analysis. In ischemic stroke, MSCs administered intracerebrally showed the highest efficacy, with intra-arterial administration ranking second and intravenous third in improving the neurological severity score.70 In myocardial infarction, infarct size was significantly reduced by transendocardial administration of MSCs, while no significant results were obtained with intravenous, intracoronary, or intramyocardial administration in swine models.71 However, results differed in human trials. The intracoronary route of MSC administration performed best, whereas the intravenous route showed some improvement, and the intramyocardial route demonstrated almost no positive effect.72
Therefore, it cannot be assumed that administering MSCs directly to the target tissue or organ would always yield the most promising results in vivo. For example, in a porcine model of emphysema, both intrathecal and intravenous routes reduced cell damage and lung inflammation.73 However, cardiovascular function improved only with intravenous administration, which also shifted lung macrophage phenotypes from M1 to M2 due to MSCs being trapped in the lung capillaries when administered intravenously.
Some studies explored transplanting MSC sheets instead of using MSC suspensions. MSC sheets consist of single layers of cells grown on cell culture plastic that detach spontaneously when the temperature decreases. Ishikane et al74 treated scarred myocardium in a rat model of chronic-stage myocardial infarction by direct transplantation of MSC sheets. Rats treated with such sheets demonstrated increased capillary density, reduced myocardial fibrosis, and improved cardiac function in the infarcted region compared to untreated control rats.
Another study examined the effectiveness of intramyocardial injection versus epicardial placement of MSC sheets in ischemic cardiomyopathy rat models.75 The authors observed increased myocardial repair with MSC sheet transplantation compared to intramyocardial injection of MSC suspensions. Kaibuchi et al76 evaluated MSC sheet transplantation for treating osteonecrosis of the jaw. They compared the results of MSC sheet transplantation with intravenous injection, finding that the MSC sheet group exhibited improved wound healing and enhanced vascularization compared to the intravenous injection group.
In general, there are 3 reasons favoring MSC sheet transplantation over MSC suspension injection: (a) improved survival of transplanted MSCs, (b) enhanced secretion of regenerative factors, and (c) no embolism risk. Although these studies highlight the significance of targeted administration, the results are highly dependent on the tissue and disease model.
Target tissue modification
Target tissue modifications can improve the homing efficiency of MSCs as shown in Figure 3. In particular, the target tissue modification aims to increase the concentration of homing factors at the target site that enhance the infiltration of MSCs.77 The target tissue modification includes direct injection of the homing factor, target tissue genetic modification, and implantation of a scaffold containing homing factor.77,78
Figure 3.
Target tissue modification for increasing MSCs homing efficiency. (A) Direct injection of homing factor, (B) target tissue genetic modification by direct injection of homing factor containing plasmid or by intravenous injection of homing factor containing microbubble injection followed by ultrasound-mediated microbubble destruction method, and (C) homing factor containing scaffold implantation. Modified from Ullah et al.31
Direct injection of homing factors
Direct injection of SDF-1 into ischemic tissue has been reported to enhance neovascularization in both the myocardium78,79 and skeletal muscle.80 Although MSCs were not used in these studies, Sasaki et al78 observed an increase in the homing of bone marrow MSCs after injecting SDF-1 directly into the ischemic myocardium. Similarly, Yamaguchi et al80 observed a 1.8-fold increase in the endogenous homing of intravenously injected endothelial progenitor cells (EPCs) following SDF-1 injection into the ischemic hind limb muscle of mice. However, SDF-1 degrades quickly due to proteolytic enzymes. To address this, Segers et al bioengineered a protease-resistant version of SDF-1 that showed prolonged binding with CXCR4. The injection of this bioengineered SDF-1 improved blood flow in cases of peripheral artery disease and enhanced cardiac function in myocardial infarction.81,82
Target tissue genetic modification
Some studies performed genetic modification by applying targeted tissue transfection with chemokines encoding constructs.77 Fujii et al83 delivered SCF-containing plasmid into the myocardium by using the UMMD (ultrasound-mediated microbubble destruction) method. In this method, the plasmids with microbubbles are injected directly into the damaged tissue. The microbubbles, which are injected along with plasmids, cavitate and generate shear stress in response to the ultrasound. This shear stress also exerts many biological effects like alteration of vascular permeability of endothelial lining that improves uptake of plasmids. This technique enhanced the expression of SDF-1 in the myocardium, as well as the homing efficiency of endogenous CXCR4-expressing progenitor cells.83 Similarly, improved cardiac function and angiogenesis were achieved by Sundararaman et al84 by injecting SDF-1 plasmid into the heart tissue of mice without using the UMMD method. Phase I clinical trials of this therapy without a control group were carried out with SDF-1 plasmid being delivered directly into the infarcted region of 17 ischemic cardiomyopathy patients.85 The patients showed quality of life and walk distance improvements after 12 months in a dose-dependent manner following the treatment. In phase II (placebo-controlled study) clinical trials of this therapy, no significant difference between treatment groups and placebo groups was observed after 6-minute walk distance.86 However, significant improvements were observed when the analysis was limited to one-third of the most severe patients. However, there are some concerns regarding target tissue transfection due to high costs, intentional mutagenesis, and immunogenicity.
MSC engraftment rate is higher in the irradiated target tissues.18,87 This increase is due to upregulated SDF-1 expression hence enhancing the activation stage of the homing process.88 However, radiation therapy in human patients raises safety concerns and thus, it cannot be used clinically. Therefore, alternatives of radiation therapy showing similar improvements in the homing process of MSCs can be used as shown in Figure 4.
Figure 4.
Overview of cell surface engineering, genetic modifications, magnetic guidance, and radiotherapeutic (ultrasound) techniques for improving MSCs homing at the target tissue. Modified from Ullah et al.31
Ultrasound has various therapeutic applications along with its usage as a diagnostic tool.89 Therapeutic ultrasound mainly focuses on the modifications of a target tissue. In particular, sound waves exert mechanical pressure at the target site, which induces many biological effects leveraging tissue regeneration. For example, ultrasound-mediated microbubble destruction techniques improve MSC homing as shown in Figure 3. Many studies applied this technique for increasing cardiac recovery in case of myocardial infarction.90-92 For example, Li et al93 observed that the ultrasound-mediated microbubble destruction (UMMMD) technique increases the proportion of CXCR4-expressing cells and enhances SDF-1 secretion in the target site. Further, the same technique promoted the homing of MSCs in the kidney by upregulating the expression of selectins, integrins, cytokines, and other trophic factors.94 In addition, the UMMD technique was shown to induce an inflammatory response in the brain that, in turn, upregulated several trophic and inflammatory factors.95 However, improper application of UTMD may produce undesired complications since previous studies reported the presence of erythrocyte extravasations, inflammation, and intracerebral hemorrhage within sonicated areas as the most common side effects.96-98 Therefore, some studies started the investigation of focused ultrasound without microbubbles. In this modified method, focused pulses of highly intense sound waves are administered by pulsed focused ultrasound (pFUS) that prevents tissue damage and high temperatures.99 Burks e al.100 demonstrated that pFUS generated a chemical gradient in the muscle leading to upregulated expression of proinflammatory cytokines and chemokines, which play a crucial role in tissue remodeling and repair processes. For example, cell adhesion molecules such as VCAM-1 and ICAM-1, growth factors, and cytokines show increased expression upon treatment. pFUS establishes a local chemical gradient by activating TNF-alpha, which further triggers the signaling cascade of cyclooxygenase-2 (Cox2) leading to upregulation of many homing factors and cytokines.101 pFUS enhanced homing of MSCs by 4-fold in limb ischemia mouse models compared to MSCs alone, hence improving clinical outcomes.102 Similar results were obtained in the kidney by using pFUS,103 which activated IL-1α and TNF-alpha in the kidney upregulating Cox2 and the NF-κB pathways.104 Moreover, Jang et al105 observed that pFUS initially increases TNF-alpha, which causes upregulation of growth factors and cytokines in the heart. However, further studies are required to determine the long-term effects of pFUS, but the research performed until now demonstrated a promising avenue for increasing the homing efficiency of MSCs by using pFUS.
Implantation of a scaffold containing chemokines increasing homing
Another method of increasing homing is to deliver chemokines in a scaffold releasing chemoattractants such as SDF-1 to the injured target site. For example, Kimura and Tabata106 generated a hydrogel showing a slow release of SDF-1. Similarly, a subcutaneous implantation of gelatin hydrogel containing SDF-1 showed improved results than SDF-1 injection alone.106 Similarly, He et al107 engineered SDF-1-loaded hydrogel, which increased the migration of bone marrow stromal cells upon implantation. Further, Goncalves et al108 engineered SDF-1 containing a chitosan/poly(g-glutamic acid) complex, which enhanced the in vitro MSCs migration. Moreover, Shen et al109 developed a silk-collagen sponge scaffold releasing SDF-1. This scaffold increased the endogenous progenitor cell migration and tendon regeneration in rat Achilles tendon injury models after implantation. Using a complex system, Thevenot et al110 engineered an SDF-1-releasing scaffold by applying a mini osmotic pump. Subcutaneous implantation of these scaffolds in mice increased the homing effect of MSCs by 3-fold.110
In vitro priming
Priming methods affect gene expression by altering the culture conditions and thus, various steps of systematic homing (tethering, activation, and transmigration) in various studies as shown in Figure 5. For example, it was shown that CD44 is upregulated at the tethering stage when MSCs are coated with hyaluronic acid.111 Further, many different soluble factors can increase the expression of CXCR4, CXCR7, CCR2, CCR3, and CCR4 during the activation step.57,112-123 The cell culture confluence also affects the migration ability of MSCs.124 Several treatments increased matrix metalloproteinase (MMP) expression, which improved the transmigration of MSCs.116 These methods aim to promote MSC’s ability to migrate toward the site of tissue damage or inflammation, which is essential for the potential therapeutic use of these cells.
Figure 5.
Overview of in vitro priming methods to enhance homing of MSCs modified from Ullah et al.31 These methods include CD44 upregulation at tethering stage, an increase in the expression of CXCR4, CXCR7, CCR2, CCR3, and CCR4 at activation step, an increase in matrix metalloproteinases (MMPs) expression at transmigration stage.
In conclusion, in vitro priming methods include CD44 upregulation at the tethering stage, increased expression of CXCR4, CXCR7, CCR2, CCR3, and CCR4 at the activation step, and increased matrix metalloproteinases (MMPs) expression at transmigration stage.
Supplementation of culture media
Some specific treatments like supplementation of culture media with HIF-1a (hypoxia-inducible factor) or coating MSCs with some specific factors can increase the expression of certain markers or genes, which increases MSC homing. For example, the selectin ligand CD44 is upregulated at the tethering stage when MSCs are coated with hyaluronic acid.111 Indeed, the CD44 upregulation increased the invasion and homing of MSCs by 2-fold, which resulted in a reduced in inflammation at the target site.111 It has been found that different soluble factors can increase the expression of CXCR4, which is linked with the activation step of the homing process. The specific combinations of soluble factors include (1) HGF, IL-6, IL-3, FLT3LG, and stem cell factor (SCF)112; (2) iron chelator deferoxamine113; (3) valproic acid (the mood stabilizer drug)114,115; (4) glycogen synthase kinase (GSK)-3b inhibitors116; (5) IL-1b117; (6) interferon (IFN)-g118; and (7) IGF-1.119
Furthermore, HIF-1a induced under hypoxic cell culture conditions, increased expression of CX3CR1,120 as well as CXCR7,121,122 and CXCR4.123 It seems that deferoxamine stabilizes HIF-1a even at the normal level of oxygen,125 that finally increases the expression of many homing genes.113 MSCs exposure to C1q (complement 1q) results in an increase in the expression of CXCR4, which enhances the migration of MSCs toward SDF-1.126 Furthermore, culture conditions can upregulate other homing receptors as well. For example, migration of MSCs toward chemokines increases when MSCs are treated with TNF-alpha because this treatment upregulates the expression of CCR2, CCR3, and CCR4.57 Other treatments can increase MMP expression, which have a role in the transmigration of MSCs. The inhibition of GSK-3 is known to induce increased expression of MMP2, a membrane-type MMP1.116 Interestingly, complement 1q treatment also enhances MMP2 secretion.126 Further, valproic acid and lithium, both drugs used to treat bipolar disease were demonstrated to enhance MMP9 activity, while valproic acid further increases the activity of MMP2.114 The combination of erythropoietin and GCSF also enhances the expression of MMP2, which improves the migration of MSCs.127 Further, sphingosine-1-phosphate-treated MSCs migration demonstrated significantly improved migration in in vitro trans well assays.128 This treatment upregulates specifically MMP9 expression, which was shown to increase the homing capacity of MSCs to the infarcted myocardium.129
The impact of culture confluence on MSC migration
The cell culture confluence can affect the migration ability of MSCs. Indeed, a study reported that highly confluent MSCs secrete a higher amount of tissue inhibitor of metalloproteinase 3 (TIMP3), an MMP inhibitor decreasing the migration of MSCs as compared to the low confluence of cultured MSCs.124 In another work, complete gene expression profiling of low and high-confluent MSCs was compared. The study demonstrated that proliferation-related genes were expressed higher levels in low-confluence MSCs, whereas genes involved in migration and regeneration (GDF15, A2M, MDK, PDFGD, VEGFA, FGF9, and WISP2), immune modulation (IL-6 and IL-1B), and activation (CXCL5, CXCL2, CXCL1, CXCL8, CXCL6, and CXCL16) related genes showed higher expression in high-confluence MSCs.130
The effect of cocultures on MSC migration
Culturing of MSCs with other cells (coculture) also affects the migration of MSCs. For example, coculturing Sertoli cells (from the testes) with MSCs upregulates homing-related factors such as CXCR4 and proliferation genes131 as well as MMP2 expression in MSCs.132 Furthermore, amniotic epithelial cells and amniotic MSCs coculturing upregulate the expression of CXCR4 and thus, increase MSCs proliferation.133 Further, the coculture of MSCs with melanoma cells dramatically improved the migratory and invasion potential of SK-Mel-5, G-361, MeWo, and A2058 melanoma cells. Furthermore, in an angiogenesis experiment in vitro, conditioned medium from all MSCs-melanoma cell cocultures stimulated tube formation.134 In addition, treatment of MSCs with endothelial cell conditioned medium promotes their migration in vitro, presumably due to the presence of cytokines such as IL-6 and IL-8.135 Interestingly, cocultures of nucleus pulposus cells and MSCs contributed to increased ECM synthesis and cell migration.136
Cell surface engineering
Many studies have performed chemical cell surface engineering of MSCs as shown in Figure 5 to enhance MSC homing. These specific modifications are temporary, but they are sufficient to improve the MSC homing because transmigration takes place within a few hours after MSC administration.137 Importantly, CD44 is the selectin ligand naturally expressed on MSCs, whereas HCELL acts as a ligand for L- and E-selectin, and the hematopoietic stem cells utilize the HCELL ligand for bone marrow homing. Sackstein et al137 converted CD44 to HCELL enzymatically by sugar modifications allowing MSCs to use L- and E-selectin for homing toward bone marrow. In the case of a diabetic mouse model, this modification increased the homing of MSCs to pancreatic islets by 3-fold after their intravenous administration and reversed the hyperglycemia condition.138 These specific modifications of sugar molecules can also occur by genetic engineering. For example, CD44 to HCELL conversion in MSCs can be triggered by a (1,3)-fucosyltransferase (FUT6 or FUT7) expression.139-141
Another method of cell surface engineering is a direct conjugation of the desired ligand on the MSCs surface instead of modifying surface glycoproteins already present on MSCs. A platform developed by Sarkar et al142 allows for any ligand attachment to the surface of MSCs. For coating MSCs in biotin, the group used biotinylated lipid vehicles. In particular, then streptavidin adaptor was attached, followed by the attachment of biotinylated SleX, which is an active site present in PSGL-1142 Moreover, Cheng et al143 used an NHS-PEG2-maleimide linker molecule for E-selectin-binding peptide conjugation with MSCs.
Moreover, the attachment of compounds the cell surface of MSCs is another popular strategy. For example, the palmityl group of palmitate protein can act as a cell membrane anchor, whereas the protein G is the site where antibody attachment occurs. Lo et al144 used this method for attaching PSGL-1 fragment bound to the tail of IgG, which helped MSCs for rolling and tethering during shear stress. Further, Ko et al145 attached anti-intercellular adhesion molecule (ICAM)-1 antibodies with MSCs by using the same system. This attachment increased MSCs’ arrest in a flow chamber. The group also attached another antibody VCAM-1 (antivascular cell adhesion molecule) on the surface of MSCs. This attachment showed a 1.3-fold improved homing efficiency of MSCs in the swollen lymph node and 1.8-fold higher MSC homing in the colon.146
Lee et al147 created bispecific antibodies, where one side bound to CD44 present on MSCs surface, while the second side recognized the light chain of myosin (MLC), expressed by infracted myocardium. The bispecific antibodies bound MSCs localized specifically to the area of the heart.147 Similarly, Gundlach et al148 also created bispecific antibodies with one part recognizing CD90 present on the surface of MSCs, while the second end recognized MLC, expressed by ischemic myocardium. Those bispecific antibodies bound MSCs became attached to the immobilized MLC in vitro. Both these studies improved the MSCs’ migration to the target tissue by targeting injury markers.
Previous studies focused mostly on the step of initial tethering; however, Won et al149 aimed to optimize the activation step by cell surface engineering. In almost all the above-cited studies, cell differentiation, adhesion, proliferation, and viability of MSCs were unaffected; however, the methods of engineering cell surfaces are very complex in clinical setting.
Genetic modification of MSC surface receptors
Homing factors can be permanently overexpressed using viral transduction, mRNA transfection, and MSCs genetic modification techniques as shown in Figure 4. For example, the expression of the SDF-1 ligand is enhanced in the ischemic myocardium can bind to CXCR4 receptors and hence enhances the homing of MSCs.150,151 Therefore, the elevated expression of CXCR4 in MSCs leads to increased MSC engraftment to ischemic myocardium due to enhanced MSC homing efficiency.150,151 Another study reported that the homing of MSCs to bone marrow in mice was improved with increased expression of CXCR4 on MSCs.152 Similarly, in the mouse model of colitis, the homing of MSCs to damaged intestinal mucosa also increased with the enhanced expression of CXCR4 on MSCs.153 Furthermore, the increase in expression of CXCR7 on MSCs promoted the homing of MSCs to injured lung tissue.48 However, in the mouse models of acute kidney injury, the homing efficiency of MSCs to the kidney did not change with the increased expression of CXCR4 or CXCR7 or both.154 The increase in expression of a4-integrin (VLA-4 integrin component) at the cell arrest stage increased the homing efficiency of MSCs to the bone marrow.155 However, in the rat model of stroke, the homing efficiency of MSCs to the heart was not increased with enhanced expression of a4-integrin, but harmful cell aggregates formation decreased.156 However, such gene raises safety concerns. For example, oncogenesis can occur during genetic modifications in case viral DNA integrates into the tumor suppressor gene.
In contrast, mRNA transfection will result in a transient protein expression while eliminating the risk of insertional mutagenesis. Therefore, several studies opted for mRNA transfection methods instead of the viral transduction. For example, Levy et al157 used the mRNA transfection method to improve MSCs tethering. They transfected MSCs with PSGL-1 and SLeX mRNA, both being ligands for P-selectin and for E-/L-selectin, respectively. Transfected MSCs showed increased homing efficiency to inflamed ear and bone marrow in the mouse experimental model. Similarly, Liao et al158 also used the same method for MSC modification. They showed that modified MSCs exhibited increased rolling, adherence, and homing to the inflamed spinal cord. Ryser et al159 used the mRNA transfection method to transiently overexpress CXCR4 mRNA in MSCs that resulted in increased migration of MSCs toward an SDF-1 gradient in vitro. However, Wiehe et al160 applying the same method, have not confirmed these results. They successfully increased the CXCR4 expression by mRNA nucleofection, but no improvement in MSCs migration was observed in vitro. Thus, the authors concluded that the possible expression of other factors may be crucial for the activation of MSCs.
Magnetic guidance for MSC targeting
Magnetic guidance is a physical approach used for MSC targeting as shown in Figure 5. In this approach, the MSCs are labeled with magnetic particles and then directed to a target tissue or organ with the help of a magnetic field provided externally. Arbab et al161 used particles of iron oxide for MSCs labeling and then injected them intravenously into the rats with and without placing the external magnet on the liver. In those rats that carried external magnets over the liver, MSCs moved deeper into the parenchyma of the liver. In contrast, in rats that did not wear magnets during MSCs targeting, MSCs were present around the portal triad. Thus, external magnet-wearing rats demonstrated 2-fold increase in the number of iron oxide-labeled MSCs in their liver. As a limitation, this study has not investigated which steps of MSCs’ homing mechanism were involved.
Further, Kobayashi et al162 treated osteochondral defects in knee joints with magnetically labeled MSCs in an ex vivo system by using an external magnetic field. In 2012, Yanai et al163 placed the external magnet within the rat orbit and targeted magnetically labeled MSCs to the retina of rats by both intravenous or intravitreal administration. A high level of growth factors and anti-inflammatory molecules was present in the retina of rats with external magnets as compared to the animal group without external magnets. Thus, the authors concluded that magnetic guidance showed a very beneficial therapeutic effect. Since then, a lot of research has been performed to explore various types of magnetic particles, which may affect MSC differentiation, gene expression, proliferation, and viability.164 Importantly, so far, no harmful effects of magnetic labeling on the functions of MSCs were observed.165,166
Conclusion and perspectives
In conclusion, MSCs hold significant promise in regenerative medicine due to their multipotent nature and ability to promote tissue healing. The natural homing mechanism of MSCs, as well as their capacity to home to injured tissues following allogenic transplantation, forms the basis for their therapeutic potential. However, attracting MSCs to the specific site of injury remains a major challenge. To optimize the homing mechanism of MSCs, various strategies have been developed. These include targeted stem cell administration, target tissue modification, in vitro priming, cell surface engineering, genetic modifications, magnetic guidance, and radiotherapeutic techniques. The majority of techniques as shown in Figure 6 are focused on systemic homing. These approaches improve the migration and localization of MSCs to the injury site, thereby enhancing their regenerative properties. By understanding the molecular pathways involved in MSC homing and utilizing these strategies, researchers can enhance the therapeutic efficacy of MSC-based therapies. Precisely guiding MSCs to the injury site will facilitate their interaction with the damaged tissue, promoting tissue repair and regeneration.
Figure 6.
Explaining MSCs homing mechanism along with various strategies used for enhancing homing efficiency of MSCs at the target site. This includes both systemic homing and nonsystemic homing mechanism improvement strategies.
The homing mechanisms of MSCs are poorly understood over several stages, including tethering, transmigration, and migration. It is also not clearly demonstrated which of these stages has a key role in MSC homing and thus, it is the most crucial for future research. Each strategy has its own set of disadvantages. Depending upon the tissue, targeted administration could not be possible or could be extremely invasive. The majority of MSC modifications do not inhibit them from spreading to nontargeted tissues. Modification of target tissue through genetic or chemical means increases the safety concerns. Such constraints pose a serious challenge to their use in clinics. Although it is still a work in progress, ultrasound usage to enhance the homing of MSCs appears to be a promising opportunity, with easy targeting for both the deep and the superficial tissues and, as far as we know, no considerable safety concerns.
Although several surface markers and integrins as well as their role in the process of homing are well described, there are still additional limitations for MSCs-based therapies. For example, senescence may occur as a negative effect of intensive cell culture expansion of MSCs, which is required for clinical applications, and it reduces the therapeutic impact.167 Further, MSCs were thought to be immune privileged; however, several studies have revealed that MScs may experience immune rejection as a result of HLA mismatches.168,169 Finally, MSCs used in clinical trials are nearly always freshly thawed; however, cryopreservation tends to reduce MSCs’ immunosuppressive characteristics and limit their in vivo survival.19 These differences may yield conflicting outcomes in both basic research and clinical trials. Therefore, understanding the fundamental processes that underlie MSC biology is crucial for further improvements. Such studies will continue to advance the area of cell-based treatments, enhancing the therapeutic efficacy of MSC transplantation across a wide range of applications, from immune regulation to regeneration.
A major obstacle is to currently attract MSCs to the injury site where they are required in regenerative medicine. This MSCs’ homing to injury site can be enhanced by various strategies to bring advancement in the field of regenerative medicine as summarized in Table 2. We described the molecular pathways involved in the homing of MSCs and different strategies for optimizing homing, which include targeted stem cell administration, target tissue modification, in vitro priming, cell surface engineering, genetic modifications, and magnetic guidance. We believe that all these strategies enhance MSC homing to target sites; however, every strategy has its limitations that need to be considered while using that strategy for improving MSC homing. The currently available technological advances allow to attract MSCs precisely to the injury site and hence improve their migration and local tissue repair properties.
Contributor Information
Umar Sajjad, National Centre of Excellence in Molecular Biology, University of the Punjab, Lahore, Pakistan.
Muhammad Ahmed, National Centre of Excellence in Molecular Biology, University of the Punjab, Lahore, Pakistan.
M Zohaib Iqbal, Tissue Biology Research Unit, Department of Surgery, University Children’s Hospital Zurich, Wagistrasse 12, CH-8952, Zurich, Switzerland; Children’s Research Center, University Children’s Hospital Zurich, Zurich, Switzerland; University of Zurich, Zurich, Switzerland.
Mahrukh Riaz, Tissue Biology Research Unit, Department of Surgery, University Children’s Hospital Zurich, Wagistrasse 12, CH-8952, Zurich, Switzerland; Children’s Research Center, University Children’s Hospital Zurich, Zurich, Switzerland; University of Zurich, Zurich, Switzerland.
Muhammad Mustafa, KAM School of Life Sciences, Forman Christian College University, Lahore, Pakistan.
Thomas Biedermann, Tissue Biology Research Unit, Department of Surgery, University Children’s Hospital Zurich, Wagistrasse 12, CH-8952, Zurich, Switzerland; Children’s Research Center, University Children’s Hospital Zurich, Zurich, Switzerland; University of Zurich, Zurich, Switzerland.
Agnes S Klar, Tissue Biology Research Unit, Department of Surgery, University Children’s Hospital Zurich, Wagistrasse 12, CH-8952, Zurich, Switzerland; Children’s Research Center, University Children’s Hospital Zurich, Zurich, Switzerland; University of Zurich, Zurich, Switzerland.
Author contributions
Umar Sajjad, Muhammad Ahmed, and M. Zohaib Iqbal conceptualized and designed the review, and contributed to drafting and revising the manuscript. Mahrukh Riaz assisted with the literature search, data analysis, and drafting. Muhammad Mustafa contributed to the review’s design, coordinated among coauthors, and helped write the manuscript. Thomas Biedermann contributed to the design, provided oversight and feedback, and assisted with final editing. Agnes S. Klar supervised the project, provided conceptual and editorial guidance, funding, and contributed to the final revision and approval of the manuscript.
Funding
This study was supported by research funding from the European Union’s Horizon 2020 Marie Skłodowska-Curie ITN project SkinTERM under grant (agreement no. 955722) and the Swiss National Science Foundation SNSF grant (no. IZCOZ0_213354; “Role of CD200-CD200 receptor interactions in human diabetic skin wounds”).
Conflicts of interest
The authors declared no potential conflicts of interest.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Sarukhan A, Zanotti L, Viola A.. Mesenchymal stem cells: myths and reality. Swiss Med Wkly. 2015;145:w14229. https://doi.org/ 10.4414/smw.2015.14229 [DOI] [PubMed] [Google Scholar]
- 2. Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP.. Heterotopic transplants of bone marrow. Transplantation. 1968;6:230-247. https://doi.org/ 10.1097/00007890-196803000-00009 [DOI] [PubMed] [Google Scholar]
- 3. Campagnoli C, Roberts IA, Kumar S, et al. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood. 2001;98:2396-2402. https://doi.org/ 10.1182/blood.v98.8.2396 [DOI] [PubMed] [Google Scholar]
- 4. Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301-313. https://doi.org/ 10.1016/j.stem.2008.07.003 [DOI] [PubMed] [Google Scholar]
- 5. Young HE, Steele TA, Bray RA, et al. Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec. 2001;264:51-62. https://doi.org/ 10.1002/ar.1128 [DOI] [PubMed] [Google Scholar]
- 6. Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. https://doi.org/ 10.1091/mbc.e02-02-0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S.. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625-13630. https://doi.org/ 10.1073/pnas.240309797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. https://doi.org/ 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- 9. Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S.. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26:1787-1795. https://doi.org/ 10.1634/stemcells.2007-0979 [DOI] [PubMed] [Google Scholar]
- 10. Snykers S, De Kock J, Rogiers V, Vanhaecke T.. In vitro differentiation of embryonic and adult stem cells into hepatocytes: state of the art. Stem Cells. 2009;27:577-605. https://doi.org/ 10.1634/stemcells.2008-0963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ullah I, Subbarao Raghavendra B, Rho Gyu J. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35. p. 1. https://doi.org/ 10.1042/bsr20150025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Volarevic V, Ljujic B, Stojkovic P, et al. Human stem cell research and regenerative medicinepresent and future. Br Med Bull. 2011;99:155-168. https://doi.org/ 10.1093/bmb/ldr027 [DOI] [PubMed] [Google Scholar]
- 13. Caplan AI, Dennis JE.. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076-1084. https://doi.org/ 10.1002/jcb.20886 [DOI] [PubMed] [Google Scholar]
- 14. Fan X-L, Zhang Z, Ma CY, Fu Q-L.. Mesenchymal stem cells for inflammatory airway disorders: promises and challenges. Biosci Rep. 2019;39: BSR20182160. p. 1. https://doi.org/ 10.1042/BSR20182160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou T, Yuan Z, Weng J, et al. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 2021;14:24. https://doi.org/ 10.1186/s13045-021-01037-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ramírez M, Lucia A, Gómez-Gallego F, et al. Mobilisation of mesenchymal cells into blood in response to skeletal muscle injury. Br J Sports Med. 2006;40:719-722. https://doi.org/ 10.1136/bjsm.2006.028639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caplan A. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318-324. https://doi.org/ 10.1002/path.2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chapel A, Bertho JM, Bensidhoum M, et al. Mesenchymal stem cells home to injured tissues when co‐infused with hematopoietic cells to treat a radiation‐induced multi‐organ failure syndrome. J Gene Med. 2003;5:1028-1038. https://doi.org/ 10.1002/jgm.452 [DOI] [PubMed] [Google Scholar]
- 19. Chinnadurai R, Copland IB, Garcia MA, et al. Cryopreserved mesenchymal stromal cells are susceptible to T-cell mediated apoptosis which is partly rescued by IFNγ licensing. Stem Cells. 2016;34:2429-2442. https://doi.org/ 10.1002/stem.2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lotfy A, AboQuella NM, Wang H.. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res Ther. 2023;14:66. https://doi.org/ 10.1186/s13287-023-03287-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rendra E, Scaccia E, Bieback K.. Recent advances in understanding mesenchymal stromal cells. F1000Res. 2020;9:156. https://doi.org/ 10.12688/f1000research.21862.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kahrizi MS, Mousavi E, Khosravi A, et al. Recent advances in pre-conditioned mesenchymal stem/stromal cell (MSCs) therapy in organ failure; a comprehensive review of preclinical studies. Stem Cell Res Ther. 2023;14:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Galipeau J. The mesenchymal stromal cells dilemma--does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy. 2013;15:2-8. https://doi.org/ 10.1016/j.jcyt.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 24. Bronckaers A, Hilkens P, Martens W, et al. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther. 2014;143:181-196. https://doi.org/ 10.1016/j.pharmthera.2014.02.013 [DOI] [PubMed] [Google Scholar]
- 25. Le Blanc K, Mougiakakos D.. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383-396. https://doi.org/ 10.1038/nri3209 [DOI] [PubMed] [Google Scholar]
- 26. Singer NG, Caplan AI.. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. 2011;6:457-478. https://doi.org/ 10.1146/annurev-pathol-011110-130230 [DOI] [PubMed] [Google Scholar]
- 27. Frenette PS, Pinho S, Lucas D, Scheiermann C.. Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annu Rev Immunol. 2013;31:285-316. https://doi.org/ 10.1146/annurev-immunol-032712-095919 [DOI] [PubMed] [Google Scholar]
- 28. Nitzsche F, Müller C, Lukomska B, et al. Concise review: MSC adhesion cascade—insights into homing and transendothelial migration. Stem Cells. 2017;35:1446-1460. https://doi.org/ 10.1002/stem.2614 [DOI] [PubMed] [Google Scholar]
- 29. Wu X, Jiang J, Gu Z, et al. Mesenchymal stromal cell therapies: immunomodulatory properties and clinical progress. Stem Cell Res Ther. 2020;11:345. https://doi.org/ 10.1186/s13287-020-01855-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Szydlak R. Biological, chemical and mechanical factors regulating migration and homing of mesenchymal stem cells. World J Stem Cells. 2021;13:619-631. https://doi.org/ 10.4252/wjsc.v13.i6.619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ullah M, Liu DD, Thakor AS.. Mesenchymal stromal cell homing: mechanisms and strategies for improvement. iScience. 2019;15:421-438. https://doi.org/ 10.1016/j.isci.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sackstein R. The lymphocyte homing receptors: gatekeepers of the multistep paradigm. Curr Opin Hematol. 2005;12:444-450. https://doi.org/ 10.1097/01.moh.0000177827.78280.79 [DOI] [PubMed] [Google Scholar]
- 33. McEver RP. Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res. 2015;107:331-339. https://doi.org/ 10.1093/cvr/cvv154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sackstein R, Merzaban JS, Cain DW, et al. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181-187. https://doi.org/ 10.1038/nm1703 [DOI] [PubMed] [Google Scholar]
- 35. Rüster B, Göttig S, Ludwig RJ, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938-3944. https://doi.org/ 10.1182/blood-2006-05-025098 [DOI] [PubMed] [Google Scholar]
- 36. Segers VFM, Riet IV, Andries LJ, et al. Mesenchymal stem cell adhesion to cardiac microvascular endothelium: activators and mechanisms. Am J Physiol Heart Circ Physiol. 2006;290:H1370-H1377. https://doi.org/ 10.1152/ajpheart.00523.2005 [DOI] [PubMed] [Google Scholar]
- 37. Steingen C, Brenig F, Baumgartner L, et al. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J Mol Cell Cardiol. 2008;44:1072-1084. https://doi.org/ 10.1016/j.yjmcc.2008.03.010 [DOI] [PubMed] [Google Scholar]
- 38. Suila H, Hirvonen T, Kotovuori A, et al. Human umbilical cord blood-derived mesenchymal stromal cells display a novel interaction between P-selectin and galectin-1. Scand J Immunol. 2014;80:12-21. https://doi.org/ 10.1111/sji.12179 [DOI] [PubMed] [Google Scholar]
- 39. Bailey AM, Lawrence MB, Shang H, Katz AJ, Peirce SM.. Agent-based model of therapeutic adipose-derived stromal cell trafficking during ischemia predicts ability to roll on P-selectin. PLoS Comput Biol. 2009;5:e1000294. https://doi.org/ 10.1371/journal.pcbi.1000294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. François S, Bensidhoum M, Mouiseddine M, et al. Local irradiation not only induces homing of human mesenchymal stem cells at exposed sites but promotes their widespread engraftment to multiple organs: a study of their quantitative distribution after irradiation damage. Stem Cells. 2006;24:1020-1029. https://doi.org/ 10.1634/stemcells.2005-0260 [DOI] [PubMed] [Google Scholar]
- 41. Lau TT, Wang DA.. Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expert Opin Biol Ther. 2011;11:189-197. https://doi.org/ 10.1517/14712598.2011.546338 [DOI] [PubMed] [Google Scholar]
- 42. Gao H, Priebe W, Glod J, Banerjee D.. Activation of signal transducers and activators of transcription 3 and focal adhesion kinase by stromal cell-derived factor 1 is required for migration of human mesenchymal stem cells in response to tumor cell-conditioned medium. Stem Cells. 2009;27:857-865. https://doi.org/ 10.1002/stem.23 [DOI] [PubMed] [Google Scholar]
- 43. Honczarenko M, Le Y, Swierkowski M, et al. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24:1030-1041. https://doi.org/ 10.1634/stemcells.2005-0319 [DOI] [PubMed] [Google Scholar]
- 44. Wynn RF, Hart CA, Corradi-Perini C, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643-2645. https://doi.org/ 10.1182/blood-2004-02-0526 [DOI] [PubMed] [Google Scholar]
- 45. Bobis-Wozowicz S, Miekus K, Wybieralska E, et al. Genetically modified adipose tissue-derived mesenchymal stem cells overexpressing CXCR4 display increased motility, invasiveness, and homing to bone marrow of NOD/SCID mice. Exp Hematol. 2011;39:686-696.e4. https://doi.org/ 10.1016/j.exphem.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 46. Lüttichau IV, Notohamiprodjo M, Wechselberger A, et al. Human adult CD34− progenitor cells functionally express the chemokine receptors CCR1, CCR4, CCR7, CXCR5, and CCR10 but not CXCR4. Stem Cells Dev. 2005;14:329-336. https://doi.org/ 10.1089/scd.2005.14.329 [DOI] [PubMed] [Google Scholar]
- 47. Li Q, Zhang A, Tao C, Li X, Jin P.. The role of SDF-1-CXCR4/CXCR7 axis in biological behaviors of adipose tissue-derived mesenchymal stem cells in vitro. Biochem Biophys Res Commun. 2013;441:675-680. https://doi.org/ 10.1016/j.bbrc.2013.10.071 [DOI] [PubMed] [Google Scholar]
- 48. Shao Y, Zhou F, He D, Zhang L, Shen J.. Overexpression of CXCR7 promotes mesenchymal stem cells to repair phosgene-induced acute lung injury in rats. Biomed Pharmacother. 2019;109:1233-1239. https://doi.org/ 10.1016/j.biopha.2018.10.108 [DOI] [PubMed] [Google Scholar]
- 49. Wang Y, Fu W, Zhang S, et al. CXCR-7 receptor promotes SDF-1α-induced migration of bone marrow mesenchymal stem cells in the transient cerebral ischemia/reperfusion rat hippocampus. Brain Res. 2014;1575:78-86. https://doi.org/ 10.1016/j.brainres.2014.05.035 [DOI] [PubMed] [Google Scholar]
- 50. Belema-Bedada F, Uchida S, Martire A, Kostin S, Braun T.. Efficient homing of multipotent adult mesenchymal stem cells depends on FROUNT-mediated clustering of CCR2. Cell Stem Cell. 2008;2:566-575. https://doi.org/ 10.1016/j.stem.2008.03.003 [DOI] [PubMed] [Google Scholar]
- 51. Schenk S, Mal N, Finan A, et al. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells. 2007;25:245-251. https://doi.org/ 10.1634/stemcells.2006-0293 [DOI] [PubMed] [Google Scholar]
- 52. Constantin G, Majeed M, Giagulli C, et al. Chemokines trigger immediate β2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity. 2000;13:759-769. https://doi.org/ 10.1016/s1074-7613(00)00074-1 [DOI] [PubMed] [Google Scholar]
- 53. Lin TH, Liu HH, Tsai TH, et al. CCL2 increases αvβ3 integrin expression and subsequently promotes prostate cancer migration. Biochim Biophys Acta Gen Subj. 2013;1830:4917-4927. https://doi.org/ 10.1016/j.bbagen.2013.06.033 [DOI] [PubMed] [Google Scholar]
- 54. Tadokoro S, Shattil SJ, Eto K, et al. Talin binding to integrin ß tails: a final common step in integrin activation. Science. 2003;302:103-106. https://doi.org/ 10.1126/science.1086652 [DOI] [PubMed] [Google Scholar]
- 55. Krampera M, Cosmi L, Angeli R, et al. Role for interferon-γ in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386-398. https://doi.org/ 10.1634/stemcells.2005-0008 [DOI] [PubMed] [Google Scholar]
- 56. Krampera M, Pasini A, Rigo A, et al. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood. 2005;106:59-66. https://doi.org/ 10.1182/blood-2004-09-3645 [DOI] [PubMed] [Google Scholar]
- 57. Ponte AL, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737-1745. https://doi.org/ 10.1634/stemcells.2007-0054 [DOI] [PubMed] [Google Scholar]
- 58. Bayo J, Real A, Fiore EJ, et al. IL-8, GRO and MCP-1 produced by hepatocellular carcinoma microenvironment determine the migratory capacity of human bone marrow-derived mesenchymal stromal cells without affecting tumor aggressiveness. Oncotarget. 2017;8:80235-80248. https://doi.org/ 10.18632/oncotarget.10288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liang-kuan B, Nan Z, Cheng L, et al. Kidney cancer cells secrete IL-8 to activate Akt and promote migration of mesenchymal stem cells. Urol Oncol. 2014;32:607-612. https://doi.org/ 10.1016/j.urolonc.2013.10.018 [DOI] [PubMed] [Google Scholar]
- 60. Hou Y, Ryu CH, Jun JA, et al. IL‐8 enhances the angiogenic potential of human bone marrow mesenchymal stem cells by increasing vascular endothelial growth factor. Cell Biol Int. 2014;38:1050-1059. https://doi.org/ 10.1002/cbin.10294 [DOI] [PubMed] [Google Scholar]
- 61. Barbash IM, Chouraqui P, Baron J, et al. Systemic delivery of bone marrow–derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863-868. https://doi.org/ 10.1161/01.cir.0000084828.50310.6a [DOI] [PubMed] [Google Scholar]
- 62. Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R.. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999-3001. https://doi.org/ 10.1182/blood-2002-06-1830 [DOI] [PubMed] [Google Scholar]
- 63. Kraitchman DL, Tatsumi M, Gilson WD, et al. Dynamic imaging of allogeneic mesenchymal stem cells trafficking to myocardial infarction. Circulation. 2005;112:1451-1461. https://doi.org/ 10.1161/circulationaha.105.537480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI.. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12-20. https://doi.org/ 10.1159/000047856 [DOI] [PubMed] [Google Scholar]
- 65. Yukawa H, Watanabe M, Kaji N, et al. Monitoring transplanted adipose tissue-derived stem cells combined with heparin in the liver by fluorescence imaging using quantum dots. Biomaterials. 2012;33:2177-2186. https://doi.org/ 10.1016/j.biomaterials.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 66. Rombouts WJC, Ploemacher RE.. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160-170. https://doi.org/ 10.1038/sj.leu.2402763 [DOI] [PubMed] [Google Scholar]
- 67. Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62-73. https://doi.org/ 10.1001/jama.2013.282909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Williams AR, Trachtenberg B, Velazquez DL, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy: functional recovery and reverse remodeling. Circ Res. 2011;108:792-796. https://doi.org/ 10.1161/circresaha.111.242610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dick AJ, Guttman MA, Raman VK, et al. Magnetic resonance fluoroscopy allows targeted delivery of mesenchymal stem cells to infarct borders in swine. Circulation. 2003;108:2899-2904. https://doi.org/ 10.1161/01.CIR.0000095790.28368.F9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vu Q, Xie K, Eckert M, Zhao W, Cramer SC.. Meta-analysis of preclinical studies of mesenchymal stromal cells for ischemic stroke. Neurology. 2014;82:1277-1286. https://doi.org/ 10.1212/wnl.0000000000000278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kanelidis AJ, Premer C, Lopez J, Balkan W, Hare JM.. Route of delivery modulates the efficacy of mesenchymal stem cell therapy for myocardial infarction: a meta-analysis of preclinical studies and clinical trials. Circ Res. 2017;120:1139-1150. https://doi.org/ 10.1161/circresaha.116.309819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jeong H, Oh TW, Song SC, Jung S.. Sense-amplifier-based flip-flop with transition completion detection for low-voltage operation. IEEE Trans Very Large Scale Integr (VLSI) Syst. 2018;26:609-620. https://doi.org/ 10.1109/tvlsi.2017.2777788 [DOI] [Google Scholar]
- 73. Antunes MA, Abreu SC, Cruz FF, et al. Effects of different mesenchymal stromal cell sources and delivery routes in experimental emphysema. Respir Res. 2014;15:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ishikane S, Hosoda H, Yamahara K, et al. Allogeneic transplantation of fetal membrane-derived mesenchymal stem cell sheets increases neovascularization and improves cardiac function after myocardial infarction in rats. Transplantation. 2013;96:697-706. https://doi.org/ 10.1097/tp.0b013e31829f753d [DOI] [PubMed] [Google Scholar]
- 75. Tano N, Narita T, Kaneko M, et al. Epicardial placement of mesenchymal stromal cell-sheets for the treatment of ischemic cardiomyopathy; in vivo proof-of-concept study. Mol Ther. 2014;22:1864-1871. https://doi.org/ 10.1038/mt.2014.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kaibuchi N, Iwata T, Yamato M, Okano T, Ando T.. Multipotent mesenchymal stromal cell sheet therapy for bisphosphonate-related osteonecrosis of the jaw in a rat model. Acta Biomater. 2016;42:400-410. https://doi.org/ 10.1016/j.actbio.2016.06.022 [DOI] [PubMed] [Google Scholar]
- 77. Penn MS, Pastore J, Miller T, Aras R.. SDF-1 in myocardial repair. Gene Ther. 2012;19:583-587. https://doi.org/ 10.1038/gt.2012.32 [DOI] [PubMed] [Google Scholar]
- 78. Sasaki T, Fukazawa R, Ogawa S, et al. Stromal cell‐derived factor‐1α improves infarcted heart function through angiogenesis in mice. Pediatr Int. 2007;49:966-971. https://doi.org/ 10.1111/j.1442-200x.2007.02491.x [DOI] [PubMed] [Google Scholar]
- 79. Saxena A, Fish JE, White MD, et al. Stromal cell–derived factor-1α is cardioprotective after myocardial infarction. Circulation. 2008;117:2224-2231. https://doi.org/ 10.1161/CIRCULATIONAHA.107.694992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Yamaguchi JI, Kusano KF, Masuo O, et al. Stromal cell–derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322-1328. https://doi.org/ 10.1161/01.cir.0000055313.77510.22 [DOI] [PubMed] [Google Scholar]
- 81. Segers VF, Revin V, Wu W, et al. Protease-resistant stromal cell–derived factor-1 for the treatment of experimental peripheral artery disease. Circulation. 2011;123:1306-1315. https://doi.org/ 10.1161/circulationaha.110.991786 [DOI] [PubMed] [Google Scholar]
- 82. Segers VF, Tokunou T, Higgins LJ, et al. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683-1692. https://doi.org/ 10.1161/circulationaha.107.718718 [DOI] [PubMed] [Google Scholar]
- 83. Fujii H, Li SH, Wu J, et al. Repeated and targeted transfer of angiogenic plasmids into the infarcted rat heart via ultrasound targeted microbubble destruction enhances cardiac repair. Eur Heart J. 2011;32:2075-2084. https://doi.org/ 10.1093/eurheartj/ehq475 [DOI] [PubMed] [Google Scholar]
- 84. Sundararaman S, Miller TJ, Pastore JM, et al. Plasmid-based transient human stromal cell-derived factor-1 gene transfer improves cardiac function in chronic heart failure. Gene Ther. 2011;18:867-873. https://doi.org/ 10.1038/gt.2011.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Penn MS, Mendelsohn FO, Schaer GL, et al. An open-label dose escalation study to evaluate the safety of administration of nonviral stromal cell-derived factor-1 plasmid to treat symptomatic ischemic heart failure. Circ Res. 2013;112:816-825. https://doi.org/ 10.1161/CIRCRESAHA.111.300440 [DOI] [PubMed] [Google Scholar]
- 86. Chung ES, Miller L, Patel AN, et al. Changes in ventricular remodelling and clinical status during the year following a single administration of stromal cell-derived factor-1 non-viral gene therapy in chronic ischaemic heart failure patients: the STOP-HF randomized phase II trial. Eur Heart J. 2015;36:2228-2238. https://doi.org/ 10.1093/eurheartj/ehv254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mouiseddine M, Francois S, Semont A, et al. Human mesenchymal stem cells home specifically to radiation-injured tissues in a non-obese diabetes/severe combined immunodeficiency mouse model. Br J Radiol. 2007;80:S49-S55. https://doi.org/ 10.1259/bjr/25927054 [DOI] [PubMed] [Google Scholar]
- 88. Ponomaryov T, Peled A, Petit I, et al. Induction of the chemokine stromal-derived factor-1 following DNA damage improves human stem cell function. J Clin Invest. 2000;106:1331-1339. https://doi.org/ 10.1172/JCI10329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Miller DL, Smith NB, Bailey MR, et al. ; Bioeffects Committee of the American Institute of Ultrasound in Medicine. Overview of therapeutic ultrasound applications and safety considerations. J Ultrasound Med. 2012;31:623-634. https://doi.org/ 10.7863/jum.2012.31.4.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zen K, Okigaki M, Hosokawa Y, et al. Myocardium-targeted delivery of endothelial progenitor cells by ultrasound-mediated microbubble destruction improves cardiac function via an angiogenic response. J Mol Cell Cardiol. 2006;40:799-809. https://doi.org/ 10.1016/j.yjmcc.2006.03.012 [DOI] [PubMed] [Google Scholar]
- 91. Ghanem A, Steingen C, Brenig F, et al. Focused ultrasound-induced stimulation of microbubbles augments site-targeted engraftment of mesenchymal stem cells after acute myocardial infarction. J Mol Cell Cardiol. 2009;47:411-418. https://doi.org/ 10.1016/j.yjmcc.2009.06.008 [DOI] [PubMed] [Google Scholar]
- 92. Xu YL, Gao YH, Liu Z, et al. Myocardium-targeted transplantation of mesenchymal stem cells by diagnostic ultrasound-mediated microbubble destruction improves cardiac function in myocardial infarction of New Zealand rabbits. Int J Cardiol. 2010;138:182-195. https://doi.org/ 10.1016/j.ijcard.2009.03.071 [DOI] [PubMed] [Google Scholar]
- 93. Li L, Wu S, Liu Z, et al. Ultrasound-targeted microbubble destruction improves the migration and homing of mesenchymal stem cells after myocardial infarction by upregulating SDF-1/CXCR4: a pilot study. Stem Cells Int. 2015;2015:1-14. https://doi.org/ 10.1155/2015/691310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang G, Zhuo Z, Yang B, et al. Enhanced homing ability and retention of bone marrow stromal cells to diabetic nephropathy by microbubble-mediated diagnostic ultrasound irradiation. Ultrasound Med Biol. 2015;41:2977-2989. https://doi.org/ 10.1016/j.ultrasmedbio.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 95. Kovacs ZI, Kim S, Jikaria N, et al. Disrupting the blood–brain barrier by focused ultrasound induces sterile inflammation. . Proc Natl Acad Sci USA. 2017;114:E75-E84. https://doi.org/ 10.1073/pnas.1614777114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Burgess A, Ayala-Grosso CA, Ganguly M, et al. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS One. 2011;6:e27877. https://doi.org/ 10.1371/journal.pone.0027877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chonpathompikunlert P, Fan CH, Ozaki Y, et al. Redox nanoparticle treatment protects against neurological deficit in focused ultrasound-induced intracerebral hemorrhage. Nanomedicine. 2012;7:1029-1043. https://doi.org/ 10.2217/nnm.12.2 [DOI] [PubMed] [Google Scholar]
- 98. Fan CH, Liu HL, Huang CY, et al. Detection of intracerebral hemorrhage and transient blood-supply shortage in focused-ultrasound-induced blood–brain barrier disruption by ultrasound imaging. Ultrasound Med Biol. 2012;38:1372-1382. https://doi.org/ 10.1016/j.ultrasmedbio.2012.03.013 [DOI] [PubMed] [Google Scholar]
- 99. Burks SR, Ziadloo A, Kim SJ, Nguyen BA, Frank JA.. Noninvasive pulsed focused ultrasound allows spatiotemporal control of targeted homing for multiple stem cell types in murine skeletal muscle and the magnitude of cell homing can be increased through repeated applications. Stem Cells. 2013;31:2551-2560. https://doi.org/ 10.1002/stem.1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Burks SR, Ziadloo A, Hancock HA, et al. Investigation of cellular and molecular responses to pulsed focused ultrasound in a mouse model. PLoS One. 2011;6:e24730. https://doi.org/ 10.1371/journal.pone.0024730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tebebi PA, Burks SR, Kim SJ, et al. Cyclooxygenase-2 or tumor necrosis factor-α inhibitors attenuate the mechanotransductive effects of pulsed focused ultrasound to suppress mesenchymal stromal cell homing to healthy and dystrophic muscle. Stem Cells. 2015;33:1173-1186. https://doi.org/ 10.1002/stem.1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tebebi PA, Kim SJ, Williams RA, et al. Improving the therapeutic efficacy of mesenchymal stromal cells to restore perfusion in critical limb ischemia through pulsed focused ultrasound. Sci Rep. 2017;7:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ziadloo A, Burks SR, Gold EM, et al. Enhanced homing permeability and retention of bone marrow stromal cells by noninvasive pulsed focused ultrasound. Stem Cells. 2012;30:1216-1227. https://doi.org/ 10.1002/stem.1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Burks SR, Nguyen BA, Bresler MN, et al. Anti-inflammatory drugs suppress ultrasound-mediated mesenchymal stromal cell tropism to kidneys. Sci Rep. 2017;7:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Jang KW, Tu TW, Nagle ME, et al. Molecular and histological effects of MR-guided pulsed focused ultrasound to the rat heart. J Transl Med. 2017;15:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kimura Y, Tabata YJ.. Controlled release of stromal-cell-derived factor-1 from gelatin hydrogels enhances angiogenesis. J Biomater Sci Polym Ed. 2010;21(1):37- 51. [DOI] [PubMed] [Google Scholar]
- 107. He X, Ma J, Jabbari E.. Migration of marrow stromal cells in response to sustained release of stromal-derived factor-1α from poly(lactide ethylene oxide fumarate) hydrogels. Int J Pharm. 2010;390:107-116. https://doi.org/ 10.1016/j.ijpharm.2009.12.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gonçalves RM, Antunes JC, Barbosa MA.. Mesenchymal stem cell recruitment by stromal derived factor-1-delivery systems based on chitosan/poly (gamma-glutamic acid) polyelectrolyte complexes. Eur Cell Mater. 2012;23:249-260. [DOI] [PubMed] [Google Scholar]
- 109. Shen W, Chen X, Chen J, et al. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials. 2010;31:7239-7249. https://doi.org/ 10.1016/j.biomaterials.2010.05.040 [DOI] [PubMed] [Google Scholar]
- 110. Thevenot PT, Nair AM, Shen J, et al. The effect of incorporation of SDF-1α into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials. 2010;31:3997-4008. https://doi.org/ 10.1016/j.biomaterials.2010.01.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Corradetti B, Taraballi F, Martinez JO, et al. Hyaluronic acid coatings as a simple and efficient approach to improve MSC homing toward the site of inflammation. Sci Rep. 2017;7:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Shi M, Li J, Liao L, et al. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92:897-904. https://doi.org/ 10.3324/haematol.10669 [DOI] [PubMed] [Google Scholar]
- 113. Najafi R, Sharifi AM.. Deferoxamine preconditioning potentiates mesenchymal stem cell homing in vitro and in streptozotocin-diabetic rats. Expert Opin Biol Ther. 2013;13:959-972. https://doi.org/ 10.1517/14712598.2013.782390 [DOI] [PubMed] [Google Scholar]
- 114. Tsai LK, Leng Y, Wang Z, Leeds P, Chuang DM.. The mood stabilizers valproic acid and lithium enhance mesenchymal stem cell migration via distinct mechanisms. Neuropsychopharmacology. 2010;35:2225-2237. https://doi.org/ 10.1038/npp.2010.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tsai LK, Wang Z, Munasinghe J, et al. Mesenchymal stem cells primed with valproate and lithium robustly migrate to infarcted regions and facilitate recovery in a stroke model. Stroke. 2011;42:2932-2939. https://doi.org/ 10.1161/strokeaha.110.612788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kim YS, Noh MY, Kim JY, et al. Direct GSK-3β inhibition enhances mesenchymal stromal cell migration by increasing expression of Beta-PIX and CXCR4. Mol Neurobiol. 2013;47:811-820. https://doi.org/ 10.1007/s12035-012-8393-3 [DOI] [PubMed] [Google Scholar]
- 117. Fan H, Zhao G, Liu L, et al. Pre-treatment with IL-1β enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell Mol Immunol. 2012;9:473-481. https://doi.org/ 10.1038/cmi.2012.40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Duijvestein M, Wildenberg ME, Welling MM, et al. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29:1549-1558. https://doi.org/ 10.1002/stem.698 [DOI] [PubMed] [Google Scholar]
- 119. Li Y, Yu X, Lin S, et al. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem Biophys Res Commun. 2007;356:780-784. https://doi.org/ 10.1016/j.bbrc.2007.03.049 [DOI] [PubMed] [Google Scholar]
- 120. Hung SC, Pochampally RR, Hsu SC, et al. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS One. 2007;2:e416. https://doi.org/ 10.1371/journal.pone.0000416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Liu H, Xue W, Ge G, et al. Hypoxic preconditioning advances CXCR4 and CXCR7 expression by activating HIF-1α in MSCs. Biochem Biophys Res Commun. 2010;401:509-515. https://doi.org/ 10.1016/j.bbrc.2010.09.076 [DOI] [PubMed] [Google Scholar]
- 122. Meng SS, Xu XP, Chang W, et al. LincRNA-p21 promotes mesenchymal stem cell migration capacity and survival through hypoxic preconditioning. Stem Cell Res Ther. 2018;9:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Annabi B, Lee YT, Turcotte S, et al. Hypoxia promotes murine bone‐marrow‐derived stromal cell migration and tube formation. Stem Cells. 2003;21:337-347. https://doi.org/ 10.1634/stemcells.21-3-337 [DOI] [PubMed] [Google Scholar]
- 124. De Becker A, Van Hummelen P, Bakkus M, et al. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica. 2007;92:440-449. https://doi.org/ 10.3324/haematol.10475 [DOI] [PubMed] [Google Scholar]
- 125. Chu K, Jung KH, Kim SJ, et al. Transplantation of human neural stem cells protect against ischemia in a preventive mode via hypoxia-inducible factor-1α stabilization in the host brain. Brain Res. 2008;1207:182-192. https://doi.org/ 10.1016/j.brainres.2008.02.043 [DOI] [PubMed] [Google Scholar]
- 126. Qiu Y, Marquez-Curtis LA, Janowska-Wieczorek A.. Mesenchymal stromal cells derived from umbilical cord blood migrate in response to complement C1q. Cytotherapy. 2012;14:285-295. https://doi.org/ 10.3109/14653249.2011.651532 [DOI] [PubMed] [Google Scholar]
- 127. Yu Q, Chen L, You Y, et al. Erythropoietin combined with granulocyte colony-stimulating factor enhances MMP-2 expression in mesenchymal stem cells and promotes cell migration. Mol Med Rep. 2011;4:31-36. https://doi.org/ 10.3892/mmr.2010.387 [DOI] [PubMed] [Google Scholar]
- 128. Kang H, Kim KH, Lim J, et al. The therapeutic effects of human mesenchymal stem cells primed with sphingosine-1 phosphate on pulmonary artery hypertension. Stem Cells Dev. 2015;24:1658-1671. https://doi.org/ 10.1089/scd.2014.0496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Chen R, Cai X, Liu J, Bai B, Li X.. Sphingosine 1-phosphate promotes mesenchymal stem cell-mediated cardioprotection against myocardial infarction via ERK1/2-MMP-9 and Akt signaling axis. Life Sci. 2018;215:31-42. https://doi.org/ 10.1016/j.lfs.2018.10.047 [DOI] [PubMed] [Google Scholar]
- 130. Kim DS, Lee MW, Yoo KH, et al. Gene expression profiles of human adipose tissue-derived mesenchymal stem cells are modified by cell culture density. PLoS One. 2014;9:e83363. https://doi.org/ 10.1371/journal.pone.0083363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Zhang F, Hong Y, Liang W, et al. Co-culture with Sertoli cells promotes proliferation and migration of umbilical cord mesenchymal stem cells. Biochem Biophys Res Commun. 2012;427:86-90. https://doi.org/ 10.1016/j.bbrc.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 132. Luo Y, Mohsin A, Xu C, et al. Co-culture with TM4 cells enhances the proliferation and migration of rat adipose-derived mesenchymal stem cells with high stemness. Cytotechnology. 2018;70:1409-1422. https://doi.org/ 10.1007/s10616-018-0235-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ran LJ, Zeng Y, Wang SC, et al. Effect of co-culture with amniotic epithelial cells on the biological characteristics of amniotic mesenchymal stem cells. Mol Med Rep. 2018;18:723-732. https://doi.org/ 10.3892/mmr.2018.9053 [DOI] [PubMed] [Google Scholar]
- 134. Castro-Oropeza R, Vazquez-Santillan K, Díaz-Gastelum C, et al. Adipose-derived mesenchymal stem cells promote the malignant phenotype of cervical cancer. Sci Rep. 2020;10:14205. https://doi.org/ 10.1038/s41598-020-69907-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Zhidkova OV, Andreeva ER, Buravkova LB.. Endothelial cells modulate differentiation potential and mobility of mesenchymal stromal cells. Bull Exp Biol Med. 2018;165:127-131. https://doi.org/ 10.1007/s10517-018-4113-y [DOI] [PubMed] [Google Scholar]
- 136. Sobajima S, Vadala G, Shimer A, et al. Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J. 2008;8:888-896. https://doi.org/ 10.1016/j.spinee.2007.09.011 [DOI] [PubMed] [Google Scholar]
- 137. Teo GSL, Ankrum JA, Martinelli R, et al. Mesenchymal stem cells transmigrate between and directly through tumor necrosis factor-α-activated endothelial cells via both leukocyte-like and novel mechanisms. Stem Cells. 2012;30:2472-2486. https://doi.org/ 10.1002/stem.1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Abdi R, Moore R, Sakai S, et al. HCELL expression on murine MSC licenses pancreatotropism and confers durable reversal of autoimmune diabetes in NOD mice. Stem Cells. 2015;33:1523-1531. https://doi.org/ 10.1002/stem.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Dykstra B, Lee J, Mortensen LJ, et al. Glycoengineering of E-selectin ligands by intracellular versus extracellular fucosylation differentially affects osteotropism of human mesenchymal stem cells. Stem Cells. 2016;34:2501-2511. https://doi.org/ 10.1002/stem.2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Lo CY, Weil BR, Palka BA, et al. Cell surface glycoengineering improves selectin-mediated adhesion of mesenchymal stem cells (MSCs) and cardiosphere-derived cells (CDCs): pilot validation in porcine ischemia-reperfusion model. Biomaterials. 2016;74:19-30. https://doi.org/ 10.1016/j.biomaterials.2015.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chou KJ, Lee PT, Chen CL, et al. CD44 fucosylation on mesenchymal stem cell enhances homing and macrophage polarization in ischemic kidney injury. Exp Cell Res. 2017;350:91-102. https://doi.org/ 10.1016/j.yexcr.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 142. Sarkar D, Vemula PK, Teo GSL, et al. Chemical engineering of mesenchymal stem cells to induce a cell rolling response. Bioconjug Chem. 2008;19:2105-2109. https://doi.org/ 10.1021/bc800345q [DOI] [PubMed] [Google Scholar]
- 143. Cheng H, Byrska-Bishop M, Zhang CT, et al. Stem cell membrane engineering for cell rolling using peptide conjugation and tuning of cell–selectin interaction kinetics. Biomaterials. 2012;33:5004-5012. https://doi.org/ 10.1016/j.biomaterials.2012.03.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Lo CY, Antonopoulos A, Dell A, et al. The use of surface immobilization of P-selectin glycoprotein ligand-1 on mesenchymal stem cells to facilitate selectin mediated cell tethering and rolling. Biomaterials. 2013;36+4:8213-8222. https://doi.org/ 10.1016/j.biomaterials.2013.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Ko IK, Kean TJ, Dennis JE.. Targeting mesenchymal stem cells to activated endothelial cells. Biomaterials. 2009;30:3702-3710. https://doi.org/ 10.1016/j.biomaterials.2009.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Ko IK, Kim BG, Awadallah A, et al. Targeting improves MSC treatment of inflammatory bowel disease. Mol Ther. 2010;18:1365-1372. https://doi.org/ 10.1038/mt.2010.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Lee RJ, Fang Q, Davol PA, et al. Antibody targeting of stem cells to infarcted myocardium. Stem Cells. 2007;25:712-717. https://doi.org/ 10.1634/stemcells.2005-0602 [DOI] [PubMed] [Google Scholar]
- 148. Gundlach CW, Caivano A, da Graca Cabreira-Hansen M, et al. Synthesis and evaluation of an anti-MLC1× anti-CD90 bispecific antibody for targeting and retaining bone- marrow-derived multipotent stromal cells in infarcted myocardium. Bioconjug Chem. 2011;22:1706-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Won YW, Patel AN, Bull DA.. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient. Biomaterials. 2014;35:5627-5635. https://doi.org/ 10.1016/j.biomaterials.2014.03.070 [DOI] [PubMed] [Google Scholar]
- 150. Cheng Z, Ou L, Zhou X, et al. Targeted migration of mesenchymal stem cells modified with cxcr4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16:571-579. https://doi.org/ 10.1038/sj.mt.6300374 [DOI] [PubMed] [Google Scholar]
- 151. Zhang D, Fan GC, Zhou X, et al. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2008;44:281-292. https://doi.org/ 10.1016/j.yjmcc.2007.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Chen W, Li M, Cheng H, et al. Overexpression of the mesenchymal stem cell Cxcr4 gene in irradiated mice increases the homing capacity of these cells. Cell Biochem Biophys. 2013;67:1181-1191. https://doi.org/ 10.1007/s12013-013-9632-6 [DOI] [PubMed] [Google Scholar]
- 153. Chen Z, Zhang Y, Ouyang C, Zhang F, Ma J.. Automated landslides detection for mountain cities using multi-temporal remote sensing imagery. Sensors (Basel). 2018;18:821. https://doi.org/ 10.3390/s18030821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Gheisari Y, Azadmanesh K, Ahmadbeigi N, et al. Genetic modification of mesenchymal stem cells to overexpress CXCR4 and cxcr7 does not improve the homing and therapeutic potentials of these cells in experimental acute kidney injury. Stem Cells Dev. 2012;21:2969-2980. https://doi.org/ 10.1089/scd.2011.0588 [DOI] [PubMed] [Google Scholar]
- 155. Kumar S, Ponnazhagan S.. Bone homing of mesenchymal stem cells by ectopic α4 integrin expression. FASEB J. 2007;21:3917-3927. https://doi.org/ 10.1096/fj.07-8275com [DOI] [PubMed] [Google Scholar]
- 156. Cui LL, Nitzsche F, Pryazhnikov E, et al. Integrin α4 overexpression on rat mesenchymal stem cells enhances transmigration and reduces cerebral embolism after intracarotid injection. Stroke. 2017;48:2895-2900. https://doi.org/ 10.1161/strokeaha.117.017809 [DOI] [PubMed] [Google Scholar]
- 157. Levy O, Zhao W, Mortensen LJ, et al. mRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammation. Blood. 2013;122:e23-e32. https://doi.org/ 10.1182/blood-2013-04-495119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Liao W, Pham V, Liu L, et al. Mesenchymal stem cells engineered to express selectin ligands and IL-10 exert enhanced therapeutic efficacy in murine experimental autoimmune encephalomyelitis. Biomaterials. 2016;77:87-97. https://doi.org/ 10.1016/j.biomaterials.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Ryser MF, Ugarte F, Thieme S, et al. mRNA transfection of CXCR4-GFP fusion—simply generated by PCR—results in efficient migration of primary human mesenchymal stem cells. Tissue Eng Part C Methods. 2008;14:179-184. https://doi.org/ 10.1089/ten.tec.2007.0359 [DOI] [PubMed] [Google Scholar]
- 160. Wiehe JM, Kaya Z, Homann JM, et al. GMP-adapted overexpression of CXCR4 in human mesenchymal stem cells for cardiac repair. Int J Cardiol. 2013;167:2073-2081. https://doi.org/ 10.1016/j.ijcard.2012.05.065 [DOI] [PubMed] [Google Scholar]
- 161. Arbab AS, Jordan EK, Wilson LB, et al. In vivo trafficking and targeted delivery of magnetically labeled stem cells. Hum Gene Ther. 2004;15:351-360. https://doi.org/ 10.1089/104303404322959506 [DOI] [PubMed] [Google Scholar]
- 162. Kobayashi T, Ochi M, Yanada S, et al. A novel cell delivery system using magnetically labeled mesenchymal stem cells and an external magnetic device for clinical cartilage repair. Arthroscopy. 2008;24:69-76. https://doi.org/ 10.1016/j.arthro.2007.08.017 [DOI] [PubMed] [Google Scholar]
- 163. Yanai A, Häfeli UO, Metcalfe AL, et al. Focused magnetic stem cell targeting to the retina using superparamagnetic iron oxide nanoparticles. Cell Transplant. 2012;21:1137-1148. https://doi.org/ 10.3727/096368911x627435 [DOI] [PubMed] [Google Scholar]
- 164. Wang W, Deng Z, Xu X, et al. Functional nanoparticles and their interactions with mesenchymal stem cells. Curr Pharm Des. 2017;23:3814-3832. https://doi.org/ 10.2174/1381612823666170622110654 [DOI] [PubMed] [Google Scholar]
- 165. Hsiao JK, Tai MF, Chu HH, et al. Magnetic nanoparticle labeling of mesenchymal stem cells without transfection agent: cellular behavior and capability of detection with clinical 1.5 T magnetic resonance at the single cell level. Magn Reson Med. 2007;58:717-724. https://doi.org/ 10.1002/mrm.21377 [DOI] [PubMed] [Google Scholar]
- 166. Song YS, Ku JH.. Monitoring transplanted human mesenchymal stem cells in rat and rabbit bladders using molecular magnetic resonance imaging. Neurourol Urodyn. 2007;26:584-593. https://doi.org/ 10.1002/nau.20351 [DOI] [PubMed] [Google Scholar]
- 167. Capasso S, Alessio N, Squillaro T, et al. Changes in autophagy, proteasome activity and metabolism to determine a specific signature for acute and chronic senescent mesenchymal stromal cells. Oncotarget. 2015;6:39457-39468. https://doi.org/ 10.18632/oncotarget.6277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Nauta AJ, Westerhuis G, Kruisselbrink AB, et al. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114-2120. https://doi.org/ 10.1182/blood-2005-11-011650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Eliopoulos N, Stagg J, Lejeune L, Pommey S, Galipeau J.. Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood. 2005;106:4057-4065. https://doi.org/ 10.1182/blood-2005-03-1004 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.