Abstract
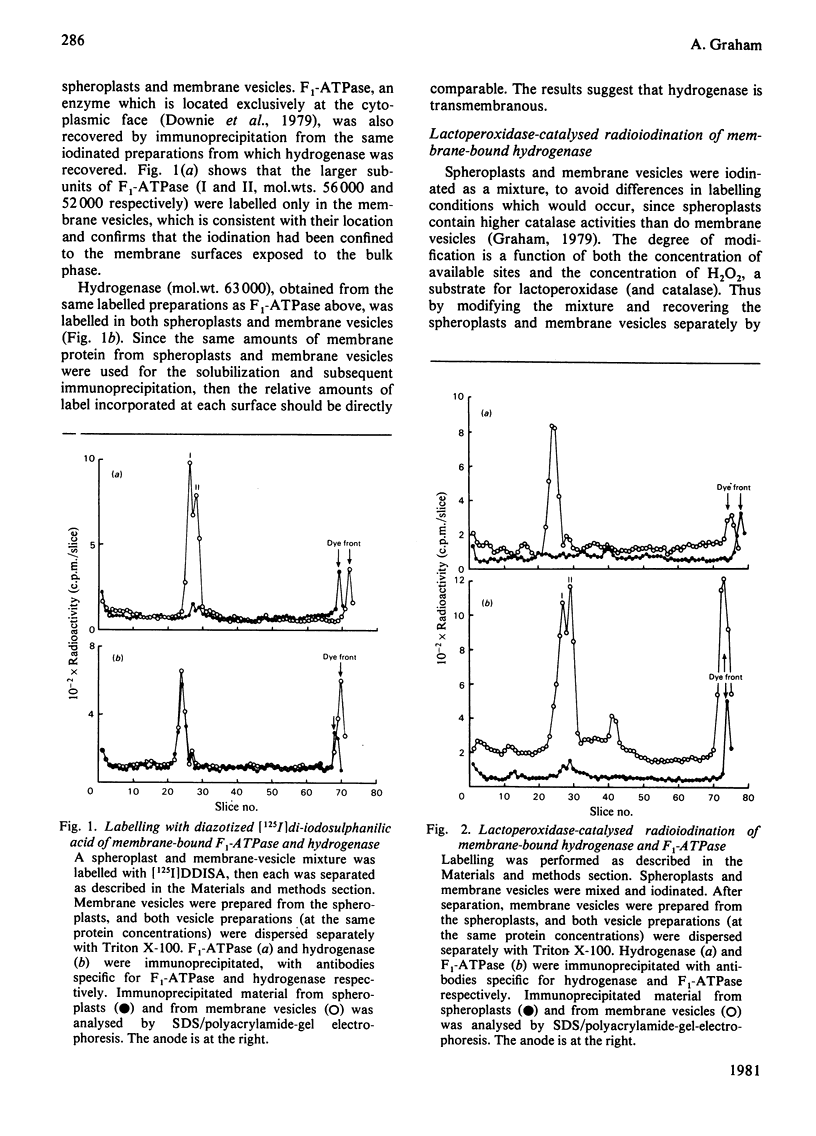
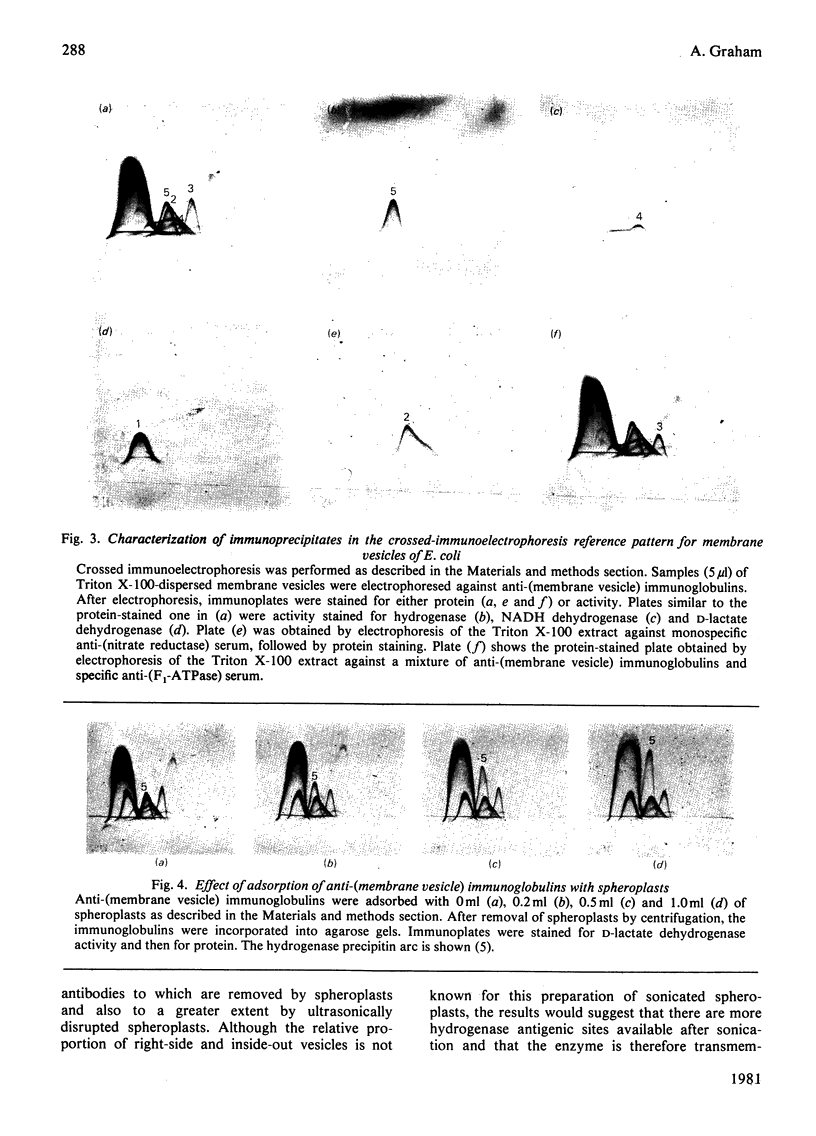
The organization of the membrane-bound hydrogenase from Escherichia coli was studied by using two membrane-impermeant probes, diazotized [125I]di-iodosulphanilic acid and lactoperoxidase-catalysed radioiodination. The labelling pattern of the enzyme obtained from labelled spheroplasts was compared with that from predominantly inside-out membrane vesicles, after recovery of hydrogenase by immunoprecipitation. The labelling pattern of F1-ATPase was used as a control for labelling at the cytoplasmic surface throughout these experiments. Hydrogenase (mol.wt. approx. 63 000) is transmembranous. Crossed immunoelectrophoresis with anti-(membrane vesicle) immunoglobulins, coupled with successive immunoadsorption of the antiserum with spheroplasts, confirmed the location of hydrogenase at the periplasmic surface. Immunoadsorption with sonicated spheroplasts suggests that the enzyme is also exposed at the cytoplasmic surface. Inside-out vesicles were prepared by agglutination of sonicated spheroplasts, and the results of immunoadsorption using these vesicles confirms the location of hydrogenase at the cytoplasmic surface.
Full text
PDF



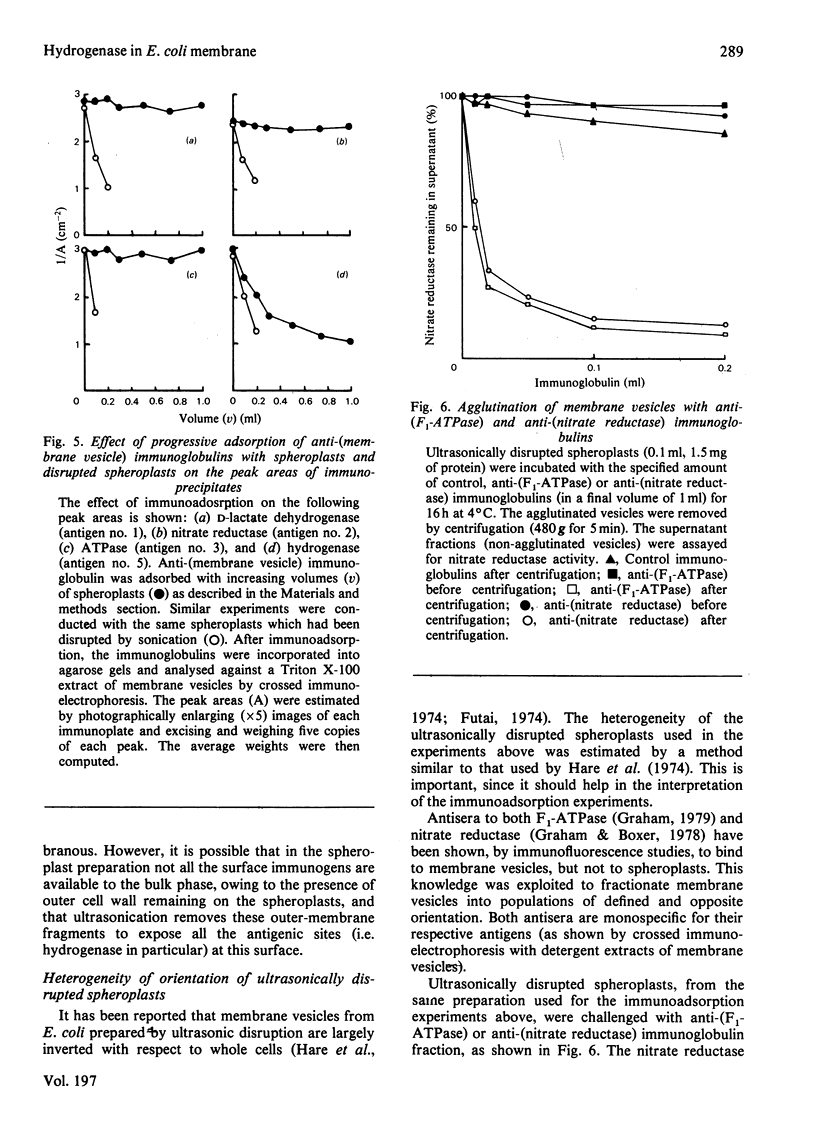


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- Downie J. A., Gibson F., Cox G. B. Membrane adenosine triphosphatases of prokaryotic cells. Annu Rev Biochem. 1979;48:103–131. doi: 10.1146/annurev.bi.48.070179.000535. [DOI] [PubMed] [Google Scholar]
- Enoch H. G., Lester R. L. The purification and properties of formate dehydrogenase and nitrate reductase from Escherichia coli. J Biol Chem. 1975 Sep 10;250(17):6693–6705. [PubMed] [Google Scholar]
- Futai M. Orientation of membrane vesicles from Escherichia coli prepared by different procedures. J Membr Biol. 1974;15(1):15–28. doi: 10.1007/BF01870079. [DOI] [PubMed] [Google Scholar]
- GRAY C. T., GEST H. BIOLOGICAL FORMATION OF MOLECULAR HYDROGEN. Science. 1965 Apr 9;148(3667):186–192. doi: 10.1126/science.148.3667.186. [DOI] [PubMed] [Google Scholar]
- Graham A., Boxer D. H. Arrangement of respiratory nitrate reductase in the cytoplasmic membrane of Escherichia coli. Location of beta subunit. FEBS Lett. 1980 Apr 21;113(1):15–20. doi: 10.1016/0014-5793(80)80484-4. [DOI] [PubMed] [Google Scholar]
- Graham A., Boxer D. H., Haddock B. A., Mandrand-Berthelot A. M., Jones R. W. Immunochemical analysis of the membrane-bound hydrogenase of Escherichia coli. FEBS Lett. 1980 May 5;113(2):167–172. doi: 10.1016/0014-5793(80)80584-9. [DOI] [PubMed] [Google Scholar]
- Graham A., Boxer D. H. Immunochemical localization of nitrate reductase in Escherichia coli [proceedings]. Biochem Soc Trans. 1978;6(6):1210–1211. doi: 10.1042/bst0061210. [DOI] [PubMed] [Google Scholar]
- Graham A., Boxer D. H. The membrane location of the beta-subunit of nitrate reductase from Escherichia coli [proceedings]. Biochem Soc Trans. 1980 Jun;8(3):331–331. doi: 10.1042/bst0080331. [DOI] [PubMed] [Google Scholar]
- Graham A., Boxer D. H. The organization of formate dehydrogenase in the cytoplasmic membrane of Escherichia coli. Biochem J. 1981 Jun 1;195(3):627–637. doi: 10.1042/bj1950627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare J. F., Olden K., Kennedy E. P. Heterogeneity of membrane vesicles from Escherichia coli and their subfractionation with antibody to ATPase. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4843–4846. doi: 10.1073/pnas.71.12.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmkamp R. W., Sears D. A. A label for the red cell membrane: diazotized diiodosulfanilic acid. Int J Appl Radiat Isot. 1970 Nov;21(11):683–685. doi: 10.1016/0020-708x(70)90127-4. [DOI] [PubMed] [Google Scholar]
- Jones R. W. Hydrogen-dependent proton translocation by membrane vesicles from Escherichia coli [proceedings]. Biochem Soc Trans. 1979 Oct;7(5):1136–1137. doi: 10.1042/bst0071136. [DOI] [PubMed] [Google Scholar]
- Jones R. W. The role of the membrane-bound hydrogenase in the energy-conserving oxidation of molecular hydrogen by Escherichia coli. Biochem J. 1980 May 15;188(2):345–350. doi: 10.1042/bj1880345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lund K., DeMoss J. A. Association-dissociation behavior and subunit structure of heat-released nitrate reductase from Escherichia coli. J Biol Chem. 1976 Apr 25;251(8):2207–2216. [PubMed] [Google Scholar]
- Macy J., Kulla H., Gottschalk G. H2-dependent anaerobic growth of Escherichia coli on L-malate: succinate formation. J Bacteriol. 1976 Feb;125(2):423–428. doi: 10.1128/jb.125.2.423-428.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Site of synthesis of lipopolysaccharide. J Biol Chem. 1972 Jun 25;247(12):3973–3986. [PubMed] [Google Scholar]
- Owen P., Kaback H. R. Molecular structure of membrane vesicles from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3148–3152. doi: 10.1073/pnas.75.7.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Siegel J., Salton M. R., Owen P. Immunochemical analysis of inner and outer membranes of Escherichia coli by crossed immunoelectrophoresis. J Bacteriol. 1978 Jan;133(1):306–319. doi: 10.1128/jb.133.1.306-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S. Isolation and characterisation of a mitochondrially synthesized precursor protein of cytochrome oxidase. Eur J Biochem. 1974 Mar 15;43(1):39–48. doi: 10.1111/j.1432-1033.1974.tb03382.x. [DOI] [PubMed] [Google Scholar]