Introduction
Heart failure is mostly caused by dilated cardiomyopathy, ischaemic heart disease, and valvular disease. Valvular degeneration is an inevitable phenomenon associated with aging bioprosthetic valves. Similarly, a previously repaired mitral valve may deteriorate, particularly in cases of ischemic mitral regurgitation. This is a problem that comes along with the improvement of the society's health level.
History of presentation
A 77‐year‐old male was referred to our tertiary centre for evaluation due to worsening degenerative primary aortic regurgitation with a EuroScore II of 12.54. He presented with dyspnoea NYHA functional class III, fatigue and atrial fibrillation. The patient had undergone mitral valve plasty and tricuspid valve plasty 20 years ago, with successful recovery. Transthoracic echocardiography revealed significant mitral and aortic regurgitation, along with severe aortic dilation, global cardiac enlargement, and left ventricular dysfunction. The patient was followed up for 1 year. Patient consent for case publication was obtained (Figure 1).
Figure 1.
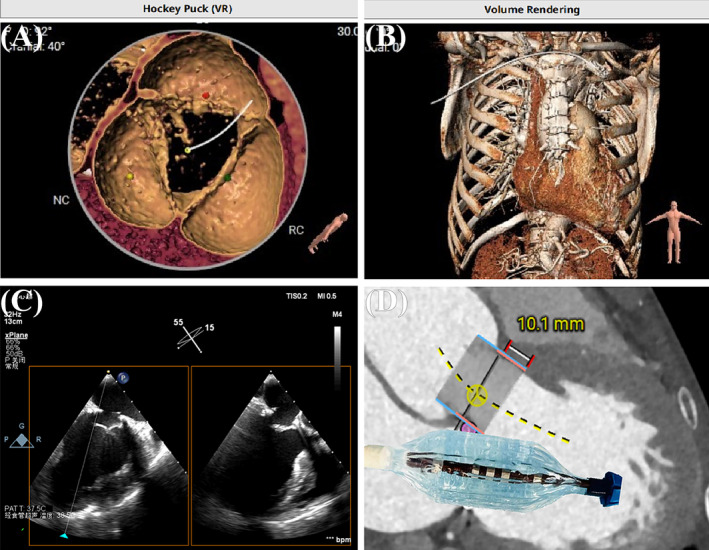
Evaluation of positioning and simulation. (A, B) VR views of the anatomical structures. (C) Utilization of xPlane technology facilitated simulations of the surgical pathway. (D) Assessing the operational feasibility and strategic approach.
Past medical history
Past medical history included coronary artery disease with multi‐vessel lesions, atrial fibrillation with an associated atrial thrombus, arteriosclerosis of both lower extremities with bilateral plaques, hypertension, prior cerebral infarction, and mild pulmonary arterial hypertension.
Investigations
An electrocardiogram revealed atrial fibrillation and ST segment changes (I aVL V5 V6). Subsequently, a transthoracic echocardiogram was performed, revealing a prominent ring echo. The annulus diameter measured 26 mm with thick dysplastic leaflets. Notably, the aortic sinus exhibited tumour‐like changes, with the inner diameter of the sinus canal junction measuring 41 mm. The aortic annulus diameter was 24 mm with dysplastic leaflets. Significant central regurgitation was observed below the valve, with vena contracta width approximately 5.3 mm (mitral regurgitant volume 15 mL; tricuspid regurgitant volume 15 mL; aortic regurgitation volume 12 mL). Both atrial and ventricular chambers showed enlargement, alongside a reduced left ventricular ejection fraction (42%). Coronary computed tomography angiography indicated an aortic annulus diameter of 27.3 mm, with a 14.3 mm‐height left coronary artery and a 26.5 mm‐height right coronary artery. Cardiac enlargement and a filling defect in the left atrial appendage were suggestive of possible thrombosis. Lower extremity vascular ultrasound demonstrated multiple plaques with varying echo intensity, particularly notable in the posterior walls of the right and left femoral arteries.
Management
Considering the prohibitive surgical risk and frailty of this patient, concurrent TA‐TAVR and TA‐TMViR were chosen after a multidisciplinary heart team discussion. The cause of the mitral regurgitation is not completely clear. It may result from degenerative or mixed mitral regurgitation. Typically, treating the aortic valve first is more common, as it can improve the LVEF and mitigate the severity of the mitral regurgitation. However, significant mitral regurgitation may not be fully resolved by addressing one valve alone. Concurrent treatment of both conditions is rare and positive. After consulting with the patient, a decision was made to proceed with both procedures. An analysis of the patient's anatomical structure influenced our procedural strategy: ring annuloplasty served as a bridge enabling the anchoring of the valve. We considered using a J‐valve bioprosthesis inversion initially; however, the presence of intact chordae tendineae muscles and proximity to the existing aortic bioprosthesis made this option unfeasible. Consequently, we selected a shorter stent valve to avoid interference with the aortic bioprosthesis. To further minimize surgical duration and trauma, transfemoral approaches such as Evolut‐Pro (Medtronic) were discounted. Therefore, we opted for the Prizvalve from NewMed Medical for the mitral valve replacement. For aortic bioprosthesis, the anchoring force was derived from two aspects: the tension caused by oversizing and the presence of three anchor claspers. The placement of both valves was meticulously planned and tested through repeated computer simulations to ensure precision during the actual procedure. The operation was conducted under general anaesthesia in a hybrid surgical suite on June 2023. Initially, the placement of a pigtail catheter at the aortic root was carried out, followed by the insertion of a temporary pacing lead into the right ventricle via the right jugular vein. The left ventricular apex was accessed via a 4 cm anterolateral minithoracotomy in the sixth intercostal space, secured with multiple U‐stitches (Prolene 3‐0, MH needle), using a standard transapical approach. Subsequently, a soft guidewire was navigated into the right pulmonary vein across the mitral valve annulus under fluoroscopic visualization following apex puncture. This wire was exchanged for a Super‐Stiff wire for further navigation using a COOK catheter. A 29 mm J‐valve (JieCheng Medical Technology Co., Suzhou, China) was then precisely positioned in the target area and deployed during rapid ventricular pacing at 180 bpm, with continuous fluoroscopic monitoring. Afterward, a 29 mm transcatheter valve (Prizvalve, NewMed Medical Technology Co., Shanghai, China), part of the domestic balloon‐expandable Prizvalve system, was inserted inversely through the apex. The atrial segment of the bioprosthetic valve was carefully deployed, adjusted against the mitral annuloplasty ring until moderate tension was achieved. The total duration of the surgery was 93 min, with an estimated blood loss of 50 mL (Figure 2).
Figure 2.
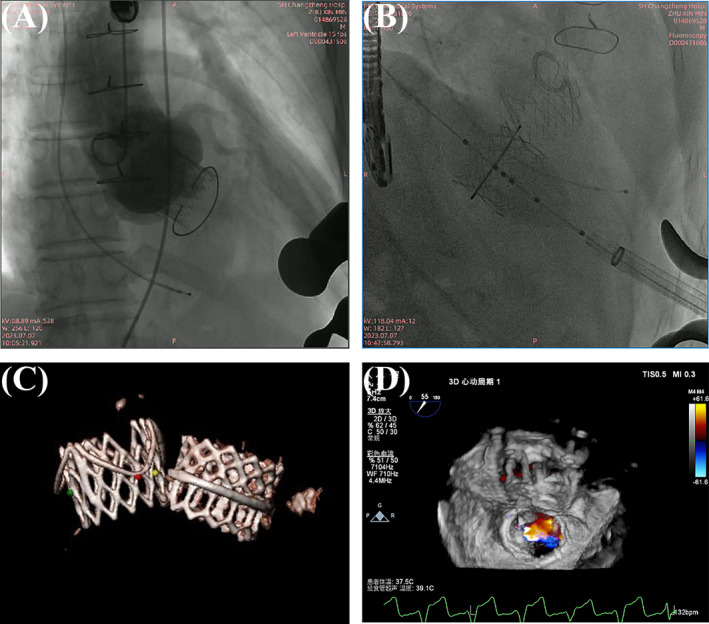
Main procedural steps for TA‐TAVR and TA‐TMViR. (A, B) Complete deployment of the aortic and mitral valves shown via angiography, demonstrating minimal paravalvular leakage. (C) The scanned model of the bioprosthetic valve 1 year later. (D) Three‐dimensional reflux imaging post‐aortic valve replacement.
Follow‐up
Two weeks post‐discharge, the patient's NYHA functional class had improved to class II. Haemodynamic data from before the operation and 1 year afterward are summarized in Table 1.
Table 1.
Comparison of patient recovery status.
| Valve type | Mitral valve | Mitral valve | Mitral valve | Aortic valve | Aortic valve | Aortic valve |
|---|---|---|---|---|---|---|
| Time of echo | Pre‐procedure | Post‐procedure | One year later | Pre‐procedure | Post‐procedure | One year later |
| Mean gradient (mmHg) | 15 | 10 | / | / | 11 | / |
| Peak gradient (mmHg) | 22 | 15 | / | / | / | / |
| Severity of regurgitation | Severe | Trace | Trace | Severe | None | None |
| Regurgitation volume (mL) | 15 | 2 | 2 | 12 | 0 | 0 |
| LVEF (%) | 49 | 48 | 55 | 49 | 48 | 55 |
Discussion
Valvular degeneration is an inevitable phenomenon associated with aging bioprosthetic valves. The degeneration of bioprosthetic valves can be accelerated by ischemic mitral regurgitation. 1 Reoperation is typically recommended for patients with a deteriorated bioprosthetic mitral valve or a failed annuloplasty. 2 On the one hand, with advances in transcatheter techniques, interventional therapy offers a viable alternative for patients unsuitable for repeat mitral valve surgery. 3 On the other hand, for patients with severe aortic stenosis, TAVR is now a popular approach. 4 Although international surgical guidelines for patients with aortic regurgitation remain under debate, some countries have developed unique competencies in this field. The applicability of transcatheter solutions for simultaneous multiple valve replacements has yet to be extensively studied. Some researchers have compared the apical approach to the ‘minimally invasive motorway’ in transcatheter valve methods, which seems like a vivid metaphor. 5 It illuminates that a valid alternative option for surgery, normally substituted for using transfemoral access, is the transapical approach. 6 The anatomic arrangement, which includes several heart structures such as the mitral valve and left ventricular outflow tract, in close vicinity and coaxiality with the left ventricular apex, makes the transapical approach a safe and feasible option. 5 This approach ensures precise and stable placement of transcatheter procedures. 7 Moreover, existing valve rings and stents can complicate further surgical interventions due to the unique challenges posed by transcatheter procedures. 8 Often, the transfemoral route is not advised for patients with specific vascular anomalies for the most part. 6 Managing complex cardiac conditions requires a determined strategy to minimize the risks associated with prolonged surgery and anaesthesia. 9 The ‘minimally invasive motorway’ is favourable compared with the transfemoral transcatheter method, offering numerous advantages over its disadvantages, particularly in scenarios involving multiple‐valve replacement. Both the feasibility of the surgical technique and the likelihood of vascular damage remain manageable while the potential for cardiac and pulmonary complications cannot be despised. Furthermore, the rapid exposure, observation, and treatment enhance safety for the patient during emergency rescue procedures. Nonetheless, further research is essential to optimize the benefits of the transapical approach and to develop strategies to decrease its associated risks. A balanced surgical strategy that focuses on risk reduction and patient safety is crucial, beyond merely minimizing incision size and invasiveness.
Conclusions
Transcatheter mitral interventions, such as valve‐in‐valve and valve‐in‐ring, prove to be effective, safe, and minimally invasive alternatives for high‐risk surgical patients. 10 The transapical technique remains a secure option for those for whom the first‐line transfemoral TAVI method is unsuitable or impractical, 6 particularly advantageous for patients with adverse vascular conditions or requiring multiple valve replacements.
Funding
This work was supported by the National Nature Science Foundation of China (No. 82070255), Military Medical Talent Project (Linghang, SL03) of Naval Medical University, Outstanding Talent Project of Health and Health Committee of Shanghai (20244Z0001), and the Innovation Team of Naval Medical University.
Conflict of interest
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Supporting information
Data S1. Supporting Information.
Data S2. Supporting Information.
Wang, G. , Wang, P. , Wang, Y. , Song, J. L. , Xu, H. , Wang, J. , Wang, J. , Qu, Y. , Xiao, J. , and Wang, Z. (2024) Concurrent transapical transcatheter aortic valve and mitral valve‐in‐ring replacement for aortic regurgitation. ESC Heart Failure, 11: 4456–4460. 10.1002/ehf2.15022.
Guoji Wang, Pei Wang, and Yizhou Wang contributed equally to this study.
[Correction added on 22 November 2024, after first online publication: The article category has been changed from ‘Clinical Correspondence’ to ‘Clinical Correspondences’ in this version.]
Contributor Information
Jian Xiao, Email: lizardxj@163.com.
Zhinong Wang, Email: wangzn007@smmu.edu.cn.
References
- 1. Murdoch DJ, Sathananthan J, Cheung A, Webb JG. Combined transapical valve‐in‐valve/valve‐in‐ring transcatheter mitral valve implantation and paravalvular leak closure for failed mitral valve surgery. Can J Cardiol 2018;34:1088.e3‐1088.e6. doi: 10.1016/j.cjca.2018.04.027 [DOI] [PubMed] [Google Scholar]
- 2. Zubarevich A, Szczechowicz M, Zhigalov K, Marx P, Lind A, Jánosi RA, et al. Transapical transcatheter mitral valve implantation in patients with degenerated mitral bioprostheses or failed ring annuloplasty. Annals of Cardiothoracic Surgery 2021;10:674‐682. doi: 10.21037/acs-2021-tviv-fs-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu J, Qiao E, Wang W. Mechanical or biologic prostheses for mitral valve replacement: a systematic review and meta‐analysis. Clin Cardiol 2022;45:701‐716. doi: 10.1002/clc.23854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee G, Chikwe J, Milojevic M, Wijeysundera HC, Biondi‐Zoccai G, Flather M, et al. ESC/EACTS vs. ACC/AHA guidelines for the management of severe aortic stenosis. Eur Heart J 2023;44:796‐812. doi: 10.1093/eurheartj/ehac803 [DOI] [PubMed] [Google Scholar]
- 5. Agostinelli A, Gallingani A, Maestri F, Grossi S, Gripshi F, de Donno L, et al. Left ventricular apex: a “minimally invasive motorway” for safe cardiovascular procedures. J. Clin Med 2021;10:3857. doi: 10.3390/jcm10173857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Useini D, Beluli B, Christ H, Schlömicher M, Ewais E, Patsalis P, et al. Transapical transcatheter aortic valve implantation in patients with aortic diseases. Eur J Cardiothorac Surg 2021;59:1174‐1181. doi: 10.1093/ejcts/ezab050 [DOI] [PubMed] [Google Scholar]
- 7. Conradi L, Silaschi M, Seiffert M, Lubos E, Blankenberg S, Reichenspurner H, et al. Transcatheter valve‐in‐valve therapy using 6 different devices in 4 anatomic positions: clinical outcomes and technical considerations. J Thorac Cardiovasc Surg 2015;150:1557‐1567. doi: 10.1016/j.jtcvs.2015.08.065 [DOI] [PubMed] [Google Scholar]
- 8. Conradi L, Blankenberg S, Seiffert M, Schaefer A. Transapical mitral valve‐in‐ring procedure with a novel self‐expandable transcatheter heart valve: first‐ and last‐in‐man report. Eur J Cardiothorac Surg 2020;58:190‐192. doi: 10.1093/ejcts/ezz350 [DOI] [PubMed] [Google Scholar]
- 9. Jelnin V, Dudiy Y, Einhorn BN, Kronzon I, Cohen HA, Ruiz CE. Clinical experience with percutaneous left ventricular transapical access for interventions in structural heart defects. J Am Coll Cardiol Intv 2011;4:868‐874. doi: 10.1016/j.jcin.2011.05.018 [DOI] [PubMed] [Google Scholar]
- 10. Owais T, el Garhy M, Elvinger S, et al. Contemporary results of transcatheter mitral valve procedures: bi‐centric retrospective analysis. The Egyptian Heart Journal 2022;74:19. doi: 10.1186/s43044-022-00257-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Data S2. Supporting Information.


