Abstract
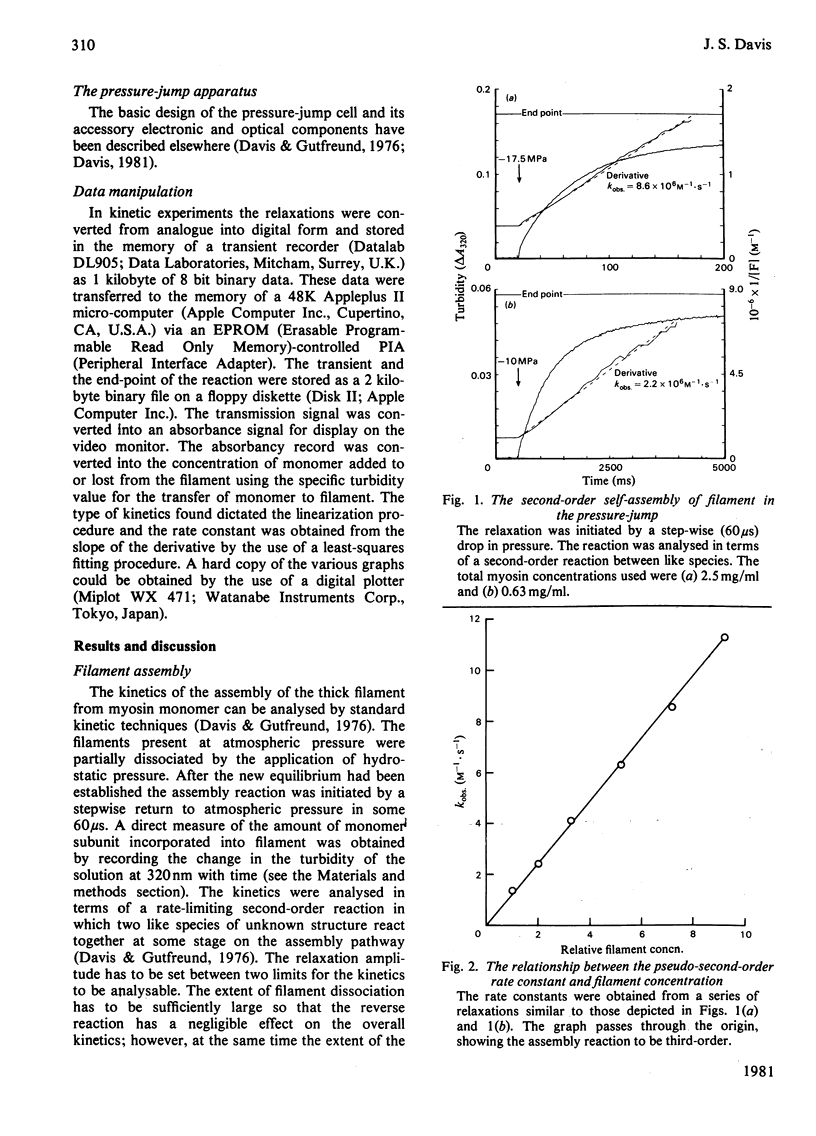
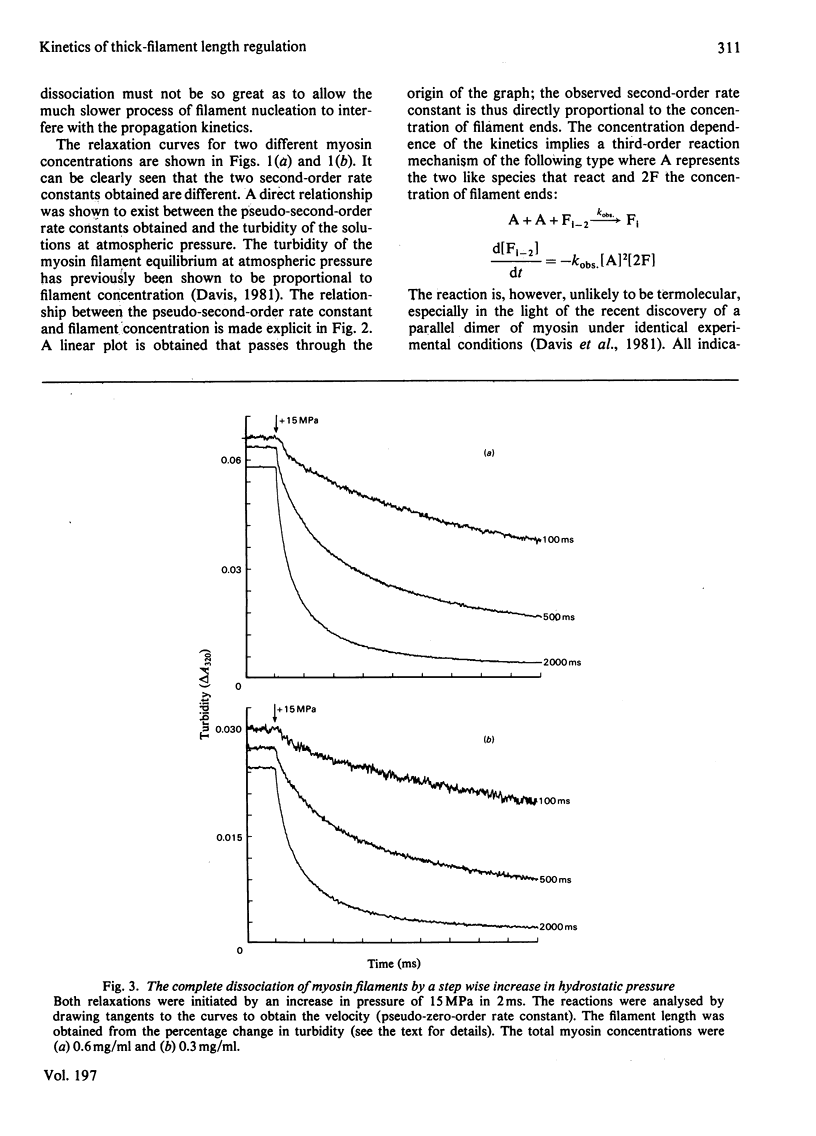
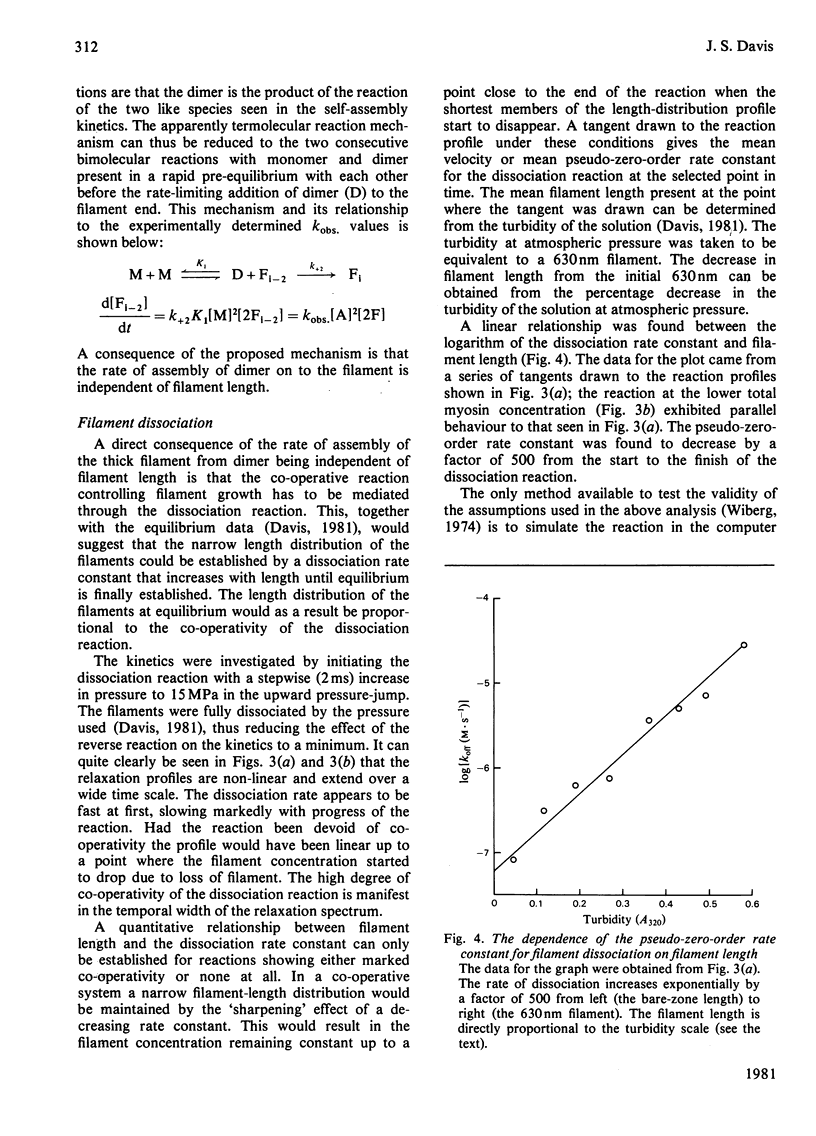
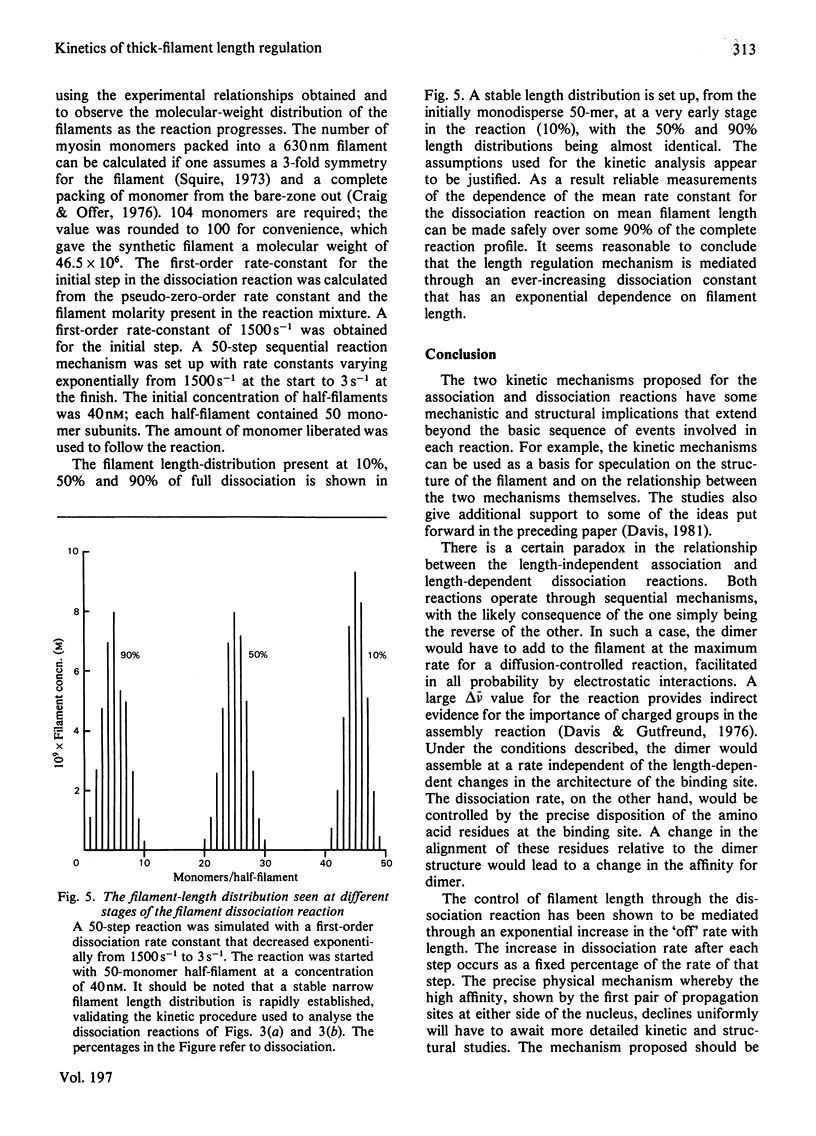
The self-assembly of myosin monomer into thick filament occurs via a two-step mechanism. At first a pair of myosin monomers reacts to form a parallel dimer; the dimer in turn adds to the filament ends at a rate that is independent of filament length. The rate of the dissociation reaction on the other hand is length-dependent. The 'off' rate constant has been shown to increase exponentially by a factor of 500 as the filament grows from the bare-zone out to its full length. The length of the filament is thus kinetically controlled; myosin is added to the filament at a fixed rate, whereas the dissociation rate increases to a point where equilibrium is established and the filament ceases to grow. The structural implications implicit in the mechanism are discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Craig R., Offer G. Axial arrangement of crossbridges in thick filaments of vertebrate skeletal muscle. J Mol Biol. 1976 Apr 5;102(2):325–332. doi: 10.1016/s0022-2836(76)80057-5. [DOI] [PubMed] [Google Scholar]
- Davis J. S., Gutfreund H. The scope of moderate pressure changes for kinetic and equilibrium studies of biochemical systems. FEBS Lett. 1976 Dec 31;72(2):199–207. doi: 10.1016/0014-5793(76)80971-4. [DOI] [PubMed] [Google Scholar]
- Godfrey J. E., Harrington W. F. Self-association in the myosin system at high ionic strength. I. Sensitivity of the interaction to pH and ionic environment. Biochemistry. 1970 Feb 17;9(4):886–893. doi: 10.1021/bi00806a025. [DOI] [PubMed] [Google Scholar]
- Godfrey J. E., Harrington W. F. Self-association in the myosin system at high ionic strength. II. Evidence for the presence of a monomer--dimer equilibrium. Biochemistry. 1970 Feb 17;9(4):894–908. doi: 10.1021/bi00806a026. [DOI] [PubMed] [Google Scholar]
- Josephs R., Harrington W. F. On the stability of myosin filaments. Biochemistry. 1968 Aug;7(8):2834–2847. doi: 10.1021/bi00848a020. [DOI] [PubMed] [Google Scholar]
- Reisler E., Burke M., Josephs R., Harrington W. F. Crosslinking of myosin and myosin filaments. J Mechanochem Cell Motil. 1973;2(3):163–179. [PubMed] [Google Scholar]
- Squire J. M. General model of myosin filament structure. 3. Molecular packing arrangements in myosin filaments. J Mol Biol. 1973 Jun 25;77(2):291–323. doi: 10.1016/0022-2836(73)90337-9. [DOI] [PubMed] [Google Scholar]


