Abstract
Aims
Mitral stenosis (MS) occasionally coexists with aortic stenosis (AS). Limited data are available regarding the functional class and clinical outcomes of patients who undergo transcatheter aortic valve implantation (TAVI) for combined AS and MS. This study compared the clinical outcomes in patients with and without MS who underwent TAVI for severe AS and assessed the impact of mitral annulus calcification (MAC) severity, transmitral gradient (TMG) and mitral valve area (MVA) on outcomes in patients with combined AS and MS.
Methods
We investigated patients in the OCEAN‐TAVI registry who underwent TAVI. MS was defined as an MVA ≤ 1.5 cm2 or TMG ≥ 5 mmHg. The composite of all‐cause death and admission for heart failure was compared between patients with and without MS. The impact of MAC, TMG and MVA on outcomes was assessed in patients with combined AS and MS.
Results
We identified 106 patients with MS (MAC 84%; TMG 6.4 ± 2.6 mmHg; MVA 1.10 ± 0.31 cm2) and 6570 without MS as controls. The MS group was older (85 ± 5 vs. 84 ± 5 years, P = 0.033), more of women (85 vs. 67%, P < 0.01), and had a higher risk of surgery (the Society of Thoracic Surgeons Mortality Score 8.7 ± 5.1 vs. 7.6 ± 5.9, P = 0.047) than the controls. In the MS group, the New York Heart Association Functional Class was 3 or 4 in 56% of the patients at baseline and 6% at 1 year after TAVI. Thirty‐day mortality (2.8% vs. 1.3%, P = 0.18) and early composite outcomes (17% vs. 15%, P = 0.56) were comparable between patients with and without MS. During a median follow‐up of 2.1 years, the presence of MS was associated with a higher incidence of adverse events compared with controls (adjusted hazard ratio [HR] 1.84; 95% confidence interval [CI] 1.34–2.51, P < 0.01), even on propensity score matched analysis (adjusted HR 1.91; 95% CI 1.14–3.22, P < 0.01). Moderate or severe MAC contributed to increased risk of adverse events in patients with MS (adjusted HR 2.89; 95% CI 1.20–6.99, P = 0.018), but TMG and MVA did not.
Conclusions
In patients undergoing TAVI for severe AS, those with moderate or severe MS experienced worse outcomes after TAVI compared with those without MS. Patients with combined AS and MS sustained symptom improvement at 1‐year post‐TAVI. MAC severity was a useful predictor of adverse events compared with MS haemodynamics such as TMG and MVA in patients with combined AS and MS.
Keywords: mitral stenosis, mitral annulus calcification, aortic stenosis, transcatheter aortic valve implantation
Introduction
Mitral stenosis (MS) frequently occurs with aortic stenosis (AS). 1 Although mitral annulus calcification (MAC) is found in nearly half of patients with severe AS, 2 its extension to the mitral leaflets reduces mitral leaflet mobility and subsequently leads to calcific MS. 3 , 4 Generally, patients with MAC are older, debilitated and have multiple comorbidities, and the presence of MAC is associated with higher cardiovascular events and mortality. 5 , 6 Additionally, an elevated transmitral gradient (TMG) has been identified as a significant risk factor for mortality in addition to a high burden of comorbidities. 3
The management of combined AS and MS remains unaddressed in the current guidelines for valvular heart diseases, 7 , 8 underscoring the need for focused research in this area. Surgical mitral valve replacement (MVR) is considered an optimal treatment for MS during aortic valve replacement (AVR). 9 However, mitral valve surgery in the presence of MAC is technically demanding and requires a prolonged cardiopulmonary bypass, leading to high operative mortality and morbidity, especially in older adults with multiple comorbidities. 9 Due to its high surgical risk, transcatheter aortic valve implantation (TAVI) has often been chosen for patients with severe AS and calcific MS; whereas, the presence of concurrent MS is associated with poorer outcomes after TAVI. 10 , 11 The effect of TAVI on the symptoms and survival of patients with combined AS and MS has not been thoroughly investigated, and the risk predictors for TAVI in these patients remain undefined. Risk stratification based on haemodynamics and the extent of MAC could improve management strategies for patients with combined AS and MS. Therefore, this study aimed to (1) compare the clinical outcomes in patients with and without MS who underwent TAVI for severe AS both in the overall and propensity‐matched cohorts and (2) assess the impact of MAC severity, TMG and mitral valve area (MVA) on outcomes in patients with combined AS and MS.
Methods
Study population
The Optimized CathEter vAlvular iNtervention‐Transcatheter Aortic Valve Implantation (OCEAN‐TAVI) registry is a Japanese multicentre prospective registry designed to observe and document the procedural results and postprocedural outcomes of patients treated with TAVI. This trial was registered with the University Hospital Medical Information Network (UMIN; UMIN000020423). Between October 2013 and December 2019, 7393 patients from 21 institutions in Japan who underwent TAVI were enrolled in the OCEAN‐TAVI registry. All patients were considered either unsuitable or at high risk for surgical AVR (SAVR) by consensus between individual centres and after discussion within multidisciplinary heart teams. In this study, 415 patients with a history of aortic or mitral valve surgery, congenital heart diseases and missing data of MS were excluded. Consequently, 6978 patients were included in the study.
The research protocol was approved by the local ethics committees at each participating institution, with written informed consent being diligently obtained from all participants before their enrolment in the registry.
Transthoracic echocardiography
Comprehensive transthoracic echocardiography (TTE) examinations were performed in accordance with guidelines. 12 , 13 , 14 Specifically, MS was defined as TMG ≥ 5 mmHg or MVA ≤ 1.5 cm2. 14 Quantitative measurements of MS and grading of MAC were further collected in patients with MS. TMG was obtained from continuous wave Doppler, and MVA was calculated using the continuity equation, planimetry method or pressure half‐time method, whichever was deemed appropriate. 15 TMG was divided into three categories; high (≥10 mmHg), medium (5–10 mmHg) and low (<5 mmHg). The increase or decrease in TMG was considered significant when it changed ≥1 mm Hg compared with that obtained from the baseline TTE. 16 MAC was characterized by the presence of dense calcium deposits at the base of the mitral leaflets between the left atrium and ventricle. Severity was defined by the circumferential involvement of the mitral ring on the short‐axis view in TTE: mild, moderate and severe with <1/3, 1/3–1/2 and >1/2 of the annulus involved, respectively. 2 Calcification of the anterior mitral leaflet (AML) was evaluated using the parasternal long‐axis view. 4 Calcific MS was identified as a bright echo‐producing structure (calcification) located in the mitral annulus and leaflets. Rheumatic MS is characterized by typical features, such as leaflet thickening, nodularity, commissural fusion and chordal fusion and shortening. Aortic, mitral and tricuspid regurgitations (AR, MR and TR, respectively) were considered significant if the grades were moderate or high.
Clinical outcomes
The study's primary endpoint was all‐cause mortality and hospitalization for heart failure after TAVI. The first event that occurred during the follow‐up period was used as the endpoint. Each hospital team obtained information on the possible occurrence and/or causes of death through telephone interviews and questionnaire surveys with families of the patient. The secondary endpoint included a composite of 30 day mortality, all strokes, life‐threatening bleeding, acute kidney injury, coronary artery obstruction requiring intervention, major vascular complications and valve‐related dysfunction that necessitated a repeat procedure such as TAVI or SAVR. 17
Statistical analysis
Continuous data are expressed as mean ± standard deviation or median (inter‐quartile range), and categorical data are expressed as frequencies or percentages. Continuous variables were compared between groups using the Student's t‐test or Wilcoxon rank‐sum test whenever appropriate. Nominal variables were compared between groups using the χ 2 test. Pre‐ and post‐operative measurements were compared using the paired t and McNemar tests. Survival analysis was performed using the Kaplan–Meier method and log‐rank test. The Cox proportional hazards model was used to identify independent predictors of all‐cause mortality or hospitalization for heart failure and to assess whether TMG or MAC severity was associated with outcomes in the MS cohort. The key clinical variables were included as candidate variables in the multivariate model. To account for confounders, a propensity score matching approach was used between patients with and without MS with a 1:1 ratio and a calliper width of ×0.2 standard deviation of the propensity score. The propensity score was calculated using a multivariate logistic regression model and matched for 21 covariates of patient characteristics and echocardiographic parameters. The pulmonary artery systolic pressure was excluded from the matching variables because of incomplete data collection. The aortic valve mean pressure and left ventricle systolic diameter were also excluded from the matching variables because of their potential multicollinearity with the aortic valve maximum velocity and left ventricle ejection fraction, respectively. The results of these analyses are presented as hazard ratios (HRs) and 95% confidence intervals (CIs). The proportional hazard assumption was evaluated visually by plotting residuals versus time and formally by testing for a correlation between the residual and time. No violations of the proportional hazard assumption were observed. Tests were two‐sided, and P < 0.05 was considered statistically significant. All statistical analyses were performed using JMP Pro 17 software (SAS Institute, Cary, North Carolina, USA).
Results
Patient characteristics
Among the 6978 patients, 106 had moderate or severe MS and 302 had mild MS. Patients with MS were compared with 6570 patients without MS (the controls). Patient characteristics and details of the TAVI procedures are presented in Table 1.
Table 1.
Patient characteristics.
| Concurrent mitral stenosis (n = 106) | Controls (n = 6570) | P | |
|---|---|---|---|
| Age | 85 ± 5 | 84 ± 5 | 0.033 |
| Women | 90 (85) | 4396 (67) | <0.01 |
| NYHA III or IV | 59 (56) | 2597 (40) | <0.01 |
| STS | 8.7 ± 5.1 | 7.6 ± 5.9 | 0.047 |
| CFS | 4.1 ± 1.4 | 3.9 ± 1.2 | 0.025 |
| Smoking history a /6378 | 1 (1.0) | 207 (3.3) | 0.20 |
| Atrial fibrillation | 21 (20) | 1345 (20) | 0.87 |
| Hypertension | 81 (76) | 5487 (84) | 0.051 |
| Dyslipidaemia | 56 (53) | 3661 (56) | 0.55 |
| Diabetes | 38 (36) | 1798 (27) | 0.052 |
| Coronary artery diseases | 29 (27) | 2181 (33) | 0.21 |
| Peripheral artery diseases | 16 (15) | 705 (11) | 0.15 |
| Chronic kidney disease | 79 (75) | 4560 (69) | 0.26 |
| COPD | 12 (11) | 635 (9.7) | 0.57 |
| Echocardiography | |||
| LV diastolic diameter, mm | 42 ± 7 | 44 ± 7 | <0.01 |
| LV systolic diameter, mm | 28 ± 6 | 29 ± 7 | 0.019 |
| LV ejection fraction, % | 60 ± 11 | 60 ± 12 | 0.86 |
| Left atrial diameter, mm | 45 ± 9 | 42 ± 7 | <0.01 |
| Pulmonary artery systolic pressure, mmHg a /5228 | 39 ± 14 | 32 ± 12 | <0.01 |
| AV max velocity, m/s | 4.9 ± 0.88 | 4.5 ± 0.80 | <0.01 |
| AV mean pressure gradient, mmHg | 57 ± 20 | 49 ± 18 | <0.01 |
| AV area, cm2 | 0.55 ± 0.17 | 0.64 ± 0.19 | <0.01 |
| Aortic regurgitation | 21 (20) | 609 (9.3) | <0.01 |
| Mitral regurgitation | 20 (19) | 711 (11) | <0.01 |
| Tricuspid regurgitation | 15 (14) | 524 (8.0) | 0.021 |
| TAVI procedure | |||
| Valve type | 0.021 | ||
| Sapien XT | 13 (12) | 1246 (19) | |
| Sapien 3 | 53 (50) | 3799 (58) | |
| Corevalve | 6 (5.7) | 176 (2.7) | |
| Evolute R | 22 (21) | 890 (14) | |
| Evolute Pro | 12 (11) | 457 (7.0) | |
| No valve | 2 (0.03) | ||
| Valve size | 0.21 | ||
| 20 mm | 7 (6.6) | 265 (3.9) | |
| 23 mm | 56 (53) | 3087 (45) | |
| 26 mm | 32 (30) | 2584 (38) | |
| 29 mm | 11 (10) | 949 (14) | |
| Access | 0.95 | ||
| Transfemoral | 96 (91) | 6003 (91) | |
| Transapical | 6 (5.7) | 370 (5.6) | |
| Transsubclavian | 3 (2.8) | 108 (1.6) | |
| Direct aorta | 1 (0.9) | 49 (0.8) | |
| Other | 0 (0) | 40 (0.6) | |
Note: Data shown are mean ± SD or n (%).
Abbreviations: AV, aortic valve; CFS, Clinical Frailty Scale; LV, left ventricle; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons; TAVI, transcatheter aortic valve implantation.
The number of available data is expressed for those variables in which some data were missing.
Mitral valve assessment
Among the 106 patients with combined AS and MS, calcific MS was observed in 80 (77%) whereas rheumatic MS was present in 21 (20%). The underlying mechanism was not identified in five patients (3%). MAC and AML calcifications were identified in 89 (84%) and 56 (53%) patients, respectively. The mean TMG, heart rate and MVA were 6.4 ± 2.6 mmHg, 73 ± 15 bpm and 1.10 ± 0.31 cm2, respectively (MVA data were unavailable in nine patients).
The distribution of MAC severity and haemodynamic profiles of MS relative to MAC severity are shown in Figure 1A. High, medium and low TMG levels were observed in 11 (11%), 64 (60%) and 31 (29%) patients, respectively (Figure 1B). Moderate or severe MAC was observed in 9 (82%), 45 (70%) and 15 (48%) patients, respectively. AML calcification was identified in 8 (73%), 36 (56%) and 12 (39%), in high, medium and low TMG categories, respectively. A higher heart rate was associated with higher TMG levels.
Figure 1.
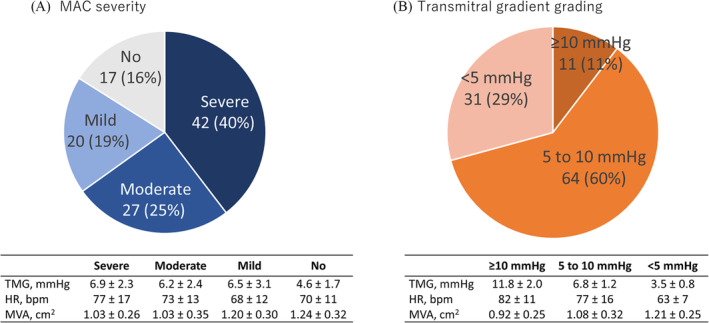
MAC severity and haemodynamics in patients with mitral stenosis Prevalence of MAC severity and mitral valve haemodynamics according to MAC severity (A). Prevalence of transmitral gradient and haemodynamics (B). MAC, mitral annular calcification.
Functional class
The New York Heart Association (NYHA) class III or IV was noted in 59 (56%) patients with MS before TAVI, which was significantly higher than the 40% observed in the control group (P < 0.01). After TAVI, 94 patients (88%) with MS had NYHA class I or II at discharge and 78 (74%) maintained NYHA class I or II 1 year after TAVI (Figure 2).
Figure 2.
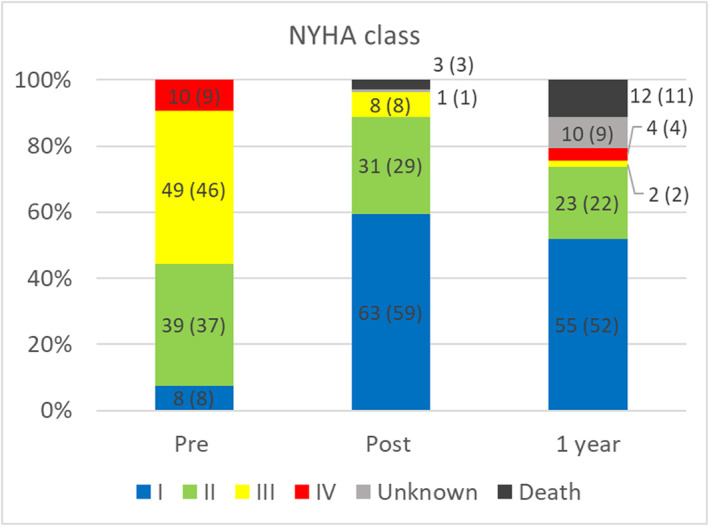
NYHA functional class. NYHA, New York Heart Association.
Short‐term outcomes
The short‐term outcomes are presented in Table 2. One patient underwent simultaneous percutaneous transvenous mitral commissurotomy (PTMC) at the time of TAVI. Thirty‐day mortality was reported in three patients, each of whom died of heart failure, cerebral haemorrhage and intestinal obstruction. Thirty‐day mortality and early composite outcomes were comparable between patients with and without MS. There were no significant differences in complications, such as myocardial infarction, stroke and bleeding. However, postoperative AR due to paravalvular leakage was significantly more frequent in patients with MS than in controls (6.7 vs. 1.9%, P < 0.01). Other than the presence of MS, Clinical Frailty Scale (CFS) was associated with early composite outcomes (HR 1.20 per 1 grade; 95% CI 1.14–1.26, P < 0.01).
Table 2.
Short‐term outcomes.
| Concurrent mitral stenosis (n = 106) | Controls (n = 6570) | P | |
|---|---|---|---|
| All‐cause mortality (30 day) | 3 (2.8) | 87 (1.3) | 0.18 |
| Early safety endpoint | 18 (17) | 979 (15) | 0.56 |
| Myocardial infarction | 1 (0.9) | 39 (0.6) | 0.64 |
| Stroke | 5 (4.7) | 159 (2.4) | 0.13 |
| All bleeding | 16 (15) | 926 (14) | 0.77 |
| Life threatening | 4 (3.8) | 189 (2.9) | 0.58 |
| Major | 7 (6.6) | 455 (6.9) | 0.90 |
| Minor | 6 (5.7) | 341 (5.2) | 0.83 |
| All vascular complication | 5 (4.7) | 474 (7.2) | 0.32 |
| Major | 4 (3.8) | 215 (3.3) | 0.77 |
| Minor | 1 (0.9) | 268 (4.1) | 0.10 |
| Acute kidney injury | 10 (9.4) | 549 (8.3) | 0.70 |
| New pacemaker implantation | 12 (11) | 527 (8.0) | 0.22 |
| New onset atrial fibrillation | 3 (3.0) | 193 (3.0) | 0.98 |
| Valve‐related dysfunction | 7 (6.7) | 369 (5.7) | 0.68 |
| Aortic regurgitation | 7 (6.7) | 126 (1.9) | <0.01 |
Note: Data shown are mean ± SD or n (%).
Long‐term outcomes
During a median follow up of 2.1 years (range: 1.2–3.4 years), 29 patients (27%) with MS and 1568 (24%) without MS died. Among these, cardiovascular deaths accounted for 13 (12%) in the MS group and 505 (8%) in the control group while 20 (19%) and 620 (9.4%) patients were admitted for heart failure, respectively. The primary endpoint (all‐cause death or hospitalization for heart failure) occurred in 41 (39%) patients with MS and 1799 (27%) without MS. Mitral valve intervention was not performed during the follow‐up in patients with MS.
Patients with MS showed significantly lower event‐free survival than the controls (HR 1.83; 95% CI 1.34–2.50, P < 0.01) (Figure 3A). As outlined in Table 3, MS was independently associated with a higher incidence of all‐cause death or hospitalization for heart failure (adjusted HR 1.84; 95% CI 1.34–2.51, P < 0.01), independent of age, sex, NYHA class, the Society of Thoracic Surgeons (STS) Mortality Score, CFS, diabetes, atrial fibrillation, coronary artery diseases, chronic kidney diseases, left ventricular ejection fraction, aortic valve max velocity, MR, TR and elevated pulmonary artery systolic pressure.
Figure 3.
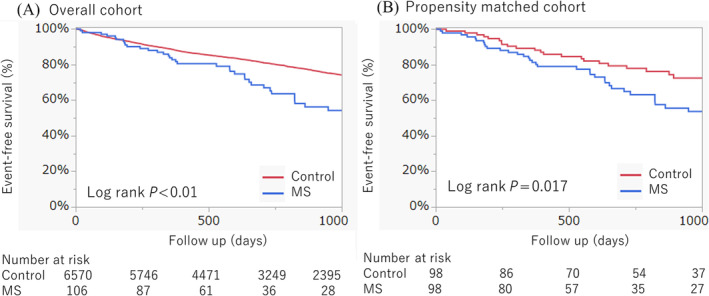
Clinical outcomes. Event‐free survival curves are described in the overall cohort (A) and propensity‐matched cohort (B). MS, mitral stenosis.
Table 3.
Variables associated with mortality or heart failure hospitalization.
| Hazard ratio | 95% CI | P | |
|---|---|---|---|
| Univariate | |||
| Age, per 1 year | 1.03 | 1.02–1.04 | <0.01 |
| Men | 1.41 | 1.28–1.54 | <0.01 |
| NYHA III or IV | 1.54 | 1.40–1.68 | <0.01 |
| STS, per 1 | 1.05 | 1.04–1.05 | <0.01 |
| CFS, per 1 grade | 1.26 | 1.22–1.31 | <0.01 |
| Smoking history | 1.42 | 1.11–1.83 | <0.01 |
| Atrial fibrillation | 1.75 | 1.58–1.93 | <0.01 |
| Hypertension | 1.03 | 0.91–1.17 | 0.64 |
| Dyslipidaemia | 0.67 | 0.62–0.74 | <0.01 |
| Diabetes | 1.20 | 1.08–1.32 | <0.01 |
| Coronary artery diseases | 1.11 | 1.01–1.22 | 0.031 |
| Peripheral artery diseases | 1.48 | 1.30–1.69 | <0.01 |
| Chronic kidney disease | 1.42 | 1.28–1.57 | <0.01 |
| COPD | 1.36 | 1.19–1.55 | <0.01 |
| LV diastolic diameter, per 1 mm | 1.00 | 0.99–1.01 | 0.63 |
| LV systolic diameter, per 1 mm | 1.01 | 1.01–1.02 | <0.01 |
| LV ejection fraction, per 1% | 0.99 | 0.99–0.99 | <0.01 |
| Left atrial diameter, per 1 mm | 1.02 | 1.01–1.02 | <0.01 |
| Pulmonary artery systolic pressure, ≥60 mmHg | 1.92 | 1.49–2.48 | <0.01 |
| AV max velocity, per 1 m/s | 0.77 | 0.73–0.82 | <0.01 |
| AV mean pressure gradient, per 1 mmHg | 0.99 | 0.99–0.99 | <0.01 |
| Aortic valve area, per 1 cm2 | 1.00 | 0.78–1.29 | 0.98 |
| Aortic regurgitation | 1.03 | 0.88–1.20 | 0.72 |
| Mitral stenosis | 1.83 | 1.34–2.50 | <0.01 |
| Mitral regurgitation | 1.37 | 1.19–1.57 | <0.01 |
| Tricuspid regurgitation | 1.71 | 1.47–1.99 | <0.01 |
| Multivariable | |||
| Age, per 1 year | 1.02 | 1.01–1.03 | <0.01 |
| Male | 1.56 | 1.41–1.72 | <0.01 |
| NYHA III or IV | 1.22 | 1.11–1.35 | <0.01 |
| STS, per 1 | 1.03 | 1.02–1.04 | <0.01 |
| CFS, per 1 grade | 1.20 | 1.15–1.24 | <0.01 |
| Diabetes | 1.10 | 0.99–1.22 | 0.071 |
| Atrial fibrillation | 1.39 | 1.25–1.55 | <0.01 |
| Coronary artery diseases | 0.97 | 0.88–1.07 | 0.53 |
| Chronic kidney diseases | 1.17 | 1.05–1.30 | <0.01 |
| LV ejection fraction, per 1% | 1.00 | 1.00–1.01 | 0.079 |
| AV max velocity, per 1 m/s | 0.81 | 0.77–0.87 | <0.01 |
| Mitral stenosis | 1.84 | 1.34–2.51 | <0.01 |
| Mitral regurgitation | 0.95 | 0.82–1.10 | 0.51 |
| Tricuspid regurgitation | 1.26 | 1.07–1.48 | <0.01 |
| Pulmonary artery systolic pressure, ≥60 mmHg | 1.35 | 1.03–1.78 | 0.028 |
Abbreviations: CI: confidence interval. Other abbreviations as in Table 1.
Propensity matched analysis
Of the 106 patients with MS, 98 were successfully matched to 98 controls without MS. The c‐statistics for this matching model was 0.81. The characteristics of the patients with and those without MS are detailed in Table S1. In line with the overall analysis, patients with MS demonstrated significantly lower event‐free survival than the controls in the propensity‐matched analysis (HR 1.80; 95% CI 1.10–2.95, P = 0.019; Figure 3B). Furthermore, multivariate analysis revealed that the incidence of all‐cause death or hospitalization for heart failure was significantly higher in patients with MS than in those without MS, adjusted for similar variables with Table 3 (adjusted HR 1.91; 95% CI 1.12–3.26, P < 0.01; Table S2).
Risk stratification of MAC grading, TMG and MVA
Analysis of outcomes based on MAC severity among patients with MS revealed that a moderate or severe MAC was significantly associated with reduced event‐free survival, (HR 2.34; 95% CI 1.11–4.91, P = 0.025; Figure 4A). However, calcification of the AML did not significantly affect event‐free survival (HR 1.27; 95% CI 0.68–2.37, P = 0.45). Additionally, when analysing outcomes according to the TMG grading, no significant association was observed between TMG and event‐free survival (Figure 4B). Multivariate analysis further confirmed that a moderate or severe MAC significantly predicted lower event‐free survival (adjusted HR 2.89; 95% CI 1.20–6.99, P = 0.018), regardless of factors such as age, sex, NYHA class, atrial fibrillation, peripheral artery disease, chronic kidney disease, aortic valve area, TMG and MR (Table S3). When comparing outcomes between MVA >1.0 cm2 and ≤1.0 cm2, MVA ≤ 1.0 cm2 was not associated with lower event‐free survival in the univariate (HR 1.76; 95% CI 0.89–3.47, P = 0.099; Figure 4C) and multivariate (adjusted HR 1.73; 95% CI 0.83–3.63, P = 0.15) analyses.
Figure 4.
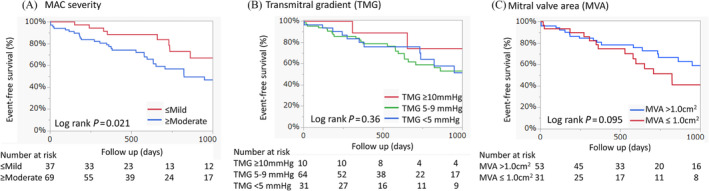
Risk stratification in patients with mitral stenosis. The event‐free survival curves are described according to MAC severity (A), transmitral gradient grading (B) and mitral valve area (C). MAC, mitral annulus calcification.
In the subgroup of nine patients with high TMG (excluding two from the initial 11 due to one receiving PTMC, another dying immediately after TAVI, and three lacking 1 year TTE records), changes in TMG post‐TAVI were analysed and are presented in Figure 5. TMG decreased in four patients post‐TAVI, remained unchanged in two, and increased in three compared with baseline TTE measurements. At 1 year, TMG decreased in three patients, remained unchanged in two, and increased in one, relative to baseline TTE data.
Figure 5.
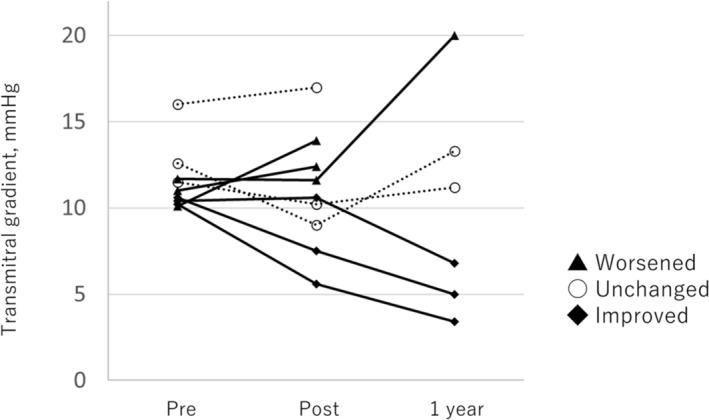
Change in transmitral gradient in patients with high transmitral gradient. TMG shows a decrease in three, no changes in two, and an increase in one at 1 year compared with TTE at baseline. Three patients show no TTE records at 1 year. TMG, transmitral gradient; TTE, transthoracic echocardiography.
Discussion
In this study, concomitant MS was associated with adverse events compared with the absence of MS in patients who underwent TAVI for AS, even after adjusting for patient characteristics including multiple comorbidities. Among patients with combined AS and MS, calcific MS was present in 80% and moderate or severe MAC contributed to increased adverse events whereas TMG and MVA did not affect the outcomes.
Several studies on patients who underwent TAVI for combined AS and MS reported poorer clinical outcomes in those with concurrent MS, concordant with our study findings. 10 , 11 , 16 , 18 The largest sample study of approximately 45 000 patients who underwent TAVI from the STS/American College of Cardiology Registry revealed that severe MS, defined by an MVA ≤ 1.5 cm2, was present in 2.7% (age 82.0 ± 8.5 years, men 36%) and associated with higher adverse events, including all‐cause mortality, stroke, hospitalization for heart failure and mitral valve intervention. 10 Severe MAC involving more than half of the mitral annulus circumference was present in 9.5% (age 82.5 ± 9.0 years, men 39%) and was an independent predictor of overall mortality among patients who underwent TAVI. 2 The issues associated with combined AS and MS are complicated because of the haemodynamics of combined valvular diseases and the multiple comorbidities symbolized by the presence of MAC. Our study successfully assessed the simultaneous impact of these problems on clinical outcomes. MAC severity provided better risk stratification compared with MS haemodynamics. Patients with combined AS and MS experienced severe symptoms more frequently than those with AS alone. Despite the poorer clinical outcomes, TAVI showed sustained improvement in symptoms after 1 year. Our results indicate that high‐risk patients with AS and MS who experience severe symptoms benefit from TAVI.
The management of combined AS and MS is often discussed in clinical settings; untreated MS at the time of AVR for AS is associated with adverse events while standard surgical MVR for calcific MS is challenging because of the increased risk of atrioventricular groove disruption, potentially caused by annular decalcification for proper prosthesis implantation. 6 Mitral valve surgery for mitral valve diseases with MAC has been selectively performed in low‐risk individuals. 19 Given that double‐valve surgery demonstrates a relatively high in‐hospital mortality rate (5.1%–12.5%) regardless of the MAC presence, 20 , 21 , 22 the presence of severe MAC further exacerbates the risk, potentially leading to even higher mortality rates. Surgical decalcification of severe MAC, as an alternative to MVR, may offer a safer approach to mitigate MS, although only a limited number of cases have been reported, with long‐term outcomes yet to be determined. 23 , 24 Considering the typically slow progression of calcific MS3 and the insights discussed previously, TAVI may represent a suitable treatment option for high‐risk patients with combined AS and MS.
A recent focus has been placed on the use of TMG for risk stratification in patients with MAC and related mitral valve dysfunction because of the association between higher TMG and increased mortality. 25 However, TMG is influenced by haemodynamic factors such as heart rate and stroke volume. 26 In particular, a higher heart rate may have led to an elevated TMG in our study population. The coexistence of AS and MS results in interdependent haemodynamic effects between the two valves. Given that approximately half of the patients with severe AS and a TMG ≥ 4 mmHg experienced a decrease in TMG of ≥1 mmHg following SAVR or TAVI, as reported previously, 16 TAVI may enhance mitral valve haemodynamics in certain patients in addition to its effects on the aortic valve. Our data also showed that post‐TAVI outcomes were similar in patients with high‐ and low‐gradient MS. MAC severity proved to be a reliable and useful parameter for risk stratification in patients with combined AS and MS undergoing TAVI. Although severe MAC does not always lead to significant obstruction of the mitral valve, it can limit the expansion of the mitral annulus during diastole, potentially leading to inflow restriction, 27 which could contribute to poor outcomes following TAVI. While AML calcification did not correlate with outcomes in our patient cohort with combined AS and MS, calcification extending to the subaortic curtain could have led to significant paravalvular leakage after TAVI. 28
Clinical implication
Our study revealed that TAVI could be safely performed in selected high‐risk patients with combined AS and MS, and it improved their symptoms. In these patients with severe symptoms, TAVI remains a feasible treatment option, even in those with an elevated TMG or decreased MVA. However, moderate or severe MAC has been linked to worse outcomes of TAVI in those patients. Thus, our study underscores the importance of assessing mitral valve calcifications for effective risk stratification in MS. Observing the extent of MAC is crucial as it serves as a significant risk factor for poorer TAVI outcomes in patients with combined AS and MS.
Limitations
Most patients with combined AS and MS were at high surgical risk in this study. It has not been determined whether TAVI alone is a better treatment option compared with surgical treatment in patients at low to intermediate surgical risk. The haemodynamic parameters of MS, including MVA and TMG, and the severity of MAC were not systematically measured in the control group. The diagnosis of MS was subject to variability among echocardiography laboratories at participating institutions. Although this was a multicentre study with a considerable sample size, it was not sufficiently powered to specifically assess the clinical outcomes in patients with high TMG or small MVA. Patients with atrial fibrillation were included and their measurements were averaged, which could have affected the accuracy of the data. Additionally, not all patients with MS underwent follow‐up TTE, limiting the assessment of long‐term valve function and disease progression.
In conclusion, although TAVI demonstrated favourable short‐term outcomes in patients with combined AS and MS and it improved their symptoms at 1 year post‐TAVI, concurrent MS was independently associated with adverse events following TAVI during the follow‐up period. Additionally, MAC severity contributed to increased adverse events regardless of MS haemodynamics among TAVI patients with combined AS and MS. TAVI is a viable treatment option for carefully selected high‐risk patients who present with symptoms of combined AS and MS, although further measures may be necessary for cases with severe MAC.
Conflict of interest
Dr. Tabata, Dr. Naganuma and Dr. Ueno are clinical proctors for Edwards Lifesciences and Medtronic. Dr. Yamamoto, Dr. Shirai, Dr. Tada, Dr. Watanabe, and Dr. Hayashida are clinical proctors for Edwards Lifesciences, Abbott Medical and Medtronic. Dr. Yashima, Dr. Ohno, Dr. Nishina and Dr. Asami are clinical proctors for Medtronic. Dr. Izumo is a screening proctor for Edwards Lifesciences. The remaining authors have nothing to disclose.
Funding
The OCEAN‐TAVI registry is supported by Edwards Lifesciences, Medtronic, Boston Scientific, Abbott and Daiichi Sankyo.
Supporting information
Table S1. Patient Characteristics in Propensity Matched Cohort.
Table S2. Variables Associated with Mortality or Heart Failure Hospitalization in Propensity Matched Cohort.
Table S3. Variables Associated with Mortality or Heart Failure Hospitalization in Patients with Mitral Stenosis.
Kato, N. , Tabata, M. , Noguchi, M. , Ito, J. , Obunai, K. , Watanabe, H. , Yashima, F. , Shirai, S. , Tada, N. , Naganuma, T. , Yamawaki, M. , Yamanaka, F. , Ueno, H. , Ohno, Y. , Izumo, M. , Nishina, H. , Asami, M. , Watanabe, Y. , Yamamoto, M. , Otsuka, T. , Hayashida, K. , and the OCEAN‐TAVI investigators (2024) Transcatheter aortic valve implantation for combined aortic and mitral stenoses: Insights from the OCEAN‐TAVI Registry. ESC Heart Failure, 11: 4257–4266. 10.1002/ehf2.15030.
References
- 1. Kato N, Guerrero M, Padang R, Amadio JM, Eleid MF, Scott CG, et al. Prevalence and natural history of mitral annulus calcification and related valve dysfunction. Mayo Clin Proc 2022;97:1094‐1107. doi: 10.1016/j.mayocp.2021.12.015 [DOI] [PubMed] [Google Scholar]
- 2. Abramowitz Y, Kazuno Y, Chakravarty T, Kawamori H, Maeno Y, Anderson D, et al. Concomitant mitral annular calcification and severe aortic stenosis: prevalence, characteristics and outcome following transcatheter aortic valve replacement. Eur Heart J 2017;38:1194‐1203. doi: 10.1093/eurheartj/ehw594 [DOI] [PubMed] [Google Scholar]
- 3. Kato N, Padang R, Scott CG, Guerrero M, Pislaru SV, Pellikka PA. The natural history of severe calcific mitral stenosis. J Am Coll Cardiol 2020;75:3048‐3057. doi: 10.1016/j.jacc.2020.04.049 [DOI] [PubMed] [Google Scholar]
- 4. Muddassir SM, Pressman GS. Mitral annular calcification as a cause of mitral valve gradients. Int J Cardiol 2007;123:58‐62. doi: 10.1016/j.ijcard.2006.11.142 [DOI] [PubMed] [Google Scholar]
- 5. Fox CS, Vasan RS, Parise H, Levy D, O'Donnell CJ, D'Agostino RB, et al. Mitral annular calcification predicts cardiovascular morbidity and mortality: the Framingham Heart Study. Circulation 2003;107:1492‐1496. doi: 10.1161/01.cir.0000058168.26163.bc [DOI] [PubMed] [Google Scholar]
- 6. Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR. Mitral annulus calcification. J Am Coll Cardiol 2015;66:1934‐1941. doi: 10.1016/j.jacc.2015.08.872 [DOI] [PubMed] [Google Scholar]
- 7. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation 2021;143:e72‐e227. doi: 10.1161/cir.0000000000000923 [DOI] [PubMed] [Google Scholar]
- 8. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. EuroIntervention 2022;17:e1126‐e1196. doi: 10.4244/EIJ-E-21-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eleid MF, Foley TA, Said SM, Pislaru SV, Rihal CS. Severe mitral annular calcification: multimodality imaging for therapeutic strategies and interventions. JACC Cardiovasc Imaging 2016;9:1318‐1337. doi: 10.1016/j.jcmg.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 10. Joseph L, Bashir M, Xiang Q, Yerokun BA, Matsouaka RA, Vemulapalli S, et al. Prevalence and outcomes of mitral stenosis in patients undergoing transcatheter aortic valve replacement: findings from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapies Registry. JACC Cardiovasc Interv 2018;11:693‐702. doi: 10.1016/j.jcin.2018.01.245 [DOI] [PubMed] [Google Scholar]
- 11. Asami M, Windecker S, Praz F, Lanz J, Hunziker L, Rothenbuhler M, et al. Transcatheter aortic valve replacement in patients with concomitant mitral stenosis. Eur Heart J 2019;40:1342‐1351. doi: 10.1093/eurheartj/ehy834 [DOI] [PubMed] [Google Scholar]
- 12. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1‐39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 13. Zoghbi WA, Adams D, Bonow RO, Enriquez‐Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303‐371. doi: 10.1016/j.echo.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 14. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr 2009;10:1‐25. doi: 10.1093/ejechocard/jen303 [DOI] [PubMed] [Google Scholar]
- 15. Sud K, Agarwal S, Parashar A, Raza MQ, Patel K, Min D, et al. Degenerative mitral stenosis: unmet need for percutaneous interventions. Circulation 2016;133:1594‐1604. doi: 10.1161/CIRCULATIONAHA.115.020185 [DOI] [PubMed] [Google Scholar]
- 16. Kato N, Padang R, Pislaru C, Miranda WR, Hoshina M, Shibayama K, et al. Hemodynamics and prognostic impact of concomitant mitral stenosis in patients undergoing surgical or transcatheter aortic valve replacement for aortic stenosis. Circulation 2019;140:1251‐1260. doi: 10.1161/CIRCULATIONAHA.119.040679 [DOI] [PubMed] [Google Scholar]
- 17. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document. J Thorac Cardiovasc Surg 2013;145:6‐23. doi: 10.1016/j.jtcvs.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 18. Mesnier J, Urena M, Chong‐Nguyen C, Fischer Q, Kikoïne J, Carrasco JL, et al. Impact of mitral annular calcium and mitral stenosis on outcomes after transcatheter aortic valve implantation. Am J Cardiol 2021;155:103‐112. doi: 10.1016/j.amjcard.2021.06.017 [DOI] [PubMed] [Google Scholar]
- 19. Kato N, Pellikka PA, Scott CG, Lee AT, Jain V, Eleid MF, et al. Impact of mitral intervention on outcomes of patients with mitral valve dysfunction and annulus calcification. Catheter Cardiovasc Interv 2022;99:1807‐1816. doi: 10.1002/ccd.30093 [DOI] [PubMed] [Google Scholar]
- 20. Galloway AC, Grossi EA, Baumann FG, LaMendola CL, Crooke GA, Harris LJ, et al. Multiple valve operation for advanced valvular heart disease: results and risk factors in 513 patients. J Am Coll Cardiol 1992;19:725‐732. doi: 10.1016/0735-1097(92)90509-l [DOI] [PubMed] [Google Scholar]
- 21. Litmathe J, Boeken U, Kurt M, Feindt P, Gams E. Predictive risk factors in double‐valve replacement (AVR and MVR) compared to isolated aortic valve replacement. Thorac Cardiovasc Surg 2006;54:459‐463. doi: 10.1055/s-2006-924247 [DOI] [PubMed] [Google Scholar]
- 22. Kreibich M, Kaier K, von Zur MC, Siepe M, Zehender M, Bode C, et al. In‐hospital outcomes of patients undergoing concomitant aortic and mitral valve replacement in Germany. Interact Cardiovasc Thorac Surg 2022;34:349‐353. doi: 10.1093/icvts/ivab352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Numaguchi R, Takaki J, Nishigawa K, Yoshinaga T, Fukui T. Outcomes of mitral valve replacement with complete annular decalcification. Asian Cardiovasc Thorac Ann 2023;31:775‐780. doi: 10.1177/02184923231206237 [DOI] [PubMed] [Google Scholar]
- 24. Brescia AA, Rosenbloom LM, Watt TMF, Bergquist CS, Williams AM, Murray SL, et al. Ultrasonic emulsification of severe mitral annular calcification during mitral valve replacement. Ann Thorac Surg 2022;113:2092‐2096. doi: 10.1016/j.athoracsur.2021.11.066 [DOI] [PubMed] [Google Scholar]
- 25. Bertrand PB, Churchill TW, Yucel E, Namasivayam M, Bernard S, Nagata Y, et al. Prognostic importance of the transmitral pressure gradient in mitral annular calcification with associated mitral valve dysfunction. Eur Heart J 2020;41:4321‐4328. doi: 10.1093/eurheartj/ehaa819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kato N, Pislaru SV, Padang R, Pislaru C, Scott CG, Nkomo VT, et al. A novel assessment using projected transmitral gradient improves diagnostic yield of Doppler hemodynamics in rheumatic and calcific mitral stenosis. JACC Cardiovasc Imaging 2021;14:559‐570. doi: 10.1016/j.jcmg.2020.12.013 [DOI] [PubMed] [Google Scholar]
- 27. Osterberger LE, Goldstein S, Khaja F, Lakier JB. Functional mitral stenosis in patients with massive mitral annular calcification. Circulation 1981;64:472‐476. doi: 10.1161/01.cir.64.3.472 [DOI] [PubMed] [Google Scholar]
- 28. Haensig M, Kuntze T, Gonzalez DL, Lapp H, Lauten P, Owais T. Extensive calcification of the mitral valve annulus in transcatheter aortic valve implants. Interact Cardiovasc Thorac Surg 2022;34:167‐175. doi: 10.1093/icvts/ivab235 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient Characteristics in Propensity Matched Cohort.
Table S2. Variables Associated with Mortality or Heart Failure Hospitalization in Propensity Matched Cohort.
Table S3. Variables Associated with Mortality or Heart Failure Hospitalization in Patients with Mitral Stenosis.


