Abstract
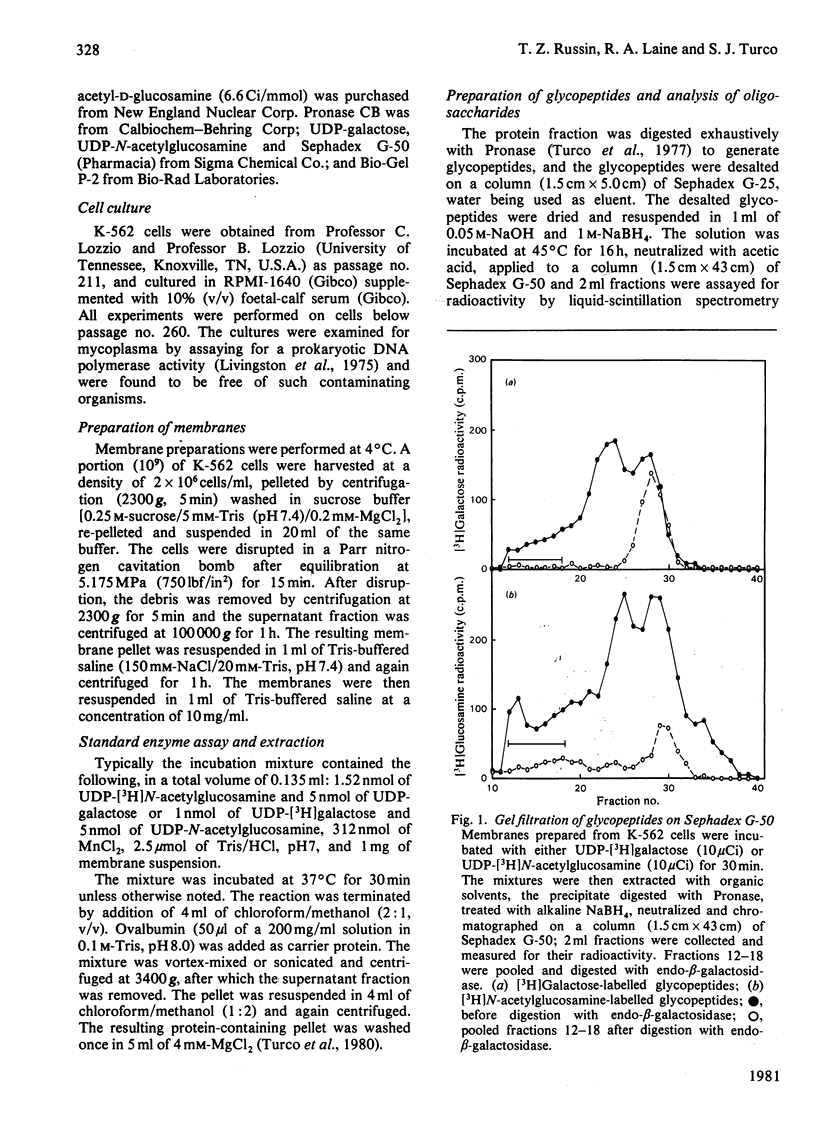
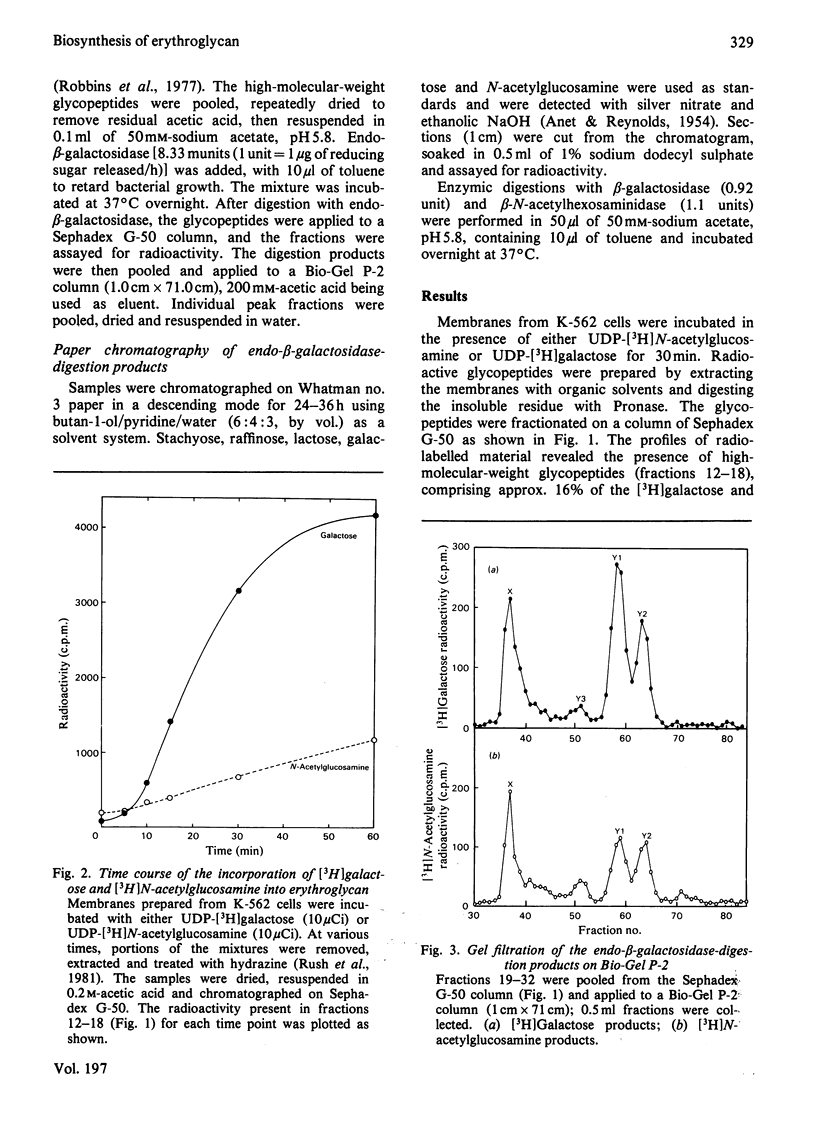
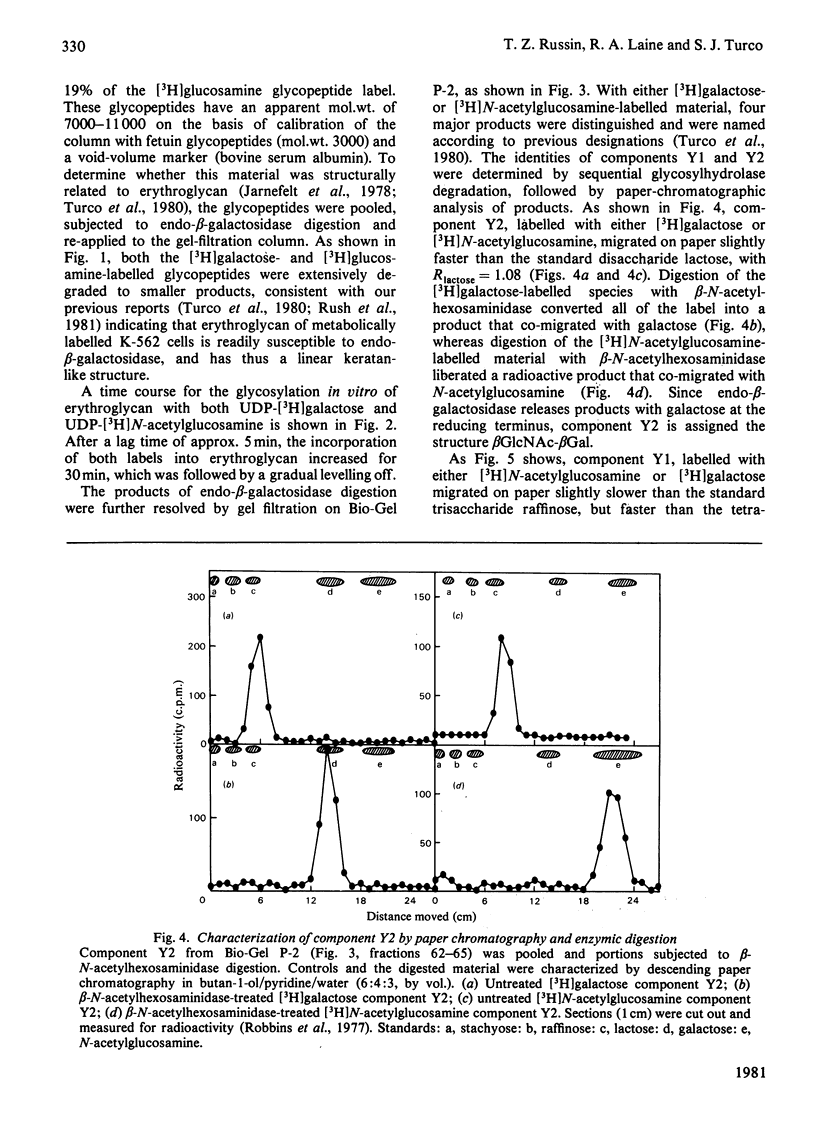
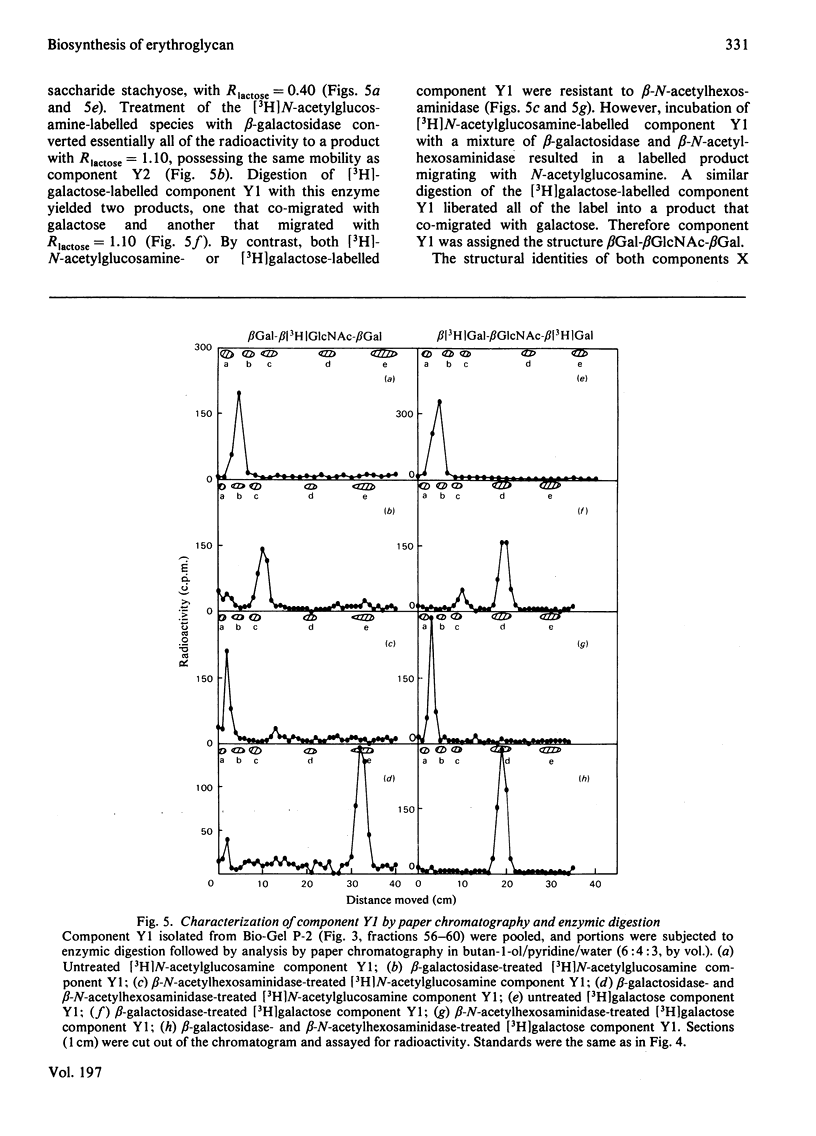
Particulate membrane preparations from K-562 [human CML (chronic-myelogenous-leukaemia)-derived] cells catalyse the transfer of [3H]galactose from UDP-[3H]-galactose and [3H]N-acetylglucosamine from UDP-[3H]N-acetylglucosamine into an endogenous product that on digestion with Pronase yields long-chain glycopeptides (mol.wt. 7000--10 000) called 'erythroglycan'. Incorporation of either labelled sugar increased up to 60 min of incubation time. The labelled erythroglycan was isolated by chromatography on Sephadex G-50 and characterized by digestion with endo-beta-galactosidase from Escherichia freundii, followed by analysis on Bio-Gel P-2 and paper chromatography. This digestion gave the following four products: (1) a disaccharide with the sequence beta GlcNAc-beta Gal; (2) a trisaccharide with the sequence betaGal-betaGlcNAc-beta Gal; (3) a larger oligosaccharide containing galactose and N-acetylglucosamine; and (4) a putative protein-linkage region.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHAPPELL J. B., GREVILLE G. D. Effect of silver ions on mitochondrial adenosine triphosphatase. Nature. 1954 Nov 13;174(4437):930–931. doi: 10.1038/174930b0. [DOI] [PubMed] [Google Scholar]
- Drickamer L. K. Orientation of the band 3 polypeptide from human erythrocyte membranes. Identification of NH2-terminal sequence and site of carbohydrate attachment. J Biol Chem. 1978 Oct 25;253(20):7242–7248. [PubMed] [Google Scholar]
- Fukuda M., Fukuda M. N., Hakomori S. Developmental change and genetic defect in the carbohydrate structure of band 3 glycoprotein of human erythrocyte membrane. J Biol Chem. 1979 May 25;254(10):3700–3703. [PubMed] [Google Scholar]
- Gahmberg C. G., Myllyla G., Leikola J., Pirkola A., Nordling S. Absence of the major sialoglycoprotein in the membrane of human En(a--) erythrocytes and increased glycosylation of band 3. J Biol Chem. 1976 Oct 10;251(19):6108–6116. [PubMed] [Google Scholar]
- Järnefelt J., Rush J., Li Y. T., Laine R. A. Erythroglycan, a high molecular weight glycopeptide with the repeating structure [galactosyl-(1 leads to 4)-2-deoxy-2-acetamido-glucosyl(1 leads to 3)] comprising more than one-third of the protein-bound carbohydrate of human erythrocyte stroma. J Biol Chem. 1978 Nov 25;253(22):8006–8009. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- Krusius T., Finne J., Rauvala H. The poly(glycosyl) chains of glycoproteins. Characterisation of a novel type of glycoprotein saccharides from human erythrocyte membrane. Eur J Biochem. 1978 Dec 1;92(1):289–300. doi: 10.1111/j.1432-1033.1978.tb12747.x. [DOI] [PubMed] [Google Scholar]
- Li E., Gibson R., Kornfeld S. Structure of an unusual complex-type oligosaccharide isolated from Chinese hamster ovary cells. Arch Biochem Biophys. 1980 Feb;199(2):393–399. doi: 10.1016/0003-9861(80)90295-7. [DOI] [PubMed] [Google Scholar]
- Livingston D. M., Hinkle D. C., Richardson C. C. Deoxyribonucleic acid polymerase III of Escherichia coli. Purification and properties. J Biol Chem. 1975 Jan 25;250(2):461–469. [PubMed] [Google Scholar]
- Parodi A. J., Leloir L. F. The role of lipid intermediates in the glycosylation of proteins in the eucaryotic cell. Biochim Biophys Acta. 1979 Apr 23;559(1):1–37. doi: 10.1016/0304-4157(79)90006-6. [DOI] [PubMed] [Google Scholar]
- Robbins P. W., Krag S. S., Liu T. Effects of UDP-glucose addition on the synthesis of mannosyl lipid-linked oligosaccharides by cell-free fibroblast preparations. J Biol Chem. 1977 Mar 10;252(5):1780–1785. [PubMed] [Google Scholar]
- Rush J. S., Turco S. J., Laine R. A. Erythroglycan biosynthesis in K-562 cells. Inhibition of synthesis by tunicamycin and lack of attachment to the G-protein of vesicular-stomatitis virus. Biochem J. 1981 Jan 1;193(1):361–365. doi: 10.1042/bj1930361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner M. J., Boxer D. H. Separation and some properties of the major proteins of the human erythrocyte membrane. Biochem J. 1972 Sep;129(2):333–347. doi: 10.1042/bj1290333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco S. J., Rush J. S., Laine R. A. Presence of erythroglycan on human K-562 chronic myelogenous leukemia-derived cells. J Biol Chem. 1980 Apr 25;255(8):3266–3269. [PubMed] [Google Scholar]
- Turco S. J., Stetson B., Robbins P. W. Comparative rates of transfer of lipid-linked oligosaccharides to endogenous glycoprotein acceptors in vitro. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4411–4414. doi: 10.1073/pnas.74.10.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]


