Abstract
Aims
Patients with obesity have an overall higher cardiovascular risk, at the same time obesity could be associated with a better outcome in a certain subgroup of patients, a phenomenon known as the obesity paradox. Data are scarce in candidates for cardiac resynchronization therapy (CRT). We aimed to investigate the association between body mass index (BMI) and all‐cause mortality in patients eligible for CRT.
Methods
Altogether 1,585 patients underwent cardiac resynchronization therapy between 2000–2020 and were categorized based on their BMI, 459 (29%) patients with normal weight (BMI < 25 kg/m2), 641 (40%) patients with overweight (BMI 25‐ < 30 kg/m2) and 485 (31%) with obesity (BMI ≥ 30 kg/m2). The primary endpoint was all‐cause mortality, heart transplantation, and left ventricular assist device implantation. We assessed periprocedural complications and 6‐month echocardiographic response.
Results
Normal‐weight patients were older compared to patients with overweight or obesity (70 years vs. 69 years vs. 68 years; P ‹0.001), respectively. Sex distribution, ischaemic aetiology, and CRT‐D implantation rates were similar in the three patient groups. Diabetes mellitus (BMI < 25 kg/m2 26% vs. BMI 25‐ < 30 kg/m2 37% vs. BMI ≥ 30 kg/m2 48%; P ‹0.001) and hypertension (BMI < 25 kg/m2 71% vs. BMI 25‐ < 30 kg/m2 74% vs. BMI ≥ 30 kg/m2 82%; P ‹0.001) were more frequent in patients with overweight and obesity.
During the mean follow‐up time of 5.1 years, 973 (61%) reached the primary endpoint, 66% in the BMI < 25 kg/m2 group, 61% in the BMI 25‐ < 30 kg/m2 group and 58% in the BMI ≥ 30 kg/m2 group (log‐rank P‹0.05). Patients with obesity showed mortality benefit over normal‐weight patients (HR 0.78; 95%CI 0.66–0.92; P = 0.003). The obesity paradox was present in patients free from diabetes, atrial fibrillation, and ischemic events. Periprocedural complication rates did not differ in the three groups (BMI < 25 kg/m2 25% vs. BMI 25‐ < 30 kg/m2 28% vs. BMI ≥ 30 kg/m2 26%; P = 0.48). Left ventricular ejection fraction improved significantly in all patient groups (BMI < 25 kg/m2 median ‐LVEF 7% vs. BMI 25‐ < 30 kg/m2 median ‐LVEF 7.5% vs. BMI ≥ 30 kg/m2 median ‐LVEF 6%; P < 0.0001) with a similar proportion of developing reverse remodeling (BMI < 25 kg/m2 58% vs. BMI 25‐ < 30 kg/m2 61% vs. BMI ≥ 30 kg/m2 57%; P = 0.48); P = 0.75).
Conclusions
The obesity paradox was present in our HF cohort at long‐term, patients underwent CRT implantation with obesity and free of comorbidities showed mortality benefit compared to normal weight patients. Patients with obesity showed similar echocardiographic response and safety outcomes compared to normal weight patients.
Keywords: obesity paradox, heart failure, mortality, cardiac resynchronization therapy
Introduction
Heart failure affects 1–2% of the general population, reaching up to 10% in the elderly. 1 Populations with obesity have a higher risk of acquiring heart failure (HF), 2 however, these patients tend to have more favourable outcomes, a phenomenon known as the obesity paradox. 3 , 4 Its pathophysiology remains unidentified as mainly hypotheses emerged, including obesity's protective factor against protein energy malnutrition and the activation of inflammatory cytokines. 5 At the end‐stage of heart failure, a catabolic state occurs often paired with malnutrition. 6 , 7 Other hypothesis mention changes in lipid metabolisms (higher cholesterol levels and lipoproteins), lower prothrombotic agents levels, an increased ghrelin production (as it may improve cardiac contractility) and elevated cytokine/adipokine production that may have cardioprotective properties. 8
Within the subset of heart failure patients, those with reduced ejection fraction (HFrEF) have the highest risk for adverse clinical outcomes, the rate of all‐cause mortality of HF can reach 50% in five years. 9 , 10 , 11 Cardiac resynchronization therapy (CRT) is known as an effective device treatment, which reduces mortality in a well‐selected HFrEF patient population. 12 , 13 The incidence of obesity in CRT candidates is high, approximately 36% have a body mass index (BMI) ≥ 30 kg/m2 defined as obese, whereas the mean BMI of the population ranges between 26.5–31.2 kg/m2. 12 , 13 , 14 A few data elucidated that obesity does not decrease the clinical benefit of CRT, 15 or may even have a positive effect on the outcome. 16 , 17 However, data are incomprehensive on the association between the optimal BMI range and such patients' survival benefit.
Our aim was to examine the association of obesity classified by BMI with all‐cause mortality, periprocedural complications, and echocardiographic response at long‐term in HF patients undergoing CRT.
Methods
Study population and categorization by body mass index
Altogether 2,656 HF patients underwent CRT implantation at the Semmelweis University, Heart and Vascular Center, between June 2000 and August 2020. Indication for CRT implantation was set up based on current ESC guidelines (symptomatic heart failure patients on optimal medical treatment, LVEF ‹35% and QRS›130 ms). 18 Patients' data were collected retrospectively into our registry „Biobankok” via hospital records. The registry consists of medical history, clinical and echocardiographic parameters, laboratory tests, and parameters of the procedures. Finally, 1,585 patients were enrolled in our study that had their weight and height available at baseline. To quantify obesity, we calculated their BMI as the ratio of weight in kilograms to the square of height in meters (BMI = kg/m2). Patients were then categorized into three patient groups based on the WHO classification, underweight and normal weight (further mentioned as normal weight group [BMI < 25 kg/m2]), patients with overweight (BMI 25.0 –<30 kg/m2) and patients with obesity (BMI ≥ 30 kg/m2). 19 , 20 Obese patients were additionally categorized into three obesity groups, also based on the WHO classification: obese I (BMI 30‐‹34.9 kg/m2), obese II (BMI ≥ 35‐‹40 kg/m2) and obese III (BMI ≥ 40 kg/m2). Survival analyses were also conducted without underweight patients, added as supplementary material.
The study complies with the Declaration of Helsinki and was approved by the Regional and Institutional Committee and Research; No. 161–0/2019.
Outcomes
The main outcomes were the composite endpoint of all‐cause mortality, heart transplantation, or left ventricular assist device (LVAD) implantation. The date of death was retrieved via the National Health Insurance Fund of Hungary, updated in December 2021. The mean follow‐up time was 5.1 years, calculated from the enrolment date into this observational study, that was defined as the date of the CRT implantation to the date of death, heart transplantation or LVAD implantation.
Secondary outcomes were periprocedural complications (bleeding, pneumothorax, haemothorax, coronary sinus dissection, pericardial tamponade, pocket infection/decubitus, infective endocarditis, lead dislodgement, lead dysfunction or phrenic nerve stimulation).
Tertiary outcomes were echocardiographic response and the development of reverse remodeling, defined as a relative increase of 15% or more in left ventricular ejection fraction (LVEF) within 6 months after CRT implantation.
Procedures
Device implantation was carried out under X‐ray, and leads were implanted through the cephalic or subclavian veins. Right ventricular leads were implanted preferably in a septal position. The optimal coronary sinus side branch was chosen by venogram routinely, left ventricular leads were preferably implanted into the lateral or posterolateral vein. Left ventricular lead implantations, if failed by the coronary sinus, were carried out by epicardial or transseptal approach. Electrical parameters such as sensing values, pacing threshold, impedance, RV‐LV activation delay were evaluated intraoperatively.
Statistical analysis
Continuous variables were described as median and interquartile range (25th–75th percentile), after Saphiro‐Wilk normality test and categorical variables as numbers and percentages. Patient characteristics were compared amongst categories using chi‐squared tests for categorical variables and the Mann–Whitney and the Kruskal‐Wallis tests for continuous variables, as appropriate. Kaplan–Meier estimates and log‐rank tests were used to evaluate unadjusted event‐free survival in each patient category. Cox multivariate regression analysis was used to assess the association between BMI status and outcomes after CRT implantation. Statistical analysis was performed using GraphPad Prism, version 8.4.2 (San Diego, CA, USA, GraphPad Software) and IBM SPSS Statistics, version 26 (Armonk, NY, USA, IBM Corp). A P‐value of less than 0.05 was considered statistically significant.
Results
Baseline clinical characteristics of the study cohort
Altogether 1,585 patients were included in our study, 459 (29%) patients were in the normal weight group (BMI < 25 kg/m2) of which 23 (5%) patients were underweight (BMI ≤ 18.5 kg/m2), 641 (40%) patients were in the overweight category (BMI 25‐ < 30 kg/m2) and 485 (31%) were patients with obesity (BMI ≥ 30 kg/m2). Patients with obesity were further categorized, of these 361 (74.4%) belonged to the obese I patient group (BMI 30‐‹34.9 kg/m2), 94 (19.4%) in the obese II group (BMI ≥ 35‐‹40 kg/m2) and 30 (6.2%) in the obese III group (BMI ≥ 40 kg/m2).
Normal‐weight patients were older compared to patients with overweight or obesity (70 years vs. 69 years vs. 68 years; p ‹0.001), respectively. Sex distribution, ischaemic aetiology, and CRT‐D implantation rates were similar in the three patient groups. Diabetes mellitus (DM) (normal weight 26% vs. patients with overweight 37% vs. patients with obesity 48%; p ‹0.001) and hypertension (normal weight 71% vs. patients with overweight 74% vs. patients with obesity 82%; p ‹0.001) were more frequent in patients with overweight and obesity.
Patients had a comparable renal function (normal weight eGFR 64 mL/min/1.73m2 vs. patients with overweight eGFR 63 mL/min/1.73m2% vs. patients with obesity eGFR 66 mL/min/1.73m2; P = 0.25) and similar N‐terminal pro‐B‐Type natriuretic peptide (NT‐proBNP) levels (normal weight 3,000 pmol/l vs. patients with overweight 2,498 pmol/l vs. patients with obesity 2,488 pmol/l; P = 0.21).
Regarding echocardiographic parameters, patients with overweight and obesity had significantly higher left ventricular ejection fractions (LVEF) (patients with obesity 30% vs. patients with overweight 28% vs. normal weight 27%; p ‹0.001).
Patients were on comparable medical treatment, but digoxin was used more in normal weight patients (normal weight 24% vs. patients with overweight 17% vs. patients with obesity 16%; P = 0.003), respectively and oral anticoagulant was used most in overweight patients (normal weight 27%, patients with overweight 33%; patients with obesity 27%; P = 0.03). (Table 1 .)
Table 1.
Baseline clinical characteristics of patients by BMI groups
| Baseline variables | All patients (n = 1,585) | BMI < 25 kg/m2 (n = 459) | BMI 25‐ < 30 kg/m2 (n = 641) | BMI ≥ 30 kg/m2 (n = 485) | p‐value |
|---|---|---|---|---|---|
| Age (yrs; median/IQR) | 69 (61–75) | 70 (62–76) | 69 (61–76) | 68 (60–73) | ‹0.001 |
| Sex (female; n; %) | 395 (25) | 144 (31) | 136 (21) | 115 (24) | 0.17 |
| NYHA III/IV (st; n; %) | 802 (50) | 238 (52) | 313 (49) | 251 (52) | 0.49 |
| Ischaemic aetiology (n; %) | 832 (52) | 232 (50) | 354 (55) | 246 (51) | 0.20 |
| CRT‐D (n; %) | 863 (54) | 238 (52) | 350 (55) | 275 (57) | 0.32 |
| BMI (kg/m2; median/IQR) | 27.4 (24.6–30.8) | 22.9 (21.1–24.2) | 27.4 (26.2–28.5) | 32.5 (31.0–35.1) | NA |
| QRS (ms; median/IQR) | 160 (140–180) | 160 (140–177) | 160 (140–178) | 160 (140–180) | 0.83 |
| Medical history | |||||
| Atrial Fibrillation (n; %) | 618 (39) | 162 (35) | 257 (40) | 199 (41) | 0.15 |
| Diabetes mellitus (n; %) | 594 (37) | 118 (26) | 241 (37) | 235 (48) | ‹0.001 |
| Hypertension (n; %) | 1,200 (76) | 328 (71) | 474 (74) | 400 (82) | ‹0.001 |
| Prior MI (n; %) | 654 (41) | 186 (40) | 276 (43) | 192 (39) | 0.47 |
| Prior PCI (n; %) | 520 (33) | 141 (31) | 228 (35) | 151 (31) | 0.15 |
| Prior CABG (n; %) | 226 (14) | 56 (12) | 100 (16) | 70 (14) | 0.28 |
| Prior COPD (n; %) | 250 (16) | 74 (16) | 88 (14) | 88 (18) | 0.13 |
| Laboratory parameters | |||||
| Serum urea (μmol/l; median/IQR) | 381 (310–469) | 409 (304–518) | 412 (333–491) | 406 (342–468) | 0.93 |
| Serum creatinine (μmol/l; median/IQR) | 110 (89–145) | 99 (79–127) | 102 (84–133) | 98 (82–128) | 0.09 |
| Serum cholesterol (mmol/l; median/IQR) | 4.3 (3.5–5.1) | 4.3 (3.4–5.1) | 4.2 (3.5–5.1) | 4.0 (3.3–5.0) | 0.27 |
| eGFR (ml/min/1.73m2; median/IQR) | 64 (48–81) | 64 (49–82) | 63 (47–78) | 66 (48–83) | 0.25 |
| NT‐proBNP (pmol/l; median/IQR) | 1,332 (509–3,365) | 3,000 (1,380–4,434) | 2,498 (1,573–3,434) | 2,488 (1,270–3,045) | 0.21 |
| Echocardiographic parameters | |||||
| LVEF (%; median/IQR) | 28 (24–33) | 27 (23–30) | 28 (24–33) | 30 (25–35) | ‹0.001 |
| LVEDV (ml; median/IQR) | 208 (154–271) | 198 (168–244) | 225 (159–225) | 205 (150–277) | 0.63 |
| LVESV (ml; median/IQR) | 153 (113–209) | 150 (117–207) | 167 (117–211) | 142 (99–212) | 0.51 |
| LVEDD (mm; median/IQR) | 63 (57–69) | 63 (57–68) | 63 (57–70) | 63 (57–70) | 0.30 |
| LVESD (mm; median/IQR) | 53 (47–60) | 53 (47–59) | 53 (46–60) | 53 (46–60) | 0.87 |
| Medical treatment | |||||
| Beta blocker (n; %) | 1,349 (85) | 404 (88) | 538 (84) | 407 (84) | 0.12 |
| ACE‐I/ARB (n; %) | 1,385 (87) | 403 (88) | 549 (86) | 433 (89) | 0.18 |
| MRA (n; %) | 1,000 (63) | 308 (67) | 393 (61) | 299 (62) | 0.11 |
| Furosemid (n; %) | 1,192 (75) | 362 (79) | 471 (73) | 359 (74) | 0.09 |
| Digoxin (n; %) | 296 (19) | 109 (24) | 110 (17) | 77 (16) | 0.003 |
| Amiodarone (n; %) | 401 (25) | 124 (27) | 164 (25) | 113 (23) | 0.41 |
| Oral anticoagulant therapy (n; %) | 469 (29) | 123 (27) | 213 (33) | 133 (27) | 0.03 |
ACE‐I, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blocker, BMI, body mass index; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; CRT‐D, cardiac resynchronization therapy defibrillator; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; LBBB, left bundle branch block; LVEDD, left ventricular end‐diastolic diameter; LVEDV, left ventricular end‐diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; LVESV, left ventricular end‐systolic volume; MI, myocardial infarction; MRA, mineralocorticoid receptor antagonists; NT‐proBNP, N‐Terminal pro‐B‐Type Natriuretic Peptide; NYHA, New York Heart Association class; PCI, percutaneous coronary intervention.
Outcomes
Main outcomes
During our mean follow‐up time of 5.1 years, 973 (61%) reached the primary endpoint, 302 (66%) in the BMI < 25 kg/m2 group, 389 (61%) in the BMI 25‐ < 30 kg/m2 group and 282 (58%) in the BMI ≥ 30 kg/m2 group (log‐rank p‹0.05). Absolute mortality rates were the lowest in the 35‐ < 40 kg/m2 BMI range, depicting a J‐shaped curve (Figure 1 .). Altogether 29 (2%) patients underwent orthotopic heart transplantation, 8 (2%) patients with normal weight, 16 (2%) patients with overweight and 5 (1%) patients with obesity. 4 (0.2%) patients reached the primary endpoint by the implantation of an LVAD, 1 (0.2%) patient with normal weight, 1 (0.1%) patient with overweight and 2 (0.4%) patients with obesity.
Figure 1.
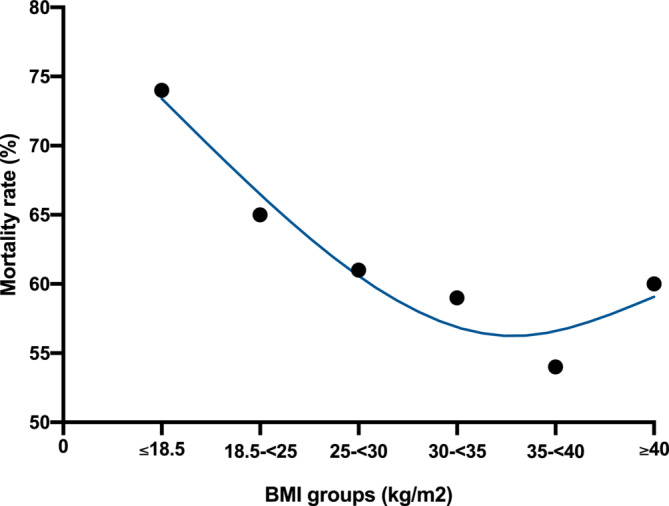
Cumulative rate of mortality by BMI groups.
Patients with obesity had a significantly lower risk of all‐cause mortality compared to normal‐weight patients (HR 0.78; 95% CI 0.66–0.92; P = 0.003) and patients with overweight showed a trend compared to normal‐weight patients (HR 0.86; 95% CI 0.74–1.00; P = 0.05). (Table 2 .) (Figure 2 .)
Table 2.
The associations of the BMI with the risk of all‐cause mortality
| Comparison of different BMI groups | |||
|---|---|---|---|
| Endpoint | All‐cause mortality | ||
| Hazard ratio | 95% CI | p‐value | |
| BMI 25‐ < 30 kg/m2 vs. BMI < 25 kg/m2 | 0.86 | 0.74–1.00 | 0.05 |
| BMI 25‐ < 30 kg/m2 vs. BMI ≥ 30 kg/m2 | 1.10 | 0.95–1.29 | 0.19 |
| BMI ≥ 30 kg/m2 vs. BMI < 25 kg/m2 | 0.78 | 0.66–0.92 | 0.003 |
BMI, body mass index; CI, confidence interval.
Figure 2.
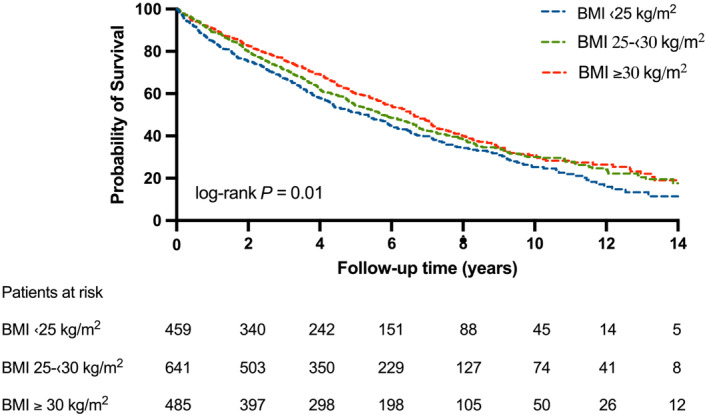
Kaplan‐Meier estimates of the probability of survival after CRT implantation by BMI groups. Patients with overweight and obesity show survival benefit compared to patients with normal weight at long‐term (log‐rank P‹0.01).
Patients in the obese II group were more likely to survive than patients in the obese III group (HR 0.51; 95% CI 0.26–1.00; P = 0.017). Survival in other patient groups did not differ significantly [obese I vs. obese II (HR 1.26; 95%CI 0.90–1.75; P = 0.20) and obese I vs. obese III (HR 0.66; 95% CI 0.37–1.20; P = 0.10)].
When underweight patients were excluded from the studied patients, statistical results did not differ notably, the risk of the primary endpoint in patients with a BMI 18.5‐‹25 kg/m2 vs. overweight patients was similar (HR 0.87; 95% CI 0.75–1.02; P = 0.08) and was significantly greater compared to obese patients (HR 0.79; 95% CI 0.67–0.94; P = 0.006). (Supplementary Table S1 and Figure S3).
At multivariate analysis, patients with a BMI ‹25 kg/m2 showed a 25% higher risk of all‐cause mortality compared to patients with overweight and obesity (HR 1.25; 95% CI 1.06–1.47; P = 0.006) after adjusting for age, sex, NYHA class, diabetes, hypertension, myocardial infarction, and atrial fibrillation.
Subgroup analyses
Patients with a BMI ≥ 25 kg/m2 show survival benefit over patients with a BMI of ‹25 kg/m2 in non‐ischaemic patients (HR 0.67; 95% CI 0.53–0.84; p ‹0.001), obesity did not protect patients with ischaemic aetiology (HR 0.94; 95% CI 0.78–1.13; P = 0.51). (Figure S1 .) Non‐diabetic patients with overweight or obesity show the best probability of survival, the lowest survival was seen in diabetic patients with a BMI ‹25 kg/m2 (p ‹0.001). Diabetic patients did not show the obesity paradox (HR 0.85; 95% CI 0.66–1.10; P = 0.20). (Figure S2 .) Both male (HR 0.87; 95% CI 0.70–0.97; P = 0.02) and female (HR 0.72; 95%CI 0.54.0–97; P = 0.02) patients experienced the obesity paradox. We did not observe survival gain in patients with overweight or obesity with atrial fibrillation (HR 0.89; 95% CI 0.77–1.11; P = 0.30). Patients with overweight or obesity had the lowest risk of all‐cause mortality with a CRT‐D device, the highest was in normal‐weighed patients with a CRT‐P device (P = 0.005), but we did not find a significant difference between patients with a BMI ≥ 25 kg/m2 and patients with a BMI ‹25 kg/m2 after CRT‐D implantation (HR 0.89; 95%CI 0.73–1.09; P = 0.28).
Regarding age, young (age ‹65 years) patients with obesity were most likely to survive and elderly (age ≥65 years) patients had the highest risk of all‐cause mortality (p‹0.001); obesity did not provide survival benefit in the elderly (HR 1.01; 95% CI 0.86–1.20; P = 0.86). (Figure 3 .)
Figure 3.
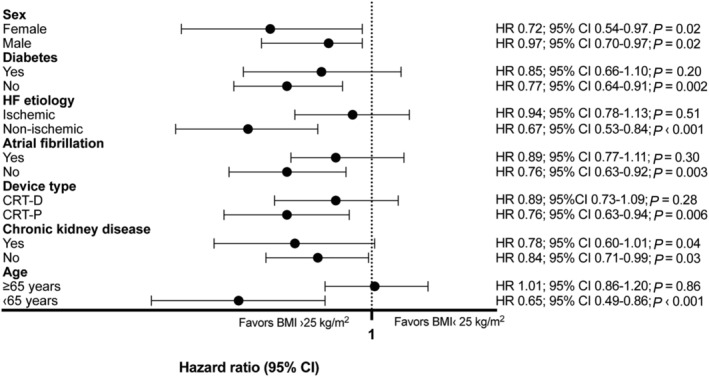
Subgroup analyses of enroled patients. Dominantly patients with obesity (BMI > 25 kg/m2) and free of comorbidities gain survival benefit.
Periprocedural complications
An equal distribution of periprocedural complications was observed, none of the examined complications occurred more frequently in any of the patient groups (BMI < 25 kg/m2 25% vs. BMI 25‐ < 30 kg/m2 28% vs. BMI ≥ 30 kg/m2 26%; P = 0.48). (Table 3 .) At the same time, bleeding (P = 0.81), pneumothorax (P = 0.19), haemothorax (P = 0.25), coronary sinus dissection (P = 0.55), pericardial tamponade (P = 0.57), pocket infection (P = 0.49), infective endocarditis (P = 0.75), lead dislodgement (P = 0.10), lead dysfunction/fracture (P = 0.53) and phrenic nerve stimulation (P = 0.57) occurred evenly across the patient groups. (Table 3 .)
Table 3.
Periprocedural complications divided by BMI groups
| Complications | All patients (n = 1,585) | BMI <25 kg/m2 (n = 459) | BMI 25‐ < 30 kg/m2 (n = 641) | BMI ≥30 kg/m2 (n = 485) | p‐value |
|---|---|---|---|---|---|
| All complications (n; %) | 420 (26) | 115 (25) | 180 (28) | 125 (26) | 0.48 |
| Bleeding (n; %) | 24 (1.5) | 8 (1.7) | 10 (1.6) | 6 (1) | 0.81 |
| Pneumothorax (n; %) | 22 (1.4) | 10 (2) | 8 (1.2) | 4 (0.8) | 0.19 |
| Haemothorax (n; %) | 4 (0.2) | 2 (0.4) | 0 (0) | 2 (0.4) | 0.25 |
| Coronary sinus dissection (n; %) | 13 (0.8) | 2 (0.4) | 6 (1) | 5 (1) | 0.55 |
| Pericardial tamponade (n; %) | 6 (0.4) | 1 (0.2) | 2 (0.3) | 3 (0.6) | 0.57 |
| Pocket infection/decubitus (n; %) | 39 (2) | 8 (1.7) | 18 (3) | 13 (3) | 0.49 |
| Infective endocarditis (n; %) | 6 (0.4) | 2 (0.2) | 3 (0.5) | 1 (0.2) | 0.75 |
| Lead dislodgement (n; %) | 112 (7) | 33 (7) | 54 (8) | 35 (7) | 0.10 |
| Lead dysfunction/fracture (n; %) | 30 (2) | 6 (1.3) | 13 (2) | 11 (2) | 0.53 |
| Phrenic nerve stimulation (n; %) | 94 (6) | 27 (6) | 34 (5) | 33 (7) | 0.57 |
BMI, body mass index
Echocardiographic response
A significant improvement in LVEF over the course of 6 months was seen in all patient categories. The mean of ‐LVEF was 7% in the normal weight group (p ‹0.001), ‐LVEF was 7.5% in patients with overweight (p ‹0.001) and 6% in patients with obesity (p ‹0.001) (Table 4 .). A similar proportion of reverse remodeling was observed across the patient groups, 58% in the normal weight, 61% in the overweight and 57% in the obese groups (P = 0.75). (Table 5 .)
Table 4.
Change of left ventricular ejection fraction 6 months after CRT implantation across patient groups
| BMI <25 kg/m2 (n = 105) | BMI 25‐ < 30 kg/m2 (n = 167) | BMI ≥30 kg/m2 (n = 111) | |
|---|---|---|---|
| Baseline LVEF (%; median/IQR) | 27 (23–30) | 28 (24–33) | 30 (25–35) |
| 6 months LVEF (%; median/IQR) | 33 (25–40) | 34 (29–40) | 37 (30–41) |
| ‐LVEF (%; median/IQR) | 7 (0–12) | 7.5 (1–13) | 6 (0.75–11) |
| p‐value | <0.001 | <0.001 | <0.001 |
BMI, body mass index; IQR, interquartile range; LVEF, left ventricular ejection fraction
Table 5.
The rate of reverse remodeling across patient groups
| BMI <25 kg/m2 (n = 105) | BMI 25‐ < 30 kg/m2 (n = 167) | BMI ≥30 kg/m2 (n = 111) | p‐value | |
|---|---|---|---|---|
|
Reverse remodeling (n; %) |
61 (58) | 102 (61) | 63 (57) | 0.75 |
BMI, body mass index
Discussion
In this large‐scale retrospective, observational study, heart failure patients with obesity free of comorbidities and selected for CRT implantation showed survival benefit compared with normal‐weight CRT candidates. Additionally, among all BMI groups the proportions of patients with reverse remodeling were similar, the incidence of peri‐ and postprocedural complications did not differ.
Based on previous observational studies, the association between obesity and a beneficial outcome in heart failure patients was partly described. 21 However, this phenomenon was uncertain in the most severe subset of heart failure patients, those undergoing CRT implantation. 17 , 22 , 23 In previous trials, the association of obesity with all‐cause mortality has been described as a J‐shaped curve in HF patients with the lowest mortality at a BMI of 25 kg/m2. 24 Others associated being overweight (BMI 25‐ < 30 kg/m2) with a more beneficial outcome compared to normal weight. 25 Despite these incomprehensive data in the literature; as some showed mortality benefit with overweight, 22 some with obesity in heart failure patients, 17 altogether higher BMI was described to associate with mortality benefit compared to normal weight or particularly with cachexia, therefore obesity may refer to a better metabolic reserve. 26
The heterogeneity of findings of previous studies might be explained by the patient selection or the time of enrolment in their course of the disease.
In the current analysis, the characteristics of our cohort are similar to prior milestone trials that enrolled HFrEF patients eligible for device implantation. 12 , 27 Patients with obesity were generally younger 22 , 26 and frequently have concomitant diseases such as diabetes, 22 , 26 hypertension 16 , 28 and are more likely to have higher LVEF than normal‐weighed patients, 3 , 16 which might also be associated with a better outcome. Moreover, the type of CRT device can also influence the risk of mortality when adding a defibrillator (CRT‐D) to further reduce the risk of sudden cardiac death. However, few data described a higher incidence of CRT‐D implantations in those with overweight or obesity, 22 , 29 in our entire study population the proportion of CRT‐D implantation did not differ across BMI groups.
The length of the follow‐up time may also influence the result. Grandin et al. investigated the ten‐year survival free from all‐cause death, orthotopic heart transplantation, or ventricular assist device implantation, which was found to be the highest in patients with obesity (36.3%), lower in overweight ones (19.2%), and the lowest in normal weight patients (12.1%). 17
Notably, we cannot know whether patients in the normal BMI group represent an end stage heart failure patient with unintentional weight loss or a normal weight one at an earlier stage of the disease. Moreover, intentional weight loss by diet or new antidiabetic drugs (e.g., GLP‐1 analogues) and its effect on outcomes is scarcely investigated in patients with device therapy, therefore further trials are warranted to investigate the effect of such new treatments on the outcome of heart failure patients selected for device implantation. As the global burden of cardiovascular diseases attributed to high BMIs is significant, acting upon prevention strategies is necessary to navigate patients into optimal BMI ranges and to avoid obtaining comorbidities. 30
Findings about the obesity paradox are influenced by several other covariates. In a recently published article, using novice anthropometric measures to define obesity, the beneficial effect could not be detected on heart failure hospitalization or all‐cause mortality. However, the protective factor could be observed when adjusted for conventional risk variables, it diminished to insignificance after adding log NT‐proBNP, 31 a biomarker known to be influenced by obesity.
The presence of concomitant diseases may have a severe influence on the outcome. Based on our analyses, only patients free of co‐morbidities gained survival benefit with obesity. The obesity paradox was present in both sexes, in patients without diabetes, without atrial fibrillation, without ischaemic aetiology, and in younger ones. In the literature, the presence of the phenomenon in the subgroup of diabetic patients is questionable. In previous studies, obesity did not provide survival benefit in patients with DM, 32 , 33 , 34 while others found that irrespective of DM status, those with obesity showed mortality benefit after CRT implantation. 17 At the same time, DM is linked to a higher burden of comorbidities and poorer outcomes in HF patients, 35 , 36 possibly mitigating obesity's protective factor. The other relevant concomitant disease, that might have influence on the presence of obesity paradox is the ischaemic aetiology. 17 Zamora et al., similarly to our findings, only observed the obesity paradox in non‐ischaemic HF. 37
As obesity's prevalence and the number of device implantations rises, 38 safety assessment is crucial in this patient group. Complication rates increase with the complexity of the devices and are also influenced by individual covariates. 18 In our analysis, periprocedural complications did not occur more frequently in patients with obesity. Device‐related complications did not differ among patient groups in a prior analysis, within 90 days after CRT‐D implantation in elderly patients. 16 But patients with obesity might face a higher rate of failed left ventricular lead placement (in patients with extreme obesity) or lead dislodgement. 39 , 40 Generally CRT implantation is considered a safe and well endured procedure in patients with higher BMIs. 41
Evaluating the response to CRT therapy within 6–12 months is essential, as positive response, the development of reverse remodeling is correlated with long‐term survival. 42 A few studies with CRT patients showed that those with obesity can experience the same or even more significant improvements in LVEF and left ventricular end‐diastolic diameter. 23 , 29 , 43 We found similar improvement in LVEF and an even proportion of developing reverse remodeling across patient groups.
Limitations
Our study has a few limitations. First, this is a single‐centre, retrospective, observational study. Second, BMI is a standardized method to quantify obesity, but it does not consider body composition nor fitness, while the fit or physically active patients with obesity show better prognosis than sarcopenic ones, 44 , 45 which was not specified in the current analysis. Third, our patient population lacked underweight or cachectic patients, who show the worst outcome. 46 Also, we do not have data on the use of drugs such as sodium glucose co‐transporter 2 inhibitors or angiotensin receptor‐neprilysin inhibitor that may influence the outcome and missed to have data on laboratory parameters (such as e.g., NT‐proBNP levels).
Conclusions
In the subset of heart failure patients eligible for cardiac resynchronization therapy implantation, those with obesity and free of comorbidities showed mortality benefit compared to normal‐weighed patients, which proved that the obesity paradox was present in our cohort.
Additionally, obesity did not infer higher rates of periprocedural complications and did not affect the efficacy of the device treatment.
Authors contributions
Study design and conceptualization: EDM, AK, BM. Data collection: WRS, AB, EDM. Data analyses: EDM, AK. Results interpretations: all authors. Manuscript writing: all authors. Manuscript proofing: all authors.
Funding
TKP2021‐EGA‐23 has been implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021‐EGA funding scheme.
Project no. RRF‐2.3.1‐21‐2022‐00003 has been implemented with the support provided by the European Union. This study was supported by the National Research, Development and Innovation Office of Hungary (NKFIA; NVKP_16‐1‐2016‐0017 National Heart Program).
The research was also supported by the Semmelweis 250 + Excellence Ph.D. Scholarship (EFOP‐3.6.3‐VEKOP‐16‐2017‐00009) by the Semmelweis University. A. Kosztin was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences.
Disclosures
AK receives consulting fees from Medtronic and Biotronik, and receives payment or honoraria for lectures from Novartis, Bayer, Boehringer Ingelheim, Astra Zeneca, Medtronic Biotronik, and Boston Scientific. BM receives grants or has contracts with Abbott, Astra Zeneca, Argint International, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol‐Myers Squibb, CSL Behring, Daiichi Sankyo, Cerenis Therapeutics SA DUKE Clinical Institut, Eli Lilly, Medtronic, Novartis, Terumo, St. Jude, Zoll and VIFOR Pharma, and receives lecture fees from Abbott, Astra Zeneca, Biotronik, Boehringer Ingelheim, and Novartis. IO received study fees from Biotronik, Medtronic, Boston Scientific, Abbott, Orchestra BioMed. LG receives lecture fees from Biotronik, Medtronic, Johnson & Johnson Medical, and Abbott outside the submitted work. E.Z. reports lecture and advisory fees outside the submitted work from Biotronik, Medtronic, Boston Scientific, Zoll Medical, Novartis, Richter, Orion Pharma outside the submitted work. RP reports lecture fees from Biotronik, Medtronic, and Abbott outside the submitted work. LM reports lecture fees from Biotronik, Medtronic, and Abbott outside the submitted work. EDM reports grants from Novartis and Boehringer Ingelheim. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supporting information
Figure S1. Kaplan Meier estimates of the probability of survival after CRT implantation by BMI groups and diabetes status. Non‐diabetic patients with overweight or obesity show the best probability of survival, the lowest survival was seen in diabetic patients with a BMI ‹25 kg/m2 (log‐rank p ‹0.001). Diabetic patients did not experience the obesity paradox (HR 0.85; 95% CI 0.66–1.10; P = 0.20).
Figure S2. Kaplan Meier estimates of the probability of survival after CRT implantation by BMI groups and heart failure aetiology. Patients with a BMI ≥ 25 kg/m2 show survival benefit over patients with a BMI of ‹25 kg/m2 in non‐ischaemic patients (HR 0.67; 95% CI 0.53–0.84; p ‹0.001), obesity did not protect patients with ischaemic aetiology (HR 0.94; 95% CI 0.78–1.13; P = 0.51).
Figure S3. Kaplan Meier estimates of the probability of survival after CRT implantation by BMI groups, underweight patients excluded.
Table S1. The associations of the BMI with the risk of all‐cause mortality.
Merkel, E. D. , Behon, A. , Masszi, R. , Schwertner, W. R. , Kuthi, L. , Veres, B. , Osztheimer, I. , Papp, R. , Molnár, L. , Zima, E. , Gellér, L. , Kosztin, A. , and Merkely, B. (2024) Obesity paradox in patients with reduced ejection fraction eligible for device implantation – an observational study. ESC Heart Failure, 11: 3616–3625. 10.1002/ehf2.14961.
Annamária Kosztin and Béla Merkely contributed equally to the coordination of the study and critical review of the manuscript.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 2. Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med 2002;347:305–313. doi: 10.1056/NEJMoa020245 [DOI] [PubMed] [Google Scholar]
- 3. Carbone S, Lavie CJ, Arena R. Obesity and Heart Failure: Focus on the Obesity Paradox. Mayo Clin Proc 2017;92:266–279. doi: 10.1016/j.mayocp.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 4. Horwich TB, Fonarow GC, Clark AL. Obesity and the Obesity Paradox in Heart Failure. Prog Cardiovasc Dis 2018;61:151–156. doi: 10.1016/j.pcad.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 5. Vareldzis R, Naljayan M, Reisin E. The Incidence and Pathophysiology of the Obesity Paradox: Should Peritoneal Dialysis and Kidney Transplant Be Offered to Patients with Obesity and End‐Stage Renal Disease? Curr Hypertens Rep 2018;20:84. doi: 10.1007/s11906-018-0882-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corsetti G, Pasini E, Romano C, Chen‐Scarabelli C, Scarabelli TM, Flati V, et al. How Can Malnutrition Affect Autophagy in Chronic Heart Failure? Focus and Perspectives. Int J Mol Sci 2021;22:3332. doi: 10.3390/ijms22073332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Esteban‐Fernández A, Villar‐Taibo R, Alejo M, Arroyo D, Bonilla Palomas JL, Cachero M, et al. Diagnosis and Management of Malnutrition in Patients with Heart Failure. J Clin Med 2023;12:3320. doi: 10.3390/jcm12093320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Donini LM, Pinto A, Giusti AM, Lenzi A, Poggiogalle E. Obesity or BMI Paradox? Beneath the Tip of the Iceberg. Front Nutr 2020;7:53. doi: 10.3389/fnut.2020.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gerber Y, Weston SA, Redfield MM, Chamberlain AM, Manemann SM, Jiang R, et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med 2015;175:996–1004. doi: 10.1001/jamainternmed.2015.0924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, et al. EURObservational Research Programme: regional differences and 1‐year follow‐up results of the Heart Failure Pilot Survey (ESC‐HF Pilot). Eur J Heart Fail 2013;15:808–817. doi: 10.1093/eurjhf/hft050 [DOI] [PubMed] [Google Scholar]
- 11. Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Køber L, Squire IB, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J 2013;34:1404–1413. doi: 10.1093/eurheartj/ehs337 [DOI] [PubMed] [Google Scholar]
- 12. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac‐resynchronization therapy for the prevention of heart‐failure events. N Engl J Med 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431 [DOI] [PubMed] [Google Scholar]
- 13. Anand IS, Carson P, Galle E, Song R, Boehmer J, Ghali JK, et al. Cardiac Resynchronization Therapy Reduces the Risk of Hospitalizations in Patients With Advanced Heart Failure. Circulation 2009;119:969–977. doi: 10.1161/CIRCULATIONAHA.108.793273 [DOI] [PubMed] [Google Scholar]
- 14. Ruschitzka F, Abraham WT, Singh JP, Bax JJ, Borer JS, Brugada J, et al. Cardiac‐Resynchronization Therapy in Heart Failure with a Narrow QRS Complex. N Engl J Med 2013;369:1395–1405. doi: 10.1056/NEJMoa1306687 [DOI] [PubMed] [Google Scholar]
- 15. Szepietowska B, Polonsky B, Sherazi S, Biton Y, Kutyifa V, McNitt S, et al. Effect of obesity on the effectiveness of cardiac resynchronization to reduce the risk of first and recurrent ventricular tachyarrhythmia events. Cardiovasc Diabetol 2016;15:93. doi: 10.1186/s12933-016-0401-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Echouffo‐Tcheugui JB, Masoudi FA, Bao H, Curtis JP, Heidenreich PA, Fonarow GC. Body mass index and outcomes of cardiac resynchronization with implantable cardioverter‐defibrillator therapy in older patients with heart failure. Eur J Heart Fail 2019;21:1093–1102. doi: 10.1002/ejhf.1552 [DOI] [PubMed] [Google Scholar]
- 17. Grandin EW, Wand A, Zamani P, Rame JE, Verdino RJ. Relation of Body Mass Index to Long‐Term Survival After Cardiac Resynchronization Therapy. Am J Cardiol 2016;118:1861–1867. doi: 10.1016/j.amjcard.2016.08.079 [DOI] [PubMed] [Google Scholar]
- 18. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IM, et al. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: Developed by the Task Force on cardiac pacing and cardiac resynchronization therapy of the European Society of Cardiology (ESC) With the special contribution of the European Heart Rhythm Association (EHRA). Eur Heart J 2021;42:3427–3520. doi: 10.1093/eurheartj/ehab364 [DOI] [PubMed] [Google Scholar]
- 19. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. Vol. 894; 2000:i–xii. 1–253 [PubMed] [Google Scholar]
- 20. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser 1995;854:1–452. [PubMed] [Google Scholar]
- 21. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007;93:1137–1146. doi: 10.1136/hrt.2003.025270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Papageorgiou N, Briasoulis A, Barra S, Sohrabi C, Lim WY, Agarwal S, et al. Long‐Term Impact of Body Mass Index on Survival of Patients Undergoing Cardiac Resynchronization Therapy: A Multi‐Centre Study. Am J Cardiol 2021;153:79–85. doi: 10.1016/j.amjcard.2021.05.024 [DOI] [PubMed] [Google Scholar]
- 23. Cai C, Hua W, Ding LG, Wang J, Chen KP, Yang XW, et al. Association of body mass index with cardiac reverse remodeling and long‐term outcome in advanced heart failure patients with cardiac resynchronization therapy. Circ J 2014;78:2899–2907. doi: 10.1253/circj.CJ-14-0812 [DOI] [PubMed] [Google Scholar]
- 24. Bhaskaran K, dos‐Santos‐Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI with overall and cause‐specific mortality: a population‐based cohort study of 3·6 million adults in the UK. Lancet Diab Endocrinol 2018;6:944–953. doi: 10.1016/S2213-8587(18)30288-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all‐cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta‐analysis. JAMA 2013;309:71–82. doi: 10.1001/jama.2012.113905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Szepietowska B, McNitt S, Polonsky B, Sherazi S, Biton Y, Kutyifa V, et al. Metabolic syndrome is associated with different clinical outcome after cardiac resynchronization therapy in patients with ischemic and non‐ischemic cardiomyopathy. Cardiol J 2016;23:344–351. doi: 10.5603/CJ.a2016.0017 [DOI] [PubMed] [Google Scholar]
- 27. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, et al. Longer‐term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization‐Heart Failure (CARE‐HF) trial extension phase]. Eur Heart J 2006;27:1928–1932. doi: 10.1093/eurheartj/ehl099 [DOI] [PubMed] [Google Scholar]
- 28. Landsberg L, Aronne LJ, Beilin LJ, Burke V, Igel LI, Lloyd‐Jones D, et al. Obesity‐Related Hypertension: Pathogenesis, Cardiovascular Risk, and Treatment. J Clin Hyper 2013;15:14–33. doi: 10.1111/jch.12049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Il'Giovine ZJ, Patel D, Kumar A, Trulock K, Ann Moennich L, Donnellan E, et al. Obesity Predicts Survival After Cardiac Resynchronization Therapy Independent of Effect on Left Ventricular Ejection Fraction. Circ Heart Fail 2020;13:e007424. doi: 10.1161/CIRCHEARTFAILURE.120.007424 [DOI] [PubMed] [Google Scholar]
- 30. Dong X‐J, Zhang X‐Q, Wang B‐B, Hou F‐F, Jiao Y, Wang J‐G. The Burden of Cardiovascular Disease Attributable to High Body Mass Index: An Observational Study. Eur Heart J 2024;10:154–167. doi: 10.1093/ehjqcco/qcad044 [DOI] [PubMed] [Google Scholar]
- 31. Butt JH, Petrie MC, Jhund PS, Sattar N, Desai AS, Køber L, et al. Anthropometric measures and adverse outcomes in heart failure with reduced ejection fraction: revisiting the obesity paradox. Eur Heart J 2023;44:1136–1153. doi: 10.1093/eurheartj/ehad083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zamora E, Lupón J, Enjuanes C, Pascual‐Figal D, de Antonio M, Domingo M, et al. No benefit from the obesity paradox for diabetic patients with heart failure. Eur J Heart Fail 2016;18:851–858. doi: 10.1002/ejhf.576 [DOI] [PubMed] [Google Scholar]
- 33. Adamopoulos C, Meyer P, Desai RV, Karatzidou K, Ovalle F, White M, et al. Absence of obesity paradox in patients with chronic heart failure and diabetes mellitus: a propensity‐matched study. Eur J Heart Fail 2011;13:200–206. doi: 10.1093/eurjhf/hfq159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pinho EM, Lourenço P, Silva S, Laszczyńska O, Leite AB, Gomes F, et al. Higher BMI in heart failure patients is associated with longer survival only in the absence of diabetes. J Cardiovasc Med (Hagerstown) 2015;16:576–582. doi: 10.2459/JCM.0b013e328364be3c [DOI] [PubMed] [Google Scholar]
- 35. MacDonald MR, Petrie MC, Varyani F, Ostergren J, Michelson EL, Young JB, et al. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur Heart J 2008;29:1377–1385. doi: 10.1093/eurheartj/ehn153 [DOI] [PubMed] [Google Scholar]
- 36. Ahmed A, Aban IB, Vaccarino V, Lloyd‐Jones DM, Goff DC Jr, Zhao J, et al. A propensity‐matched study of the effect of diabetes on the natural history of heart failure: variations by sex and age. Heart 2007;93:1584–1590. doi: 10.1136/hrt.2006.113522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zamora E, Lupón J, de Antonio M, Urrutia A, Coll R, Díez C, et al. The obesity paradox in heart failure: Is etiology a key factor? Int J Cardiol 2013;166:601–605. doi: 10.1016/j.ijcard.2011.11.022 [DOI] [PubMed] [Google Scholar]
- 38. Valzania C, Torbica A, Tarricone R, Leyva F, Boriani G. Implant rates of cardiac implantable electrical devices in Europe: A systematic literature review. Health Policy 2016;120:1–15. doi: 10.1016/j.healthpol.2015.11.001 [DOI] [PubMed] [Google Scholar]
- 39. Nishimura M, Marcus GM, Varosy PD, Bao H, Wang Y, Curtis JP, et al. Association of body mass index with cardiac resynchronization therapy intention and left ventricular lead implantation failure: insights from the NCDR implantable cardioverter‐defibrillator registry. J Interv Card Electrophysiol 2020;57:279–288. doi: 10.1007/s10840-019-00550-x [DOI] [PubMed] [Google Scholar]
- 40. Kawata H, Patel J, McGarry T, Joshi R, Krummen D, Feld G, et al. Obese female patients have higher rates of lead dislodgement after ICD or CRT‐D implantation. Int J Cardiol 2014;172:e522–e524. doi: 10.1016/j.ijcard.2014.01.076 [DOI] [PubMed] [Google Scholar]
- 41. Attanasio P, Lacour P, Ernert A, Pieske B, Haverkamp W, Blaschke F, et al. Cardiac device implantations in obese patients: Success rates and complications. Clin Cardiol 2017;40:230–234. doi: 10.1002/clc.22650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gold MR, Daubert C, Abraham WT, Ghio S, St John Sutton M, Hudnall JH, et al. The effect of reverse remodeling on long‐term survival in mildly symptomatic patients with heart failure receiving cardiac resynchronization therapy: results of the REVERSE study. Heart Rhythm 2015;12:524–530. doi: 10.1016/j.hrthm.2014.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yanagisawa S, Inden Y, Shimano M, Yoshida N, Ishikawa S, Kato H, et al. Impact of cardiac resynchronization therapy‐defibrillator implantation on the association between body mass index and prognosis in patients with heart failure. J Interv Card Electrophysiol 2015;43:269–277. doi: 10.1007/s10840-015-0015-3 [DOI] [PubMed] [Google Scholar]
- 44. Piepoli MF, Corrà U, Veglia F, Bonomi A, Salvioni E, Cattadori G, et al. Exercise tolerance can explain the obesity paradox in patients with systolic heart failure: data from the MECKI Score Research Group. Eur J Heart Fail 2016;18:545–553. doi: 10.1002/ejhf.534 [DOI] [PubMed] [Google Scholar]
- 45. Li X, Chen K, Hua W, Su Y, Yang J, Liang Z, et al. Association of the Obesity Paradox With Objective Physical Activity in Patients at High Risk of Sudden Cardiac Death. J Clin Endocrinol Metab 2020;105:e4801–e4810. doi: 10.1210/clinem/dgaa659 [DOI] [PubMed] [Google Scholar]
- 46. Anker SD, Negassa A, Coats AJ, Afzal R, Poole‐Wilson PA, Cohn JN, et al. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin‐converting‐enzyme inhibitors: an observational study. Lancet 2003;361:1077–1083. doi: 10.1016/S0140-6736(03)12892-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Kaplan Meier estimates of the probability of survival after CRT implantation by BMI groups and diabetes status. Non‐diabetic patients with overweight or obesity show the best probability of survival, the lowest survival was seen in diabetic patients with a BMI ‹25 kg/m2 (log‐rank p ‹0.001). Diabetic patients did not experience the obesity paradox (HR 0.85; 95% CI 0.66–1.10; P = 0.20).
Figure S2. Kaplan Meier estimates of the probability of survival after CRT implantation by BMI groups and heart failure aetiology. Patients with a BMI ≥ 25 kg/m2 show survival benefit over patients with a BMI of ‹25 kg/m2 in non‐ischaemic patients (HR 0.67; 95% CI 0.53–0.84; p ‹0.001), obesity did not protect patients with ischaemic aetiology (HR 0.94; 95% CI 0.78–1.13; P = 0.51).
Figure S3. Kaplan Meier estimates of the probability of survival after CRT implantation by BMI groups, underweight patients excluded.
Table S1. The associations of the BMI with the risk of all‐cause mortality.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.


