Abstract
Aims
Hospitalized patients with heart failure (HF) are a heterogeneous population, with multiple phenotypes proposed. Prior studies have not examined the biological phenotypes of critically ill patients with HF admitted to the contemporary cardiac intensive care unit (CICU). We aimed to leverage unsupervised machine learning to identify previously unknown HF phenotypes in a large and diverse cohort of patients with HF admitted to the CICU.
Methods
We screened 6008 Mayo Clinic CICU patients with an admission diagnosis of HF from 2007 to 2018 and included those without missing values for common laboratory tests. Consensus k‐means clustering was performed based on 10 common admission laboratory values (potassium, chloride, anion gap, blood urea nitrogen, haemoglobin, red blood cell distribution width, mean corpuscular volume, platelet count, white blood cell count and neutrophil‐to‐lymphocyte ratio). In‐hospital mortality was evaluated using logistic regression, and 1 year mortality was evaluated using Cox proportional hazard models after multivariable adjustment.
Results
Among 4877 CICU patients with HF who had complete admission laboratory data (mean age 69.4 years, 38.4% females), we identified five clusters with divergent demographics, comorbidities, laboratory values, admission diagnoses and use of critical care therapies. We labelled these clusters based on the characteristic laboratory profile of each group: uncomplicated (25.7%), iron‐deficient (14.5%), cardiorenal (18.4%), inflamed (22.3%) and hypoperfused (19.2%). In‐hospital mortality occurred in 10.7% and differed between the phenotypes: uncomplicated, 2.7% (reference); iron‐deficient, 8.1% [adjusted odds ratio (OR) 2.18 (1.38–3.48), P < 0.001]; cardiorenal, 10.3% [adjusted OR 2.11 (1.37–3.32), P < 0.001]; inflamed, 12.5% [adjusted OR 1.79 (1.18–2.76), P = 0.007]; and hypoperfused, 21.9% [adjusted OR 4.32 (2.89–6.62), P < 0.001]. These differences in mortality between phenotypes were consistent when patients were stratified based on demographics, aetiology, admission diagnoses, mortality risk scores, shock severity and systolic function. One‐year mortality occurred in 31.5% and differed between the phenotypes: uncomplicated, 11.9% (reference); inflamed, 26.8% [adjusted hazard ratio (HR) 1.56 (1.27–1.92), P < 0.001]; iron‐deficient, 33.8% [adjusted HR 2.47 (2.00–3.04), P < 0.001]; cardiorenal, 41.2% [adjusted HR 2.41 (1.97–2.95), P < 0.001]; and hypoperfused, 52.3% [adjusted HR 3.43 (2.82–4.18), P < 0.001]. Similar findings were observed for post‐discharge 1 year mortality.
Conclusions
Unsupervised machine learning clustering can identify multiple distinct clinical HF phenotypes within the CICU population that display differing mortality profiles both in‐hospital and at 1 year. Mortality was lowest for the uncomplicated HF phenotype and highest for the hypoperfused phenotype. The inflamed phenotype had comparatively higher in‐hospital mortality yet lower post‐discharge mortality, suggesting divergent short‐term and long‐term prognosis.
Keywords: cardiac intensive care unit, cardiogenic shock, heart failure, machine learning, mortality, phenotyping
Introduction
The heterogeneity of the modern cardiac intensive care unit (CICU) has expanded, reflecting a transition from patients with acute coronary syndrome (ACS) to patients with circulatory failure. 1 , 2 Heart failure (HF) is becoming the most common diagnosis in contemporary CICU populations. 1 , 2 , 3 Patients with HF in the CICU encompass a wide spectrum of acuity and chronic illness severity and are at elevated risk of adverse outcomes (particularly the important minority with advanced HF). 4 , 5 The prognostic variables relevant to hospitalized HF patients with lower acuity may differ from critically ill HF patients in the CICU. 6 , 7 Better understanding of the clinical profile and outcomes of diverse groups of HF patients within the CICU population is needed to identify subgroups of interest that may require unique management approaches. 7
Historically, clinicians have tried to simplify the diverse acute HF population by defining physiologic phenotypes based on haemodynamic variables (e.g., cardiac output, peripheral vascular tone and filling pressures) or left ventricular systolic function, but these data are not always available at initial evaluation in the CICU. 4 , 8 , 9 Other phenotypes have been defined for hospitalized HF patients according to the clinical features that define the overarching disease process. 10 , 11 These traditional phenotyping approaches overlook the distinct pathophysiology and biological heterogeneity that exists within these broad clinical profiles and could affect treatment responses.
Unsupervised machine learning can be used to distinguish HF subphenotypes based on relevant clinical variables and can evaluate more complex interactions than standard statistical analyses in a data‐driven manner. 12 These occult subphenotypes identified using machine learning may differ in underlying pathophysiology, prognosis and response to therapy, in turn potentially enabling individualization of care. 12 This in silico approach has been examined in patients with acute and chronic HF, as well as patients with cardiogenic shock (CS), but has not been applied to critically ill patients with HF requiring CICU admission. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 We sought to leverage machine learning in a large CICU population as a proof‐of‐concept analysis to determine whether we could identify occult HF subphenotypes with differing clinical profiles and outcomes based on commonly available laboratory data from the time of CICU admission. We hypothesized that previously unrecognized subphenotypes with divergent characteristics could be identified, resulting in differences in short‐term and long‐term survival.
Methods
Patient population
This retrospective observational cohort study was approved by the institutional review board (IRB) of Mayo Clinic under a waiver of informed consent for patients who had provided consent for their medical records to be used for research. 1 We retrospectively analysed consecutive unique patients admitted to the Mayo Clinic (Rochester, MN) CICU from January 2007 to April 2018 with an admission diagnosis of acute or chronic HF; only the first admission was considered for patients who had multiple admissions during the time period to minimize potential bias due to readmissions. 1 , 23 The CICU at Mayo Clinic admits patients with medical critical illness focusing on those with acute or chronic cardiac disease but does not admit post‐cardiotomy patients or patients with extracorporeal membrane oxygenation (ECMO) or durable left ventricular assist devices (LVADs). We examined the availability of all admission laboratory values and selected laboratory values with <20% missingness for further analysis. We then excluded patients with missing values for any of these common laboratory tests (complete‐case analysis) to create the final study population (Figure 1 ), as previously utilized. 13 , 20
Figure 1.
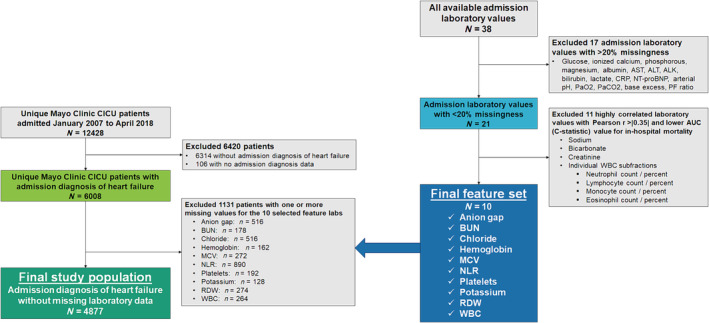
Flow diagram demonstrating inclusion and exclusion criteria for the final study population as well as selection of admission laboratory values as features for clustering. ALK, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUC, area under the receiver operating characteristic curve; BUN, blood urea nitrogen; CICU, cardiac intensive care unit; CRP, C‐reactive protein; MCV, mean corpuscular volume; NLR, neutrophil‐to‐lymphocyte ratio; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PaCO2, arterial partial pressure of carbon dioxide; PaO2, arterial partial pressure of oxygen; PF ratio, ratio of PaO2 to fraction of inspired oxygen; RDW, red blood cell distribution width; WBC, white blood cell count.
Data sources and definitions
Clinical, diagnosis, laboratory, treatment and outcome data were extracted electronically from the electronic health record and relevant Mayo Clinic databases, as previously described. 1 Vital signs at the time of admission were available only for patients admitted from 2007 to 2015. 6 , 24 Admission diagnoses (including HF) were defined as all International Classification of Diseases (ICD)‐9/10 diagnosis codes documented within 1 day of CICU admission. 3 Admission laboratory values were those that were obtained closest to CICU admission. Data from the first 24 h of the CICU stay were used to calculate the Acute Physiology and Chronic Health Evaluation (APACHE)‐III/IV and Sequential Organ Failure Assessment (SOFA) scores using validated electronic algorithms. 3 , 25 , 26 The Mayo CICU Admission Risk Score (M‐CARS) was calculated based on data from the time of admission and has been shown to outperform either APACHE or SOFA for prediction of in‐hospital mortality in this Mayo Clinic CICU population. 23 Current and prior diagnoses were used to determine the Charlson Comorbidity Index (CCI) using a validated electronic algorithm. 1 For patients admitted from 2007 to 2015, the Society for Cardiovascular Angiography and Interventions (SCAI) Shock Classification (i.e., SCAI shock stages A through E) was assigned based on data from the first 24 h of CICU admission, and the Get With The Guidelines Heart Failure (GWTG‐HF) risk score was calculated based on admission variables. 6 , 24 Echocardiographic data were extracted from the Mayo Clinic Cardiovascular DataMart for patients who had a transthoracic echocardiogram (TTE) within 1 day of CICU admission (n = 2998). 4 The severity of left ventricular systolic dysfunction (LVSD) was classified based on the left ventricular ejection fraction (LVEF) according to American Society of Echocardiography (ASE) guidelines. The severity of right ventricular (RV) dysfunction (RVSD) was assigned holistically by the board‐certified cardiologist interpreting the TTE based on the entirety of available data.
Selection of feature variables for clustering
All the candidate admission laboratory values without excessive missingness were derived from the basic metabolic panel (BMP) and complete blood count (CBC) with differential; all other laboratory values had >30% missingness (Table S1 ). Because k‐means clustering compares group means and can be sensitive to highly correlated feature variables, we examined Pearson product–moment correlations between candidate laboratory values (Figure S1 ), including the neutrophil‐to‐lymphocyte ratio (NLR), which was calculated based on white blood cell (WBC) subfractions from the CBC differential. 27 We then performed univariable logistic regression to evaluate the association between these laboratory variables and in‐hospital mortality (Table S1 ) and determine the area under the receiver operating characteristic curve (AUC, C‐statistic). As in prior analyses, when two laboratory values were substantially correlated, as demonstrated by Pearson r correlation coefficients >|0.35|, we selected the one with a higher AUC for in‐hospital mortality and excluded the other, as previously described—this resulted in the exclusion of sodium, bicarbonate, creatinine and the individual WBC subtypes. 21 Ultimately, 10 admission laboratory values were selected as features for the clustering analysis: potassium, chloride, anion gap, blood urea nitrogen (BUN), haemoglobin, red blood cell distribution width (RDW), mean corpuscular volume (MCV), platelet count, WBC count and NLR (Figure 1 ).
Clustering analysis
The methodology for the clustering analysis mirrored prior analyses in patients with CS as described by Zweck et al. 21 Prior to clustering, the 10 selected laboratory values were natural log transformed and then centred and scaled using the mean and standard deviation (SD) to determine the Z score. Extreme values >2 SDs from the mean (Z score >|2|) were trimmed to |2|. Consensus k‐means clustering was performed using 2000 repetitions with 10 starting seeds for between 2 and 10 clusters. The total within‐clusters and between‐clusters sum of squares values (Y axis) were plotted according to the number of clusters (X axis) to generate an elbow plot (Figure S2), which had a subtle inflection point at five clusters, which we used as evidence to support this as the optimal number of clusters. We wanted to avoid any excessively small groups accounting for substantially <15% of the population, and we wanted the highest risk and lowest risk clusters to diverge substantially (>4‐fold) in the risk of in‐hospital mortality; these criteria further justified the use of five clusters. The mean Z scores for each laboratory feature in each cluster (i.e., cluster centroids) were plotted to describe the clusters (Figure 2 ).
Figure 2.
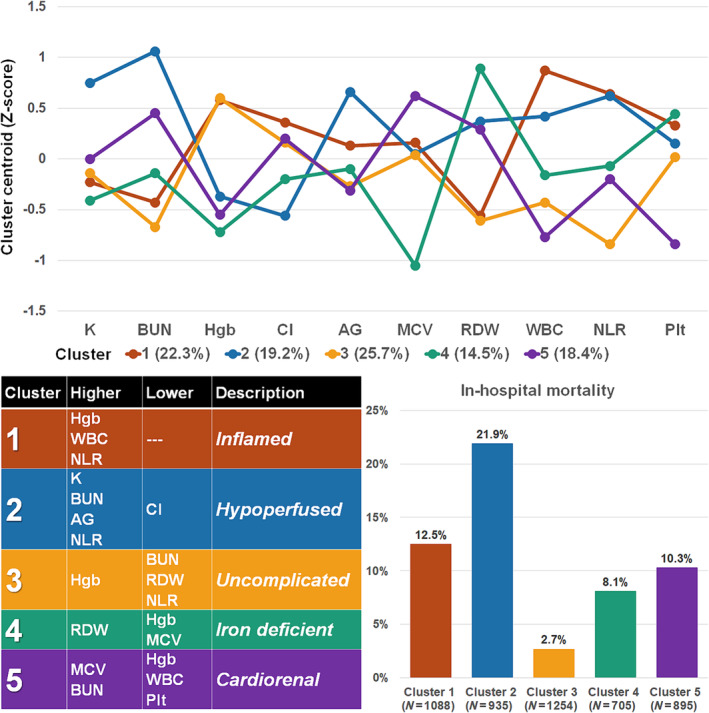
Characteristics, description and outcomes of clusters within the cohort. The cluster centroids (top) were used to develop proposed descriptions of the identified clusters based on salient patterns of laboratory variables (bottom left) compared with population means. Marked differences in in‐hospital mortality are observed between clusters (bottom right). AG, anion gap; BUN, blood urea nitrogen; Cl, chloride; Hgb, haemoglobin; K, potassium; MCV, mean corpuscular volume; NLR, neutrophil‐to‐lymphocyte ratio; Plt, platelet count; RDW, red blood cell distribution width; WBC, white blood cell count.
Statistical analysis
To demonstrate that cluster assignments were clinically relevant, we examined survival outcomes; the primary outcome was all‐cause in‐hospital mortality (including CICU mortality), and the secondary outcome was all‐cause 1 year mortality based on electronic chart review. Patients lost to follow‐up were analysed based on their vital status at the last known follow‐up. Continuous variables were summarized as mean and SD, and differences across groups were evaluated using Student's t tests or analysis of variance (ANOVA) as appropriate. Categorical variables were summarized as number (per cent), and differences across groups were evaluated using the Pearson chi‐squared test. Odds ratio (OR) and 95% confidence interval (CI) values for prediction of in‐hospital mortality were estimated using logistic regression, before and after multivariable adjustment. Survival to 1 year after CICU admission (overall and for hospital survivors) was estimated using Kaplan–Meier curves, with groups compared using the log‐rank test. Hazard ratio (HR) and 95% CI values for prediction of 1 year mortality were estimated using Cox proportional hazard regression, before and after multivariable adjustment. Covariates for multivariable models were selected a priori based on clinical relevance: age, sex, CCI, Day 1 SOFA score, admission Braden score, and admission diagnoses of ACS, shock, cardiac arrest and respiratory failure. 3 , 23 , 26 , 28 Two‐tailed P values <0.05 were considered significant. All statistical analyses were performed using BlueSky Version 10.3.1 Pro (BlueSky LLC, Chicago, IL). The authors declare that all supporting data are available within the article and its supporting information.
Results
Study population
Out of a data set of 12 428 unique CICU patient admissions, 6008 (48.3%) had an admission diagnosis of HF. Among these, 4877 (81.2%) had available data for all laboratory values from the BMP and CBC with differential and comprised the final study population (Figure 1 ). The final study population had modest differences from excluded HF patients, although mean laboratory values were similar (Table S2 ). The final study population had a mean age of 69.4 (14.9), and 1872 (38.4%) were females (Table 1 ). Other admission diagnoses included ACS in 36.5%, shock in 22.2%, cardiac arrest in 12.6%, sepsis in 9.3% and respiratory failure in 38.1%. Vasoactive drugs were administered in 35.9% of all patients, and invasive mechanical ventilation was needed in 22.4%. Among patients with available vital sign data (n = 3688), the distribution of SCAI shock stages was as follows: A, 37.3%; B, 33.9%; C, 17.1%; D, 10.8%; and E, 0.8%. Among those with LVEF data (n = 2397), the mean LVEF was 39.8% (16.9%), and 33.8% had LVEF ≥ 50% [HF with preserved ejection fraction (HFpEF)].
Table 1.
Baseline characteristics according to cluster assignment.
|
Cluster 1 ‘Inflamed’ (N = 1088) |
Cluster 2 ‘Hypoperfused’ (N = 935) |
Cluster 3 ‘Uncomplicated’ (N = 1254) |
Cluster 4 ‘Iron‐deficient’ (N = 705) |
Cluster 5 ‘Cardiorenal’ (N = 895) |
Total (N = 4877) | P value | |
|---|---|---|---|---|---|---|---|
| Demographics and nonfatal outcomes | |||||||
| Age | 69.7 (14.5) | 71.4 (14.3) | 66.7 (15.1) | 66.5 (16.1) | 73.2 (13.2) | 69.4 (14.9) | <0.001 |
| Female | 420 (38.6%) | 354 (37.9%) | 433 (34.5%) | 328 (46.5%) | 337 (37.7%) | 1872 (38.4%) | <0.001 |
| White race | 1025 (94.2%) | 861 (92.1%) | 1150 (91.7%) | 629 (89.2%) | 825 (92.2%) | 4490 (92.1%) | 0.005 |
| ICU length of stay | 3.7 (5.6) | 3.9 (4.0) | 2.7 (7.0) | 3.8 (5.9) | 3.5 (7.0) | 3.5 (6.1) | <0.001 |
| Hospital length of stay | 9.5 (11.9) | 11.9 (13.6) | 8.8 (13.4) | 14.2 (27.2) | 13.5 (22.8) | 11.2 (17.9) | <0.001 |
| 30 day readmission | 77 (8.1%) | 101 (13.8%) | 133 (10.9%) | 80 (12.4%) | 97 (12.1%) | 488 (11.2%) | 0.004 |
| 1 year readmission | 309 (32.7%) | 276 (37.8%) | 461 (37.9%) | 262 (40.5%) | 293 (36.5%) | 1601 (36.9%) | 0.021 |
| Comorbidities | |||||||
| CCI | 2.2 (2.4) | 4.3 (3.0) | 2.1 (2.1) | 3.1 (2.6) | 4.0 (2.9) | 3.0 (2.8) | <0.001 |
| Prior heart failure | 245 (22.6%) | 449 (48.1%) | 363 (29.0%) | 295 (42.1%) | 440 (49.3%) | 1792 (36.8%) | <0.001 |
| Prior MI | 205 (18.9%) | 238 (25.5%) | 268 (21.4%) | 139 (19.9%) | 250 (28.0%) | 1100 (22.6%) | <0.001 |
| Diabetes mellitus | 292 (26.9%) | 475 (50.9%) | 357 (28.5%) | 256 (36.6%) | 340 (38.1%) | 1720 (35.3%) | <0.001 |
| Lung disease | 216 (19.9%) | 281 (30.1%) | 236 (18.8%) | 180 (25.7%) | 238 (26.7%) | 1151 (23.7%) | <0.001 |
| CKD | 138 (12.7%) | 495 (53.0%) | 173 (13.8%) | 218 (31.1%) | 438 (49.0%) | 1462 (30.0%) | <0.001 |
| Prior dialysis | 29 (2.7%) | 165 (17.6%) | 18 (1.4%) | 42 (6.0%) | 111 (12.4%) | 365 (7.5%) | <0.001 |
| Ischaemic HF aetiology | 689 (63.4%) | 452 (48.4%) | 613 (48.9%) | 267 (38.1%) | 395 (44.2%) | 2416 (49.6%) | <0.001 |
| Admission ICD‐9/10 diagnoses | |||||||
| Cardiac arrest | 256 (23.5%) | 108 (11.6%) | 111 (8.9%) | 63 (8.9%) | 77 (8.6%) | 615 (12.6%) | <0.001 |
| Shock | 363 (33.4%) | 283 (30.3%) | 153 (12.2%) | 115 (16.3%) | 169 (18.9%) | 1083 (22.2%) | <0.001 |
| CS | 317 (29.1%) | 235 (25.1%) | 135 (10.8%) | 92 (13.0%) | 121 (13.5%) | 900 (18.5%) | <0.001 |
| Sepsis | 129 (11.9%) | 131 (14.0%) | 40 (3.2%) | 60 (8.5%) | 94 (10.5%) | 454 (9.3%) | <0.001 |
| Respiratory failure | 566 (52.0%) | 477 (51.0%) | 247 (19.7%) | 257 (36.5%) | 311 (34.7%) | 1858 (38.1%) | <0.001 |
| ACS | 603 (55.4%) | 314 (33.6%) | 465 (37.1%) | 174 (24.7%) | 223 (24.9%) | 1779 (36.5%) | <0.001 |
| STEMI | 371 (34.1%) | 120 (12.8%) | 242 (19.3%) | 70 (9.9%) | 80 (8.9%) | 883 (18.1%) | <0.001 |
| Severity of illness | |||||||
| GWTG‐HF risk score | 42.0 (7.9) | 51.0 (8.5) | 39.2 (6.9) | 43.2 (7.7) | 46.4 (7.7) | 43.9 (8.7) | <0.001 |
| APACHE‐III | 70.8 (27.4) | 80.4 (21.4) | 53.8 (17.4) | 63.6 (19.0) | 72.6 (19.6) | 67.6 (23.3) | <0.001 |
| Day 1 SOFA | 5.0 (3.4) | 6.2 (3.3) | 2.4 (2.0) | 3.8 (2.6) | 5.3 (3.1) | 4.4 (3.2) | <0.001 |
| M‐CARS | 3.4 (2.4) | 4.3 (1.9) | 1.6 (1.6) | 3.0 (1.7) | 3.1 (1.7) | 3.0 (2.1) | <0.001 |
| Braden score | 16.2 (3.6) | 16.3 (3.2) | 18.9 (2.8) | 17.2 (3.2) | 16.9 (3.2) | 17.2 (3.4) | <0.001 |
| SCAI shock stage | <0.001 | ||||||
| A | 225 (27.2%) | 150 (23.1%) | 515 (53.6%) | 214 (39.7%) | 272 (38.3%) | 1376 (37.3%) | |
| B | 293 (35.4%) | 199 (30.7%) | 316 (32.9%) | 193 (35.8%) | 251 (35.3%) | 1252 (33.9%) | |
| C | 155 (18.7%) | 169 (26.0%) | 107 (11.1%) | 83 (15.4%) | 116 (16.3%) | 630 (17.1%) | |
| D | 142 (17.1%) | 119 (18.3%) | 21 (2.2%) | 48 (8.9%) | 69 (9.7%) | 399 (10.8%) | |
| E | 13 (1.6%) | 12 (1.8%) | 2 (0.2%) | 1 (0.2%) | 3 (0.4%) | 31 (0.8%) | |
| Procedures and therapies | |||||||
| IMV | 427 (39.2%) | 241 (25.8%) | 119 (9.5%) | 151 (21.4%) | 155 (17.3%) | 1093 (22.4%) | <0.001 |
| NIPPV | 291 (26.7%) | 365 (39.0%) | 195 (15.6%) | 196 (27.8%) | 248 (27.7%) | 1295 (26.6%) | <0.001 |
| CRRT | 18 (1.7%) | 100 (10.7%) | 2 (0.2%) | 25 (3.5%) | 36 (4.0%) | 181 (3.7%) | <0.001 |
| Dialysis | 38 (3.5%) | 163 (17.4%) | 32 (2.6%) | 66 (9.4%) | 85 (9.5%) | 384 (7.9%) | <0.001 |
| Vasopressors | 394 (36.2%) | 356 (38.1%) | 192 (15.3%) | 192 (27.2%) | 238 (26.6%) | 1372 (28.1%) | <0.001 |
| Inotropes | 105 (9.7%) | 174 (18.6%) | 195 (15.6%) | 159 (22.6%) | 172 (19.2%) | 805 (16.5%) | <0.001 |
| Any vasoactives | 410 (37.7%) | 403 (43.1%) | 330 (26.3%) | 270 (38.3%) | 337 (37.7%) | 1750 (35.9%) | <0.001 |
| IABP | 200 (18.4%) | 83 (8.9%) | 157 (12.5%) | 74 (10.5%) | 86 (9.6%) | 600 (12.3%) | <0.001 |
| Other MCS | 7 (0.6%) | 16 (1.7%) | 21 (1.7%) | 11 (1.6%) | 12 (1.3%) | 67 (1.4%) | 0.192 |
| PAC | 153 (14.1%) | 162 (17.3%) | 223 (17.8%) | 158 (22.4%) | 169 (18.9%) | 865 (17.7%) | <0.001 |
| Coronary angiography | 709 (65.2%) | 410 (43.9%) | 784 (62.5%) | 369 (52.3%) | 424 (47.4%) | 2696 (55.3%) | <0.001 |
| PCI | 405 (37.2%) | 166 (17.8%) | 334 (26.6%) | 140 (19.9%) | 199 (22.2%) | 1244 (25.5%) | <0.001 |
| Transfusion | 120 (11.0%) | 199 (21.3%) | 40 (3.2%) | 142 (20.1%) | 193 (21.6%) | 694 (14.2%) | <0.001 |
| IHCA | 40 (3.7%) | 29 (3.1%) | 18 (1.4%) | 17 (2.4%) | 16 (1.8%) | 120 (2.5%) | 0.004 |
Note: Continuous variables are reported as mean (standard deviation).
Abbreviations: ACS, acute coronary syndrome; APACHE‐III, Acute Physiology and Chronic Health Evaluation‐III; CCI, Charlson Comorbidity Index; CKD, chronic kidney disease; CRRT, continuous renal replacement therapy; CS, cardiogenic shock; GWTG‐HF, Get With The Guidelines Heart Failure; HF, heart failure; IABP, intra‐aortic balloon pump; ICD‐9/10, International Classification of Diseases‐9/10; ICU, intensive care unit; IHCA, in‐hospital cardiac arrest; IMV, invasive mechanical ventilation; M‐CARS, Mayo Cardiac Intensive Care Unit Admission Risk Score; MCS, mechanical circulatory support; MI, myocardial infarction; NIPPV, noninvasive positive pressure ventilation; PAC, pulmonary artery catheter; PCI, percutaneous coronary intervention; SCAI, Society for Cardiovascular Angiography and Interventions; SOFA, Sequential Organ Failure Assessment; STEMI, ST‐elevation myocardial infarction.
Clusters
The distribution of the five clusters was as follows: 1, 22.3%; 2, 19.2%; 3, 25.7%; 4, 14.5%; and 5, 18.4% (Figure 2 ). These clusters differed substantially in terms of baseline demographics, comorbidities, admission diagnoses, illness severity and need for critical care therapies (Table 1 ). Most admission laboratory values, including those that were not used as features for clustering, differed substantially across clusters (Table 2 ). Echocardiographic features likewise varied across clusters, including markers of LVSD, RVSD and calculated haemodynamics (Table 3 ). The distribution of clusters varied modestly by age or sex and more substantially by admission diagnosis, as well as by SCAI shock stage and the severity of LVSD (Figure S3).
Table 2.
Admission laboratory values according to cluster assignment.
|
Cluster 1 ‘Inflamed’ (N = 1088) |
Cluster 2 ‘Hypoperfused’ (N = 935) |
Cluster 3 ‘Uncomplicated’ (N = 1254) |
Cluster 4 ‘Iron‐deficient’ (N = 705) |
Cluster 5 ‘Cardiorenal’ (N = 895) |
Total (N = 4877) | P value | |
|---|---|---|---|---|---|---|---|
| Basic chemistry | |||||||
| Sodium | 138.4 (4.3) | 135.2 (5.7) | 138.2 (3.9) | 136.9 (5.0) | 138.4 (4.6) | 137.5 (4.8) | <0.001 |
| Potassium | 4.2 (0.6) | 4.9 (0.8) | 4.2 (0.5) | 4.0 (0.6) | 4.3 (0.7) | 4.3 (0.7) | <0.001 |
| Bicarbonate | 22.5 (4.1) | 23.1 (5.6) | 24.9 (3.7) | 25.2 (4.7) | 25.1 (5.3) | 24.1 (4.8) | <0.001 |
| Chloride | 103.0 (5.2) | 97.0 (6.5) | 101.7 (4.7) | 99.4 (6.0) | 102.0 (5.9) | 100.8 (6.0) | <0.001 |
| Anion gap | 13.6 (3.4) | 16.0 (4.2) | 12.1 (3.0) | 12.7 (3.2) | 11.9 (3.3) | 13.2 (3.7) | <0.001 |
| BUN | 23.2 (10.0) | 57.2 (24.5) | 19.9 (8.2) | 29.2 (17.3) | 40.5 (20.4) | 32.9 (21.5) | <0.001 |
| Creatinine | 1.2 (0.5) | 2.8 (2.0) | 1.1 (0.4) | 1.4 (0.8) | 1.9 (1.2) | 1.6 (1.3) | <0.001 |
| Glucose | 179.4 (79.5) | 170.4 (83.3) | 139.9 (67.0) | 141.6 (60.1) | 138.4 (57.7) | 154.4 (72.9) | <0.001 |
| Ionized calcium | 4.6 (0.5) | 4.6 (0.5) | 4.8 (0.3) | 4.7 (0.3) | 4.7 (0.4) | 4.7 (0.4) | <0.001 |
| Phosphorous | 3.1 (1.1) | 4.1 (1.6) | 3.4 (0.8) | 3.7 (1.1) | 3.4 (1.1) | 3.5 (1.2) | <0.001 |
| Magnesium | 2.0 (0.3) | 2.2 (0.4) | 2.0 (0.3) | 2.0 (0.3) | 2.1 (0.4) | 2.1 (0.4) | <0.001 |
| Other chemistries | |||||||
| Albumin | 3.3 (0.5) | 3.2 (0.6) | 3.6 (0.5) | 3.2 (0.6) | 3.3 (0.6) | 3.3 (0.6) | <0.001 |
| AST | 238.7 (626.7) | 402.8 (1265.3) | 68.9 (114.2) | 115.7 (537.1) | 142.2 (651.1) | 204.4 (764.7) | <0.001 |
| ALT | 138.1 (397.5) | 281.0 (758.1) | 55.1 (107.5) | 80.9 (300.7) | 101.9 (349.0) | 138.2 (459.3) | <0.001 |
| Alkaline phosphatase | 90.1 (50.8) | 123.7 (86.1) | 82.0 (35.1) | 120.9 (87.9) | 109.6 (78.4) | 104.4 (71.4) | <0.001 |
| Bilirubin | 0.9 (1.2) | 1.3 (2.7) | 0.8 (0.7) | 1.1 (1.6) | 1.3 (1.6) | 1.1 (1.7) | <0.001 |
| C‐reactive protein | 72.1 (83.0) | 84.8 (80.8) | 44.1 (66.7) | 68.3 (75.6) | 65.0 (67.6) | 68.1 (77.2) | 0.008 |
| NT‐proBNP | 9015.8 (11 395.9) | 17 150.2 (16 459.2) | 4366.7 (5961.7) | 10 463.4 (12 767.7) | 11 581.8 (13 323.2) | 10 649.3 (13 263.2) | <0.001 |
| Initial troponin T | 1.76 (3.45) | 1.02 (2.22) | 0.89 (2.54) | 0.68 (1.72) | 0.80 (2.97) | 1.11 (2.79) | <0.001 |
| Peak troponin T | 3.13 (5.26) | 1.64 (3.24) | 1.42 (3.22) | 1.17 (2.73) | 1.42 (4.42) | 1.91 (4.10) | <0.001 |
| Complete blood count | |||||||
| Haemoglobin | 13.1 (1.9) | 10.9 (2.1) | 13.0 (1.6) | 10.1 (1.5) | 10.5 (1.7) | 11.8 (2.2) | <0.001 |
| Platelets | 234.0 (84.0) | 220.7 (92.3) | 201.9 (66.1) | 250.7 (103.3) | 139.1 (51.0) | 208.2 (87.6) | <0.001 |
| WBC | 15.3 (5.6) | 12.9 (8.1) | 8.3 (2.8) | 9.9 (4.9) | 7.2 (2.5) | 10.8 (6.0) | <0.001 |
| Neutrophils | 12.7 (4.9) | 10.6 (5.0) | 5.6 (1.9) | 7.5 (3.6) | 5.4 (2.2) | 8.4 (4.8) | <0.001 |
| Lymphocytes | 1.1 (0.6) | 1.0 (1.2) | 1.7 (1.4) | 1.4 (3.1) | 1.0 (0.7) | 1.3 (1.6) | <0.001 |
| Monocytes | 1.0 (0.5) | 0.9 (0.6) | 0.7 (0.3) | 0.8 (0.4) | 0.6 (0.3) | 0.8 (0.5) | <0.001 |
| Eosinophils | 0.1 (0.2) | 0.1 (0.1) | 0.1 (0.2) | 0.1 (0.2) | 0.1 (0.2) | 0.1 (0.2) | <0.001 |
| NLR | 15.0 (12.7) | 16.1 (14.9) | 4.0 (2.3) | 8.9 (9.7) | 7.5 (5.8) | 10.1 (11.1) | <0.001 |
| Acid–base | |||||||
| Lactate | 2.7 (2.1) | 2.7 (2.5) | 1.9 (1.8) | 1.9 (1.7) | 1.8 (1.4) | 2.4 (2.1) | <0.001 |
| Arterial pH | 7.3 (0.1) | 7.4 (0.1) | 7.4 (0.1) | 7.4 (0.1) | 7.4 (0.1) | 7.4 (0.1) | <0.001 |
| Arterial PaCO2 | 41.6 (10.0) | 41.2 (12.8) | 42.3 (11.3) | 40.8 (10.5) | 43.6 (13.3) | 41.9 (11.6) | 0.019 |
| Arterial base excess | −2.8 (4.7) | −1.7 (6.2) | 0.8 (4.8) | 0.8 (5.4) | 0.8 (5.9) | −0.7 (5.7) | <0.001 |
Note: Continuous variables are reported as mean (standard deviation). Note that the prevalence of missing values is reported in Table S1 .
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; NLR, neutrophil‐to‐lymphocyte ratio; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PaCO2, arterial partial pressure of carbon dioxide; WBC, white blood cell count.
Table 3.
Echocardiographic findings for patients with a TTE within 1 day of CICU admission according to cluster assignment.
|
Cluster 1 ‘Inflamed’ (N = 808) |
Cluster 2 ‘Hypoperfused’ (N = 587) |
Cluster 3 ‘Uncomplicated’ (N = 745) |
Cluster 4 ‘Iron‐deficient’ (N = 396) |
Cluster 5 ‘Cardiorenal’ (N = 462) |
Total (N = 2998) |
N with data |
P value | |
|---|---|---|---|---|---|---|---|---|
| Vital signs | ||||||||
| Systolic BP | 114.73 (21.86) | 113.24 (22.69) | 118.88 (22.83) | 114.93 (21.01) | 114.05 (22.65) | 115.39 (22.37) | 2854 | <0.001 |
| Diastolic BP | 64.66 (14.72) | 60.90 (14.71) | 68.21 (14.34) | 63.87 (13.32) | 60.80 (14.15) | 64.10 (14.63) | 2851 | <0.001 |
| Heart rate | 83.16 (20.33) | 83.75 (19.84) | 76.13 (18.25) | 83.94 (19.23) | 78.93 (19.60) | 80.98 (19.73) | 2774 | <0.001 |
| AF | 112 (15.9%) | 146 (28.5%) | 81 (12.3%) | 90 (26.9%) | 122 (32.0%) | 551 (21.3%) | 2590 | <0.001 |
| LV structure and function | ||||||||
| LVEF | 37.29 (15.73) | 40.02 (18.00) | 39.26 (15.69) | 41.38 (17.70) | 43.29 (17.86) | 39.75 (16.89) | 2397 | <0.001 |
| LVSD group | 2397 | <0.001 | ||||||
| Normal | 160 (20.7%) | 163 (29.0%) | 161 (23.2%) | 118 (32.9%) | 162 (37.9%) | 764 (27.1%) | ||
| Mild LVSD | 103 (13.3%) | 89 (15.8%) | 130 (18.7%) | 43 (12.0%) | 58 (13.6%) | 423 (15.0%) | ||
| Moderate LVSD | 246 (31.9%) | 129 (22.9%) | 197 (28.3%) | 102 (28.4%) | 94 (22.0%) | 768 (27.3%) | ||
| Severe LVSD | 263 (34.1%) | 182 (32.3%) | 207 (29.8%) | 96 (26.7%) | 114 (26.6%) | 862 (30.6%) | ||
| HFpEF (LVEF ≥ 50%) | 242 (26.3%) | 270 (37.3%) | 248 (28.1%) | 205 (39.5%) | 278 (43.9%) | 1243 (33.8%) | 2397 | <0.001 |
| LVEDD | 52.82 (8.81) | 54.54 (9.78) | 55.48 (9.64) | 54.22 (10.86) | 54.08 (9.19) | 54.21 (9.59) | 2550 | <0.001 |
| LVESD | 41.26 (10.80) | 42.36 (12.90) | 43.29 (11.82) | 41.66 (13.17) | 41.23 (11.97) | 42.05 (12.03) | 2123 | 0.037 |
| LV mass index | 115.23 (35.81) | 126.00 (41.50) | 123.28 (38.33) | 123.56 (44.29) | 126.75 (37.38) | 122.22 (39.16) | <0.001 | |
| Fractional shortening | 22.33 (10.17) | 23.56 (11.77) | 22.72 (10.04) | 24.13 (11.42) | 24.71 (11.47) | 23.29 (10.88) | 2120 | 0.010 |
| LV WMS index | 2.08 (0.45) | 2.05 (0.50) | 2.02 (0.47) | 2.05 (0.44) | 2.00 (0.48) | 2.04 (0.47) | 1793 | 0.193 |
| Forward flow | ||||||||
| LVOT peak velocity | 0.95 (0.20) | 0.96 (0.22) | 0.96 (0.20) | 0.98 (0.25) | 0.99 (0.23) | 0.97 (0.22) | 2453 | 0.024 |
| LVOT VTI | 16.95 (4.59) | 17.60 (5.62) | 18.44 (5.39) | 17.73 (5.00) | 19.15 (5.87) | 17.90 (5.32) | 2452 | <0.001 |
| Stroke volume | 66.23 (21.09) | 68.74 (23.50) | 74.22 (22.72) | 68.91 (22.49) | 75.48 (24.93) | 70.57 (23.06) | 2425 | <0.001 |
| Stroke volume index | 34.04 (10.28) | 35.42 (12.21) | 37.49 (10.62) | 35.49 (10.89) | 39.41 (12.51) | 36.23 (11.35) | 2397 | <0.001 |
| Cardiac output | 5.17 (1.57) | 5.46 (1.78) | 5.40 (1.59) | 5.55 (1.82) | 5.57 (1.81) | 5.39 (1.70) | 2392 | 0.001 |
| Cardiac index | 2.66 (0.76) | 2.81 (0.91) | 2.73 (0.72) | 2.87 (0.93) | 2.90 (0.88) | 2.77 (0.83) | 2370 | <0.001 |
| CPO | 0.94 (0.34) | 0.95 (0.37) | 1.02 (0.36) | 1.00 (0.39) | 0.97 (0.35) | 0.97 (0.36) | 2351 | <0.001 |
| LVSWI | 30.77 (11.04) | 28.77 (12.77) | 36.15 (12.66) | 30.74 (12.63) | 32.81 (13.42) | 32.25 (12.65) | 1773 | <0.001 |
| MCF | 0.34 (0.14) | 0.32 (0.15) | 0.35 (0.14) | 0.33 (0.14) | 0.36 (0.14) | 0.34 (0.14) | 1947 | 0.004 |
| SVRI | 28.62 (10.33) | 26.21 (19.28) | 29.59 (9.97) | 26.11 (9.16) | 24.67 (9.32) | 27.44 (12.43) | 2139 | <0.001 |
| Diastolic function | ||||||||
| Medial mitral e′ | 5.27 (2.19) | 4.92 (1.77) | 5.44 (2.15) | 5.44 (2.53) | 5.13 (2.01) | 5.25 (2.13) | 2031 | 0.003 |
| Mitral E wave | 0.81 (0.31) | 0.99 (0.32) | 0.84 (0.30) | 0.99 (0.33) | 1.00 (0.34) | 0.90 (0.33) | 2052 | <0.001 |
| Mitral E/A ratio | 1.22 (0.79) | 1.47 (0.85) | 1.23 (0.70) | 1.60 (1.07) | 1.50 (1.04) | 1.34 (0.85) | 1467 | <0.001 |
| Medial E/e′ ratio | 17.18 (9.20) | 22.28 (10.44) | 17.30 (8.73) | 21.00 (10.72) | 21.95 (9.88) | 19.28 (9.86) | 1899 | <0.001 |
| Right ventricular function and pulmonary haemodynamics | ||||||||
| TR velocity | 2.81 (0.50) | 2.97 (0.54) | 2.80 (0.50) | 3.05 (0.62) | 3.04 (0.59) | 2.91 (0.55) | 2264 | <0.001 |
| RA pressure | 11.16 (5.26) | 13.20 (5.42) | 9.46 (4.97) | 12.46 (5.34) | 13.15 (5.33) | 11.64 (5.44) | 2464 | <0.001 |
| RVSP | 44.08 (13.38) | 49.96 (14.76) | 42.16 (13.88) | 51.71 (18.95) | 51.46 (16.17) | 47.08 (15.61) | 2234 | <0.001 |
| TASV | 11.05 (3.43) | 9.86 (3.43) | 11.33 (3.51) | 10.10 (3.52) | 10.30 (3.90) | 10.68 (3.58) | 1786 | <0.001 |
| Tricuspid annulus to RVSP ratio | 0.27 (0.11) | 0.21 (0.10) | 0.30 (0.14) | 0.21 (0.11) | 0.22 (0.11) | 0.25 (0.12) | 1509 | <0.001 |
| PA elastance | 0.74 (0.34) | 0.83 (0.40) | 0.65 (0.35) | 0.85 (0.44) | 0.77 (0.41) | 0.76 (0.39) | 2003 | <0.001 |
| Pressure‐adjusted heart rate | 11.56 (6.78) | 14.43 (7.48) | 8.99 (6.12) | 13.18 (7.23) | 13.78 (7.62) | 12.06 (7.26) | 2322 | <0.001 |
| Global RV function | 2046 | <0.001 | ||||||
| Normal or borderline RV function | 183 (35.1%) | 86 (19.4%) | 192 (40.2%) | 82 (28.9%) | 83 (26.1%) | 626 (30.6%) | ||
| Mild RV dysfunction | 174 (33.3%) | 154 (34.7%) | 168 (35.1%) | 85 (29.9%) | 105 (33.0%) | 686 (33.5%) | ||
| Moderate to severe RV dysfunction | 165 (31.6%) | 204 (45.9%) | 118 (24.7%) | 117 (41.2%) | 130 (40.9%) | 734 (35.9%) | ||
| Biventricular dysfunction | 2817 | <0.001 | ||||||
| No RV/LV dysfunction | 264 (34.2%) | 215 (38.2%) | 302 (43.5%) | 130 (36.2%) | 184 (43.0%) | 1095 (38.9%) | ||
| LV dysfunction | 346 (44.8%) | 147 (26.1%) | 277 (39.9%) | 114 (31.8%) | 117 (27.3%) | 1001 (35.5%) | ||
| RV dysfunction | 40 (5.2%) | 59 (10.5%) | 30 (4.3%) | 50 (13.9%) | 50 (11.7%) | 229 (8.1%) | ||
| Biventricular dysfunction | 122 (15.8%) | 142 (25.2%) | 86 (12.4%) | 65 (18.1%) | 77 (18.0%) | 492 (17.5%) | ||
Note: Continuous variables are reported as mean (standard deviation).
Abbreviations: AF, atrial fibrillation; BP, blood pressure; CICU, cardiac intensive care unit; CPO, cardiac power output; HFpEF, heart failure with preserved ejection fraction; LV, left ventricular; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; LVOT, left ventricular outflow tract; LVSD, left ventricular systolic dysfunction; LVSWI, left ventricular stroke work index; MCF, myocardial contraction fraction; PA, pulmonary artery; RA, right atrial; RV, right ventricular; RVSP, right ventricular systolic pressure; SVRI, systemic vascular resistance index; TASV, tricuspid annular systolic velocity; TR, tricuspid regurgitation; TTE, transthoracic echocardiogram; VTI, velocity time integral; WMS, wall motion score.
In‐hospital mortality
A total of 524 (10.7%) patients died during hospitalization, including 276 (5.7%) dying in the CICU. In‐hospital mortality differed substantially between clusters (Figure 2 ), rising incrementally for Cluster 3 (lowest), Cluster 4, Cluster 5, Cluster 1 and Cluster 2 (highest). This pattern was similar when patients were stratified by age/sex (Figure 3 ), admission diagnosis (Figure 3 ), GWTG‐HF risk score (Figure 4 A ), SCAI shock stage (Figure 4 B ), or the aetiology (ischaemic vs. nonischaemic) or pattern (de novo vs. acute on chronic) of HF (Figure S4). In‐hospital mortality differed across clusters when stratified into low‐risk and high‐risk groups by the M‐CARS (Figure 5 A ) and when stratified according to the severity of LVSD (Figure 5 B ) and RVSD (Figure S5). When compared with the low‐risk Cluster 3, in‐hospital mortality was higher in each other cluster both before and after multivariable adjustment, with the risk remaining highest in Cluster 2 (Table 4 ).
Figure 3.
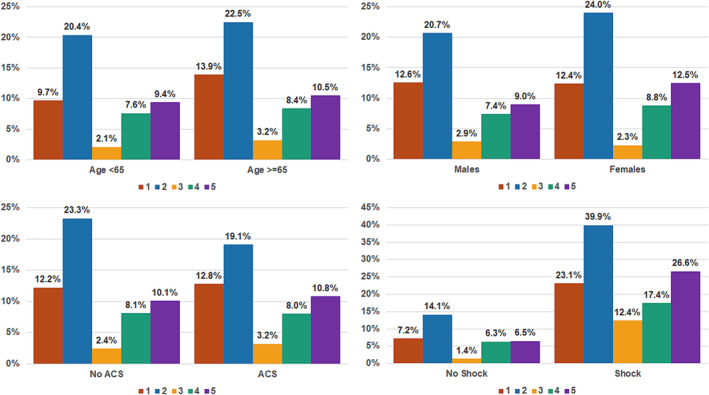
In‐hospital mortality by cluster according to age, sex and admission diagnosis.
Figure 4.
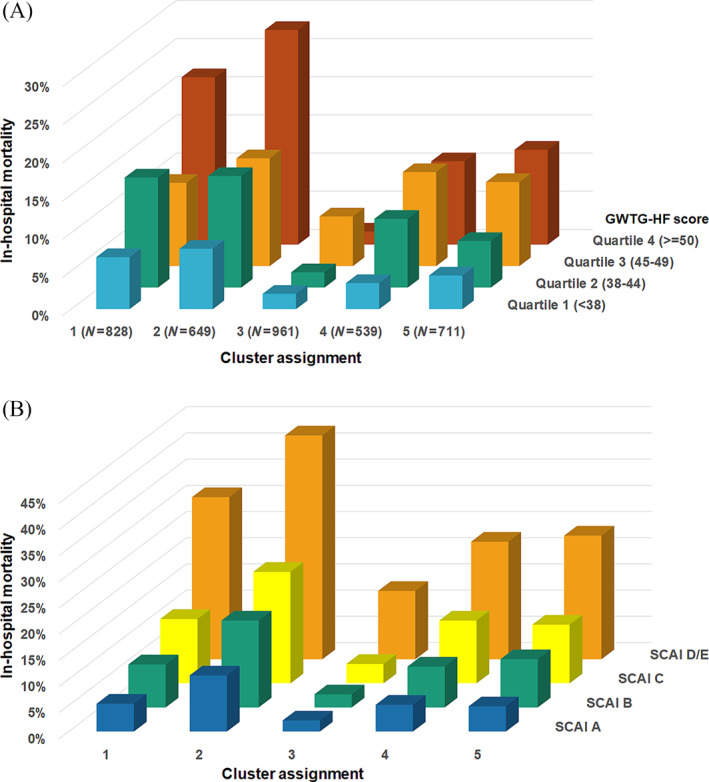
In‐hospital mortality by cluster according to Get With The Guidelines Heart Failure (GWTG‐HF) risk score quartile (A) and Society for Cardiovascular Angiography and Interventions (SCAI) shock stage (B).
Figure 5.
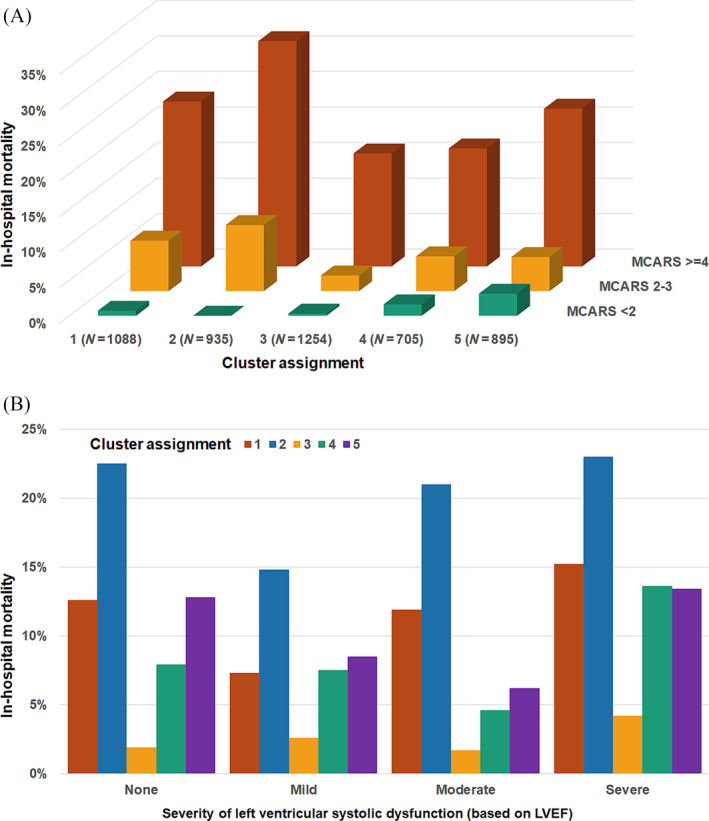
In‐hospital mortality by cluster assignment according to the Mayo Cardiac Intensive Care Unit Admission Risk Score (M‐CARS) risk group (A) and severity of left ventricular systolic dysfunction by the American Society of Echocardiography (ASE) guidelines (B). LVEF, left ventricular ejection fraction.
Table 4.
Results of regression models for prediction of in‐hospital (logistic) and 1 year (Cox) mortality by the cluster assignment, before and after adjustment for age, sex, CCI, Day 1 SOFA score, admission Braden score, and admission diagnoses of ACS, shock, cardiac arrest and respiratory failure.
| In‐hospital mortality | ||||
|---|---|---|---|---|
| Cluster | Unadjusted OR | P value | Adjusted OR | P value |
| Uncomplicated | Referent | — | Referent | — |
| Iron‐deficient | 3.156 (2.054–4.921) | <0.001 | 2.177 (1.381–3.478) | <0.001 |
| Cardiorenal | 4.111 (2.774–6.232) | <0.001 | 2.112 (1.371–3.317) | <0.001 |
| Inflamed | 5.126 (3.530–7.647) | <0.001 | 1.788 (1.182–2.765) | 0.007 |
| Hypoperfused | 10.077 (7.026–14.888) | <0.001 | 4.318 (2.891–6.615) | <0.001 |
| One‐year mortality—Overall | ||||
|---|---|---|---|---|
| Cluster | Unadjusted HR | P value | Adjusted HR | P value |
| Uncomplicated | Referent | — | Referent | — |
| Iron‐deficient | 3.293 (2.683–4.042) | <0.001 | 2.469 (2.002–3.045) | <0.001 |
| Cardiorenal | 4.107 (3.395–4.969) | <0.001 | 2.411 (1.970–2.949) | <0.001 |
| Inflamed | 2.560 (2.101–3.118) | <0.001 | 1.561 (1.269–1.920) | <0.001 |
| Hypoperfused | 6.215 (5.172–7.467) | <0.001 | 3.434 (2.817–4.184) | <0.001 |
| One‐year mortality—Hospital survivors | ||||
|---|---|---|---|---|
| Cluster | Unadjusted HR | P value | Adjusted HR | P value |
| Uncomplicated | Referent | — | Referent | — |
| Iron‐deficient | 3.443 (2.725–4.350) | <0.001 | 2.733 (2.150–3.473) | <0.001 |
| Cardiorenal | 4.259 (3.427–5.294) | <0.001 | 2.657 (2.109–3.348) | <0.001 |
| Inflamed | 1.826 (1.435–2.324) | <0.001 | 1.451 (1.130–1.864) | 0.004 |
| Hypoperfused | 5.241 (4.220–6.510) | <0.001 | 3.264 (2.584–4.123) | <0.001 |
Abbreviations: ACS, acute coronary syndrome; CCI, Charlson Comorbidity Index; HR, hazard ratio; OR, odds ratio; SOFA, Sequential Organ Failure Assessment.
One‐year mortality
A total of 1537 (31.5%) patients died within 1 year of CICU admission, including in‐hospital deaths. Among the 4353 hospital survivors, 1013 (23.3%) died by 1 year, and 460 had follow‐up <1 year and were alive at last follow‐up. One‐year survival by the Kaplan–Meier method differed between clusters, both overall (Figure 6 A ) and for hospital survivors (Figure 6 B ), increasing incrementally for Cluster 3, Cluster 1, Cluster 4, Cluster 5 and Cluster 2. Fewer than half of patients in Cluster 2 survived to 1 year (median survival: 8.1 months). On Cox proportional hazard regression, 1 year mortality differed between clusters before and after multivariable adjustment, both overall and for hospital survivors (Table 4 ).
Figure 6.
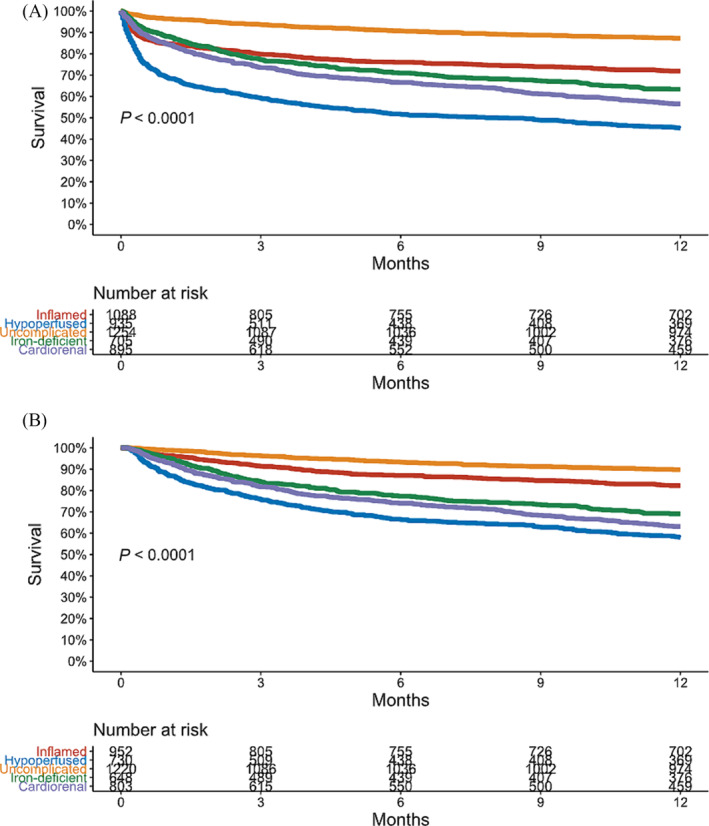
Kaplan–Meier curves demonstrating 1 year survival by cluster, overall (A) and among hospital survivors (B).
Discussion
Within a cohort of nearly 5000 CICU patients with HF, we identified five distinct subphenotypes based on admission laboratory values using unsupervised machine learning clustering. These subphenotypes differed across a multitude of clinical, laboratory and echocardiographic variables even beyond those features used for clustering. Both in‐hospital and 1 year mortality (including among hospital survivors) differed substantially between subphenotypes, even after adjusting for severity of illness and other relevant covariates. We identified one high‐risk phenotype, one low‐risk phenotype and three intermediate‐risk phenotypes. Differences in mortality between subphenotypes were observed even when stratified for a variety of important characteristics including admission diagnosis, shock severity and overall illness severity. The subphenotype grouping outperformed traditional HF classifiers (e.g., aetiology or chronicity) for mortality risk stratification and provided added risk stratification beyond established prognostic markers. This proof‐of‐concept analysis demonstrates that prognostically important occult patient subgroups can be identified within the heterogeneous CICU HF population beyond traditional labels. Clustering patients based on patterns of common admission laboratory values can identify clinically relevant subphenotypes with distinct underlying pathophysiology.
The admission laboratory values we used to define our subphenotypes (potassium, chloride, anion gap, BUN, haemoglobin, RDW, MCV, platelet count, WBC count and NLR) are widely available for hospitalized patients and have shown an association with outcomes in CICU patients or patients with HF reflecting the effects of acute and chronic diseases on end‐organ function. 4 , 23 , 27 , 29 , 30 , 31 , 32 , 33 We named the subphenotypes based on our interpretation of their characteristic patterns of laboratory findings based on cluster centroids (Figure 1 ), recognizing that these subjective labels may not describe the true underlying pathophysiology. Our algorithm assigned patients to the cluster they most closely resembled, recognizing that many patients had features of two or more clusters.
The inflamed subphenotype (Cluster 1) displayed leucocytosis and an elevated NLR with a relatively high severity of illness. This subphenotype had an intermediate risk of short‐term mortality and a more favourable long‐term outcome among hospital survivors, despite frequent evidence of hypoperfusion/shock with poor echocardiographic haemodynamics. This could relate to the predominance of ACS patients, who presumably had a reversible disease process and were at lower risk of adjusted mortality. The hypoperfused subphenotype (Cluster 2) had severe kidney dysfunction, multi‐organ dysfunction, and anion gap acidosis with the worst TTE haemodynamics and RV dysfunction. This cluster had the highest risk of death at all time points despite a similar prevalence and severity of shock versus Cluster 1, perhaps representing the development of haemometabolic shock or cardiorenal syndrome with multi‐organ dysfunction. 21 , 22 Low chloride levels, as observed in this group, can be associated with advanced HF, cardiorenal syndrome, poor diuretic response and adverse outcomes. 5 , 29 , 33 This group with extensive physiological abnormalities and poor outcome could be identified using machine learning based on routine laboratory values available on initial evaluation in the CICU, in a manner that could be leveraged using modern electronic health record systems.
The low‐risk uncomplicated subphenotype (Cluster 3) was the largest cluster and had favourable values of most clinical, laboratory and echocardiography variables (despite a greater prevalence of LVSD and frequent use of vasoactive drugs), resulting in the best outcomes at all time points. The two least prevalent subphenotypes, iron‐deficient (Cluster 4) and cardiorenal (Cluster 5), both had more anaemia and differed from each other primarily based on other haematologic indices, along with worse renal function in the cardiorenal cluster. Both anaemic subphenotypes were at intermediate risk of short‐term mortality but had worse long‐term survival than the more acutely ill inflamed subphenotype, implying a higher level of chronic illness and perhaps reflecting a greater prevalence of advanced HF. The similar outcomes and presence of anaemia could justify combining these two phenotypes, but the divergent haematologic and renal parameters support keeping them separate as per the clustering algorithm.
Prior analyses have used unsupervised machine learning clustering methods to define subphenotypes in acute HF populations, although ours is the first to focus on a large cohort of CICU patients with HF. Horiuchi et al. used clinical, laboratory, echocardiographic and electrocardiogram (ECG) variables to describe three clusters in 345 patients with acute HF. 16 The identified clusters were characterized as ‘vascular failure’, with hypertension and pulmonary oedema; ‘cardiac failure’, with cardiorenal syndrome; and a third group consistent with chronic HFpEF. Murray et al. did a post hoc analysis of 812 hospitalized HFpEF patients in the ASCEND‐HF trial, reporting four clusters based on clinical variables that differed in terms of cardiac and non‐cardiac organ function as well as long‐term prognosis. 20 Unlike our analysis, the differences in reported laboratory values were comparatively modest, and clusters differed most notably in terms of demographic factors and vital signs. Several prior studies in chronic HF patients highlight the potential usefulness of unsupervised machine learning for identifying potential phenotypes with different prognosis and response to treatment. 13 , 14 , 17 , 18 , 19
Zweck et al. used k‐means clustering to define subphenotypes in patients with CS based on admission laboratory values [WBC, bicarbonate, glomerular filtration rate (GFR), lactate, alanine aminotransferase (ALT) and platelet count]. 21 The three proposed phenotypes (noncongested, cardiorenal and haemometabolic) were associated with differences in mortality even after stratification for shock severity, including in a validation study from this CICU cohort, with marked differences in clinical profile, echocardiographic findings, and both short‐ and long‐term outcomes observed. 22 In the current analysis, we used a different set of admission laboratory values in a larger cohort of CICU patients with HF including predominantly those without CS. Despite only a minority being labelled with CS, our highest risk subphenotype (hypoperfused/Cluster 2) carries many similarities with the highest risk CS phenotype (haemometabolic), including poor kidney function, metabolic acidosis and transaminitis. 21 , 22 These analyses emphasize the importance of hypoperfusion and end‐organ dysfunction (particularly acute and chronic cardiorenal syndrome) as key determinants of adverse outcomes in critically ill patients with circulatory failure. Underlying echocardiographic and haemodynamic features shared by the haemometabolic CS subphenotype and our hypoperfused (Cluster 2) subphenotype suggest RV congestion and dysfunction as a driver of end‐organ injury. 21 , 22
Limitations
Our study carries the same inherent limitations of all retrospective cohort studies and cannot infer causal relationships. We chose to perform a complete‐case analysis similar to prior authors, which could have resulted in selection bias by excluding those with missing data who likely differed from included patients as data were not missing at random; we chose not to perform multiple imputation because of the apparent violation of the missing‐at‐random assumption. 13 , 20 The degree of missingness (i.e., >50% missing values) forced us to exclude several potentially relevant laboratory values as features in the clustering, such as transaminases, lactate, albumin and inflammatory markers. We did not include laboratory values that are used to define conditions such as ACS (e.g., troponins) or HF (e.g., natriuretic peptides), which enabled us to demonstrate conservation of subphenotypes across diagnosis groups. We focused only on commonly available basic laboratory values as features for clustering, while prior analyses have used a broader array of clinical and patient‐level variables. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 This approach could have excluded important clinical variables that might have improved subphenotype definitions but has the strength of being simple and objective, allowing automatic application using an electronic health record system. We used prediction of in‐hospital mortality as a criterion to select between highly correlated candidate feature variables, which could have resulted in bias resulting from data leakage with resultant overfitting for prediction of in‐hospital mortality; we used logistic regression for this task, recognizing that this method may be insensitive to nonlinear associations. Only a minority of candidate feature variables (i.e., 4 of 10) were selected in this manner, but this could have exaggerated differences in mortality between subphenotypes. Our heterogeneous CICU cohort represents those with both acute and chronic HF, which includes patients with ACS, shock and other acute conditions, and may be less relevant for a pure acute HF population. Cluster assignment showed similar associations with mortality across all relevant subgroups, and the clusters we identified showed similarities to those described in prior cohorts. 14 , 21 The clusters we identified in our population may be unique to this specific CICU population and would not necessarily be the same in a non‐intensive care unit (ICU) HF population or in HF patients admitted to a different type of ICU. In any unsupervised machine learning clustering analysis, groups will be identified, but this does not guarantee that the groups are reproducible or meaningful, and a different clustering method could have divergent results necessitating external validation; the lack of an external validation cohort makes our findings exploratory and hypothesis‐generating. 12 , 20 Selecting the optimal number of clusters involved some subjectivity, and we may not have chosen the ideal number; it is conceivable that no true occult subphenotypes exist in this cohort. 12 There are numerous methods to define the optimal number of clusters in a data‐driven manner, with limited consensus about the ideal approach. We chose to use the elbow plot based on its simplicity, recognizing that identification of the inflection point can be subjective; we specifically wanted to ensure that the identified subgroups were of suitable size and divergent mortality risk. We could not calculate other potential metrics, such as the gap statistic or silhouette values, which can be used for this purpose. While the clusters identified in our analysis had systematic differences in mean values of feature variables (i.e., cluster centroids), there was substantial overlap between clusters on most laboratory values, and many individual patients fell on the border between clusters. This proof‐of‐concept analysis is hypothesis‐generating with the goal of identifying patterns within a heterogeneous group that may hold insights into underlying disease processes that might have a differential response to therapy. We do not have sufficient data on in‐hospital or post‐discharge treatments that could have impacted outcomes and cannot comment on whether the subphenotypes we observed responded differently to treatments.
Conclusions
Within a large, diverse population of CICU patients spanning the spectrum of HF, we identified five clinically relevant subphenotypes based on standard admission laboratory values. These subphenotypes defined distinct patient profiles that differed not only in their laboratory findings but also in clinical variables and echocardiographic measurements. These subphenotypes stratified the risk of short‐term and long‐term mortality across subgroups, with one low‐risk subphenotype, one high‐risk subphenotype and three intermediate‐risk subphenotypes. The mortality risk stratification provided by the subphenotype assignment was additive to established prognostic marker and outperformed traditional HF phenotype assignments. Unsupervised machine learning can be applied to common laboratory values for HF patients at the time of CICU admission to identify subphenotypes with divergent underlying pathophysiology. Future studies will be needed to externally validate these subphenotypes and to determine whether they are truly associated with differences in underlying disease mechanisms and treatment responses. If heterogeneity of treatment effect for specific therapies can be demonstrated across these subphenotypes, then the simple laboratory subphenotypes could improve risk stratification and facilitate individualized therapy for critically ill patients with HF.
Conflict of interest statement
The authors have no relevant financial disclosures or conflicts of interest related to this manuscript.
Supporting information
Table S1. Univariable logistic regression models for prediction of in‐hospital mortality with all available admission laboratory variables in the final study population.
Table S2. Baseline characteristics of the final study population and CICU patients with HF who were excluded due to one or missing laboratory variable of interest.
Figure S1. Correlation matrix showing Pearson correlations among selected candidate laboratory feature variables in the final study cohort, with web plot demonstrating these same data.
Figure S2. Elbow plot demonstrating the between‐clusters and within‐clusters sum of squares, demonstrating a subtle inflection point at 5 clusters.
Figure S3. Distribution of clusters according to patient characteristics. Cluster 1 = Inflamed; Cluster 2 = Hypoperfused; Cluster 3 = Uncomplicated; Cluster 4 = Iron‐Deficient; Cluster 5 = Cardiorenal.
Figure S4. In‐hospital mortality according to subphenotype and etiology (ischemic versus nonischemic) or pattern (de novo versus acute on chronic) HF.
Figure S5. In‐hospital mortality according to subphenotype and pattern of ventricular dysfunction based on moderate or greater RV and LV dysfunction by TTE.
Jentzer, J. C. , Reddy, Y. N. V. , Soussi, S. , Crespo‐Diaz, R. , Patel, P. C. , Lawler, P. R. , Mebazaa, A. , and Dunlay, S. M. (2024) Unsupervised machine learning to identify subphenotypes among cardiac intensive care unit patients with heart failure. ESC Heart Failure, 11: 4242–4256. 10.1002/ehf2.15027.
References
- 1. Jentzer JC, van Diepen S, Barsness GW, Katz JN, Wiley BM, Bennett CE, et al. Changes in comorbidities, diagnoses, therapies and outcomes in a contemporary cardiac intensive care unit population. Am Heart J 2019;215:12‐19. doi: 10.1016/j.ahj.2019.05.012 [DOI] [PubMed] [Google Scholar]
- 2. Bohula EA, Katz JN, van Diepen S, Alviar CL, Baird‐Zars VM, Park JG, et al. Demographics, care patterns, and outcomes of patients admitted to cardiac intensive care units: The Critical Care Cardiology Trials Network prospective North American multicenter registry of cardiac critical illness. JAMA Cardiol 2019;4:928‐935. doi: 10.1001/jamacardio.2019.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jentzer JC, van Diepen S, Murphree DH, Ismail AS, Keegan MT, Morrow DA, et al. Admission diagnosis and mortality risk prediction in a contemporary cardiac intensive care unit population. Am Heart J 2020;224:57‐64. doi: 10.1016/j.ahj.2020.02.018 [DOI] [PubMed] [Google Scholar]
- 4. Jentzer JC, Reddy YN, Rosenbaum AN, Dunlay SM, Borlaug BA, Hollenberg SM. Outcomes and predictors of mortality among cardiac intensive care unit patients with heart failure. J Card Fail 2022;28:1088‐1099. doi: 10.1016/j.cardfail.2022.02.015 [DOI] [PubMed] [Google Scholar]
- 5. Jentzer JC, Redfield MM, Killian J, Katz JN, Roger VL, Dunlay SM. Advanced heart failure in the cardiac intensive care unit: A community‐based study. JACC Heart Fail 2023;11:252‐254. doi: 10.1016/j.jchf.2022.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lyle M, Wan SH, Murphree D, Bennett C, Wiley BM, Barsness G, et al. Predictive value of the Get With The Guidelines Heart Failure risk score in unselected cardiac intensive care unit patients. J Am Heart Assoc 2020;9:e012439. doi: 10.1161/JAHA.119.012439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O'Connor KD, Yamamoto Y, Sen S, Samsky MD, Wilson FP, Desai N, et al. Risk prediction for heart failure patients admitted to the intensive care unit: Insights from REVeAL‐HF. JACC Heart Fail 2023;11:727‐728. doi: 10.1016/j.jchf.2023.01.021 [DOI] [PubMed] [Google Scholar]
- 8. Javaloyes P, Miro O, Gil V, Martín‐Sánchez FJ, Jacob J, Herrero P, et al. Clinical phenotypes of acute heart failure based on signs and symptoms of perfusion and congestion at emergency department presentation and their relationship with patient management and outcomes. Eur J Heart Fail 2019;21:1353‐1365. doi: 10.1002/ejhf.1502 [DOI] [PubMed] [Google Scholar]
- 9. Nowak RM, Reed BP, DiSomma S, Nanayakkara P, Moyer M, Millis S, et al. Presenting phenotypes of acute heart failure patients in the ED: Identification and implications. Am J Emerg Med 2017;35:536‐542. doi: 10.1016/j.ajem.2016.12.003 [DOI] [PubMed] [Google Scholar]
- 10. Gonzalez‐Pacheco H, Alvarez‐Sangabriel A, Martinez‐Sanchez C, Briseño‐Cruz JL, Altamirano‐Castillo A, Mendoza‐García S, et al. Clinical phenotypes, aetiologies, management, and mortality in acute heart failure: A single‐institution study in Latin‐America. ESC Heart Fail 2021;8:423‐437. doi: 10.1002/ehf2.13092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chioncel O, Mebazaa A, Harjola VP, Coats AJ, Piepoli MF, Crespo‐Leiro MG, et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: The ESC Heart Failure Long‐Term Registry. Eur J Heart Fail 2017;19:1242‐1254. doi: 10.1002/ejhf.890 [DOI] [PubMed] [Google Scholar]
- 12. Jentzer JC, Rayfield C, Soussi S, Berg DD, Kennedy JN, Sinha SS, et al. Machine learning approaches for phenotyping in cardiogenic shock and critical illness: Part 2 of 2. JACC: Advances 2022;1:1‐14. doi: 10.1016/j.jacadv.2022.100126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ahmad T, Pencina MJ, Schulte PJ, O'Brien E, Whellan DJ, Piña IL, et al. Clinical implications of chronic heart failure phenotypes defined by cluster analysis. J Am Coll Cardiol 2014;64:1765‐1774. doi: 10.1016/j.jacc.2014.07.979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 2015;131:269‐279. doi: 10.1161/CIRCULATIONAHA.114.010637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahmad T, Lund LH, Rao P, Ghosh R, Warier P, Vaccaro B, et al. Machine learning methods improve prognostication, identify clinically distinct phenotypes, and detect heterogeneity in response to therapy in a large cohort of heart failure patients. J Am Heart Assoc 2018;7:7. doi: 10.1161/JAHA.117.008081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Horiuchi Y, Tanimoto S, Latif A, Urayama KY, Aoki J, Yahagi K, et al. Identifying novel phenotypes of acute heart failure using cluster analysis of clinical variables. Int J Cardiol 2018;262:57‐63. doi: 10.1016/j.ijcard.2018.03.098 [DOI] [PubMed] [Google Scholar]
- 17. Cohen JB, Schrauben SJ, Zhao L, Basso MD, Cvijic ME, Li Z, et al. Clinical phenogroups in heart failure with preserved ejection fraction: Detailed phenotypes, prognosis, and response to spironolactone. JACC Heart Fail 2020;8:172‐184. doi: 10.1016/j.jchf.2019.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Segar MW, Patel KV, Ayers C, Basit M, Tang WHW, Willett D, et al. Phenomapping of patients with heart failure with preserved ejection fraction using machine learning‐based unsupervised cluster analysis. Eur J Heart Fail 2020;22:148‐158. doi: 10.1002/ejhf.1621 [DOI] [PubMed] [Google Scholar]
- 19. Choy M, Liang W, He J, Fu M, Dong Y, He X, et al. Phenotypes of heart failure with preserved ejection fraction and effect of spironolactone treatment. ESC Heart Fail 2022;9:2567‐2575. doi: 10.1002/ehf2.13969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murray EM, Greene SJ, Rao VN, Sun JL, Alhanti BA, Blumer V, et al. Machine learning to define phenotypes and outcomes of patients hospitalized for heart failure with preserved ejection fraction: Findings from ASCEND‐HF. Am Heart J 2022;254:112‐121. doi: 10.1016/j.ahj.2022.08.009 [DOI] [PubMed] [Google Scholar]
- 21. Zweck E, Thayer KL, Helgestad OKL, Kanwar M, Ayouty M, Garan AR, et al. Phenotyping cardiogenic shock. J Am Heart Assoc 2021;10:e020085. doi: 10.1161/JAHA.120.020085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jentzer JC, Soussi S, Lawler PR, Kennedy JN, Kashani KB. Validation of cardiogenic shock phenotypes in a mixed cardiac intensive care unit population. Catheter Cardiovasc Interv 2022;99:1006‐1014. doi: 10.1002/ccd.30103 [DOI] [PubMed] [Google Scholar]
- 23. Jentzer JC, Anavekar NS, Bennett C, Murphree DH, Keegan MT, Wiley B, et al. Derivation and validation of a novel cardiac intensive care unit admission risk score for mortality. J Am Heart Assoc 2019;8:e013675. doi: 10.1161/JAHA.119.013675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jentzer JC, van Diepen S, Barsness GW, Henry TD, Menon V, Rihal CS, et al. Cardiogenic shock classification to predict mortality in the cardiac intensive care unit. J Am Coll Cardiol 2019;74:2117‐2128. doi: 10.1016/j.jacc.2019.07.077 [DOI] [PubMed] [Google Scholar]
- 25. Bennett CE, Wright RS, Jentzer J, Gajic O, Murphree DH, Murphy JG, et al. Severity of illness assessment with application of the APACHE IV predicted mortality and outcome trends analysis in an academic cardiac intensive care unit. J Crit Care 2019;50:242‐246. doi: 10.1016/j.jcrc.2018.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jentzer JC, Bennett C, Wiley BM, Murphree DH, Keegan MT, Gajic O, et al. Predictive value of the Sequential Organ Failure Assessment score for mortality in a contemporary cardiac intensive care unit population. J Am Heart Assoc 2018;7:7. doi: 10.1161/JAHA.117.008169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jentzer JC, Szekely Y, Burstein B, Ballal Y, Kim EY, van Diepen S, et al. Peripheral blood neutrophil‐to‐lymphocyte ratio is associated with mortality across the spectrum of cardiogenic shock severity. J Crit Care 2022;68:50‐58. doi: 10.1016/j.jcrc.2021.12.005 [DOI] [PubMed] [Google Scholar]
- 28. Jentzer JC, Anavekar NS, Brenes‐Salazar JA, Wiley B, Murphree DH, Bennett C, et al. Admission Braden skin score independently predicts mortality in cardiac intensive care patients. Mayo Clin Proc 2019;94:1994‐2003. doi: 10.1016/j.mayocp.2019.04.038 [DOI] [PubMed] [Google Scholar]
- 29. Breen TJ, Brueske B, Sidhu MS, Kashani KB, Anavekar NS, Barsness GW, et al. Abnormal serum chloride is associated with increased mortality among unselected cardiac intensive care unit patients. PLoS ONE 2021;16:e0250292. doi: 10.1371/journal.pone.0250292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brueske B, Sidhu MS, Schulman‐Marcus J, Kashani KB, Barsness GW, Jentzer JC. Hyperkalemia is associated with increased mortality among unselected cardiac intensive care unit patients. J Am Heart Assoc 2019;8:e011814. doi: 10.1161/JAHA.118.011814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rayes HA, Vallabhajosyula S, Barsness GW, Anavekar NS, Go RS, Patnaik MS, et al. Association between anemia and hematological indices with mortality among cardiac intensive care unit patients. Clin Res Cardiol 2020;109:616‐627. doi: 10.1007/s00392-019-01549-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patel SM, Jentzer JC, Alviar CL, Baird‐Zars VM, Barsness GW, Berg DD, et al. A pragmatic lab‐based tool for risk assessment in cardiac critical care: Data from the Critical Care Cardiology Trials Network (CCCTN) registry. Eur Heart J Acute Cardiovasc Care 2022;11:252‐257. doi: 10.1093/ehjacc/zuac012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ter Maaten JM, Damman K, Hanberg JS, Givertz MM, Metra M, O'Connor CM, et al. Hypochloremia, diuretic resistance, and outcome in patients with acute heart failure. Circ Heart Fail 2016;9: doi: 10.1161/CIRCHEARTFAILURE.116.003109 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Univariable logistic regression models for prediction of in‐hospital mortality with all available admission laboratory variables in the final study population.
Table S2. Baseline characteristics of the final study population and CICU patients with HF who were excluded due to one or missing laboratory variable of interest.
Figure S1. Correlation matrix showing Pearson correlations among selected candidate laboratory feature variables in the final study cohort, with web plot demonstrating these same data.
Figure S2. Elbow plot demonstrating the between‐clusters and within‐clusters sum of squares, demonstrating a subtle inflection point at 5 clusters.
Figure S3. Distribution of clusters according to patient characteristics. Cluster 1 = Inflamed; Cluster 2 = Hypoperfused; Cluster 3 = Uncomplicated; Cluster 4 = Iron‐Deficient; Cluster 5 = Cardiorenal.
Figure S4. In‐hospital mortality according to subphenotype and etiology (ischemic versus nonischemic) or pattern (de novo versus acute on chronic) HF.
Figure S5. In‐hospital mortality according to subphenotype and pattern of ventricular dysfunction based on moderate or greater RV and LV dysfunction by TTE.


