Abstract
The efficacy and safety of new‐generation devices (NGDs) for severe aortic regurgitation (AR) have mostly been based on single‐arm studies with limited sample sizes. Our goal was to summarize the current evidence on NGDs and compare the safety and efficacy of ‘off‐label’ and ‘on‐label’ devices in NGDs. We searched MEDLINE, Embase, Cochrane Library, and Scopus for articles on transcatheter aortic valve replacement in patients with AR. A total of 31 studies that included 1851 patients were identified through April 2023. Among these, 1067 (57.6%) patients received treatment with ‘on‐label’ devices (JenaValve and J‐Valve). For NGDs, the total device success rate at 30 days was 94.5% (on‐label: 97.8%, off‐label: 89.9%; P < 0.001), the all‐cause mortality was 4.2% (on‐label: 2.6%, off‐label: 5.1%; P = 0.006), permanent pacemaker implantation (PPI) was 8.8% (on‐label: 6.9%, off‐label: 18.4%; P < 0.001), and the rate of greater‐than‐mild paravalvular leak (PVL) was 1.2% (on‐label: 0.9%, off‐label: 3.8%; P = 0.003). On‐label devices showed significantly better safety and efficacy in terms of the success rate, PPI, greater‐than‐mild PVL, and 30 day mortality than off‐label devices.
Keywords: Aortic regurgitation, Transcatheter aortic valve replacement, Transcatheter aortic valve implantation, Meta‐analysis
Introduction
The prevalence of aortic regurgitation (AR) increases with advancing age, affecting up to 2% of individuals aged more than 75 years. Not uncommonly, patients with severe symptomatic AR may not be appropriate for surgery because of the high surgical risk due to their advanced age and comorbidities. According to data from the Euro Heart Survey on Valvular Heart Disease, patients with severe AR and an ejection fraction (EF) of <30% showed that mortality is as high as 20% for these patients; however, only 5% underwent surgical aortic valve replacement (SAVR). 1 There is an urgent need to treat these patients with a less invasive approach.
Transcatheter aortic valve replacement (TAVR) has been a standard treatment option for patients with severe symptomatic aortic stenosis (AS) regardless of the surgical risk. 2 , 3 , 4 However, the treatment of TAVR for severe AR has not been as successful as for AS. The reasons were likely because of the differences in pathological anatomy between the two conditions. The anatomic characteristics of pure AR frequently include a dilated, non‐calcified aortic annulus and root and a lack of a stable anchoring zone for prosthesis, which, consequently, increase the risk of valve malposition and migration, conversion to surgery, paravalvular leak (PVL), and valve embolization.
New‐generation devices (NGDs) with design features, such as recyclability, repositioning, and anchoring mechanisms, have been used in an ‘off‐label’ setting to treat AR and have shown reduction in mortality and improved quality of life. 5 , 6 , 7 , 8 , 9 Two ‘on‐label’ devices—JenaValve 10 , 11 and J‐Valve 12 , 13 —with a unique paper clip‐like anchorage mechanism and U‐shaped anchoring claspers, respectively, have shown promising outcomes in clinical trials. However, most of the current studies of AR were presented either with a limited sample size or without a comparison arm, which limits the generalizability and accuracy of estimating effectiveness. Moreover, the stage of development of transcatheter intervention technologies in various countries and regions varies significantly, which further limited the capacity to fully understand what are the optimal time and clinical criteria to optimize TAVR in AR treatment. To comprehensively investigate the generalizability and clinical outcomes of NGDs in treating AR, we performed a systematic review and meta‐analysis to evaluate the outcomes of NGDs and compare the efficacy and safety of on‐label vs. off‐label NGDs in patients with severe AR.
Methods
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) 14 and A Measurement Tool to Assess systematic Reviews (AMSTAR) 15 guidelines. The project has been registered in PROSPERO (https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=413990; CRD42023413990).
Literature search
Four databases—MEDLINE, Embase, Cochrane Library, and Scopus—were searched by two independent reviewers for relevant studies. Publicly available clinical trial registries such as ClinicalTrials.gov were also searched. Search strategies based on the following keywords were used: (‘TAVI’ OR ‘TAVR’ OR ‘Transcatheter aortic valve replacement’ OR ‘Transcatheter aortic valve implantation’ OR ‘transcatheter’) And (‘aortic insufficiency’ OR ‘aortic regurgitation’ OR ‘aortic incompetence’ OR ‘TAVR insufficiency’ OR ‘aortic valve regurgitation’ OR ‘TAVR regurgitation’ OR ‘TAVI regurgitation’ OR ‘TAVI insufficiency’). Databases were searched on 8 April 2023. All available data from these four databases were included in the study analysis.
Studies
We searched for all randomized controlled trials and observational studies including cohort studies, case‐controlled studies, and case series with at least 10 cases. Studies not reporting the desired outcomes or from which summary data could not be extracted were excluded.
Study outcomes (reported according to the Valve Academic Research Consortium‐3 definition)
The outcomes included the following events that occurred within 30 days or 1 year after the TAVR procedure:
all‐cause mortality at 30 days and 1 year;
device success defined by the Valve Academic Research Consortium‐3 (VARC‐3) criteria 16 at 30 days;
permanent pacemaker implantation (PPI) at 30 days;
conversion to SAVR at 30 days;
annulus rupture in procedure;
reintervention: repeat procedure for second prosthetic heart valve at 30 days;
greater‐than‐mild PVL at 30 days;
mild PVL at 30 days; and
no/trace PVL at 30 days.
Selection of studies and data extraction
An initial screening was independently conducted by two reviewers who conducted the initial literature searching, and studies that did not meet the initial inclusion criteria were excluded after reviewing titles, keywords, and abstracts. For the remaining publications, two reviewers further read the full articles to document the eligibility of each; the reasons why studies were included or excluded were documented. A third reviewer resolved any disagreements between the two reviewers regarding study inclusion/exclusion criteria.
The two independent reviewers extracted the following information from the included studies:
characteristics of the study such as publication date, country, and number of patients;
characteristics of patients: age, sex, medical history, left ventricular EF, annulus diameter, ascending aortic diameter, aortic root diameter, concomitant greater‐than‐moderate mitral regurgitation, Society of Thoracic Surgeons score, logistic EuroSCORE I, logistic EuroSCORE II, and inclusion criteria of each study;
aforementioned outcomes such as the rate of device success, 30 day all‐cause mortality, 1 year all‐cause mortality, and PPI;
type and characteristics of transcatheter heart valves (THVs);
access route: transfemoral access (TF) or transapical access (TA) during TAVR.
The NGDs were defined as the second‐generation TAVR devices (e.g., JenaValve, J‐Valve, Evolut, SAPIEN 3, Direct Flow, ACURATE, Lotus, Engager, Portico, and Symetis) in contrast to the first‐generation TAVR devices [CoreValve (Medtronic, Minneapolis, MN, USA) and SAPIEN XT (Edwards Lifesciences, Irvine, CA, USA)]. The J‐Valve and JenaValve were the only two NGDs designated for AR and were referred to as the on‐label devices. The off‐label devices included the remaining NGDs that did not belong to the on‐label category of devices.
Data synthesis and analysis
Meta‐analyses of specific results were performed only when at least two studies were available. We summarized the binary variables with proportion and 95% confidence interval (CI) and the mean difference with the 95% CI for continuous variables. A P value of <0.05 was considered to indicate statistical significance. In this analysis, I 2 was used to evaluate the heterogeneity of pooled outcomes. Values of I 2 > 50% indicated considerable heterogeneity, whereas I 2 < 50% represented mild or moderate heterogeneity. DerSimonian and Laird's method for pooled outcomes was used. In addition, we performed a subgroup analysis of the on‐label devices for AR. The studies that used off‐label devices or studies that used both on‐label and off‐label devices were excluded due to mixed data. We conducted similar subgroup analyses for off‐label devices. STATA SE statistical software (Version 16.0; StataCorp, College Station, TX, USA) and the ‘metaprop’ package in STATA were used for data analysis. χ 2 or Fisher's exact test was used to compare the outcomes in subgroup analysis (SPSS software, Version 24.0).
Results
Study selection and study characteristics
The search terms yielded 4037 studies from the four main electronic databases, among which 5976 studies were excluded after the initial screen due to non‐related research topic. The remaining 61 full‐text articles were retrieved, and two reviewers conducted full‐text reviews. Thirty studies were excluded due to mixed data of AS and AR, mixed devices of early‐generation devices (EGDs) and NGDs, or the number of patients in the study was <10. A total of 1851 patients from 31 unique studies met inclusion criteria and were included in the final analysis 5 , 6 , 9 , 10 , 11 , 12 , 13 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 (Figure 1 ). Patient characteristics were summarized in Table 1 . J‐Valve was the most commonly used device (949, 51.3%), followed by JenaValve (307, 16.6%), Evolut (284, 15.3%), SAPIEN 3 (61, 3.3%), Direct Flow (90, 4.9%), ACURATE (76, 4.1%), Lotus (34, 1.8%), Engager (26, 1.4%), Portico (9, 0.5%), and Symetis (15, 0.8%). The valve sizes were summarized in Table 2 . Valve size was reported in 706 cases (38.1%), in which the 27 mm valves were mostly used (44.1%). The detailed information of valve size was provided in Supporting Information, Table S1 . Most of the studies reported criteria of outcomes used the Valve Academic Research Consortium‐2 (VARC‐2) (20/23). Risk of bias was evaluated using index for non‐randomized studies (MINORS) criteria, with most pooled studies having a low risk of bias (Supporting Information, Table S2 ).
Figure 1.
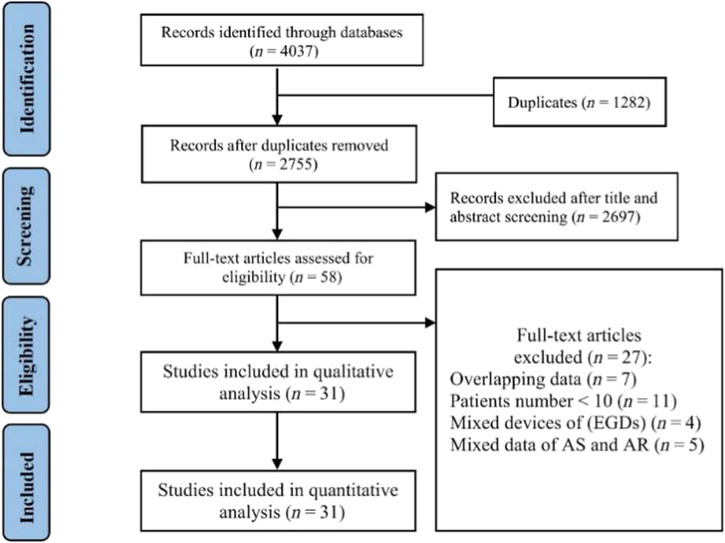
Flow diagram of the literature search for the meta‐analysis. AR, aortic regurgitation; AS, aortic stenosis; EGDs, early‐generation devices.
Table 1.
Baseline characteristics
| Study | Device | Access | Country | Patients (n) | Age (years ± SD/IQR) | Male (n, %) | LES I (%) | LES II (%) | STS (%) | Definition | Inclusion criteria |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vahl et al. (2021) | JenaValve | Transfemoral | The United States | 71 | 74 | NA | NA | NA | NA | VARC‐2 | Symptomatic patients with greater‐than‐moderate AR with high surgical risk |
| Gogia et al. (2020) | JenaValve | Transfemoral | The United States | 11 | 77.6 | NA | NA | NA | NA | NA | Pure severe AR with high surgical risk |
| Silaschi et al. (2018) | JenaValve | Transfemoral | Germany | 30 | 74.4 ± 9.3 | 12 (40) | 17.7 ± 14.8 | 6.9 ± 6.5 | 4.9 ± 3.5 | VARC‐1 | Severe AR with high surgical risk |
| Seiffert et al. (2014) | JenaValve | Transapical | Germany | 31 | 73.8 ± 9.1 | 20 (64.5) | 23.6 ± 14.5 | 9.3 ± 6.4 | 5.4 ± 3.6 | VARC‐2 | Severe AR with high surgical risk |
| Schlingloff et al. (2014) | JenaValve | Transapical | Germany | 10 | 79 ± 9 | 6 | 28.3 ± 17.1 | NA | NA | VARC‐2 | Severe AR with high or prohibitive surgical risk |
| Liu et al. (2022) | J‐Valve | Transapical | China | 161 | NA | NA | NA | NA | NA | NA | Symptomatic severe AR with high surgical risk |
| Liu et al. (2022) | J‐Valve | Transapical | China | 134 | 73.1 ± 6.4 | 100 | NA | 11.5 ± 6.8 | 9.8 ± 5.3 | VARC‐2 | Severe AR with high surgical risk |
| Kong et al. (2022) | J‐Valve | Transapical | China | 69 | 71.46 ± 7.92 | 52 (75.4) | NA | NA | 3.76 ± 3.93 | VARC‐2 | Moderate‐to‐severe AR with high surgical risk |
| Shi et al. (2021) | J‐Valve | Transapical | China | 44 | 76.2 ± 5.5 | 30 (68.2) | 25.3 ± 8.5 | NA | NA | VARC‐2 | Severe AR with high surgical risk |
| Shen et al. (2020) | J‐Valve | Transapical | China | 123 | 72.1 ± 6.4 | NA | NA | NA | NA | VARC‐2 | Pure non‐calcified native AR with high surgical risk |
| Zhu et al. (2018) | J‐Valve | Transapical | China | 44 | 73.8 ± 5.6 | 13 | 25.4 ± 5.3 | NA | 9.1 ± 3.6 | NA | Severe AR with high surgical risk and an annulus diameter within the 19–27 mm range |
| Tung et al. (2018) | J‐Valve | Transapical | China | 43 | 73.8 ± 5.7 | 30 (69.8) | 25.3 ± 5.3 | NA | NA | VARC‐2 | Severe AR with high surgical risk |
| Guo et al. (2018) | J‐Valve | Transapical | China | 58 | 72.3 ± 5.7 | 39 | 20.1 ± 3.3 | NA | NA | VARC‐2 | Severe pure AR with high surgical risk |
| Zhu et al. (2016) | J‐Valve | Transapical | China | 33 | 74.2 ± 5.2 | 26 | 24.4 ± 5.1 | NA | NA | VARC‐2 | Severe pure AR with high surgical risk |
| Zhu et al. (2016) | J‐Valve | Transapical | China | 28 | 74.1 ± 4.6 | NA | 23.2 ± 3.3 | NA | NA | VARC‐2 | Severe pure AR with high surgical risk |
| Zhu et al. (2015) | J‐Valve | Transapical | China | 11 | 74.5 ± 4.7 | NA | NA | NA | NA | NA | Severe pure/dominant AR with high surgical risk |
| Guo et al. (2015) | J‐Valve | Transapical | China | 18 | 73.8 ± 3.7 | NA | 24.1 ± 4.5 | NA | NA | NA | Severe pure AR with high surgical risk |
| Liu et al. (2018) | J‐Valve | Transapical | China | 43 | 73.9 ± 5.7 | 30 (69.8) | 25.5 ± 5.3 | NA | NA | VARC‐2 | Severe pure AR with high surgical risk |
| Liu et al. (2019) | J‐Valve | Transapical | China | 82 | NA | NA | NA | NA | NA | VARC 1 | Severe pure AR with high surgical risk |
| Liu et al. (2019) | J‐Valve | Transapical | China | 53 | 76.4 ± 5.2 | NA | NA | NA | 6.3 ± 1.8 | VARC‐2 | Severe pure AR with high surgical risk |
| Delhomme et al. (2023) | SAPIEN 3 | Transfemoral | France | 37 | 81 (69–85) | 27 | 12.29 (8.1–22.26) | 4.82 (2.46–6.86) | NA | VARC‐2 | Severe pure AR with high or prohibitive surgical risk |
| Yin et al. (2022) | Evolut R, J‐Valve | Transfemoral, transapical | China | 10 | 72.8 ± 11.7 | 7 | NA | NA | 6.2 ± 4.1 | VARC‐2 | Severe pure native AR with high surgical risk |
| Pracon et al. (2022) | Evolut R, SAPIEN 3 | Transfemoral | The United Kingdom | 17 | NA | NA | NA | NA | NA | VARC‐3 | Severe pure aortic regurgitation with high surgical risk |
| Purita et al. (2020) | ACURATE neo | Transfemoral | Italy | 24 | 79.4 (50–88) | 10 (41.6) | NA | 5 ± 4.05 | 3.9 ± 2.37 | VARC‐2 | Severe pure aortic regurgitation with high or prohibitive surgical risk |
| Anwaruddin et al. (2019) | Evolut R | Transfemoral | The United States | 149 | 68.9 ± 15.1 | 80 (54) | NA | NA | 8.6 ± 9.9 | NA | Pure or mixed, with predominantly moderate or severe AR |
| Toggweiler et al. (2018) | ACURATE neo | Transfemoral | Switzerland | 20 | 79 ± 8 | NA | NA | NA | 8.3 ± 9.3 | NA | Severe pure AR with high surgical risk |
| De Backer et al. (2018) | Evolut, ACURATE, Portico, SAPIEN 3, Lotus, Direct Flow, JenaValve, Engager | Transapical, transformal | Denmark | 145 | 75 ± 10 | 69 (49) | NA | NA | 6.2 ± 4.9 | VARC‐2 | Severe native AR without significant stenosis with prohibitive surgical risk |
| De Backer et al. (2017) | JenaValve, Evolut R, Direct Flow, Symetis, Lotus, Engager, SAPIEN 3, Portico | Transapical, transformal | Denmark | 118 | NA | NA | NA | NA | 5.7 ± 5.2 | NA | Severe native AR without significant stenosis |
| Yoon et al. (2017) | SAPIEN 3, Evolut R, JenaValve, Direct Flow, J‐Valve, Engager, Portico, ACURATE, Lotus | Transapical, transformal | The United States | 212 | 74.5 ± 11.6 | 104 | NA | 8.9 ± 9.4 | 6.2 ± 6.7 | VARC‐2 | Severe pure AR with high surgical or prohibitive risk |
| Sawaya et al. (2017) | Evolut R, JenaValve, Direct Flow, Lotus, SAPIEN 3 | Transapical, transformal, transsubclavian | Denmark | 41 | NA | NA | NA | NA | NA | VARC‐2 | Severe pure native AR with high surgical risk |
| Schofer et al. (2015) | Direct Flow | Transfemoral | Germany | 11 | 74.7 ± 12.9 | 4 | 19.9 ± 7.1 | NA | 8.84 ± 8.9 | VARC‐2 | Symptomatic severe pure AR with high or prohibitive surgical risk |
AR, aortic regurgitation; IQR, inter‐quartile range; LES, logistic EuroSCORE; NA, not available; STS, Society of Thoracic Surgeons score; VARC‐1, Valve Academic Research Consortium‐1; VARC‐2, Valve Academic Research Consortium‐2; VARC‐3, Valve Academic Research Consortium‐3.
Table 2.
Valve size of pooled studies
| THV | Total (n) | Valve size | ||||||
|---|---|---|---|---|---|---|---|---|
| 21 mm | 23 mm | 25 mm | 26 mm | 27 mm | 29 mm | 34 mm | ||
| JenaValve | 139 | 0 | 25 | 32 | 0 | 82 | 0 | 0 |
| J‐Valve | 351 | 3 | 14 | 88 | 0 | 215 | 31 | 0 |
| SAPIEN 3 | 37 | 0 | 2 | 0 | 9 | 0 | 26 | 0 |
| Evolut R | 144 | 0 | 1 | 0 | 33 | 0 | 54 | 56 |
| Direct Flow | 11 | 0 | 0 | 2 | 0 | 4 | 0 | 5 |
| S | L | M | ||||||
| ACURATE neo | 24 | 1 | 11 | 12 | ||||
THV, transcatheter heart valve.
Among the pooled studies, 20 studies (including 1067 individuals) used the on‐label devices (5 studies used JenaValve 10 , 11 , 20 , 33 , 36 and 15 studies 12 , 13 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 31 , 32 , 35 , 38 , 39 , 40 used J‐Valve) for patients with severe AR. The remaining 11 studies 5 , 6 , 9 , 17 , 18 , 19 , 28 , 29 , 30 , 34 , 37 (including 784 individuals) used the NGDs SAPIEN 3, Evolut R, JenaValve, Direct Flow, J‐Valve, Engager, Portico, ACURATE, Symetis, and Lotus. The study design was shown in Figure 2 .
Figure 2.
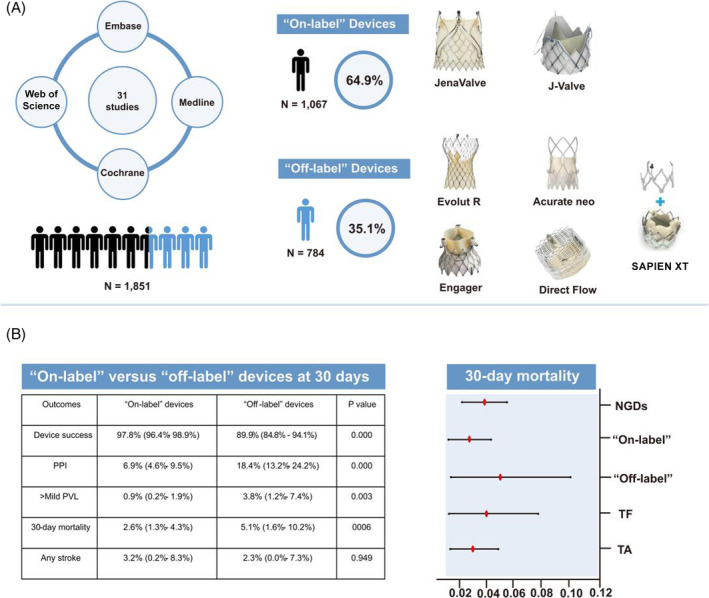
Illustration showing the study design and outcomes. (A) Study design and on‐label and off‐label devices for the treatment of severe aortic regurgitation. (B) The clinical outcomes of on‐label vs. off‐label devices at 30 days (left) and the 30 day mortality of new‐generation devices (NGDs), on‐label devices, off‐label devices, transfemoral access (TF), and transapical access (TA), respectively (right). The X axis indicates the occurrence of 30 day mortality. The Y axis represents the corresponding devices and procedural access. PPI, permanent pacemaker implantation; PVL, paravalvular leak.
Meta‐analysis of procedural and clinical outcomes of all patients
In our analysis, the rate of device success defined by VARC‐3 was 94.5% (95% CI: 91.3–97.1%, I 2 = 76.8%) (Figure 3 ). The estimated rate of PPI was 8.8% (95% CI: 6.1–11.9%, I 2 = 57.0%) (Figure 4 ); the rate of conversion to SAVR was 2.2% (95% CI: 0.9–3.8%, I 2 = 0.0%); the rate of annulus rupture was 0.2% (95% CI: 0.0–1.7%, I 2 = 0.0%); the rate of reintervention was 2.3% (95% CI: 0.7–4.5%, I 2 = 13.9%); the rate of greater‐than‐mild PVL was 1.2% (95% CI: 0.4–2.2%, I 2 = 0.0%); the rate of mild PVL was 20.9% (95% CI: 17.6–24.4%, I 2 = 12.8%); and the rate of no or trace PVL was 77.4% (95% CI: 70.8–83.5%, I 2 = 71.3%).
Figure 3.
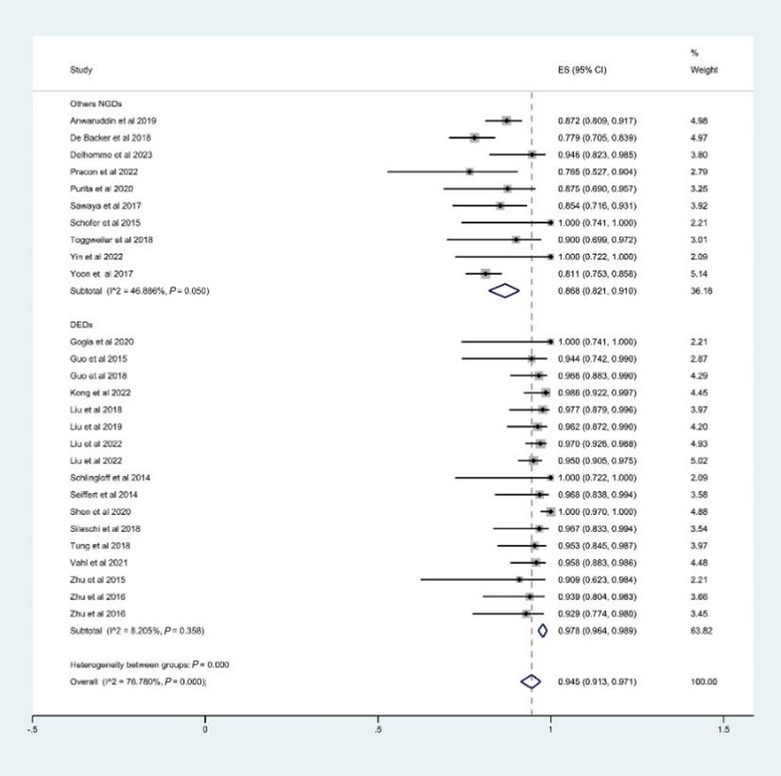
The estimated rate of device success. CI, confidence interval; DEDs, dedicated devices for aortic regurgitation (on‐label devices); ES, effect size; NGDs, new‐generation devices.
Figure 4.
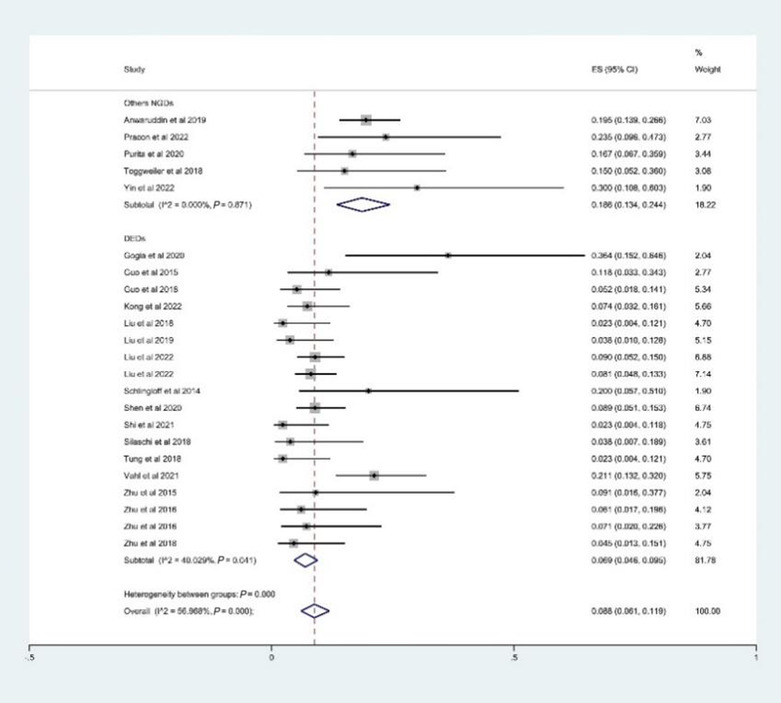
The estimated rate of permanent pacemaker implantations. CI, confidence interval; DEDs, dedicated devices for aortic regurgitation (on‐label devices); ES, effect size; NGDs, new‐generation devices.
The estimated 30 day all‐cause mortality was 4.2% (95% CI: 2.7–5.9%, I 2 = 43.8%) (Figure 5 ), and the estimated 1 year mortality was 8.1% (95% CI: 5.1–11.7%, I 2 = 67.3%). The above outcomes are summarized in Table 3 .
Figure 5.
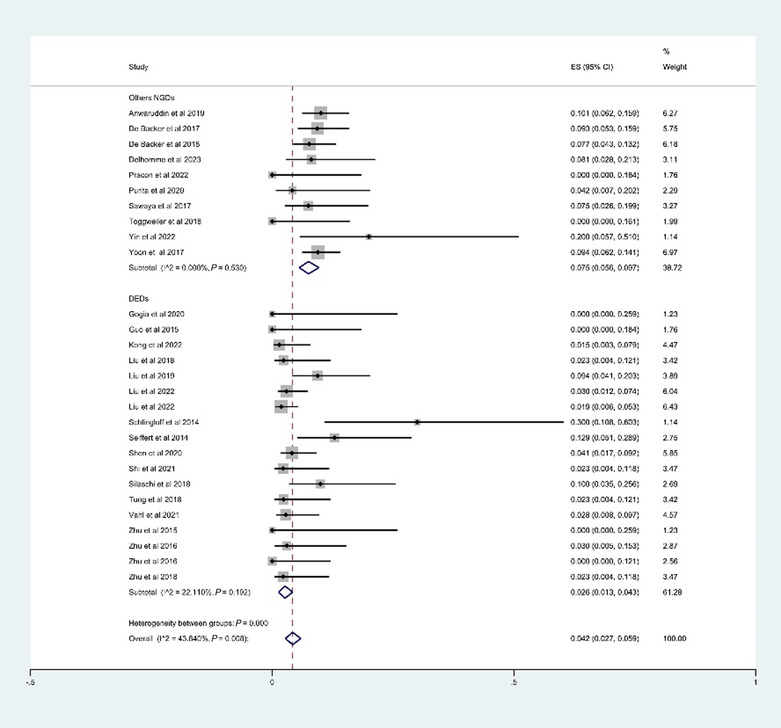
The estimated rate of 30 day all‐cause mortality. CI, confidence interval; DEDs, dedicated devices for aortic regurgitation (on‐label devices); ES, effect size; NGDs, new‐generation devices.
Table 3.
The outcomes of new‐generation devices
| Outcomes | NGDs | On‐label devices | Off‐label devices | P ** | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ES (95% CI) | I 2 (%) | P * | ES (95% CI) | I 2 (%) | P * | ES (95% CI) | I 2 (%) | P * | ||
| 30 day procedural outcomes | ||||||||||
| Device success |
0.945 (0.913–0.971) |
76.8 | 0.000 |
0.978 (0.964–0.989) |
8.2 | 0.358 |
0.899 (0.848–0.941) |
10.3 | 0.350 | <0.001 |
| Permanent pacemaker implantation |
0.088 (0.061–0.119) |
57.0 | 0.000 |
0.069 (0.046–0.095) |
40.0 | 0.041 |
0.184 (0.132–0.242) |
0.0 | 0.928 | <0.001 |
| Conversion to SAVR |
0.022 (0.009–0.038) |
0.0 | 0.981 |
0.025 (0.012–0.042) |
0.0 | 0.957 | NA | NA | NA | NA |
| Annulus rupture |
0.002 (0.000–0.017) |
0.0 | 0.941 | NA | NA | NA | NA | NA | NA | NA |
| Reintervention |
0.023 (0.007–0.045) |
13.9 | 0.324 | NA | NA | NA |
0.028 (0.000–0.114) |
54.6 | 0.111 | NA |
| Greater‐than‐mild PVL |
0.012 (0.004–0.022) |
0.0 | 0.713 |
0.009 (0.002–0.019) |
0.0 | 0.942 |
0.038 (0.012–0.074) |
0.0 | 0.611 | 0.003 |
| Mild PVL |
0.209 (0.176–0.244) |
12.8 | 0.304 |
0.203 (0.165–0.243) |
19.0 | 0.241 | NA | NA | NA | NA |
| None/trace PVL |
0.774 (0.708–0.835) |
71.3 | 0.000 |
0.780 (0.705–0.847) |
73.4 | 0.000 | NA | NA | NA | NA |
| 30 day clinical outcomes | ||||||||||
| 30 day mortality |
0.042 (0.027–0.059) |
43.8 | 0.008 |
0.026 (0.013–0.043) |
22.1 | 0.192 |
0.051 (0.016–0.102) |
30.4 | 0.219 | 0.006 |
| 1 year clinical outcomes | ||||||||||
| 1 year mortality |
0.081 (0.051–0.117) |
67.3 | 0.001 |
0.059 (0.035–0.087) |
23.3 | 0.251 | NA | NA | NA | NA |
CI, confidence interval; ES, effect size; I 2, the variation attributable to heterogeneity; NA, not available; NGDs, new‐generation devices including on‐label devices and off‐label devices; PVL, paravalvular leak; SAVR, surgical aortic valve replacement.
P value for significance of I 2.
P value for significance of the χ 2 or Fisher's exact test between off‐label and on‐label devices.
Procedural and clinical outcomes of the on‐label devices
A total of 1067 patients from 20 studies underwent TAVR with on‐label devices. The device success rate was 97.8% (95% CI: 96.4–98.9%, I 2 = 8.2%) (Figure 3 ); PPI was 6.9% (95% CI: 4.6–9.5%, I 2 = 40.0%) (Figure 4 ); conversion to SAVR was 2.5% (95% CI: 1.2–4.2%, I 2 = 0.0%); greater‐than‐mild PVL was 0.9% (95% CI: 0.2–1.9%, I 2 = 0.0%); mild PVL was 20.3% (95% CI: 16.5–24.3%, I 2 = 19.0%); and no or trace PVL was 78.0% (95% CI: 70.5–84.7%, I 2 = 71.3%).
The estimated 30 day all‐cause mortality was 2.6% (95% CI: 1.3–4.3%, I 2 = 22.1%) (Figure 5 ), and the estimated 1 year mortality was 5.9% (95% CI: 3.5–8.7%, I 2 = 23.3%). The outcomes are summarized in Table 3 .
Meta‐analysis of the off‐label devices
The outcomes only reported by less than three studies were not included due to the inability to estimate heterogeneity score. Finally, 258 patients from six studies who used SAPIEN 3, Evolut R, ACURATE neo, and Direct Flow were included. The estimated device success rate, PPI, reintervention, greater‐than‐mild PVL, and 30 day mortality were 89.9% (95% CI: 84.8–94.1%, I 2 = 10.3%), 18.4% (95% CI: 13.2–24.2%, I 2 = 0.0), 2.8% (95% CI: 0.0–11.4%, I 2 = 54.6%), 3.8% (95% CI: 1.2–7.4%, I 2 = 0.0%), and 5.1% (95% CI: 1.6–10.2%, I 2 = 30.4%), respectively. Our analysis also showed significant differences between the off‐label and on‐label devices on device success, PPI, greater‐than‐mild PVL, and 30 day mortality (P < 0.001). The results are summarized in Table 3 .
Subgroup analysis of puncture approach of transapical access and transfemoral access
Sixteen studies (873 patients) that used TA access and eight studies (340 patients) that used TF access were included. The outcomes only reported by less than three studies in either subgroup were not included in the summary table (Table 4 ). Comparing TA and TF, TA showed a higher device success rate (96.1% vs. 92.5%, P < 0.001) and slightly higher PVL (21.6% vs. 18.4%, P = 0.314) than TF; however, PPI (6% vs. 19.4%, P < 0.001), greater‐than‐mild PVL (0.8% vs. 3.4%, P = 0.002), no/trace PVL (76.9% vs. 78.1%, P = 0.897), and 30 day mortality (2.9% vs. 4%, P = 0.052) were lower for the TA group than the TF group. The outcomes are summarized in Table 4 .
Table 4.
Outcomes of transfemoral vs. transapical access
| Outcomes | Transfemoral | Transapical | P ** | ||||
|---|---|---|---|---|---|---|---|
| ES (95% CI) | I 2 (%) | P * | ES (95% CI) | I 2 (%) | P * | ||
| 30 day procedural outcomes | |||||||
| Device success | 0.925 (0.875–0.964) | 35.6 | 0.144 | 0.961 (0.939–0.979) | 50.4 | 0.003 | 0.000 |
| Permanent pacemaker implantation | 0.194 (0.148–0.244) | 0.0 | 0.814 | 0.060 (0.043–0.078) | 0.0 | 0.787 | 0.000 |
| Greater‐than‐mild PVL | 0.034 (0.012–0.063) | 0.0 | 0.791 | 0.008 (0.001–0.018) | 0.0 | 0.960 | 0.002 |
| Mild PVL | 0.184 (0.115–0.263) | 29.5 | 0.235 | 0.216 (0.117–0.259) | 13.1 | 0.314 | 0.314 |
| No/trace PVL | 0.781 (0.685–0.866) | 42.9 | 0.154 | 0.769 (0.683–0.846) | 76.2 | 0.000 | 0.897 |
| 30 day clinical outcomes | |||||||
| 30 day mortality | 0.040 (0.012–0.078) | 28.9 | 0.208 | 0.029 (0.014–0.047) | 30.7 | 0.117 | 0.052 |
CI, confidence interval; ES, effect size; I 2, the variation attributable to heterogeneity; PVL, paravalvular leak.
P value for significance of I 2.
P value for significance of the χ 2 or Fisher's exact test.
Discussion
The current meta‐analysis, which included 1851 patients from 31 studies, compared the safety and efficacy of off‐label and on‐label NGDs in patients with pure AR. Nearly 70% of patients were treated with TA access, whereas 30% were treated with TF access. We found that off‐label NGDs are technically acceptable in patients with pure AR in terms of procedure success and mortality. Because the JenaValve and J‐Valve devices have comparable features for the anatomical characteristics of pure AR, our results showed a lower occurrence of complications such as 30 day mortality, PPI, and greater‐than‐mild PVL for on‐label devices than off‐label devices.
AR affects ~13% of patients with isolated native left‐sided valvular heart disease and occurs in up to 2% of people aged over 70 years. 1 It is usually accompanied by a dilated aortic annulus and ascending aorta, which challenge the proper positioning and stability of THVs and may lead to complications such as PVL, conduction disorders, or annular rupture. Generally, the anchoring of an off‐label prosthesis mainly depends on the radial forces via 10–20% THV oversizing, which allows for adequate adjustment of an individual's anatomy and prosthetic system. Cumulative clinical evidence and the advance in new‐generation THV devices result in a reduction in off‐label use of TAVR in patients with pure AR, but lack of anchoring and the possibility of migration remain major problems of THV and need to be addressed in future studies.
On‐label devices with secured fixation via a clip or a clasper‐like mechanism have become a superior interventional strategy for severe AR. The JenaValve TAVR System was the first self‐expanding dedicated device for pure AR; it is anchored on the native leaflets by a paper clip‐like anchor mechanism. The fixation is independent of the extent of the dilated and non‐calcified aortic annulus, and it roots and provides adequate sealing to enhance the anchoring and decrease the risk of PVL. The JenaValve received a CE‐mark approval for the treatment of AR. Another device, the J‐Valve, is designed with claspers (U‐shaped anchor rings) and tactile feedback, which help lock the self‐expandable porcine valve to the native valve leaflets to ensure correct positioning. Evidence supporting the safety and efficacy of on‐label devices through TF has increased since the debut of TF in clinical use in 2019. 38 The J‐Valve has been approved by the National Medical Products Administration of China. Recent few studies have highlighted the great potential benefits of TAVR for patients with AR. The results of three studies that included 51 patients showed a 30 day mortality of 11%, major bleeding of 4%, major vascular complications of 4%, and PPI of 6%. Wernly et al. 8 conducted a meta‐analysis of on‐label devices for TAVR for pure AR. They combined the outcomes of using these two valve prosthesis systems in 203 patients. The rate of procedural success was 93.0%, the 30 day mortality was 9.1%, and the major bleeding rate was 3.0%. Our study extends to cover more recent studies. By including 20 latest studies (1067 patients) that used J‐Valve and JenaValve to treat pure AR, our results show similar or better outcomes for on‐label devices (Table 3 ). The improvement may be attributed to accumulated clinical experience, advanced preprocedural measurement, refined devices, and improved procedural techniques.
Studies conducted in recent years have shown that on‐label devices implanted via the TF approach were associated with lower rates of vascular complications and successful outcomes than those implanted via the TA approach. However, our analysis revealed a significantly better outcome with TA in terms of device success, PPI, greater‐than‐mild PVL, and any stroke within the 30 day follow‐up period. The better outcomes may be related to advances in operative visualization technology, precise coaxiality, and the deployment of THVs via TA access. However, those findings were limited to the short‐term follow‐up. Further investigation is needed regarding how the clip or clasper‐like design impacts the available effective orifice area in off‐label NGDs. In the pooled studies of on‐label devices, the mean transvalvular pressure gradient ranged from 4.0 to 11.4 mmHg immediately after the procedure, 11 , 12 , 13 , 23 , 28 , 30 , 31 , 33 , 35 , 40 from 5.5 to 11.2 mmHg at 6 months, 13 , 20 , 23 , 26 , 35 and from 9.5 to 12.8 mmHg after a 1–2 year follow‐up. 21 , 23 , 25 , 32 , 33 , 35 , 40 Collectively, this evidence supports the favourable haemodynamic outcomes after on‐label NGD implants in both the short‐term and long‐term follow‐up periods.
Surgical aortic valve replacement is the primary choice of treatment for patients with severe AR and is recommended by multiple international guidelines; however, a significant proportion of patients who are at high or prohibitive surgical risk do not receive a replacement valve. As an alternative, TAVR has been used in an off‐label setting. Mentias et al. 41 reported the utilization of SAVR for 9880 patients (mean age: 72.9 ± 5.1 years); their results showed that patients had a lower prevalence of most comorbidities and lower frailty scores [3.0, inter‐quartile range (IQR): 1.5–6.4] than those in the TAVR group. In our study, the outcomes with on‐label devices were comparable with SAVR with respect to 30 day mortality (SAVR: 2.7%, on‐label devices: 2.6%), 1 year mortality (SAVR: 5.7%, on‐label devices: 5.9%), and PPI (SAVR: 6.7%, on‐label devices: 6.9%). More evidence is needed to compare the efficacy and safety of SAVR and TAVR (especially on‐label devices) in patients with severe AR who are not feasible for surgery. In addition, optimized timing for valve replacement that takes into consideration the extent of left ventricular remodelling and systolic cardiac function, as determined via echocardiography or cardiac magnetic resonance imaging, will benefit the long‐term outcomes for patients with AR.
Limitations
First, most of the included studies were single‐armed retrospective observational studies with small sample sizes, which limits the capacity to interpret the size of the effect. Furthermore, due to the majority included studies being observation studies, the patients included in the studies are heterogeneous. The heterogeneity may not be sufficiently controlled in the analysis. Second, patients with pure AR, AR derived from bicuspid aortic valve, and AR combined with AS were recruited, potentially increasing the heterogeneity of the study population. Third, pooled multicentre studies conducted in the same country may lead to the risk of overlapped study cohorts, especially for the J‐Valve in China. Rigorous selection criteria for studies can decrease the influence of the risk of an overlapped population. Finally, this study did not differ between self‐expanding prostheses (SE) and balloon‐expandable prostheses (BE) devices due to the inability to differentiate between SE and BE in the articles.
Conclusions
This meta‐analysis investigated recent studies that used NGDs in patients with pure AR. The results showed that both on‐label and off‐label devices are safe and feasible for patients with severe AR and who are unfit for open chest surgery. On‐label devices showed significantly higher procedural success rates and better clinical outcomes than off‐label devices and should be the preferred choice. To expand the indication of TAVR for AR, future studies should focus on optimal interventional timing, device selection, long‐term haemodynamic outcomes, and left ventricular function.
Conflict of interest
None declared.
Funding
This work was supported by the Capital Funds for Health Improvement and Research (Grant Number 2022‐2‐2066).
Supporting information
Table S1. The detailed information of valve size.
Table S2. MINORS Criteriaa.
Liu, R. , Fu, Z. , Jiang, Z. , Yan, Y. , Yao, J. , Liu, X. , Yan, X. , and Song, G. (2024) Transcatheter aortic valve replacement for aortic regurgitation: a systematic review and meta‐analysis. ESC Heart Failure, 11: 3488–3500. 10.1002/ehf2.14832.
References
- 1. Iung B, Baron G, Butchart EG, Delahaye F, Gohlke‐Bärwolf C, Levang OW, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231‐1243. doi: 10.1016/s0195-668x(03)00201-x [DOI] [PubMed] [Google Scholar]
- 2. Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, et al. Transcatheter aortic‐valve replacement with a self‐expanding prosthesis. N Engl J Med 2014;370:1790‐1798. doi: 10.1056/NEJMoa1400590 [DOI] [PubMed] [Google Scholar]
- 3. Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O'Hair D, et al. Transcatheter aortic‐valve replacement with a self‐expanding valve in low‐risk patients. N Engl J Med 2019;380:1706‐1715. doi: 10.1056/NEJMoa1816885 [DOI] [PubMed] [Google Scholar]
- 4. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic‐valve replacement in high‐risk patients. N Engl J Med 2011;364:2187‐2198. doi: 10.1056/NEJMoa1103510 [DOI] [PubMed] [Google Scholar]
- 5. De Backer O, Pilgrim T, Simonato M, Mackensen GB, Fiorina C, Veulemanns V, et al. Usefulness of transcatheter aortic valve implantation for treatment of pure native aortic valve regurgitation. Am J Cardiol 2018;122:1028‐1035. doi: 10.1016/j.amjcard.2018.05.044 [DOI] [PubMed] [Google Scholar]
- 6. Sawaya FJ, Deutsch MA, Seiffert M, Yoon SH, Codner P, Wickramarachchi U, et al. Safety and efficacy of transcatheter aortic valve replacement in the treatment of pure aortic regurgitation in native valves and failing surgical bioprostheses: Results from an international registry study. JACC Cardiovasc Interv 2017;10:1048‐1056. doi: 10.1016/j.jcin.2017.03.004 [DOI] [PubMed] [Google Scholar]
- 7. Takagi H, Hari Y, Kawai N, Ando T. Meta‐analysis and meta‐regression of transcatheter aortic valve implantation for pure native aortic regurgitation. Heart Lung Circ 2020;29:729‐741. doi: 10.1016/j.hlc.2019.04.012 [DOI] [PubMed] [Google Scholar]
- 8. Wernly B, Eder S, Navarese EP, Kretzschmar D, Franz M, Alushi B, et al. Transcatheter aortic valve replacement for pure aortic valve regurgitation: “On‐label” versus “off‐label” use of TAVR devices. Clin Res Cardiol 2019;108:921‐930. doi: 10.1007/s00392-019-01422-0 [DOI] [PubMed] [Google Scholar]
- 9. Yin WH, Lee YT, Tsao TP, Lee KC, Hsiung MC, Wei J. Outcomes of transcatheter aortic valve replacement for pure native aortic regurgitation with the use of newer‐ vs. early‐generation devices. Ann Transl Med 2022;10:24. doi: 10.21037/atm-21-6936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schlingloff F, Schäfer U, Frerker C, Schmoeckel M, Bader R. Transcatheter aortic valve implantation of a second‐generation valve for pure aortic regurgitation: Procedural outcome, haemodynamic data and follow‐up. Interact Cardiovasc Thorac Surg 2014;19:388‐393. doi: 10.1093/icvts/ivu155 [DOI] [PubMed] [Google Scholar]
- 11. Seiffert M, Bader R, Kappert U, Rastan A, Krapf S, Bleiziffer S, et al. Initial German experience with transapical implantation of a second‐generation transcatheter heart valve for the treatment of aortic regurgitation. JACC Cardiovasc Interv 2014;7:1168‐1174. doi: 10.1016/j.jcin.2014.05.014 [DOI] [PubMed] [Google Scholar]
- 12. Guo Y, Zhu D. TCT‐105 Transcatheter aortic valve implantation using a new second‐generation TAVI system: J‐ValveTM for high‐risk patients with pure aortic regurgitation. J Am Coll Cardiol 2015;66:B48‐B49. [DOI] [PubMed] [Google Scholar]
- 13. Zhu D, Chen Y, Guo Y, Hu J, Zhang J, Wei X, et al. Transapical transcatheter aortic valve implantation using a new second‐generation TAVI system—J‐Valve™ for high‐risk patients with aortic valve diseases: Initial results with 90‐day follow‐up. Int J Cardiol 2015;199:155‐162. doi: 10.1016/j.ijcard.2015.07.037 [DOI] [PubMed] [Google Scholar]
- 14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non‐randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. doi: 10.1136/bmj.j4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Généreux P, Piazza N, Alu MC, Nazif T, Hahn RT, Pibarot P, et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research. J Am Coll Cardiol 2021;77:2717‐2746. doi: 10.1016/j.jacc.2021.02.038 [DOI] [PubMed] [Google Scholar]
- 17. Anwaruddin S, Desai ND, Szeto WY, Hermiller JB Jr, Sorajja P, Kodali S, et al. Self‐expanding valve system for treatment of native aortic regurgitation by transcatheter aortic valve implantation (from the STS/ACC TVT registry). Am J Cardiol 2019;124:781‐788. doi: 10.1016/j.amjcard.2019.05.045 [DOI] [PubMed] [Google Scholar]
- 18. De Backer O, Pilgrim T, Sondergaard L, Dos Santos MS, Mackensen GB, Cerillo A, et al. TCT‐448 Transcatheter aortic valve replacement for isolated severe native aortic valve regurgitation—Results from the TAVR‐NAVR registry. J Am Coll Cardiol 2017;70:B184. [Google Scholar]
- 19. Delhomme C, Urena M, Zouaghi O, Campelo‐Parada F, Ohlmann P, Rioufol G, et al. Transcatheter aortic valve implantation using the SAPIEN 3 valve to treat aortic regurgitation: The French multicentre S3AR study. Arch Cardiovasc Dis 2023;116:98‐105. doi: 10.1016/j.acvd.2022.12.003 [DOI] [PubMed] [Google Scholar]
- 20. Gogia S, Vahl T, Khalique O, Hamid N, Borden S, Chung C, et al. TCT CONNECT‐92 initial single‐center experience with transfemoral transcatheter aortic valve replacement in patients with symptomatic severe aortic regurgitation. J Am Coll Cardiol 2020;76:B41. [Google Scholar]
- 21. Guo Y, Zhu D, Wei L, Wang C, Wang W, Wang X. Treatment of pure aortic regurgitation using a novel second‐generation transcatheter aortic valve implantation system: Real‐world experience. Innov: Technol Tech Cardiothorac Vasc Surg 2018;13:S66. [Google Scholar]
- 22. Kong M, Hong Z, Liu X, Zhu X, Wang J, Dong A. 30‐day outcomes after surgical or transapical aortic valve replacement in symptomatic aortic regurgitation. J Cardiovasc Dev Dis 2022;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu H, Yang Y, Wang W, Zhu D, Wei L, Guo K, et al. Transapical transcatheter aortic valve replacement for aortic regurgitation with a second‐generation heart valve. J Thorac Cardiovasc Surg 2018;156:106‐116. doi: 10.1016/j.jtcvs.2017.12.150 [DOI] [PubMed] [Google Scholar]
- 24. Liu L, Guo Y, Zhu D, Qin C, Shi J. Treatment of pure aortic regurgitation using a novel second‐generation transcatheter aortic valve implantation system real‐world experience. Heart Lung Circ 2019;28:S91. doi: 10.1016/j.hlc.2019.02.076 [DOI] [Google Scholar]
- 25. Liu L, Peng Y, Shi J, Qian H, Guo Y. Initial experience with repositionable J‐Valve for severe aortic regurgitation: A single‐center experience. J Cardiovasc Surg (Torino) 2022;63:521‐528. doi: 10.23736/S0021-9509.22.11260-7 [DOI] [PubMed] [Google Scholar]
- 26. Liu L, Yao X, Peng Y, Huang W, Jun S, Qian H, et al. One‐year outcome after transcatheter aortic valve replacement for aortic regurgitation: A single‐center study. J Card Surg 2022;37:882‐892. doi: 10.1111/jocs.16238 [DOI] [PubMed] [Google Scholar]
- 27. Liu W, Zhou YJ, Zhang HB, Meng XU, Gao YN. P1852 The clinical experience of J valve transapical transcatheter aortic valve replacement system in high‐risk patients with severe pure aortic regurgitation. Eur Heart J 2019;40:1146.30770702 [Google Scholar]
- 28. Pracon R, Dimitrov A, McGarvey M, Cadiz S, Kabir T, Dalby M, et al. TCT‐428 Outcomes of patients undergoing TAVI for purely regurgitant native aortic valves with the current generation transcatheter aortic valve platforms. J Am Coll Cardiol 2022;80:B173‐B174. [Google Scholar]
- 29. Purita PAM, Tahoces LS, Fraccaro C, Nai Fovino L, Kim WK, Espada‐Guerreiro C, et al. Transcatheter treatment of native aortic valve regurgitation: Results from an international registry using the transfemoral ACURATE neo valve. Int J Cardiol Heart Vasc 2020;27:100480. doi: 10.1016/j.ijcha.2020.100480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schofer J, Nietlispach F, Bijuklic K, Colombo A, Gatto F, De Marco F, et al. Transfemoral implantation of a fully repositionable and retrievable transcatheter valve for noncalcified pure aortic regurgitation. JACC Cardiovasc Interv 2015;8:1842‐1849. doi: 10.1016/j.jcin.2015.08.022 [DOI] [PubMed] [Google Scholar]
- 31. Shen C, Peng Y, Liu L, Shi J, Guo Y, Rao L. Safety and efficacy of transapical transcatheter aortic valve replacement in the treatment of pure non‐calcified native aortic regurgitation. Circulation 2020;142: doi: 10.1161/circ.142.suppl_3.16322 [DOI] [Google Scholar]
- 32. Shi J, Wei L, Chen Y, Wang X, Ye J, Qin C, et al. Transcatheter aortic valve implantation with J‐Valve: 2‐year outcomes from a multicenter study. Ann Thorac Surg 2021;111:1530‐1536. doi: 10.1016/j.athoracsur.2020.06.139 [DOI] [PubMed] [Google Scholar]
- 33. Silaschi M, Conradi L, Wendler O, Schlingloff F, Kappert U, Rastan AJ, et al. The JUPITER registry: One‐year outcomes of transapical aortic valve implantation using a second generation transcatheter heart valve for aortic regurgitation. Catheter Cardiovasc Interv 2018;91:1345‐1351. doi: 10.1002/ccd.27370 [DOI] [PubMed] [Google Scholar]
- 34. Toggweiler S, Cerillo AG, Kim WK, Biaggi P, Lloyd C, Hilker M, et al. Transfemoral implantation of the Acurate neo for the treatment of aortic regurgitation. J Invasive Cardiol 2018;30:329‐333. [PubMed] [Google Scholar]
- 35. Tung M, Wang X, Li F, Wang H, Guo Y, Wang C, et al. A versatile transapical device for aortic valvular disease: One‐year outcomes of a multicenter study on the J‐Valve system. J Cardiol 2018;72:377‐384. doi: 10.1016/j.jjcc.2018.05.020 [DOI] [PubMed] [Google Scholar]
- 36. Vahl T, Makkar R, Kodali S, Baldus S, Treede H, Daniels D, et al. 30‐day outcomes of transfemoral transcatheter aortic valve replacement for aortic regurgitation with a novel self‐expanding prosthesis. J Am Coll Cardiol 2021;77:919. [Google Scholar]
- 37. Yoon SH, Schmidt T, Bleiziffer S, Schofer N, Fiorina C, Munoz‐Garcia AJ, et al. Transcatheter aortic valve replacement in pure native aortic valve regurgitation. J Am Coll Cardiol 2017;70:2752‐2763. [DOI] [PubMed] [Google Scholar]
- 38. Zhu D, Guo Y. Treatment of pure aortic regurgitation using a novel second‐generation TAVI system (J‐ValveTM System): Initial clinical experience. EuroIntervention 2016;391. [Google Scholar]
- 39. Zhu D, Wei L, Cheung A, Guo Y, Chen Y, Zhu L, et al. Treatment of pure aortic regurgitation using a second‐generation transcatheter aortic valve implantation system. J Am Coll Cardiol 2016;67:2803‐2805. [DOI] [PubMed] [Google Scholar]
- 40. Zhu L, Guo Y, Wang W, Liu H, Yang Y, Wei L, et al. Transapical transcatheter aortic valve replacement with a novel transcatheter aortic valve replacement system in high‐risk patients with severe aortic valve diseases. J Thorac Cardiovasc Surg 2018;155:588‐597. doi: 10.1016/j.jtcvs.2017.09.015 [DOI] [PubMed] [Google Scholar]
- 41. Mentias A, Saad M, Menon V, Reed GW, Popovic Z, Johnston D, et al. Transcatheter vs surgical aortic valve replacement in pure native aortic regurgitation. Ann Thorac Surg 2023;115:870‐876. doi: 10.1016/j.athoracsur.2022.09.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The detailed information of valve size.
Table S2. MINORS Criteriaa.


