Introduction
Pelvic exenteration is the standard of care, and only potentially curative treatment option, for selected patients with locally advanced or recurrent rectal cancer. This complex procedure involves surgical resection of all anatomical structures involved by the tumour and typically requires the removal of multiple pelvic viscera, as well as major pelvic bone, blood vessels, and nerves, generally followed by complex reconstruction of these systems. Although potentially curative, such radical surgery may be associated with major morbidity (up to 60%), functional impairments, and reduction in quality of life1,2. Therefore, whether or not pelvic exenteration should be recommended for an individual patient is a major and often difficult decision. The consequences of surgery must be weighed against the potential for cure, with consideration given to a patient’s individual treatment goals and priorities.
Currently, decision-making around whether to recommend pelvic exenteration is based on individual surgeon or team experiences and does not follow an evidence-based approach. These decisions have traditionally been made with anticipated survival benefit as the main outcome of interest, with relative neglect of quality-of-life and functional outcomes. Furthermore, the views of patients, carers, and clinicians regarding which outcomes are most important and should guide decision-making have not been previously determined. It is also clear from recent comparative studies that there are dramatic differences in practices between specialist exenteration centres with respect to both patient selection and treatment approach3,4. This unwarranted variation in treatment decision-making may be, at least in part, due to lack of a standardized, reproducible, evidence-based approach to decision-making in this group of patients.
The aim of this study was to identify the pelvic exenteration outcomes that are considered most important by patients with locally advanced or recurrent rectal cancer, their carers, and treating clinicians. This will inform the development of an evidence-based surgical decision-making tool that can be used at the time of diagnosis to predict a range of individual patient outcomes (beyond survival alone).
Methods
This study was coordinated by the Surgical Outcomes Research Centre (SOuRCe) in conjunction with the Institute of Academic Surgery (IAS) at Royal Prince Alfred Hospital, Sydney, New South Wales, Australia. The study involved two phases. First, a longlist of outcomes of pelvic exenteration was generated by systematically reviewing the literature5 and conducting in-depth interviews with patients who had undergone exenteration surgery and their carers6. Second, longlisted outcomes were reviewed by a multidisciplinary committee and used to populate a three-round Delphi survey of patients, carers, and clinicians to identify the outcomes of highest priority to these stakeholder groups. The study protocol was published a priori7 and was reported according to the Conducting and REporting of DElphi Studies (CREDES) recommendations8. Ethical approval for this study was granted by the Royal Prince Alfred Hospital Human Research Ethics Committee (X22-0422 and 2022/ETH02659).
Phase one: outcomes longlist
A systematic review of the contemporary literature (published between 1990 and 2023) was conducted to identify all reported outcomes of extended or exenterative multivisceral resections for locally advanced primary or recurrent rectal cancer5. Semi-structured interviews were then conducted to explore the experiences of patients who had undergone pelvic exenteration and their carers in depth, with a focus on the outcomes of surgery that they considered important6. Outcomes identified by the systematic review and interviews were then combined to generate a longlist. The longlist was reviewed by a multidisciplinary committee that included specialist surgeons, an experienced surgical outcomes researcher, a pelvic exenteration nurse specialist, and two patient advocates. During this review process, outcomes addressing the same or similar concepts were combined as ‘standardized outcomes’. Standardized outcomes were excluded when they were considered to be of minimal clinical relevance by the committee. Standardized outcomes were assigned a domain and a lay definition, and were used to populate the first round of the Delphi survey. Domains were based on those proposed by the Core Outcome Measures in Effectiveness Trials Initiative, but modified for applicability to pelvic exenteration patients for the purposes of this study9.
Phase two: Delphi survey
The Delphi process involved three iterative surveys, which were distributed using the online software known as Research Electronic Data Capture (REDCap), with reminders sent at 10 and 20 days for each round. Participants were recruited from three stakeholder groups:
Clinicians with experience in pelvic exenteration for locally advanced and recurrent rectal cancer. An invitation to participate was distributed to members of the PelvEx Collaborative (an international collaborative of clinicians with experience managing advanced pelvic tumours), Australia and New Zealand pelvic exenteration multidisciplinary teams and to corresponding authors of publications identified in the systematic review described above5. Although the first-round invitation was sent to approximately 350 clinicians, participants were encouraged to forward the first-round invitation e-mail to their local networks, including multidisciplinary teams, and therefore the total number of invited clinicians is unknown.
Patients who had undergone pelvic exenteration for locally advanced primary or recurrent rectal cancer. A total of 50 patients who had indicated an interest in participating in the second phase during the semi-structured interviews conducted in the first phase (see above) were invited to participate.
Carers for patients who had undergone pelvic exenteration for locally advanced primary or recurrent rectal cancer. Patients who participated were invited to forward the invitation e-mail to their carers.
First-round survey
The first-round survey was piloted on a patient representative, an experienced qualitative researcher, and two clinicians, and was then revised before distribution. The first section of the survey captured participant demographic information, including type of rectal cancer (primary or recurrent) and time since exenteration surgery (for patients), and information about specialty training and level of experience managing exenteration patients (for clinicians). The second section of the survey included a list of standardized outcomes identified during the first phase, with associated lay definitions. Participants rated the importance of each outcome using a nine-point Likert scale (limited importance, 1–3; important, but not critical, 4–6; and critically important, 7–9). A final open question allowed participants to list additional outcomes using free text.
Second-round survey
Outcomes with a mean score of one to three during the first round were discarded, whereas those scoring four to nine (important, but not critical, or critically important), and any additional unique outcomes suggested by participants, were included in the second-round survey. For each outcome, participants were provided with their score for the first round, as well as the mean score from their stakeholder group, and were asked to reflect on this information before scoring each outcome using the same nine-point Likert scale. Before the third round, each outcome was assessed for consensus according to criteria proposed by Williamson et al.10, which were defined a priori7:
Consensus in: 70% or more of respondents within a participant group rate the outcome as critically important (7–9) and 15% or fewer rate the outcome as of limited importance (1–3).
Consensus out: 70% or more of respondents within a participant group rate the outcome as of limited importance and 15% or fewer rate the outcome as critically important (7–9).
No consensus: neither of the above criteria are met.
Third-round survey
Outcomes meeting ‘consensus in’ criteria within all stakeholder groups during the second round were used to populate the third-round survey. Participants were asked to give each outcome a score between 0 (less important) and 100 (more important) to indicate its relative importance in relation to the other listed outcomes.
Statistical analysis
Demographic information and survey results are summarized using descriptive statistics. During the third-round survey, outcomes were ranked from one to nine for each stakeholder group according to the median score (of importance) attributed by participants within that group (where the outcome with the highest median score was assigned a rank of ‘1’ and the outcome with the lowest median score was assigned a rank of ‘9’). For each outcome, the ranks provided by stakeholder groups were summed to give a combined rank for the entire cohort, where the lowest value represented the most important outcome. This approach was adopted to ensure the final priority outcomes were equally representative of the three stakeholder groups, despite uneven numbers of participants within these groups.
Results
Phase one: outcomes longlist
Results of the systematic review and interviews with patients and their carers have been previously reported5. In brief, 2765 outcomes were identified from the literature (1157 after exclusion of duplicates) and combined with 46 outcomes extracted from transcripts of interviews with 34 patients and 5 carers. During the multidisciplinary committee evaluation process duplicates were removed before outcomes were reviewed, merged, and piloted. At the conclusion of this process, there were 70 standardized outcomes that were carried forward to the Delphi process and used to populate the first-round survey.
Phase two: Delphi survey
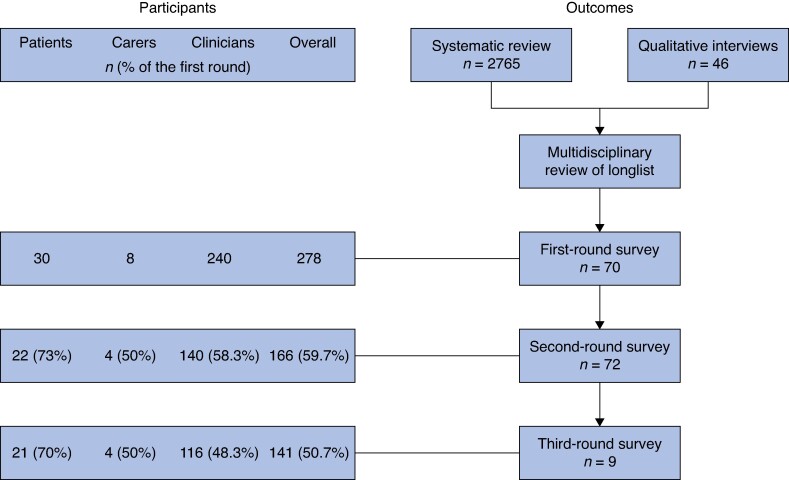
A total of 278 participants (30 patients, 8 carers, and 240 clinicians) responded to the invitation to participate and completed the first round of the survey, of which 166 (59.7%) completed the second round of the survey and 141 (50.7%) completed the third round of the survey (Fig. 1). Demographic information of the participating patients and carers who completed all three rounds of the Delphi survey are presented in Table 1 and the demographic information of the participating clinicians who completed all three rounds of the Delphi survey are presented in Table 2. In total, 11 (52%) and 10 (48%) patients had locally advanced primary and locally recurrent rectal cancer respectively and the median time since exenteration surgery was 36 (interquartile range 24–50) months. All four carers were spouses of patients who had undergone exenteration and all were female. The majority of clinicians were surgeons (78%); however, there was a range of multidisciplinary experts, including oncologists, nurses, radiologists, and dietitians.
Fig. 1.
Flow chart outlining the numbers of participants and outcomes included in each round of the Delphi survey
Table 1.
Demographic information of the participating patients and carers who completed all three rounds of the Delphi survey (n = 25)
| Characteristic | Value |
|---|---|
| Patients (n = 21) | |
| Age (years) | |
| 30–50 | 3 (14) |
| 51–70 | 13 (62) |
| >70 | 5 (24) |
| Sex | |
| Male | 10 (48) |
| Female | 11 (52) |
| Type of tumour | |
| Locally advanced primary rectal cancer | 11 (52) |
| Locally recurrent rectal cancer | 10 (48) |
| Time since exenteration surgery (months), median (interquartile range) | 36 (24–50) |
| Carers (n = 4) | |
| Age (years) | |
| 30–50 | 0 |
| 51–70 | 2 (50) |
| >70 | 2 (50) |
| Sex | |
| Male | 0 |
| Female | 4 (100) |
| Relationship to patient who underwent exenteration | |
| Spouse | 4 (100) |
| Immediate family | 0 |
| Other | 0 |
Values are n (%) unless otherwise indicated.
Table 2.
Demographic information of the participating clinicians who completed all three rounds of the Delphi survey (n = 116)
| Characteristic | Value |
|---|---|
| Age (years) | |
| <31 | 3 (2.6) |
| 31–50 | 66 (56.9) |
| 51–70 | 47 (40.5) |
| Sex | |
| Male | 84 (72.4) |
| Female | 32 (27.6) |
| Profession | |
| Surgeon | 91 (78.4) |
| Nurse | 7 (6.0) |
| Radiation oncologist | 4 (3.4) |
| Anaesthetist | 4 (3.4) |
| Medical oncologist | 3 (2.6) |
| Dietitian | 3 (2.6) |
| Radiologist | 2 (1.7) |
| Nuclear medicine specialist | 1 (0.8) |
| Administrator | 1 (0.8) |
| Region | |
| Europe and Central Asia | 57 (49.1) |
| Australasia and Pacific | 31 (26.7) |
| North America | 20 (17.2) |
| East Asia | 3 (2.6) |
| Latin America | 3 (2.6) |
| South Asia | 2 (1.7) |
| Dedicated exenteration training (surgeons only) | |
| Yes | 58 (50.0) |
| No | 28 (24.1) |
| Not applicable | 30 (25.9) |
| Experience treating advanced and recurrent rectal cancer (years) | |
| <5 | 29 (25.0) |
| 5–10 | 29 (25.0) |
| 11–20 | 38 (32.8) |
| >20 | 20 (17.2) |
| Access to dedicated pelvic oncology MDT meeting | |
| Yes | 106 (91.4) |
| No | 10 (8.6) |
| Annual centre pelvic exenteration case volume, median (interquartile range) | 30 (18–50) |
Values are n (%) unless otherwise indicated. MDT, multidisciplinary team.
First-round survey
The mean score according to each stakeholder group for the 70 outcomes during the first round (and their lay definitions) is presented in Table S1. The participants suggested 46 additional outcomes in response to the open text question of the first round, from which 2 unique outcomes were identified and included in the second round (‘decisional regret’ and ‘cost of treatment’). Because all outcomes had a mean score of 4–9, with no outcomes meeting the predefined criterion for exclusion after the first round (mean score of 1–3), 72 outcomes were carried forward to the second round.
Second-round survey
The consensus status for the 72 outcomes included in the second round are included in Table S2. There were nine outcomes that met ‘consensus in’ criteria among all stakeholder groups (patients, carers, and clinicians) and were therefore considered the final priority outcomes.
Third-round survey
The nine priority outcomes were distributed in the third round and scores of relative importance attributed to these outcomes by each stakeholder group are presented in Table 3. The outcomes of highest importance to patients were ‘overall survival’ (median score = 90.0), ‘disease-free survival’ (median score = 89.0), ‘mobility’ (median score = 88.0), and ‘resection margins’ (median score = 88.0). The outcomes of highest importance to carers were ‘distant recurrence’ (median score = 94.5), ‘resection margins’ (median score = 92.5), and ‘local recurrence-free survival’ (median score = 91.0). The outcomes of highest importance to clinicians were ‘resection margins’ (median score = 90.0), ‘overall survival’ (median score = 85.0), ‘local recurrence-free survival’ (median score = 85.0), and ‘global quality of life’ (median score = 85.0). Patient responses demonstrated less discrimination in importance between outcomes (median score range 83.0–90.0) compared with carers (77.5–94.5) and clinicians (70.0–90.0). When the ranks of individual stakeholder groups were combined, the outcomes of highest importance to the overall cohort were ‘resection margins’, ‘local recurrence-free survival’, and ‘overall survival’.
Table 3.
Final priority outcomes of pelvic exenteration, which met ‘consensus in’ criteria among patient, carer, and clinician stakeholder groups
| Priority outcome | Lay description | Patients (n = 21) | Carers (n = 4) | Clinicians (n = 116) | Combined rank | |||
|---|---|---|---|---|---|---|---|---|
| Median | Rank | Median | Rank | Median | Rank | |||
| Survival | ||||||||
| Overall survival | The length of time until death from any cause (including cancer and non-cancer causes) | 90.0 | 1 | 77.5 | 9 | 85.0 | 2 | 3 |
| Disease-free survival | The length of time a person lives without cancer coming back | 89.0 | 2 | 88.5 | 5 | 80.0 | 5 | 4 |
| Local recurrence-free survival | The length of time a person lives without cancer coming back in the pelvis | 86.0 | 6 | 91.0 | 3 | 85.0 | 2 | 2 |
| Cancer recurrence | ||||||||
| Distant recurrence | The cancer coming back elsewhere in the body, other than the pelvis | 83.0 | 9 | 94.5 | 1 | 70.0 | 8 | 8 |
| Patient-reported outcomes and functioning | ||||||||
| Psychological functioning | Ability to think, reason, remember, and make decisions, as well as experience and cope with emotions, including fear of the cancer coming back | 84.0 | 8 | 80.5 | 8 | 76.0 | 7 | 9 |
| Physical functioning | Ability to perform daily activities, such as walking, climbing stairs, and carrying out self-care tasks | 87.0 | 5 | 88.0 | 6 | 80.0 | 5 | 5 |
| Global quality of life | Overall well-being, including physical health, emotional well-being, social relationships, and life satisfaction | 85.0 | 7 | 81.5 | 7 | 85.0 | 2 | 5 |
| Mobility | Being able to walk or get around | 88.0 | 3 | 90.5 | 4 | 70.0 | 9 | 5 |
| Pathological outcomes | ||||||||
| Resection margins | Surgical removal of the entire tumour in one piece, with clear margins | 88.0 | 3 | 92.5 | 2 | 90.0 | 1 | 1 |
The median score of relative importance and corresponding rank for each stakeholder group is presented, as well as the combined rank.
Discussion
This study identifies nine outcomes of pelvic exenteration surgery that are considered of highest priority by patients, carers, and clinicians. These nine ‘priority outcomes’ cover four broad domains: survival (overall survival, disease-free survival, and local recurrence-free survival), cancer recurrence (distant recurrence), patient-reported outcomes and functioning (psychological functioning, physical functioning, global quality of life, and mobility) and pathological outcomes (resection margins). Priority outcomes were considered critically important by 70% or more of participants in each stakeholder group and were selected after a comprehensive review of 1157 unique outcomes reported in the contemporary literature (published between 1990 and 2023) and 46 additional outcomes from patient and carer interviews. Importantly, the rigorous consensus methodology used to identify the high-priority outcomes in this study ensured that patients, carers, and clinicians were equally represented.
When scored and ranked by participants according to relative importance, ‘resection margins’, ‘local recurrence-free survival’, and ‘overall survival’ were considered the most important outcomes of exenteration surgery for locally advanced primary and recurrent rectal cancer. Importantly, this rank order may be less discriminatory than it appears because all nine outcomes were, by definition, already considered critically important and were scored highly in the third-round survey, particularly by patients, with little discrimination as to the relative importance of outcomes. Therefore, it is difficult to draw conclusions about differences in priorities between stakeholder groups. Nonetheless, the finding that ‘resection margins’ was ranked highest overall by participants in this study is noteworthy. R0 resection is strongly associated with improved overall survival11,12, disease-free survival13, and local recurrence-free survival14, as well as lower patient-reported long-term pain scores15 and quality of life16. On this basis, achieving clear resection margins has become the ‘holy grail’ of exenterative surgery and it is likely that patients and their carers are counselled extensively by their treating clinicians about the importance of pathology margin status. However, resection margins remain only a surrogate marker for the more clinically meaningful outcomes identified in this study: survival, disease recurrence, and quality of life.
The priority outcomes identified in this study will form the outcomes of interest of a risk prediction tool being developed for patients with locally advanced primary or recurrent rectal cancer7. Developed using prospectively collected data from Royal Prince Alfred Hospital, a high-volume exenteration centre, this tool aims to predict the priority outcomes of exenteration surgery for individual patients based on preoperative information (such as demographic and disease characteristics). The goal of the risk prediction tool is to support reproducible, evidence-based decision-making for patients with locally advanced and recurrent rectal cancer, by quantifying the predicted outcomes of surgery for individual patients. Importantly, unlike many risk prediction tools and nomograms, which generally predict survival as the single outcome of interest17,18, the outcomes identified in this study and included in the proposed risk prediction tool address a range of outcome domains considered critically important by patients and carers, as well as clinicians. Although treatment decision-making must always be individualized, with consideration given to a specific patient’s goals and priorities, the ability to predict the priority outcomes identified in this study may serve as a framework for decision-making. The broader goal is to address the issue of unwarranted variation in treatment decision-making both at the time of diagnosis (that is whether an individual patient should be referred to a specialist exenteration centre) and by exenteration multidisciplinary teams (that is whether an individual patient should be offered surgery).
A possible secondary application of the priority outcomes is to direct future research, including selection of endpoints and outcomes in database and trial design. The priority outcomes identified in this study address four domains: survival, cancer recurrence, patient-reported outcomes and functioning, and pathological outcomes. In the systematic review component of this study, which included 156 studies of exenterative or multivisceral resections for locally advanced primary or recurrent rectal cancer, the most commonly reported domain was ‘complications’ (146 studies, 94%); however, no complication-related outcomes reached consensus criteria to be included as priorities in this study. Conversely, four of nine priority outcomes were categorized as ‘patient-reported outcomes and functioning’, which was the least commonly reported domain in the literature (26% of studies)5. This highlights the disparity between consensus-based priority outcomes (that is considering the views of patients and carers) and those commonly reported in the contemporary literature (that is by clinicians). In future, the identified priority outcomes should be considered during study design and, in particular, prospective patient-reported outcome measurement should be considered a mandatory component of audit and quality assessment at specialist exenteration units.
The strengths of this study include patient involvement in its design and conduct, particularly in the multidisciplinary review of outcomes and piloting of the first-round survey. Importantly, for participating patients, the median time since exenteration surgery was 36 months, which ensured that the views of those living with the long-term consequences of surgery were captured (beyond the immediate recuperation interval). The outcome longlist development process was comprehensive, involving an extensive review of outcomes reported in the contemporary literature (2756 outcomes from 156 studies) and those extracted from in-depth interviews with a large sample of patients and carers (34 patients and 5 carers). This ensured that a wide range of outcomes was captured and considered during the Delphi phase of the study. The consensus criteria, which were defined a priori, ensured that the final priority outcomes reflected the shared priorities of patients, carers, and an international group of exenteration clinicians, despite unequal numbers of participants in each stakeholder group. The attrition rate across rounds was acceptable at 49% for the overall cohort. A limitation of the study is that the patients (and their carers) invited to participate were from one country (Australia) due to practical limitations with regard to seeking ethical approval in multiple countries. Importantly, the clinician cohort represented 22 countries. The number of clinicians invited to participate in the surveys (and therefore the denominator in the calculation of response rate) is unknown due to the use of snowball sampling, where participants were asked to forward the invitation to their local multidisciplinary teams.
The priority outcomes identified in this study reflect the shared priorities of patients who have undergone pelvic exenteration for locally advanced or recurrent rectal cancer, their carers, and treating clinicians. These priorities include survival, cancer recurrence, quality of life, and resection margin status. These findings will inform the development of a risk prediction tool, which aims to predict these consensus-based priority outcomes for individual patients using information available at the point of diagnosis and to facilitate reproducible, evidence-based decision-making, thus addressing the issue of unwarranted variation in treatment decision-making in this group of patients.
Collaborators
EviSurg Collaborative Group
Nabila Ansari (Royal Prince Alfred Hospital, Sydney, Australia); Kirk Austin (Royal Prince Alfred Hospital, Sydney, Australia); Wendy Brown (Royal Prince Alfred Hospital, Sydney, Australia); Phyllis Butow (University of Sydney, Sydney, Australia); Chris Byrne (Royal Prince Alfred Hospital, Sydney, Australia); Sharon Carey (Royal Prince Alfred Hospital, Sydney, Australia); Alix Dumitrescu (Royal Prince Alfred Hospital, Sydney, Australia); Sophie Hatcher (Royal Prince Alfred Hospital, Sydney, Australia); Lisa Horvath (Chris O‘Brien Lifehouse Cancer Centre, Sydney, Australia); Cherry Koh (Royal Prince Alfred Hospital, Sydney, Australia); Peter Lee (Royal Prince Alfred Hospital, Sydney, Australia); Kate Mahon (Chris O‘Brien Lifehouse Cancer Centre, Sydney, Australia); Kate McBride (Royal Prince Alfred Hospital, Sydney, Australia); Glen Salkeld (University of University of Wollongong, Wollongong, Australia); Charbel Sandroussi (Royal Prince Alfred Hospital, Sydney, Australia); David Yeo (Royal Prince Alfred Hospital, Sydney, Australia).
Supplementary Material
Acknowledgements
The authors would like to thank Don Robertson and Alexandra Crawford, for participating in the multidisciplinary advisory meeting, and Mr Michael Kelly from the PelvEx Collaborative, for distributing the survey invitation.
Contributor Information
Kilian G M Brown, Department of Colorectal Surgery, Royal Prince Alfred Hospital, Sydney, New South Wales, Australia; Surgical Outcomes Research Centre (SOuRCe), Royal Prince Alfred Hospital, Sydney, New South Wales, Australia; Institute of Academic Surgery (IAS), Royal Prince Alfred Hospital, Sydney, New South Wales, Australia; Faculty of Medicine and Health, Central Clinical School, University of Sydney, Sydney, New South Wales, Australia.
James Morkaya, Surgical Outcomes Research Centre (SOuRCe), Royal Prince Alfred Hospital, Sydney, New South Wales, Australia.
Michael J Solomon, Department of Colorectal Surgery, Royal Prince Alfred Hospital, Sydney, New South Wales, Australia; Surgical Outcomes Research Centre (SOuRCe), Royal Prince Alfred Hospital, Sydney, New South Wales, Australia; Institute of Academic Surgery (IAS), Royal Prince Alfred Hospital, Sydney, New South Wales, Australia; Faculty of Medicine and Health, Central Clinical School, University of Sydney, Sydney, New South Wales, Australia.
Kheng-Seong Ng, Department of Colorectal Surgery, Royal Prince Alfred Hospital, Sydney, New South Wales, Australia; Surgical Outcomes Research Centre (SOuRCe), Royal Prince Alfred Hospital, Sydney, New South Wales, Australia; Institute of Academic Surgery (IAS), Royal Prince Alfred Hospital, Sydney, New South Wales, Australia; Faculty of Medicine and Health, Central Clinical School, University of Sydney, Sydney, New South Wales, Australia.
Kate White, Sydney Nursing School, University of Sydney, Sydney, New South Wales, Australia; The Daffodil Centre, University of Sydney, a joint venture with Cancer Council NSW, Sydney, New South Wales, Australia.
Paul Sutton, Surgical Outcomes Research Centre (SOuRCe), Royal Prince Alfred Hospital, Sydney, New South Wales, Australia; Colorectal and Peritoneal Oncology Centre, The Christie NHS Foundation Trust, Manchester, UK; Division of Cancer Sciences, University of Manchester, Manchester, UK.
Desmond C Winter, Department of Surgery, St Vincent’s University Hospital, Dublin, Ireland.
Daniel Steffens, Surgical Outcomes Research Centre (SOuRCe), Royal Prince Alfred Hospital, Sydney, New South Wales, Australia; Institute of Academic Surgery (IAS), Royal Prince Alfred Hospital, Sydney, New South Wales, Australia; Faculty of Medicine and Health, Central Clinical School, University of Sydney, Sydney, New South Wales, Australia.
EvigSurg Collaborative Group:
Nabila Ansari, Kirk Austin, Wendy Brown, Phyllis Butow, Chris Byrne, Sharon Carey, Alix Dumitrescu, Sophie Hatcher, Lisa Horvath, Cherry Koh, Peter Lee, Kate Mahon, Kate McBride, Glen Salkeld, Charbel Sandroussi, and David Yeo
Funding
K.G.M.B. is the recipient of the Mitchell J Notaras Fellowship in Colorectal Surgery from the University of Sydney. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contributions
Kilian G. M. Brown (Conceptualization, Data curation, Formal analysis, Writing—original draft), James Morkaya (Conceptualization, Data curation, Formal analysis, Writing—review & editing), Michael J. Solomon (Conceptualization, Methodology, Supervision, Writing—review & editing), Kheng-Seong Ng (Conceptualization, Formal analysis, Methodology, Writing—review & editing), Kate White (Conceptualization, Data curation, Methodology, Writing—review & editing), Paul Sutton (Conceptualization, Methodology, Writing—review & editing), Desmond C. Winter (Conceptualization, Data curation, Writing—review & editing), Daniel Steffens (Conceptualization, Methodology, Data curation, Supervision, Writing—review & editing), and EviSurg Collaborative Group (Conceptualization, Methodology)
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS online.
Data availability
Data can be made available, upon reasonable request, but requires ethical approval.
References
- 1. PelvEx Collaborative . Contemporary results from the PelvEx Collaborative: improvements in surgical outcomes for locally advanced and recurrent rectal cancer. Colorectal Dis 2024;26:926–931 [DOI] [PubMed] [Google Scholar]
- 2. Brown KGM, Solomon MJ, Koh CE, Sutton PA, Aguiar S Jr, Bezerra TS et al. Defining benchmarks for pelvic exenteration surgery: a multicentre analysis of patients with locally advanced and recurrent rectal cancer. Ann Surg 2024; DOI: 10.1097/SLA.0000000000006348 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3. Denost Q, Solomon M, Tuech J-J, Ghouti L, Cotte E, Panis Y et al. International variation in managing locally advanced or recurrent rectal cancer: prospective benchmark analysis. Br J Surg 2020;107:1846–1854 [DOI] [PubMed] [Google Scholar]
- 4. Nordkamp S, Voogt ELK, van Zoggel DMGI, Martling A, Holm T, Jansson Palmer G et al. Locally recurrent rectal cancer: oncological outcomes with different treatment strategies in two tertiary referral units. Br J Surg 2022;109:623–631 [DOI] [PubMed] [Google Scholar]
- 5. Brown KGM, Pisaniello J, Ng KS, Solomon MJ, Sutton PA, Hatcher S et al. Variation in outcome measurement and reporting in studies of pelvic exenteration for locally advanced and recurrent rectal cancer: a systematic review. Colorectal Dis 2024;26:272–280 [DOI] [PubMed] [Google Scholar]
- 6. Brown KGM, White K, Solomon MJ, Sutton PA, Ng KS, Koh CE et al. A matter of survival - Patients' and carers' perspectives on the decision to undergo pelvic exenteration surgery for locally advanced and recurrent rectal cancer. Colorectal Dis 2024; (In press) [DOI] [PubMed] [Google Scholar]
- 7. Brown K, Solomon M, Ng K-S, Sutton P, Koh C, White K et al. Development of a risk prediction tool for patients with locally advanced and recurrent rectal cancer undergoing pelvic exenteration: protocol for a mixed-methods study. BMJ Open 2023;13:e075304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jünger S, Payne SA, Brine J, Radbruch L, Brearley SG. Guidance on Conducting and REporting DElphi Studies (CREDES) in palliative care: recommendations based on a methodological systematic review. Palliat Med 2017;31:684–7068 [DOI] [PubMed] [Google Scholar]
- 9. Dodd S, Clarke M, Becker L, Mavergames C, Fish R, Williamson PR. A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J Clin Epidemiol 2018;96:84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E et al. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. PelvEx Collaborative . Surgical and survival outcomes following pelvic exenteration for locally advanced primary rectal cancer: results from an international collaboration. Ann Surg 2019;269:315–321 [DOI] [PubMed] [Google Scholar]
- 12. Bhangu A, Ali SM, Darzi A, Brown G, Tekkis P. Meta-analysis of survival based on resection margin status following surgery for recurrent rectal cancer. Colorectal Dis 2012;14:1457–1466 [DOI] [PubMed] [Google Scholar]
- 13. Simillis C, Baird DLH, Kontovounisios C, Pawa N, Brown G, Rasheed S et al. A systematic review to assess resection margin status after abdominoperineal excision and pelvic exenteration for rectal cancer. Ann Surg 2017;265:291–299 [DOI] [PubMed] [Google Scholar]
- 14. Koh CE, Brown KGM, Steffens D, Young J, Salkeld G, Solomon MJ. What constitutes a clear margin in patients with locally recurrent rectal cancer undergoing pelvic exenteration? Ann Surg 2022;275:157–165 [DOI] [PubMed] [Google Scholar]
- 15. Vuong K, Alchin LM, Solomon MJ, Koh CE, Steffens D. A prospective investigation of pain and fatigue following pelvic exenteration. Eur J Surg Oncol 2021;47:3137–3143 [DOI] [PubMed] [Google Scholar]
- 16. Austin KKS, Young JM, Solomon MJ. Quality of life of survivors after pelvic exenteration for rectal cancer. Dis Colon Rectum 2010;53:1121–1126 [DOI] [PubMed] [Google Scholar]
- 17. Varey AHR, Li I, El Sharouni M-A, Simon J, Dedeilia A, Ch'ng S et al. Predicting recurrence-free and overall survival for patients with stage II melanoma: the MIA calculator. J Clin Oncol 2024;42:1169–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Candido Dos Reis FJ, Wishart GC, Dicks EM, Greenberg D, Rashbass J, Schmidt MK et al. An updated PREDICT breast cancer prognostication and treatment benefit prediction model with independent validation. Breast Cancer Res 2017;19:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data can be made available, upon reasonable request, but requires ethical approval.
References
- 1. PelvEx Collaborative . Contemporary results from the PelvEx Collaborative: improvements in surgical outcomes for locally advanced and recurrent rectal cancer. Colorectal Dis 2024;26:926–931 [DOI] [PubMed] [Google Scholar]
- 2. Brown KGM, Solomon MJ, Koh CE, Sutton PA, Aguiar S Jr, Bezerra TS et al. Defining benchmarks for pelvic exenteration surgery: a multicentre analysis of patients with locally advanced and recurrent rectal cancer. Ann Surg 2024; DOI: 10.1097/SLA.0000000000006348 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3. Denost Q, Solomon M, Tuech J-J, Ghouti L, Cotte E, Panis Y et al. International variation in managing locally advanced or recurrent rectal cancer: prospective benchmark analysis. Br J Surg 2020;107:1846–1854 [DOI] [PubMed] [Google Scholar]
- 4. Nordkamp S, Voogt ELK, van Zoggel DMGI, Martling A, Holm T, Jansson Palmer G et al. Locally recurrent rectal cancer: oncological outcomes with different treatment strategies in two tertiary referral units. Br J Surg 2022;109:623–631 [DOI] [PubMed] [Google Scholar]
- 5. Brown KGM, Pisaniello J, Ng KS, Solomon MJ, Sutton PA, Hatcher S et al. Variation in outcome measurement and reporting in studies of pelvic exenteration for locally advanced and recurrent rectal cancer: a systematic review. Colorectal Dis 2024;26:272–280 [DOI] [PubMed] [Google Scholar]
- 6. Brown KGM, White K, Solomon MJ, Sutton PA, Ng KS, Koh CE et al. A matter of survival - Patients' and carers' perspectives on the decision to undergo pelvic exenteration surgery for locally advanced and recurrent rectal cancer. Colorectal Dis 2024; (In press) [DOI] [PubMed] [Google Scholar]
- 7. Brown K, Solomon M, Ng K-S, Sutton P, Koh C, White K et al. Development of a risk prediction tool for patients with locally advanced and recurrent rectal cancer undergoing pelvic exenteration: protocol for a mixed-methods study. BMJ Open 2023;13:e075304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jünger S, Payne SA, Brine J, Radbruch L, Brearley SG. Guidance on Conducting and REporting DElphi Studies (CREDES) in palliative care: recommendations based on a methodological systematic review. Palliat Med 2017;31:684–7068 [DOI] [PubMed] [Google Scholar]
- 9. Dodd S, Clarke M, Becker L, Mavergames C, Fish R, Williamson PR. A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J Clin Epidemiol 2018;96:84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E et al. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. PelvEx Collaborative . Surgical and survival outcomes following pelvic exenteration for locally advanced primary rectal cancer: results from an international collaboration. Ann Surg 2019;269:315–321 [DOI] [PubMed] [Google Scholar]
- 12. Bhangu A, Ali SM, Darzi A, Brown G, Tekkis P. Meta-analysis of survival based on resection margin status following surgery for recurrent rectal cancer. Colorectal Dis 2012;14:1457–1466 [DOI] [PubMed] [Google Scholar]
- 13. Simillis C, Baird DLH, Kontovounisios C, Pawa N, Brown G, Rasheed S et al. A systematic review to assess resection margin status after abdominoperineal excision and pelvic exenteration for rectal cancer. Ann Surg 2017;265:291–299 [DOI] [PubMed] [Google Scholar]
- 14. Koh CE, Brown KGM, Steffens D, Young J, Salkeld G, Solomon MJ. What constitutes a clear margin in patients with locally recurrent rectal cancer undergoing pelvic exenteration? Ann Surg 2022;275:157–165 [DOI] [PubMed] [Google Scholar]
- 15. Vuong K, Alchin LM, Solomon MJ, Koh CE, Steffens D. A prospective investigation of pain and fatigue following pelvic exenteration. Eur J Surg Oncol 2021;47:3137–3143 [DOI] [PubMed] [Google Scholar]
- 16. Austin KKS, Young JM, Solomon MJ. Quality of life of survivors after pelvic exenteration for rectal cancer. Dis Colon Rectum 2010;53:1121–1126 [DOI] [PubMed] [Google Scholar]
- 17. Varey AHR, Li I, El Sharouni M-A, Simon J, Dedeilia A, Ch'ng S et al. Predicting recurrence-free and overall survival for patients with stage II melanoma: the MIA calculator. J Clin Oncol 2024;42:1169–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Candido Dos Reis FJ, Wishart GC, Dicks EM, Greenberg D, Rashbass J, Schmidt MK et al. An updated PREDICT breast cancer prognostication and treatment benefit prediction model with independent validation. Breast Cancer Res 2017;19:58. [DOI] [PMC free article] [PubMed] [Google Scholar]