Abstract
Mother–child closeness, a mutually trusting and affectionate bond, is an important factor in shaping positive youth development. However, little is known about the neural pathways through which mother–child closeness is related to brain organization. Utilizing a longitudinal sample primarily from low-income families (N = 181; 76% African American youth and 54% female), this study investigated the associations between mother–child closeness at ages 9 and 15 years and structural connectivity organization (network integration, robustness, and segregation) at age 15 years. The assessment of mother–child closeness included perspectives from both mother and child. The results revealed that greater mother–child closeness is linked with increased global efficiency and transitivity, but not with modularity. Specifically, both the mother’s and child’s reports of closeness at age 15 years predicted network metrics, but report at age 9 years did not. Our findings suggest that mother–child closeness is associated with neural white matter organization, as adolescents who experienced greater mother–child closeness displayed topological properties indicative of more integrated and robust structural networks.
Keywords: maternal closeness, positive parenting, adolescent brain development, structural connectivity organizations
Introduction
Adolescence is marked by significant neurodevelopmental growth, shaped by influences from surrounding social contexts (Dahl 2004). Within developmental neuroscience, there is growing interest in identifying promotive social factors, such as positive parenting and close relationship with others, that are linked to neural correlates of positive youth outcomes (Telzer et al. 2018, Farber et al. 2022). This line of research aims to complement our understanding of the neural mechanisms of early-life stress by identifying potential routes for positive youth development (Belsky and De Haan 2011). While the social landscapes of youth evolve from early childhood, close relationships with primary caregivers, often mothers, continue to play a pivotal role in positive youth development (Youngblade et al. 2007, Telzer et al. 2018). Despite the prominence of the mother–child relationship in development, little research has examined links between this relationship and brain development.
Mother–child closeness, influenced by both parent and child attributes including positive parenting practices, signifies a relationship characterized by trusting interactions between mutually affectionate dyads (Collins and Laursen 2004, McWayne et al. 2017). This strong dyadic relationship has been observed across families of diverse racial–ethnic and socioeconomic backgrounds, particularly among those under-represented in research on positive parenting, such as families of color, single-parent households, and low-income families (Aronowitz and Morrison‐Beedy 2004, Brody et al. 2005, King et al. 2018). Furthermore, studies across diverse groups of children and adolescents show that greater mother–child closeness relates to higher prosocial behavior and lower depression and risk-taking behaviors (Ackard et al. 2006, Day and Padilla-Walker 2009, Ge et al. 2009, Fagan 2022, Lawrence 2022, Jones et al. 2023). Therefore, further examination is warranted to elucidate the neural processes through which quality mother–child dyadic relationships “get under the skin” and shape developmental trajectories.
Research using animal models has long suggested a possible pathway where parent–child relationship may influence a child’s brain development (Knop et al. 2017). Furthermore, these findings offer possible insights into how mother–child relationships specifically could be associated with distinct neural patterns in youth. For example, implications from the studies on the influences of the postnatal environment on rodents’ brain development indicate that a high level of mother–child closeness during childhood and adolescence may be a significant social context for optimal human brain development (Pryce and Feldon 2003). Previous work has shown that maternal bonding of dams with their pups, through behaviors such as licking and nursing, leads to significant alterations in the offspring rodents’ neurobiological systems related to stress responses (Weaver et al. 2004, Moriceau and Sullivan 2006).
A few studies have investigated the neural outcomes of the mother–child dyadic relationship in humans. For instance, one study found that adolescents who reported more positive relationships with their parents showed a greater decrease in ventral striatum activation during risk-taking tasks in functional magnetic resonance imaging (fMRI) scans from the baseline to a follow-up (Qu et al. 2015). Another showed that a high level of secure attachment between the mother and the child during adolescence was associated with increased activation in brain regions commonly associated with cognitive, affective, and reward processing in adulthood (Lin et al. 2024). Lastly, a study on brain structure found that youth who experienced high levels of maternal warmth and cooperation showed faster maturation in the left and right orbitofrontal cortices, which are associated with adaptive emotion and behavior regulation abilities (Whittle et al. 2014). Building upon these earlier studies, additional work is necessary to examine the contribution of mother–child closeness to indices of overall brain development.
The network organization of structural brain connectivity is an important aspect of adolescent brain development. Brain maturation involves enhanced communication between neural regions, with the myelination process playing a pivotal role (Ladouceur et al. 2012). Studies examining white matter structures have predominantly focused on examining white matter volume, density, and connectivity, which are reported to generally increase from childhood to young adulthood, albeit with some region-specific variations (Schmithorst and Yuan 2010, Goetschius et al. 2020, Hardi et al. 2022). Moreover, studies have expanded our methodology of estimating structural connectivity using diffusion neuroimaging data using network approaches that can evaluate the characteristics of whole-brain connectomes (Yeh et al. 2021).
Application of graph analytic methods to extract network organization metrics has provided insight into the spatial characteristics of structural networks, revealing a comprehensive map of information-processing pathways related to the integration, robustness, and segregation of neural connectivity within the brain (Bullmore and Sporns 2009, Menon 2011). Three commonly assessed topological properties of network analyses include global network efficiency, transitivity, and modularity. Global network efficiency quantifies how quickly information can travel from one end of the network to the other, where greater efficiency signifies faster information transfer within the network. Transitivity indicates the degree of complexity with which the nodes of the networks cluster together, where greater transitivity suggests network robustness. Finally, modularity represents to what extent a network distinctively subdivides into separate modules or segregated networks, where greater modularity indicates a more segregated network (Bullmore and Sporns 2009, Menon 2011).
Previous studies utilizing network metrics have shown that white matter organization is sensitive to parenting (a process that influences and is influenced by the parent–child relationship), although these studies have primarily focused on the effects of harsh parenting. For example, two studies found that a high degree of negative maternal behaviors was associated with reduced modularity among young children (age 8 years), and a history of maltreatment by caregivers was linked to reduced global efficiency among young adults (ages 18–25 years) (Ohashi et al. 2017, Richmond et al. 2021). Based on existing evidence, mother–child closeness may also shape structural network organization, potentially revealing a diverging pattern to that observed with harsh parenting. While harsh parenting may be stressful and shape brain development to adapt to a stressful and uncertain environment, nurturing from a mother with whom the child identifies as having a close and supportive relationship may provide a buffer against other stressors and help scaffold positive and supportive relationships, which can, in turn, promote positive brain development. Thus, greater mother–child closeness may be linked to more efficient, clustered, and segregated brain networks.
An important factor to consider when investigating the neural processes linked to mother–child closeness is that both the mother and child play pivotal roles in creating a dyadic bond. Thus, using reports from both parties provides a more comprehensive evaluation than relying on a single informant. In addition, the quality of the mother–child relationship may vary from preadolescence to adolescence due to shifts in time spent together, increased autonomy, and potential conflicts (De Goede et al. 2009, Fang et al. 2021). However, as adolescents develop better perspective-taking skills, they may gain a deeper understanding and appreciation of their parents, which could improve relationship quality (Collins and Laursen 2004, De Goede et al. 2009, Hou et al. 2020). Therefore, delineating the neural processes linked to mother–child closeness can benefit from considering reports from both members of the mother–child dyad, as well as differential level of closeness across developmental periods.
Expanding on prior work that examined the neural correlates of positive parenting and quality of mother–child relationships (Whittle et al. 2014, Qu et al. 2015, Lin et al. 2024), a related, though distinct construct, the present investigation sought to delineate how the mother’s and child’s appraisal of their closeness contributes to the youth neural development. Specifically, given that no study has examined how mother–child closeness is associated with structural connectivity organization, the current investigation examined the whole-brain structural network organization to complement prior studies focusing on specific regions. This understanding may enhance our knowledge of normative and positive parenting effects on brain development, which remains relatively understudied and warrants further research (Farber et al. 2022). The present study investigated associations between mother–child closeness at ages 9 and 15 years with characteristics of structural network organization (global efficiency, transitivity, and modularity) at age 15 years. We also examined whether mother or child report had stronger associations with the white matter network metrics. We hypothesized that greater mother–child closeness at ages 9 and 15 years would be related to more efficient, clustered, and segregated structural organization in adolescence. In addition, we hypothesized that both the mother’s and child’s evaluation of closeness would equally contribute to the youth network metrics.
Methods
Sample
Participants were recruited from the Future of Families and Child Wellbeing Study (FFCWS; N = 4898), a population-based longitudinal study of children born in 20 large US cities between 1998 and 2000 (Reichman et al. 2001). The FFCWS is oversampled (3:1) for nonmarital births and low-income families. When the children reached ages 15–17 years, they were invited to participate in the Study of Adolescent Neurodevelopment (SAND), a follow-up study examining the impact of the environment on adolescent brain development (Hein et al. 2018). A cohort of 237 families from Detroit, MI, Toledo, OH, and Chicago, IL, participated in the study. Given the demographics of these cities, most families in the SAND subsample identified as African American (76%), with 54.4% identifying as female. The University of Michigan Institutional Review Board approved the study, and all families provided written consent to participate. In the current study, we restricted our longitudinal dataset to individuals with usable neuroimaging data, culminating in a final sample size of 181 (Supplementary 1). Refer to Table 1 for demographic details.
Table 1.
Demographic characteristics of the study participants (N = 181).
| n (%) or M (s.d., min–max) | |
|---|---|
| Child’s gender (female) | 99 (54.7) |
| Child’s race/ethnicity | |
| African American | 137 (77.8) |
| European American | 20 (11.4) |
| Hispanic/Latinx | 12 (6.8) |
| Other | 7 (4.0) |
| Maternal education status | |
| ≤High school | 76 (42.0) |
| Average household income | 2.86 (1.22, 1–5) |
| Maternal marital status (no) | 140 (77.3) |
| Maternal age at birth | 25.66 (6.21, 17–43) |
| Maternal depression | |
| Age 9 years | 35 (19.3) |
| Age 15 years | 45 (24.9) |
| Pubertal scores | 3.26 (0.58, 1.33–4) |
| Mother–child closeness | |
| Child’s appraisal (age 9 years) | 2.35 (0.69, 0.5–3) |
| Mother’s appraisal (age 9 years) | 2.72 (0.55, 0–3) |
| Child’s appraisal (age 15 years) | 2.10 (0.72, 0–3) |
| Mother’s appraisal (age 15 years) | 2.53 (0.73, 0–3) |
Measures
Mother–child closeness was assessed through mother and child self-reports collected at ages 9 and 15 years. The assessment of closeness was based on the Caregiver–Child Relationship construct created by FFCWS. This construct utilized a modified brief version of the Family Functioning and Adolescent sections from the National Survey of Children’s Health (National Survey of Children’s Health 2003). We used items that were consistently asked across two time points. This survey encompassed the following questions: “How close do you feel to your mom/child?” and “How well do you and your mom share ideas or talk about things that matter?” These items have been shown to be strong indicators of the quality of a dyadic relationship (Blumberg et al. 2005, Bandy and Moore 2008, De Luca et al. 2018) and have been used in other datasets with nationally representative samples (King et al. 2018). The child’s report of mother–child closeness was created using two items, and the mother’s report was created using one item. All three items (two child-reported and one mother-reported) were rated on a four-point Likert scale, with the scales reverse-coded so that a higher score denoted stronger closeness (“0 = not very close” to “3 = extremely close”). Given that there were two items for children and one for the mother, we used a scaled score to ensure both that the mother and child’s responses were equally weighted. In total, four scores were created: child’s and mother’s reports of mother–child closeness at ages 9 and 15 years.
Covariates were added to our analyses to account for possible demographic influences: household income [average poverty threshold at ages 9 and 15 years determined by the US Census Bureau average income-to-needs ratio (United States Census Bureau 2020)], maternal age, maternal educational status (“0 = less than high school/ high school”, “1 = more than high school”), maternal depression at age 9 and 15 years (“0 = no depression”, “1 = depression”), children’s race (European American, African American, Hispanic/Latinx, and Other; dummy coded) and gender (“0 = male”, “1 = female”), and pubertal development at age 15 years (self-report on the Pubertal Development Scale (Petersen et al. 1988)) to account for robust biological changes occurring during adolescence and its implication on structural brain development (Herting and Sowell 2017, Roberts et al. 2020). We used race as a social construct variable reflecting differential experiences of exposure to structural racism-related adversity (Shonkoff et al. 2021). We adjusted for maternal depression as it is documented to have an effect on the mother–child relationship (Lovejoy et al. 2000). We additionally adjusted for marital status (“0 = non-married”, “1 = married”) to account for the original sampling strategy (Reichman et al. 2001).
Neuroimaging measures
Data acquisition and preprocessing
Magnetic resonance imaging (MRI) scans were obtained using a 3-T GE Discovery MR750 scanner with an eight-channel head coil at the University of Michigan Functional MRI Laboratory. Participants were provided with detailed instructions to minimize head movement, and head paddings were used. T1-weighted gradient echo images were acquired with the following parameters: TR (repetition time) = 12 ms, TE (echo time) = 5 ms, TI (inversion time) = 500 ms, flip angle = 15°, field of view = 26 cm, slice thickness = 1.44 mm, 256 × 192 matrix, and 110 slices. Subsequently, diffusion MRI (dMRI) data were collected using a spin-echo echo-planar imaging (EPI) diffusion sequence with a repetition time of 7250 ms, minimum echo time, 128 × 128 acquisition matrix, a field of view of 22 cm, 3-mm no-gap thick slices with 40 slices acquired using alternating increasing order, a b-value of 1000 s/mm2, 64 nonlinear directions b-value of 1000 s/mm2, and 64 nonlinear diffusion directions. An initial visual inspection of the dMRI images was performed to ensure quality. Slices with an average intensity below 4 standard deviations were marked as outliers and replaced using predicted models (Andersson et al. 2016). Participants were excluded if >5% of slices were replaced, and the images of 10 participants with the highest number of replaced slices underwent further visual scrutiny.
Structural connectivity organization estimation
The dMRI data were then processed to estimate structural connectivity using the MRtrix pipeline, which utilizes a novel tensor-fitting method called the constrained spherical deconvolution (Tournier et al. 2004, 2007, Farquharson et al. 2013), which performs especially well in connectivity estimation in regions of crossing fibers (Tournier et al. 2012). A probabilistic tractography approach was employed to generate 10 million streamlines, which were subsequently used to estimate structural connectivity between the AAL2 atlas (Rolls et al. 2015) to create a 94 × 94 connectome matrix, representing the count of streamlines or structural connectivity between distinct brain regions. Graph analysis was then applied to the resulting weighted, undirected, and unthresholded connectome matrixes using the Brain Connectivity Toolbox (Rubinov and Sporns 2010) in MATLAB. Three graph network metrics were computed: global network efficiency (how efficient information travels from one end of the network to the other; greater efficiency signifies faster information transfer), transitivity (the presence of triangles in the network; greater transitivity suggests increased network clustering/robustness), and modularity (to what extent network distinctively subdivides into separate modules; greater modularity represents more segregated networks).
Statistical analysis
We conducted path models to examine the associations between mother–child closeness and whole-brain structural connectivity metrics (global efficiency, transitivity, and modularity). Each model used the mother’s and child’s reports of closeness at ages 9 and 15 years as predictor variables (4 in total). Then, we conducted the Z-test to examine whether mother or child perceptions matter most to network metrics. In addition, we evaluated the unique variance predicted by each reporter’s response while controlling for the response from the other developmental period. We created one path model with the child’s reports of mother–child closeness at ages 9 and 15 years as separate predictor variables and another path model with the mother’s reports of mother–child closeness at ages 9 and 15 years as separate predictor variables. All analyses were conducted using R to perform path analyses (R Core Team 2021), and the full information maximum likelihood estimation method was used to account for missing data (Kline, 2023). All models controlled for covariates.
Results
The descriptive findings from the zero-order correlations (Supplementary 2) suggest that there is some discordance between the mother’s and child’s reports. There were positive associations between mother–child closeness and the topological properties of two network metrics. Specifically, the mother’s report of greater mother–child closeness at age 15 years and the child’s report of greater mother–child closeness at age 15 years were associated with greater global network efficiency (mother: β = 0.196, P = .009; child: β = 0.147, P = .043) and greater transitivity (mother: β = 0.186, P = .013; child: β = 0.20, P = .005) but not modularity (mother: β = −0.035, P = .659; child: β = −0.023, P = .759) (Table 3, Fig. 1). No significant difference was observed when statistically comparing the outcomes based on reporters (Z-test) (global efficiency: z = 0.43 P = .66; transitivity: z = 0.48 P = .95). There was no significant association between reports at age 9 years and topological properties of network metrics (Table 2, Fig. 1). See Fig. 2 for a visual representation of the differential patterns of structural networks between individuals with varying global efficiency and transitivity. Lastly, analyses examining appraisals of mother–child closeness separately by reporter across two developmental periods in one path model indicated that reports from age 15 years had significant associations with the network metrics, even after controlling for reports from age 9 years. The mother’s report at age 15 years remained positively associated with global efficiency (β = 0.201, P = .015) and transitivity (β = 0.177, P = .031) (Table 3). This pattern was consistently observed for the child’s report at age 15 years as well (β = 0.149, P = .043; β = 0.196, P = .007) (Table 3). Thus, greater mother–child closeness at age 15 years is significantly associated with greater global network efficiency and transitivity, independent of the influence of mother–child closeness at age 9 years.
Table 3.
Results of path analyses with mother’s and child’s reports from two time points in a single model.
| b | β | SE | z | P | |
|---|---|---|---|---|---|
| Global efficiency | |||||
| Mother’s closeness report (age 15 years) | 0.027 | 0.201 | 0.011 | 2.427 | .015 |
| Mother’s closeness report (age 9 years) | −0.002 | −0.010 | 0.015 | −0.116 | .908 |
| Pubertal score | −0.033 | −0.195 | 0.015 | −2.203 | .028 |
| Maternal age | −0.001 | −0.060 | 0.001 | −0.783 | .434 |
| Maternal marital status | −0.048 | −0.203 | 0.019 | −2.516 | .012 |
| Household income | −0.004 | −0.044 | 0.007 | −0.513 | .608 |
| Maternal education | −0.001 | −0.005 | 0.015 | −0.070 | .944 |
| Child’s gender | 0.025 | 0.125 | 0.018 | 1.380 | .168 |
| Race/ethnicity (White) | −0.035 | −0.114 | 0.023 | −1.508 | .132 |
| Race/ethnicity (Hispanic/Latinx) | −0.002 | −0.006 | 0.029 | −0.081 | .936 |
| Race/ethnicity (Other) | −0.042 | −0.085 | 0.037 | −1.136 | .256 |
| Maternal depression (9) | −0.011 | −0.049 | 0.017 | −0.644 | .520 |
| Maternal depression (15) | −0.005 | −0.020 | 0.020 | −0.249 | .803 |
| Transitivity | |||||
| Mother’s closeness report (age 15 years) | 0.129 | 0.177 | 0.060 | 2.153 | .031 |
| Mother’s closeness report (age 9 years) | 0.021 | 0.022 | 0.081 | 0.263 | .793 |
| Pubertal score | −0.193 | −0.211 | 0.081 | −2.369 | .018 |
| Maternal age | −0.006 | −0.065 | 0.007 | −0.840 | .401 |
| Maternal marital status | −0.269 | −0.211 | 0.104 | −2.596 | .009 |
| Household income | −0.026 | −0.060 | 0.037 | −0.698 | .485 |
| Maternal education | 0.074 | 0.069 | 0.082 | 0.895 | .371 |
| Child’s gender | 0.212 | 0.201 | 0.097 | 2.197 | .028 |
| Race/ethnicity (White) | −0.122 | −0.074 | 0.125 | −0.977 | .328 |
| Race/ethnicity (Hispanic/Latinx) | 0.079 | 0.038 | 0.154 | 0.512 | .609 |
| Race/ethnicity (Other) | −0.148 | −0.055 | 0.199 | −0.744 | .457 |
| Maternal depression (9) | −0.097 | −0.080 | 0.093 | −1.047 | .295 |
| Maternal depression (15) | 0.042 | 0.032 | 0.104 | 0.400 | .689 |
| Modularity | |||||
| Mother’s closeness report (age 15 years) | 0.012 | 0.042 | 0.019 | 0.131 | .896 |
| Mother’s closeness report (age 9 years) | 0.000 | 0.005 | 0.025 | −1.415 | .157 |
| Pubertal score | 0.044 | 0.111 | 0.026 | 0.455 | .649 |
| Maternal age | 0.001 | 0.005 | 0.002 | 0.065 | .948 |
| Maternal marital status | 0.016 | 0.049 | 0.034 | 1.284 | .199 |
| Household income | −0.025 | −0.076 | 0.012 | 0.051 | .960 |
| Maternal education | 0.046 | 0.090 | 0.026 | 0.613 | .540 |
| Child’s gender | −0.051 | −0.078 | 0.031 | −0.800 | .424 |
| Race/ethnicity (White) | 0.141 | 0.168 | 0.041 | 1.131 | .258 |
| Race/ethnicity (Hispanic/Latinx) | 0.040 | 0.105 | 0.050 | −1.013 | .311 |
| Race/ethnicity (Other) | −0.039 | −0.097 | 0.065 | 2.175 | .030 |
| Maternal depression (9) | 0.012 | 0.042 | 0.030 | 1.336 | .182 |
| Maternal depression (15) | 0.000 | 0.005 | 0.033 | −1.186 | .236 |
| Global efficiency | |||||
| Child’s closeness report (age 15 years) | 0.017 | 0.149 | 0.008 | 2.021 | .043 |
| Child’s closeness report (age 9 years) | −0.001 | −0.010 | 0.011 | −0.134 | .893 |
| Pubertal score | −0.032 | −0.187 | 0.015 | −2.100 | .036 |
| Maternal age | −0.001 | −0.044 | 0.001 | −0.573 | .567 |
| Maternal marital status | −0.052 | −0.218 | 0.020 | −2.639 | .008 |
| Household income | −0.004 | −0.049 | 0.007 | −0.559 | .576 |
| Maternal education | −0.003 | −0.015 | 0.015 | −0.194 | .846 |
| Child’s gender | 0.028 | 0.141 | 0.018 | 1.527 | .127 |
| Race/ethnicity (White) | −0.031 | −0.099 | 0.024 | −1.298 | .194 |
| Race/ethnicity (Hispanic/Latinx) | −0.004 | −0.011 | 0.029 | −0.154 | .878 |
| Race/ethnicity (Other) | −0.039 | −0.078 | 0.038 | −1.044 | .297 |
| Maternal depression (9) | −0.019 | −0.082 | 0.017 | −1.089 | .276 |
| Maternal depression (15) | −0.004 | −0.017 | 0.020 | −0.210 | .834 |
| Transitivity | |||||
| Child’s closeness report (age 15 years) | 0.121 | 0.196 | 0.045 | 2.689 | .007 |
| Child’s closeness report (age 9 years) | 0.019 | 0.025 | 0.059 | 0.325 | .745 |
| Pubertal score | −0.194 | −0.212 | 0.081 | −2.393 | .017 |
| Maternal age | −0.005 | −0.060 | 0.007 | −0.783 | .434 |
| Maternal marital status | −0.283 | −0.222 | 0.104 | −2.715 | .007 |
| Household income | −0.025 | −0.057 | 0.037 | −0.660 | .509 |
| Maternal education | 0.070 | 0.066 | 0.082 | 0.856 | .392 |
| Child’s gender | 0.230 | 0.217 | 0.097 | 2.375 | .018 |
| Race/ethnicity (White) | −0.085 | −0.051 | 0.125 | −0.682 | .495 |
| Race/ethnicity (Hispanic/Latinx) | 0.068 | 0.033 | 0.151 | 0.452 | .651 |
| Race/ethnicity (Other) | −0.125 | −0.046 | 0.198 | −0.630 | .529 |
| Maternal depression (9) | −0.134 | −0.110 | 0.091 | −1.471 | .141 |
| Maternal depression (15) | 0.050 | 0.039 | 0.103 | 0.491 | .624 |
| Modularity | |||||
| Child’s closeness report (age 15 years) | −0.005 | −0.028 | 0.015 | −0.371 | .710 |
| Child’s closeness report (age 9 years) | 0.006 | 0.026 | 0.019 | 0.326 | .744 |
| Pubertal score | 0.011 | 0.040 | 0.026 | 0.435 | .664 |
| Maternal age | 0.000 | −0.008 | 0.002 | −0.096 | .923 |
| Maternal marital status | 0.047 | 0.120 | 0.035 | 1.355 | .175 |
| Household income | 0.002 | 0.016 | 0.012 | 0.177 | .859 |
| Maternal education | 0.021 | 0.063 | 0.027 | 0.785 | .433 |
| Child’s gender | −0.027 | −0.081 | 0.031 | −0.845 | .398 |
| Race/ethnicity (White) | 0.046 | 0.088 | 0.041 | 1.100 | .271 |
| Race/ethnicity (Hispanic/Latinx) | −0.040 | −0.062 | 0.050 | −0.804 | .421 |
| Race/ethnicity (Other) | 0.133 | 0.158 | 0.066 | 2.022 | .043 |
| Maternal depression (9) | 0.039 | 0.103 | 0.030 | 1.322 | .186 |
| Maternal depression (15) | −0.029 | −0.072 | 0.033 | −0.889 | .374 |
Figure 1.
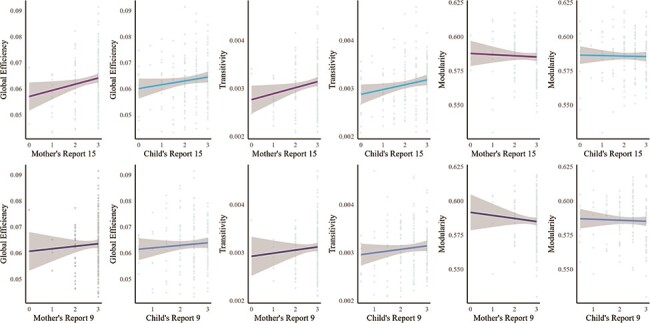
The association between mother–child closeness and the topological properties of the structural neural network architecture was investigated.
Table 2.
Results of path analyses with individual reports from mothers and children at two developmental time points (ages 9 and 15 years).
| b | β | SE | z | P | |
|---|---|---|---|---|---|
| Global efficiency | |||||
| Mother’s closeness report (age 15 years) | 0.026 | 0.196 | 0.010 | 2.603 | .009 |
| Pubertal score | −0.033 | −0.195 | 0.015 | −2.201 | .028 |
| Maternal age | −0.001 | −0.060 | 0.001 | −0.782 | .434 |
| Maternal marital status | −0.048 | −0.204 | 0.019 | −2.519 | .012 |
| Household income | −0.003 | −0.043 | 0.007 | −0.498 | .619 |
| Maternal education | −0.001 | −0.004 | 0.015 | −0.054 | .957 |
| Child’s gender | 0.025 | 0.125 | 0.018 | 1.371 | .170 |
| Race/ethnicity (White) | −0.036 | −0.115 | 0.024 | −1.520 | .128 |
| Race/ethnicity (Hispanic/Latinx) | −0.002 | −0.006 | 0.029 | −0.076 | .939 |
| Race/ethnicity (Other) | −0.043 | −0.085 | 0.037 | −1.145 | .252 |
| Maternal depression (9) | −0.011 | −0.049 | 0.017 | −0.652 | .514 |
| Maternal depression (15) | −0.005 | −0.020 | 0.019 | −0.250 | .803 |
| Transitivity | |||||
| Mother’s closeness report age 15 years | 0.135 | 0.186 | 0.054 | 2.491 | .013 |
| Pubertal score | −0.193 | −0.211 | 0.081 | −2.373 | .018 |
| Maternal age | −0.005 | −0.064 | 0.007 | −0.827 | .408 |
| Maternal marital status | −0.268 | −0.210 | 0.104 | −2.589 | .010 |
| Household income | −0.027 | −0.062 | 0.037 | −0.713 | .476 |
| Maternal education | 0.072 | 0.068 | 0.082 | 0.885 | .376 |
| Child’s gender | 0.211 | 0.200 | 0.097 | 2.183 | .029 |
| Race/ethnicity (White) | −0.124 | −0.075 | 0.125 | −0.986 | .324 |
| Race/ethnicity (Hispanic/Latinx) | 0.072 | 0.034 | 0.152 | 0.471 | .637 |
| Race/ethnicity (Other) | −0.144 | −0.053 | 0.199 | −0.724 | .469 |
| Maternal depression (9) | −0.095 | −0.078 | 0.093 | −1.030 | .303 |
| Maternal depression (15) | 0.034 | 0.026 | 0.102 | 0.333 | .739 |
| Modularity | |||||
| Mother’s closeness report (age 15 years) | −0.008 | −0.035 | 0.018 | −0.442 | .659 |
| Pubertal score | 0.012 | 0.043 | 0.026 | 0.462 | .644 |
| Maternal age | 0.000 | 0.000 | 0.002 | 0.003 | .998 |
| Maternal marital status | 0.043 | 0.109 | 0.035 | 1.248 | .212 |
| Household income | 0.002 | 0.014 | 0.012 | 0.151 | .880 |
| Maternal education | 0.019 | 0.058 | 0.026 | 0.728 | .466 |
| Child’s gender | −0.025 | −0.075 | 0.031 | −0.786 | .432 |
| Race/ethnicity (White) | 0.046 | 0.089 | 0.041 | 1.121 | .262 |
| Race/ethnicity (Hispanic/Latinx) | −0.042 | −0.064 | 0.050 | −0.835 | .404 |
| Race/ethnicity (Other) | 0.134 | 0.160 | 0.065 | 2.062 | .039 |
| Maternal depression (9) | 0.037 | 0.099 | 0.030 | 1.252 | .211 |
| Maternal depression (15) | −0.030 | −0.073 | 0.033 | −0.904 | .366 |
| Global efficiency | |||||
| Child’s closeness report (age 15 years) | 0.017 | 0.147 | 0.008 | 2.022 | .043 |
| Pubertal score | −0.032 | −0.188 | 0.015 | −2.111 | .035 |
| Maternal age | −0.001 | −0.046 | 0.001 | −0.596 | .551 |
| Maternal marital status | −0.051 | −0.216 | 0.019 | −2.641 | .008 |
| Household income | −0.004 | −0.048 | 0.007 | −0.554 | .580 |
| Maternal education | −0.003 | −0.014 | 0.015 | −0.185 | .853 |
| Child’s gender | 0.027 | 0.139 | 0.018 | 1.517 | .129 |
| Race/ethnicity (White) | −0.031 | −0.099 | 0.024 | −1.299 | .194 |
| Race/ethnicity (Hispanic/Latinx) | −0.004 | −0.011 | 0.029 | −0.152 | .879 |
| Race/ethnicity (Other) | −0.039 | −0.078 | 0.037 | −1.050 | .294 |
| Maternal depression (9) | −0.019 | −0.082 | 0.017 | −1.096 | .273 |
| Maternal depression (15) | −0.004 | −0.017 | 0.020 | −0.208 | .835 |
| Transitivity | |||||
| Child’s closeness report (age 15 years) | 0.123 | 0.200 | 0.044 | 2.778 | .005 |
| Pubertal score | −0.193 | −0.211 | 0.081 | −2.383 | .017 |
| Maternal age | −0.005 | −0.057 | 0.007 | −0.746 | .456 |
| Maternal marital status | −0.288 | −0.226 | 0.103 | −2.781 | .005 |
| Household income | −0.025 | −0.059 | 0.037 | −0.683 | .495 |
| Maternal education | 0.067 | 0.063 | 0.081 | 0.824 | .410 |
| Child’s gender | 0.232 | 0.219 | 0.097 | 2.400 | .016 |
| Race/ethnicity (White) | −0.089 | −0.054 | 0.125 | −0.715 | .474 |
| Race/ethnicity (Hispanic/Latinx) | 0.065 | 0.031 | 0.151 | 0.432 | .666 |
| Race/ethnicity (Other) | −0.136 | −0.051 | 0.197 | −0.691 | .489 |
| Maternal depression (9) | −0.132 | −0.108 | 0.091 | −1.453 | .146 |
| Maternal depression (15) | 0.050 | 0.038 | 0.103 | 0.484 | .628 |
| Modularity | |||||
| Child’s closeness report (age 15 years) | −0.004 | −0.023 | 0.014 | −0.306 | .759 |
| Pubertal score | 0.012 | 0.041 | 0.026 | 0.447 | .655 |
| Maternal age | 0.000 | −0.004 | 0.002 | −0.048 | .962 |
| Maternal marital status | 0.045 | 0.112 | 0.035 | 1.284 | .199 |
| Household income | 0.002 | 0.015 | 0.012 | 0.164 | .870 |
| Maternal education | 0.020 | 0.060 | 0.026 | 0.756 | .450 |
| Child’s gender | −0.025 | −0.077 | 0.031 | −0.812 | .417 |
| Race/ethnicity (White) | 0.045 | 0.088 | 0.041 | 1.101 | .271 |
| Race/ethnicity (Hispanic/Latinx) | −0.040 | −0.062 | 0.050 | −0.803 | .422 |
| Race/ethnicity (Other) | 0.132 | 0.157 | 0.065 | 2.031 | .042 |
| Maternal depression (9) | 0.040 | 0.105 | 0.029 | 1.342 | .180 |
| Maternal depression (15) | −0.029 | −0.072 | 0.033 | −0.893 | .372 |
| Global efficiency | |||||
| Mother’s closeness report (age 9 years) | 0.011 | 0.059 | 0.015 | 0.732 | .464 |
| Pubertal score | −0.030 | −0.175 | 0.015 | −1.956 | .050 |
| Maternal age | −0.001 | −0.036 | 0.001 | −0.460 | .646 |
| Maternal marital status | −0.049 | −0.205 | 0.020 | −2.491 | .013 |
| Household income | −0.005 | −0.060 | 0.007 | −0.686 | .493 |
| Maternal education | −0.004 | −0.018 | 0.015 | −0.233 | .816 |
| Child’s gender | 0.025 | 0.127 | 0.018 | 1.370 | .171 |
| Race/ethnicity (White) | −0.036 | −0.115 | 0.024 | −1.499 | .134 |
| Race/ethnicity (Hispanic/Latinx) | −0.005 | −0.012 | 0.029 | −0.156 | .876 |
| Race/ethnicity (Other) | −0.038 | −0.077 | 0.038 | −1.016 | .309 |
| Maternal depression (9) | −0.019 | −0.084 | 0.017 | −1.108 | .268 |
| Maternal depression (15) | −0.006 | −0.025 | 0.020 | −0.304 | .761 |
| Transitivity | |||||
| Mother’s closeness report (age 9 years) | 0.082 | 0.085 | 0.076 | 1.083 | .279 |
| Pubertal score | −0.177 | −0.194 | 0.082 | −2.159 | .031 |
| Maternal age | −0.004 | −0.043 | 0.007 | −0.558 | .577 |
| Maternal marital status | −0.271 | −0.212 | 0.105 | −2.574 | .010 |
| Household income | −0.032 | −0.074 | 0.038 | −0.844 | .399 |
| Maternal education | 0.062 | 0.058 | 0.083 | 0.747 | .455 |
| Child’s gender | 0.214 | 0.202 | 0.098 | 2.184 | .029 |
| Race/ethnicity (White) | −0.124 | −0.075 | 0.127 | −0.980 | .327 |
| Race/ethnicity (Hispanic/Latinx) | 0.067 | 0.032 | 0.155 | 0.432 | .666 |
| Race/ethnicity (Other) | −0.132 | −0.049 | 0.201 | −0.657 | .511 |
| Maternal depression (9) | −0.135 | −0.111 | 0.092 | −1.461 | .144 |
| Maternal depression (15) | 0.037 | 0.028 | 0.106 | 0.351 | .726 |
| Modularity | |||||
| Mother’s closeness report (age 9 years) | −0.031 | −0.103 | 0.023 | −1.325 | .185 |
| Pubertal score | 0.012 | 0.043 | 0.026 | 0.471 | .637 |
| Maternal age | 0.000 | 0.005 | 0.002 | 0.062 | .950 |
| Maternal marital status | 0.044 | 0.110 | 0.034 | 1.266 | .205 |
| Household income | 0.001 | 0.006 | 0.012 | 0.061 | .951 |
| Maternal education | 0.016 | 0.049 | 0.026 | 0.619 | .536 |
| Child’s gender | −0.025 | −0.077 | 0.031 | −0.812 | .417 |
| Race/ethnicity (White) | 0.047 | 0.091 | 0.041 | 1.147 | .252 |
| Race/ethnicity (Hispanic/Latinx) | −0.048 | −0.075 | 0.050 | −0.966 | .334 |
| Race/ethnicity (Other) | 0.141 | 0.168 | 0.065 | 2.167 | .030 |
| Maternal depression (9) | 0.040 | 0.105 | 0.029 | 1.349 | .177 |
| Maternal depression (15) | −0.039 | −0.097 | 0.033 | −1.183 | .237 |
| Global efficiency | |||||
| Child’s closeness report (age 9 years) | 0.002 | 0.013 | 0.011 | 0.011 | .171 |
| Pubertal score | −0.030 | −0.174 | 0.015 | 0.015 | −1.931 |
| Maternal age | −0.001 | −0.032 | 0.001 | 0.001 | −.406 |
| Maternal marital status | −0.048 | −0.203 | 0.020 | 0.020 | −2.442 |
| Household income | −0.005 | −0.066 | 0.007 | 0.007 | −.753 |
| Maternal education | −0.005 | −0.024 | 0.015 | 0.015 | −.307 |
| Child’s gender | 0.025 | 0.125 | 0.018 | 0.018 | 1.343 |
| Race/ethnicity (White) | −0.035 | −0.112 | 0.024 | 0.024 | −1.450 |
| Race/ethnicity (Hispanic/Latinx) | −0.007 | −0.018 | 0.029 | 0.029 | −.243 |
| Race/ethnicity (Other) | −0.034 | −0.068 | 0.038 | 0.038 | −.897 |
| Maternal depression (9) | −0.019 | −0.085 | 0.017 | 0.017 | −1.124 |
| Maternal depression (15) | −0.010 | −0.040 | 0.020 | 0.020 | −.491 |
| Transitivity | |||||
| Child’s closeness report (age 9 years) | 0.044 | 0.057 | 0.059 | 0.745 | .456 |
| Pubertal score | −0.178 | −0.194 | 0.082 | −2.156 | .031 |
| Maternal age | −0.004 | −0.044 | 0.007 | −0.556 | .578 |
| Maternal marital status | −0.260 | −0.204 | 0.106 | −2.449 | .014 |
| Household income | −0.034 | −0.079 | 0.038 | −0.901 | .368 |
| Maternal education | 0.058 | 0.054 | 0.083 | 0.696 | .486 |
| Child’s gender | 0.208 | 0.197 | 0.098 | 2.115 | .034 |
| Race/ethnicity (White) | −0.112 | −0.068 | 0.127 | −0.880 | .379 |
| Race/ethnicity (Hispanic/Latinx) | 0.052 | 0.025 | 0.154 | 0.337 | .736 |
| Race/ethnicity (Other) | −0.088 | −0.033 | 0.202 | −0.438 | .662 |
| Maternal depression (9) | −0.140 | −0.115 | 0.093 | −1.509 | .131 |
| Maternal depression (15) | 0.012 | 0.009 | 0.103 | 0.120 | .904 |
| Modularity | |||||
| Child’s closeness report (age 9 years) | 0.005 | 0.021 | 0.019 | 0.273 | .785 |
| Pubertal score | 0.011 | 0.037 | 0.026 | 0.407 | .684 |
| Maternal age | 0.000 | −0.009 | 0.002 | −0.112 | .911 |
| Maternal marital status | 0.045 | 0.113 | 0.035 | 1.291 | .197 |
| Household income | 0.003 | 0.020 | 0.012 | 0.217 | .828 |
| Maternal education | 0.021 | 0.064 | 0.026 | 0.805 | .421 |
| Child’s gender | −0.025 | −0.077 | 0.031 | −0.810 | .418 |
| Race/ethnicity (White) | 0.046 | 0.089 | 0.041 | 1.115 | .265 |
| Race/ethnicity (Hispanic/Latinx) | −0.040 | −0.061 | 0.050 | −0.798 | .425 |
| Race/ethnicity (Other) | 0.133 | 0.158 | 0.065 | 2.030 | .042 |
| Maternal depression (9) | 0.040 | 0.105 | 0.030 | 1.341 | .180 |
| Maternal depression (15) | −0.029 | −0.070 | 0.033 | −0.875 | .381 |
Figure 2.
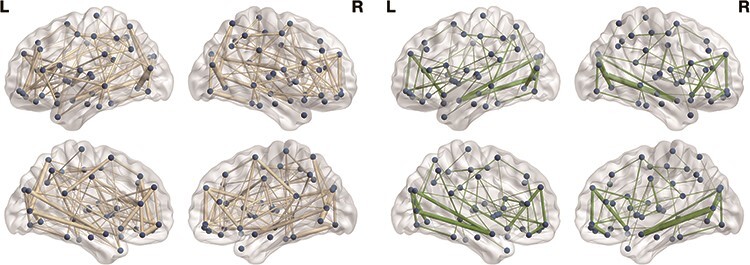
Visual representation of the white matter connectome at age 15 years. The left side represents individuals with greater mother–child closeness, characterized by high global efficiency and transitivity, while the right side represents those with less mother–child closeness, characterized by low global efficiency and transitivity. The circles represent nodes located in different brain regions, including frontal lateral, frontal medial, orbitofrontal, temporal, limbic, subcortical, parietal, and occipital. The lines represent the edges, which denote structural connectivity between brain regions.
Discussion
Our study examined the associations between mother–child closeness and structural brain network organization using a sample from a population-based study, primarily consisting of low-income, African American families. Specifically, this paper aimed to investigate how mother–child closeness, as assessed by both mother and child during childhood (age 9 years) and adolescence (age 15 years), may be meaningfully associated with structural network metrics. Overall, greater mother–child closeness was associated with increased global neural network efficiency and transitivity in the structural organization of the adolescent brain. Both mother’s and child’s appraisals of closeness at age 15 years, but not at age 9 years, consistently showed significant positive associations with global efficiency and transitivity, but not modularity. This structural organizational pattern facilitates faster information flow (global network efficiency), which may enhance interconnectedness among brain regions, while also providing greater robustness against potential disruptions (transitivity) (Bullmore and Sporns 2009, Farahani et al. 2019).
The observed age-specific (age 15 years) pattern of increased global efficiency and increased transitivity may reflect brain maturation, indicative of a developmental trajectory toward enhanced structural connectivity that often correlates with cognitive and emotional development (Huang et al. 2015, Vértes and Bullmore 2015, Khundrakpam et al. 2016). For example, preliminary studies on white matter tracts, as well as more recent research on white matter organization, have observed that children and adolescents exhibit a more integrated structural organization as cognitive functioning, intelligence, and academic attainment improve (Nagy et al. 2004, Schmithorst et al. 2005, Bathelt et al. 2019). Thus, mother–child closeness, a salient influence within their social environment, may contribute to distinct organizational patterns within the structural networks, thereby leading to maturation.
Both the mother’s and the child’s reports of closeness at age 15 years independently showed significant positive associations with global efficiency and transitivity, but not modularity. Furthermore, the association between mother–child closeness and these two topological properties of network metrics at age 15 years remained significant for both reports, even after accounting for mother–child closeness at age 9 years. This suggests that regardless of the mother–child closeness at age 9 years, mother–child closeness at age 15 years is an influential social context for adolescent structural organization and that change over time (e.g. from ages 9 to 15 years) may be particularly important. Our findings highlight the salient role of youth experiencing close relationships with their mother during adolescence, often characterized by receiving maternal love, care, and support in relationships (Collins and Laursen 2004, McWayne et al. 2017) in their brain development (Telzer et al. 2018).
Furthermore, at age 15 years, both the mother’s and the child’s reports were equivalently associated with the network metrics, emphasizing their similar importance in relation to structural networks. This is particularly interesting since these reports were only moderately correlated (r = 0.17 at age 9 years, r = 0.47 at age 15 years). Thus, even if youth and parents are disagreeing about levels, each unique perspective still seems to be associated with structural organization. Leveraging our findings from the multi-informant approach, the current study suggests that the child’s experience of the high level of closeness with their mother and the mother’s experience of closeness with their child during adolescence are equally crucial for the more efficient and clustered white matter organization. Previous literature underscores that both the mother and the child actively contribute to cultivating their closeness, as emotional bonds and shared experiences often co-occur between the dyad (Hou et al. 2020). Consequently, a child who feels close to their mother is likely to have a mother who feels close to the child. Our work supports previous findings that positive mother–child relationships are a crucial contextual factor in brain development (Butterfield, et al., 2021, Whittle et al. 2014, Qu et al. 2015) and extends this literature by suggesting that information on the dyad’s relationship quality, whether obtained from the mother or the child, may yield similar results.
The study’s focus on the role of mother–child closeness in adolescent brain development aligns with the increasing interest in elucidating neural mechanisms linked to normative development occurring within positive social contexts and complementing existing work on the role of early-life adversity in brain development (Belsky and De Haan 2011, Farber et al. 2022). Thus, leveraging reports of mother–child closeness to investigate adolescent brain development contributes to the ongoing conversation around the importance of examining the mechanisms underlying positive youth development, especially among adolescents of color, who have been primarily studied in research on social determinants of maladaptive development (Gaylord-Harden et al. 2018, Green et al. 2022). This approach is crucial as it enhances our understanding of normative developmental trajectories, especially for those who are often studied within the framework of marginalization and increased developmental risks, with limited representation in research on positive youth development (Gaylord-Harden et al. 2018).
The study’s results should be interpreted with several limitations and recommendations for future research in mind. The sample size (n = 181) was relatively modest; thus, replication with a larger sample is needed. Our measurement of mother–child closeness was very brief. The data used in this study were part of a larger population-based longitudinal study, which only had a limited set of items on parental relationship quality with their children. Follow-up study with more elaborated data on mother–child relationships from both parties is recommended. Our data on mother–child closeness were collected at only two time points, and imaging data were available from just one time point. This limitation restricts our ability to draw comprehensive conclusions about brain development. Research indicates that developmental patterns of the brain observed in cross-sectional studies or those with only two time points may differ from those seen in studies with at least three time points (Keresztes et al. 2022). Thus, extending the longitudinal data by following participants into adulthood and including additional time points could offer a more nuanced understanding of changes in structural organization related to mother–child closeness within the context of normative brain development.
For the future direction, linking our findings to neural mechanisms through which mother–child closeness may influence the onset of psychopathology and behavioral outcomes in adulthood could further deepen our understanding of the human brain. However, it is important to note that increasing evidence suggests that there may not be one-to-one associations between brain structures and psychological or behavioral outcomes, as individuals exhibit diverse patterns in their brain systems (Gratton et al. 2022, Monk and Hardi 2023). Lastly, ongoing research is needed to elucidate how white matter development relates to socioenvironmental influences across different developmental periods, given that the field has observed inconsistent findings regarding the association between various socioenvironmental contexts and structural brain development (Hanson et al. 2013, Keding et al. 2021, Richmond et al. 2022, Hardi et al. 2023).
Conclusion
The present study suggests that mother–child closeness, characterized by trusting interactions between mutually affectionate dyads, may be an important contextual contributor to adolescence brain development. We observed neural correlates associated with mother–child closeness, specifically noting more efficient and clustered brain structural networks via white matter organization. Our work contributes to ongoing efforts to delineate the neural mechanisms associated with normative and positive youth development, with a focus on under-represented populations in this field (Gaylord-Harden et al. 2018, Farber et al. 2022). Lastly, while our study examined neural correlates related to a familial-level contextual factor, it is crucial to recognize that efforts to investigate the influence of social context on brain development, especially for youth of color, must extend beyond family-level factors. Addressing systemic barriers that hinder positive caregiver–child relationships, such as poverty-related stressors perpetuated by racialized policies, is essential to complement research on neural correlates of family-level factors and enhance our understanding of positive youth development, particularly among youth of color (McLoyd 1990, Murry et al. 2022).
Supplementary Material
Acknowledgements
We sincerely appreciate the families that participated in our study for generously sharing their experiences with us, as well as the project staff for their dedication in making this study possible.
Contributor Information
Sunghyun H Hong, Department of Psychology, University of Michigan, Ann Arbor, MI 48109, United States; School of Social Work, University of Michigan, Ann Arbor, MI 48109, United States.
Felicia A Hardi, Department of Psychology, University of Michigan, Ann Arbor, MI 48109, United States.
Scott Tillem, Department of Psychology, University of Michigan, Ann Arbor, MI 48109, United States.
Leigh G Goetschius, The Hilltop Institute, University of Maryland Baltimore County, Baltimore, MD 21250, United States.
Jeanne Brooks-Gunn, Teachers College, Columbia University, New York, NY 10027, United States; College of Physicians and Surgeons, Columbia University, New York, NY 10032, United States.
Vonnie McLoyd, Department of Psychology, University of Michigan, Ann Arbor, MI 48109, United States.
Nestor L Lopez-Duran, Department of Psychology, University of Michigan, Ann Arbor, MI 48109, United States.
Colter Mitchell, Survey Research Center, Institute for Social Research, University of Michigan, Ann Arbor, MI 48104, United States; Population Studies Center, Institute for Social Research, University of Michigan, Ann Arbor, MI 48106, United States.
Luke W Hyde, Department of Psychology, University of Michigan, Ann Arbor, MI 48109, United States; Survey Research Center, Institute for Social Research, University of Michigan, Ann Arbor, MI 48104, United States.
Christopher S Monk, Department of Psychology, University of Michigan, Ann Arbor, MI 48109, United States; Survey Research Center, Institute for Social Research, University of Michigan, Ann Arbor, MI 48104, United States; Michigan Neuroscience Institute, University of Michigan, Ann Arbor, MI 48109, United States; Department of Psychiatry, University of Michigan, Ann Arbor, MI 48109, United States.
Supplementary data
Supplementary data is available at SCAN online.
Conflicts of interest
None declared.
Funding
This study was supported by the National Institute of Mental Health [R01MH103761 (PI: C.S.M.), R01 MH121079 (Principal Investigatorss: L.W.H., C.M., and C.S.M.)], Eunice Kennedy Shriver National Institute of Child Health and Human Development [T32 HD007109 (PIs: Gelman, C.S.M.)], and National Institute of Health Office of the Director [1S10OD012240 (PI: Noll)].
Data availability
The data underlying this article are publicly available.
References
- Ackard DM, Neumark-Sztainer D, Story M. et al. Parent–child connectedness and behavioral and emotional health among adolescents. Am J Preventive Med 2006;30:59–66. doi: 10.1016/j.amepre.2005.09.013 [DOI] [PubMed] [Google Scholar]
- Andersson JL, Graham MS, Zsoldos E. et al. Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage 2016;141:556–72. doi: 10.1016/j.neuroimage.2016.06.058 [DOI] [PubMed] [Google Scholar]
- Aronowitz T, Morrison‐Beedy D. Resilience to risk‐taking behaviors in impoverished African American girls: the role of mother–daughter connectedness. Res Nurs Health 2004;27:29–39. doi: 10.1002/nur.20004 [DOI] [PubMed] [Google Scholar]
- Bandy T, and Moore KA. The Parent-Child Relationship: A Family Strength. Fact Sheet. (Publication# 2008-27). Child Trends. 2008. https://www.childtrends.org/publications/the-parent-child-relationship-a-family-strength (1 August 2004, date last accessed). [Google Scholar]
- Bathelt J, Scerif G, Nobre A. et al. Whole-brain white matter organization, intelligence, and educational attainment. Trends Neurosci Educ 2019;15:38–47. doi: 10.1016/j.tine.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, De Haan M. Annual research review: parenting and children’s brain development: the end of the beginning. J Child Psychol Psychiatry 2011;52:409–28. doi: 10.1111/j.1469-7610.2010.02281.x [DOI] [PubMed] [Google Scholar]
- Blumberg SJ, Olson L, Frankel MR. et al. Design and Operation of the National Survey of Children’s Health, 2003 Vital Health Stat 2005; 1–131. [PubMed] [Google Scholar]
- Brody GH, Murry VM, McNair L. et al. Linking changes in parenting to parent–child relationship quality and youth self‐control: the Strong African American Families Program. J Res Adolesc 2005;15:47–69. doi: 10.1111/j.1532-7795.2005.00086.x [DOI] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 2009;10:186–98. doi: 10.1038/nrn2575 [DOI] [PubMed] [Google Scholar]
- Butterfield RD, Silk JS, Lee KH. et al. Parents still matter! Parental warmth predicts adolescent brain function and anxiety and depressive symptoms 2 years later. Dev Psychopathol 2021;33:226–239. doi: 10.1017/S0954579419001718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins WA, and Laursen B. Parent‐adolescent relationships and influencesLerner Richard and Steinberg Laurence. In: Handbook of Adolescent Psychology (Hoboken, New Jersey, United States: John Wiley & Sons, Inc.)3. 2004, 331–61. [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann NY Acad Sci 2004;1021:1–22. doi: 10.1196/annals.1308.001 [DOI] [PubMed] [Google Scholar]
- Day RD, Padilla-Walker LM. Mother and father connectedness and involvement during early adolescence. J Family Psychol 2009;23:900. doi: 10.1037/a0016438 [DOI] [PubMed] [Google Scholar]
- De Goede IH, Branje SJ, Meeus WH. Developmental changes in adolescents’ perceptions of relationships with their parents. J Youth Adolesc 2009;38:75–88. doi: 10.1007/s10964-008-9286-7 [DOI] [PubMed] [Google Scholar]
- De Luca SM, Yueqi Y, DiCorcia D. et al. A longitudinal study of Latino and non-Hispanic mothers’ and fathers’ depressive symptoms and its association with parent-child communication. J Affect Disord 2018;227:580–87. doi: 10.1016/j.jad.2017.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan J. Longitudinal associations among low-income mothers’ and fathers’ parenting and relationships with children and adolescent depression. Res Child Adolesc Psychopathol 2022;50:1339–50. doi: 10.1007/s10802-022-00918-0 [DOI] [PubMed] [Google Scholar]
- Fang S, Galambos NL, Johnson MD. Parent–child contact, closeness, and conflict across the transition to adulthood. J Marr Family 2021;83:1176–93. doi: 10.1111/jomf.12760 [DOI] [Google Scholar]
- Farahani FV, Karwowski W, Lighthall NR. Application of graph theory for identifying connectivity patterns in human brain networks: a systematic review. Front Neurosci 2019;13:439505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber MJ, Gee DG, Hariri AR. Normative range parenting and the developing brain: a scoping review and recommendations for future research. Eur J Neurosci 2022;55:2341–58. doi: 10.1111/ejn.15003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquharson S, Tournier J-D, Calamante F. et al. White matter fiber tractography: why we need to move beyond DTI. J Neurosurg 2013;118:1367–77. doi: 10.3171/2013.2.JNS121294 [DOI] [PubMed] [Google Scholar]
- Gaylord-Harden NK, Barbarin O, Tolan PH. et al. Understanding development of African American boys and young men: moving from risks to positive youth development. Am Psychologist 2018;73:753. doi: 10.1037/amp0000300 [DOI] [PubMed] [Google Scholar]
- Ge X, Natsuaki MN, Neiderhiser JM. et al. The longitudinal effects of stressful life events on adolescent depression are buffered by parent–child closeness. Dev Psychopathol 2009;21:621–35. doi: 10.1017/S0954579409000339 [DOI] [PubMed] [Google Scholar]
- Goetschius LG, Hein TC, Mitchell C. et al. Childhood violence exposure and social deprivation predict adolescent amygdala-orbitofrontal cortex white matter connectivity. Dev Cogn Neurosci 2020;45:100849. doi: 10.1016/j.dcn.2020.100849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C, Nelson SM, Gordon EM. Brain-behavior correlations: two paths toward reliability. Neuron 2022;110:1446–49. doi: 10.1016/j.neuron.2022.04.018 [DOI] [PubMed] [Google Scholar]
- Green KH, Van De Groep IH, Te Brinke LW. et al. A perspective on enhancing representative samples in developmental human neuroscience: connecting science to society. Front Integr Neurosci 2022;16:981657. doi: 10.3389/fnint.2022.981657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Hair N, Shen DG. et al. Family poverty affects the rate of human infant brain growth. PLoS One 2013;8:e80954. doi: 10.1371/journal.pone.0080954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardi FA, Goetschius LG, Peckins MK. et al. Differential developmental associations of material hardship exposure and adolescent amygdala–prefrontal cortex white matter connectivity. J Cognitive Neurosci 2022;34:1866–91. doi: 10.1162/jocn_a_01801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardi FA, Goetschius LG, Tillem S. et al. Early childhood household instability, adolescent structural neural network architecture, and young adulthood depression: a 21-year longitudinal study. Dev Cogn Neurosci 2023;61:101253. doi: 10.1016/j.dcn.2023.101253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein TC, Mattson WI, Dotterer HL. et al. Amygdala habituation and uncinate fasciculus connectivity in adolescence: a multi-modal approach. Neuroimage 2018;183:617–26. doi: 10.1016/j.neuroimage.2018.08.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Sowell ER. Puberty and structural brain development in humans. Front Neuroendocrinol 2017;44:122–37. doi: 10.1016/j.yfrne.2016.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Benner AD, Kim SY. et al. Discordance in parents’ and adolescents’ reports of parenting: a meta-analysis and qualitative review. Am Psychologist 2020;75:329. doi: 10.1037/amp0000463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Shu N, Mishra V. et al. Development of human brain structural networks through infancy and childhood. Cereb. Cortex 2015;25:1389–404. doi: 10.1093/cercor/bht335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B, Durtschi J, Keilholtz B. Maternal engagement, relational closeness, and adolescent internalizing symptoms: the association of engaged mothering with adolescent depression and anxiety. J Marital Fam Ther 2023;49:861–78. doi: 10.1111/jmft.12662 [DOI] [PubMed] [Google Scholar]
- Keding TJ, Heyn SA, Russell JD. et al. Differential patterns of delayed emotion circuit maturation in abused girls with and without internalizing psychopathology. Am J Psychiatry 2021;178:1026–36. doi: 10.1176/appi.ajp.2021.20081192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keresztes A, Raffington L, Bender AR. et al. Longitudinal developmental trajectories do not follow cross-sectional age associations in hippocampal subfield and memory development. Developmental Cognitive Neuroscience 2022;54:101085. doi: 10.1016/j.dcn.2022.101085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khundrakpam BS, Lewis JD, Zhao L. et al. Brain connectivity in normally developing children and adolescents. Neuroimage 2016;134:192–203. doi: 10.1016/j.neuroimage.2016.03.062 [DOI] [PubMed] [Google Scholar]
- King V, Boyd LM, Pragg B. Parent–adolescent closeness, family belonging, and adolescent well-being across family structures. J Family Issues 2018;39:2007–36. doi: 10.1177/0192513X17739048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline R. Data Preparation. In: Little T (ed.), Principles and Practice of Structural Equation Modeling. 5th edn. New York: Guilford Publications, 2023, 46–66. [Google Scholar]
- Knop J, Joëls M, van der Veen R. The added value of rodent models in studying parental influence on offspring development: opportunities, limitations and future perspectives. Curr Opin Psychol 2017;15:174–81. doi: 10.1016/j.copsyc.2017.02.030 [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Peper JS, Crone EA. et al. White matter development in adolescence: The influence of puberty and implications for affective disorders. Developmental Cognitive Neuroscience 2012;2:36–54. doi: 10.1016/j.dcn.2011.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence TI. Parental support, marital conflict, and stress as predictors of depressive symptoms among African American adolescents. Clin Child Psychol Psychiatry 2022;27:630–43. doi: 10.1177/13591045211070163 [DOI] [PubMed] [Google Scholar]
- Lin J, Stern JA, Allen JP. et al. Does attachment in adolescence predict neural responses to handholding in adulthood? A functional magnetic resonance imaging study. J Soc Pers Relat 2024;41:2276–96. doi: 10.1177/02654075241239604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O’Hare E. et al. Maternal depression and parenting behavior: a meta-analytic review. Clinic Psychol Rev 2000;20:561–92. doi: 10.1016/S0272-7358(98)00100-7 [DOI] [PubMed] [Google Scholar]
- McLoyd VC. The impact of economic hardship on Black families and children: psychological distress, parenting, and socioemotional development. Child Dev 1990;61:311–46. doi: 10.2307/1131096 [DOI] [PubMed] [Google Scholar]
- McWayne CM, Mattis JS, Green Wright LE. et al. An emic, mixed-methods approach to defining and measuring positive parenting among low-income Black families. Early Educ Dev 2017;28:182–206. doi: 10.1080/10409289.2016.1208601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Monk CS, Hardi FA. Poverty, brain development, and mental health: progress, challenges, and paths forward. Annu Rev Devel Psychol 2023;5:309–30. doi: 10.1146/annurev-devpsych-011922-012402 [DOI] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci 2006;9:1004–06. doi: 10.1038/nn1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry VM, Bradley C, Cruden G. et al. Re-envisioning, retooling, and rebuilding prevention science methods to address structural and systemic racism and promote health equity. Prevent Sci 2022;25:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy Z, Westerberg H, Klingberg T. Maturation of white matter is associated with the development of cognitive functions during childhood. J Cognitive Neurosci 2004;16:1227–33. doi: 10.1162/0898929041920441 [DOI] [PubMed] [Google Scholar]
- National Survey of Children’s Health . Middle Childhood and Adolescence Section. 2003. https://www.childhealthdata.org/learn-about-the-nsch/archive-prior-year-data-documents-and-resources/2003-nsch#S7 (1 August 2024, date last accessed).
- Ohashi K, Anderson CM, Bolger EA. et al. Childhood maltreatment is associated with alteration in global network fiber-tract architecture independent of history of depression and anxiety. Neuroimage 2017;150:50–59. doi: 10.1016/j.neuroimage.2017.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M. et al. A self-report measure of pubertal status: reliability, validity, and initial norms. J Youth Adolesc 1988;17:117–33. doi: 10.1007/BF01537962 [DOI] [PubMed] [Google Scholar]
- Pryce CR, Feldon J. Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neurosci Biobehav Rev 2003;27:57–71. doi: 10.1016/S0149-7634(03)00009-5 [DOI] [PubMed] [Google Scholar]
- Qu Y, Fuligni AJ, Galvan A. et al. Buffering effect of positive parent–child relationships on adolescent risk taking: a longitudinal neuroimaging investigation. Dev Cogn Neurosci 2015;15:26–34. doi: 10.1016/j.dcn.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R: A Language and Environment for Statistical Computing. 2021. https://www.R-project.org/ (1 August 2025, date last accessed).
- Reichman NE, Teitler JO, Garfinkel I. et al. Fragile families: sample and design. Child Youth Services Rev 2001;23:303–26. doi: 10.1016/S0190-7409(01)00141-4 [DOI] [Google Scholar]
- Rex K. Data Preparation. In: Little T (ed.), Principles and practice of structural equation modeling, 5th edn. New York, NewYork, United States: Guilford Press, 2023. [Google Scholar]
- Richmond S, Beare R, Johnson KA. et al. Towards understanding neurocognitive mechanisms of parenting: maternal behaviors and structural brain network organization in late childhood. Human Brain Mapp 2021;42:1845–62. doi: 10.1002/hbm.25334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond S, Beare R, Johnson KA. et al. Maternal warmth is associated with network segregation across late childhood: a longitudinal neuroimaging study. Front Psychol 2022;13:917189. doi: 10.3389/fpsyg.2022.917189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SO, Bareket-Shavit C, Dollins FA. et al. Racial inequality in psychological research: trends of the past and recommendations for the future. Perspect Psychol Sci 2020;15:1295–309. doi: 10.1177/1745691620927709 [DOI] [PubMed] [Google Scholar]
- Rolls ET, Joliot M, Tzourio-Mazoyer N. Implementation of a new parcellation of the orbitofrontal cortex in the automated anatomical labeling atlas. Neuroimage 2015;122:1–5. doi: 10.1016/j.neuroimage.2015.07.075 [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010;52:1059–69. doi: 10.1016/j.neuroimage.2009.10.003 [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ. et al. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Human Brain Mapp 2005;26:139–47. doi: 10.1002/hbm.20149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Yuan W. White matter development during adolescence as shown by diffusion MRI. Brain Cogn 2010;72:16–25. doi: 10.1016/j.bandc.2009.06.005 [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Slopen N, Williams DR. Early childhood adversity, toxic stress, and the impacts of racism on the foundations of health. Ann Rev Public Health 2021;42:115–34. doi: 10.1146/annurev-publhealth-090419-101940 [DOI] [PubMed] [Google Scholar]
- Telzer EH, Van Hoorn J, Rogers CR. et al. Social influence on positive youth development: a developmental neuroscience perspective. Adv Child Dev Behav 2018;54:215–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier J-D, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage 2007;35:1459–72. doi: 10.1016/j.neuroimage.2007.02.016 [DOI] [PubMed] [Google Scholar]
- Tournier JD, Calamante F, Connelly A. MRtrix: diffusion tractography in crossing fiber regions. Int J Imaging Syst Technol 2012;22:53–66. doi: 10.1002/ima.22005 [DOI] [Google Scholar]
- Tournier J-D, Calamante F, Gadian DG. et al. Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage 2004;23:1176–85. doi: 10.1016/j.neuroimage.2004.07.037 [DOI] [PubMed] [Google Scholar]
- United States Census Bureau . How the Census Bureau Measures Poverty. The United States Census Bureau. 2020. https://www.census.gov/topics/income-poverty/poverty/guidance/poverty-measures.html (15 July 2021, date last accessed).
- Vértes PE, Bullmore ET. Annual research review: growth connectomics—the organization and reorganization of brain networks during normal and abnormal development. J Child Psychol Psychiatry 2015;56:299–320. doi: 10.1111/jcpp.12365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA. et al. Epigenetic programming by maternal behavior. Nat Neurosci 2004;7:847–54. doi: 10.1038/nn1276 [DOI] [PubMed] [Google Scholar]
- Whittle S, Simmons JG, Dennison M. et al. Positive parenting predicts the development of adolescent brain structure: a longitudinal study. Dev Cogn Neurosci 2014;8:7–17. doi: 10.1016/j.dcn.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh CH, Jones DK, Liang X. et al. Mapping structural connectivity using diffusion MRI: challenges and opportunities. J Magn Reson Imaging 2021;53:1666–82. doi: 10.1002/jmri.27188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngblade LM, Theokas C, Schulenberg J. et al. Risk and promotive factors in families, schools, and communities: a contextual model of positive youth development in adolescence. Pediatrics 2007;119:S47–S53. doi: 10.1542/peds.2006-2089H [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are publicly available.


