Abstract
Objective
To assess the impact of the COVID-19 pandemic on the presentation, treatment, and survival of patients with early breast cancer (Stage I–III).
Methods
This study utilized data from the Surveillance, Epidemiology, and End Results database from January 1, 2018, to December 31, 2020. Patients diagnosed with primary breast cancer in 2020 were compared to those diagnosed in 2018 or 2019. The primary outcomes were stage distribution and changes in the treatment modalities for early breast cancer. The secondary outcomes were overall survival (OS) and breast cancer-specific survival (BCSS).
Results
We analyzed 142,038 patients. There has been a decrease in breast cancer diagnoses in 2020, as well as the smaller number of surgeries. The distribution of breast cancer stages among patients exhibited a notable shift in 2020, with a decrease in the proportion of Stage 0-I and an increase in advanced-stage. Additionally, there was a significant decrease in the proportion of breast-conserving surgery (BCS) performed in 2020. The proportion of patients undergoing radiation decreased, while that of chemotherapy cases increased significantly in 2020. Patients showed a shorter treatment delay in 2020 than in 2018 or 2019 (2018: hazard ratio [HR] = 0.969, 95% confidence interval [CI] = 0.956–0.982, p < 0.001; 2019: HR=0.959, 95% CI = 0.946–0.972, p < 0.001). Diagnosis in 2020 showed a significant correlation with worse OS than diagnosis in 2018 (HR = 0.861, 95% CI = 0.743-0.996, p = 0.045).
Conclusion
We observed a shift to advanced-stage and a change of treatment modalities of early breast cancer in 2020. The OS of patients with breast cancer was worse during the pandemic than before the pandemic. The findings could provide empirical basis for optimizing cancer prevention and control strategies in future public health emergencies.
Keywords: COVID-19 pandemic, overall survival, cancer diagnosis, breast cancer, cancer treatment
1. Introduction
COVID-19 triggers SARS by inflaming the respiratory tract and activating immune cells to produce excessive pro-inflammatory cytokines (1). By the beginning of 2023, the World Health Organization reported approximately 750 000 000 confirmed cases of COVID-19 and more than 6 800 000 deaths. The Covid-19 pandemic spread worldwide and place a significant strain on the healthcare systems.
Breast cancer screenings were largely disrupted during the onset of the COVID-19 pandemic (2–4), leading to a decrease in the number of diagnosed cases in 2020 (5–7). Additionally, socioeconomic disparities may have aggravated this risk (8, 9). Various studies have shown that the pandemic led to patients presenting with advanced stages of breast cancer (10, 11). However, Tonneson et al. published contrasting findings that the breast cancer stage at diagnosis did not differ significantly between the COVID-19 and pre-COVID-19 periods (12). The study from the Netherlands Cancer Registry supported these conclusions (5). So far, it is unclear whether COVID-19 affected the stage of breast cancer. Meanwhile, few studies reported the effect of the pandemic on the treatment changes and prognosis of breast cancer.
This study aimed to investigate whether the COVID-19 pandemic affected the presentation, treatment and survival of patients with early breast cancer (Stage I–III).
2. Materials and methods
2.1. Study participants
The Surveillance, Epidemiology, and End Results (SEER) database is a systematic population-based cancer registry covering approximately 48% of the United States population. Clinically relevant data of patients diagnosed with breast cancer from 2018 to 2020 were extracted from the SEER 18 registry database (1975–2020) using SEER*Stat 8.4.2. STROCSS criteria was followed in this work (13). The need for ethical approval was waived because the SEER is a publicly accessible and freely available database. The year 2020 marks the onset of the COVID-19 pandemic, and we utilized data from 2018 and 2019 as control data. Patients with primary breast cancer were included in the study. The following variables were extracted: patient ID, race, age, grade, stage as per the American Joint Committee on Cancer (8th edition), subtype, radiation, chemotherapy, surgery code, vital status, cause of death, time to treatment initiation (TTI), and survival in months. Patients with unclear tumor grade or stage were excluded.
2.2. Outcomes of interest
The primary outcomes were cancer stage distribution, changes in the treatment modalities in early breast cancer, and treatment delay. Treatment modalities included surgery, radiation, and chemotherapy. Treatment delay was measured according to the TTI. The secondary outcomes were overall survival (OS) and breast cancer-specific survival (BCSS) in patients with Stage I–III tumors. OS was defined as the time from diagnosis to death or the last follow-up. BCSS was defined as the time from cancer to death due to breast cancer.
2.3. Statistical analyses
Patient demographics, tumor characteristics, and treatment information were compared among the patients diagnosed in 2018, 2019, and 2020 using the chi-square test with Bonferroni correction. Time-to-event analysis was performed to evaluate the TTI recorded. The multivariable Cox proportional hazards model was applied to evaluate the covariates associated with OS and BCSS. Considering the different follow-up durations among patients diagnosed in 2020 and 2018 or 2019, we used the modified Cox proportional hazards model. Patients were classified as “alive,” if they had not yet died 1 year after diagnosis. These results were presented as hazard ratios (HRs) with 95% confidence intervals (CIs). P-values <0.05 were considered statistically significant, and all tests were two-sided. All statistical analyses were performed using Statistical Product and Service Solutions (version 22.0; IBM, Armonk, NY, USA).
3. Results
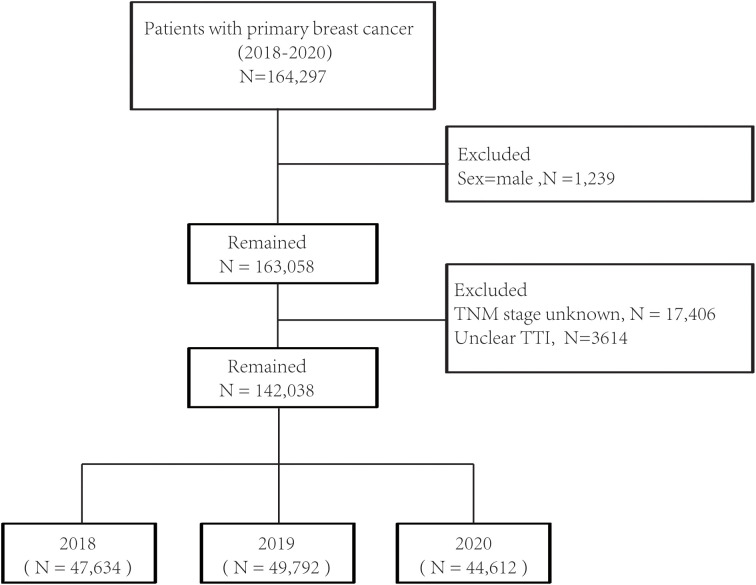
In total, 142,038 patients diagnosed with primary breast cancer between 2018 and 2020 were included in the final analysis, of which 47,634 (33.5%) were diagnosed in 2018, 49792 (35.1%) in 2019, and 44612 (31.4%) in 2020 ( Figure 1 ). The median follow-up duration was 29 months (interquartile range, 25–32) in 2018 and 17 months (interquartile range, 14–20) in 2019, and it decreased to 5 months (interquartile range, 2–9) in 2020. As shown in Table 1 , patients diagnosed with breast cancer in 2020 tended to be younger than those diagnosed in 2018 or 2019. Fewer patients diagnosed in 2020 underwent surgery and radiation but more patients received chemotherapy as compared to those diagnosed in 2018 or 2019. Moreover, treatment initiation in one month was more common among patients diagnosed in 2020 than those diagnosed in previous years.
Figure 1.
Flowchart for the SEER (Surveillance, Epidemiology, and End Results) data screening.
Table 1.
Demographics and clinical characteristics of patients diagnosed in 2018, 2019 and 2020.
| Characteristics | 2018 (n=47634) | 2019 (n=49792) | 2020 (n=44612) | Total (n=142038) | P value | |
|---|---|---|---|---|---|---|
| Race | <0.001 | |||||
| White | 36610 (76.9%) | 38177 (76.7%) | 33944 (76.1%) | 108731 | ||
| Black | 5104 (10.7%) | 5452 (10.9%) | 5016 (11.2%) | 15572 | ||
| Other | 5479 (11.5%) | 5728 (11.5%) | 5120 (11.5%) | 16327 | ||
| Unknown | 441 (0.9%) | 435 (0.9%) | 532 (1.2%) | 1408 | ||
| Age | <0.001 | |||||
| <50 | 9915 (20.8%) | 10382 (20.9%) | 9806 (22%) | 30103 | ||
| 50≤;<70 | 24514 (51.5%) | 25513 (51.2%) | 22559 (50.6%) | 72586 | ||
| ≥70 | 13205 (27.7%)_ | 13897 (27.9%) | 12247 (27.5%) | 39349 | ||
| Grade | <0.001 | |||||
| I | 10843 (22.8%) | 11200 (22.5%) | 9394 (21.1%) | 31437 | ||
| II | 21629 (45.4%) | 23182 (46.6%) | 20786 (46.6%) | 65597 | ||
| III | 14500 (30.4%) | 14729 (29.6%) | 13779 (30.9%) | 43008 | ||
| Unknown | 662 (1.4%) | 681 (1.4%) | 653 (1.5%) | 1996 | ||
| Subtype | <0.001 | |||||
| HR+/Her2- | 35728 (75%) | 38055 (76.4%) | 33422 (74.9%) | 107205 | ||
| HR+/Her2+ | 4945 (10.4%) | 4778 (9.6%) | 4526 (10.1%) | 14249 | ||
| HR-/Her2+ | 1899 (4%) | 1804 (3.6%) | 1796 (4%) | 5499 | ||
| HR-/Her2- | 4872 (10.2%) | 4931 (9.9%) | 4673 (10.5%) | 14476 | ||
| Unknown | 190 (0.4%) | 224 (0.4%) | 195 (0.4%) | 609 | ||
| Stage | <0.001 | |||||
| 0 | 33 (0.1%) | 42 (0.1%) | 52 (0.1%) | 127 | ||
| I | 34542 (72.5%) | 36723 (73.8%) | 32049 (71.8%) | 103314 | ||
| II | 6933 (14.6%) | 6773 (13.6%) | 6432 (14.4%) | 20138 | ||
| III | 3459 (7.3%) | 3539 (7.1%) | 3431 (7.7%) | 10429 | ||
| IV | 2667 (5.6%) | 2715 (5.5%) | 2648 (5.9%) | 8030 | ||
| Surgery | <0.001 | |||||
| Aspiration/biopsy only | 3087 (6.5%) | 3535 (7.1%) | 3755 (8.4%) | 10377 | ||
| Mastectomy | 16578 (34.8%) | 16734 (33.6%) | 15268 (34.2%) | 48580 | ||
| Breast conserving | 27944 (58.7%) | 29465 (59.2%) | 25554 (57.3%) | 82963 | ||
| Unknown | 25 (0.1%) | 58 (0.1%) | 35 (0.1%) | 118 | ||
| Radiation | <0.001 | |||||
| No | 19363 (40.6%) | 20700 (41.6%) | 19526 (43.8%) | 59589 | ||
| Yes | 28271 (59.4%) | 29092 (58.4%) | 25086 (56.2%) | 82449 | ||
| Chemotherapy | <0.001 | |||||
| No | 28797 (60.5%) | 30675 (61.6%) | 26258 (58.9%) | 85730 | ||
| Yes | 18837 (39.5%) | 19117 (38.4%) | 18354 (41.1%) | 56308 | ||
| Time to treatment initiation (month) | <0.001 | |||||
| ≤1 | 32374 (68%) | 33091 (66.5%) | 31063 (69.6%) | 96528 | ||
| >1 | 15260 (32%) | 16701 (33.5%) | 13549 (30.4%) | 45510 |
3.1. Stage distribution
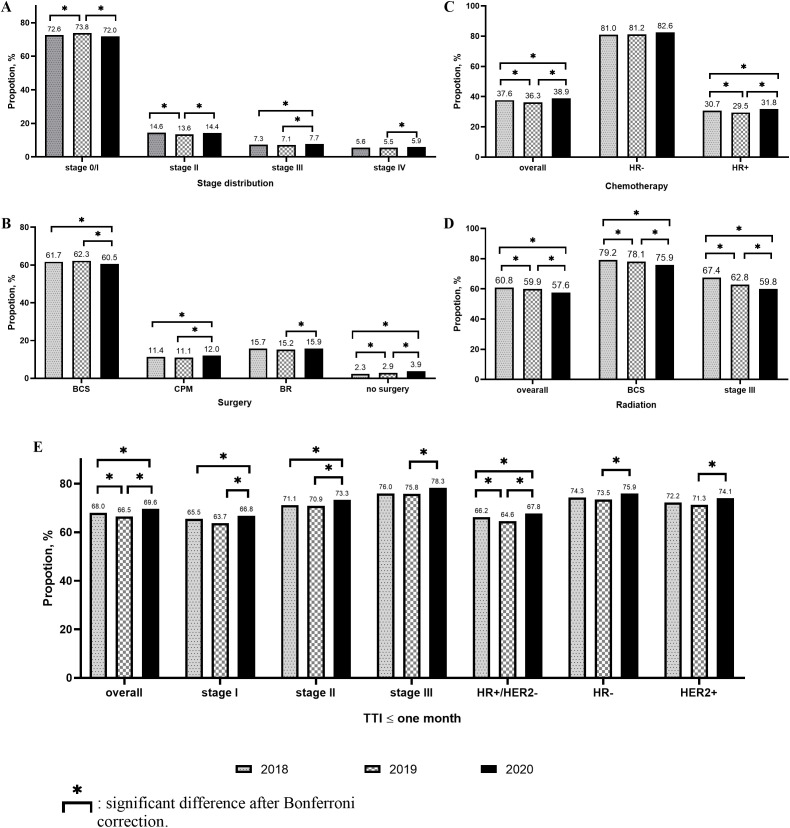
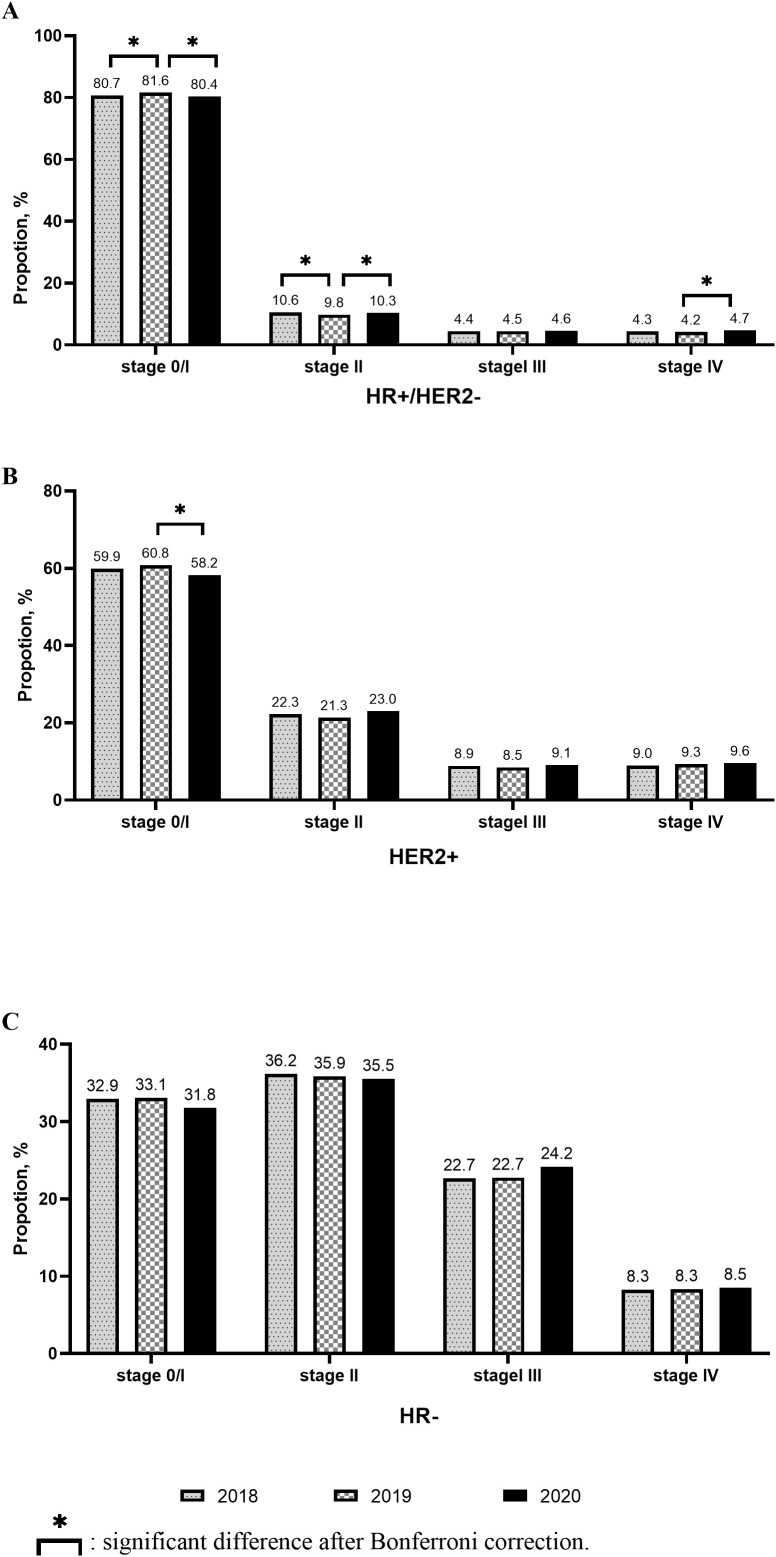
Compared to 2018, the proportion of Stage 0–I breast cancer patients increased, while that of Stage II breast cancer patients decreased in 2019. Meanwhile, no significant change was observed in the proportion of Stage III–IV breast cancer patients between 2018 and 2019. However, in 2020, the proportion of Stage 0–I breast cancer patients decreased significantly, while that of Stage II–IV breast cancer patients increased significantly ( Figure 2A ). Similar results were observed in the hormone receptor (HR) positive (+)/human epidermal growth factor receptor (HER) 2 negative (−) populations ( Figure 3A ). However, only the HER2+ patients showed a statistically significant reduction in the proportion of Stage 0–I breast cancer patients in 2020 ( Figure 3B ). In patients with aggressive subtypes of breast cancer, i.e., HER2+ or HR− breast cancer, no significant change was observed in the proportion of patients with Stage III–IV breast cancer ( Figures 3B, C ).
Figure 2.
Changes of stage distribution and treatment received for early breast cancer. (A. Stage distribution; (B) Surgery; (C) Chemotherapy; (D) Radiation; (E) Time to treatment initiation (TTI) ≤ one month.
Figure 3.
Changes of stage distribution in different subtypes.
3.2. Changes in treatment modalities for early breast cancer
In early breast cancer patients, the proportion of patients without surgery increased significantly. The proportion of breast-conserving surgery (BCS) cases decreased significantly in 2020 than in 2018 or 2019. The proportion of contralateral prophylactic mastectomy (CPM) and immediate breast reconstruction (IBR) cases increased significantly in 2020 than in 2019 ( Figure 2B ).
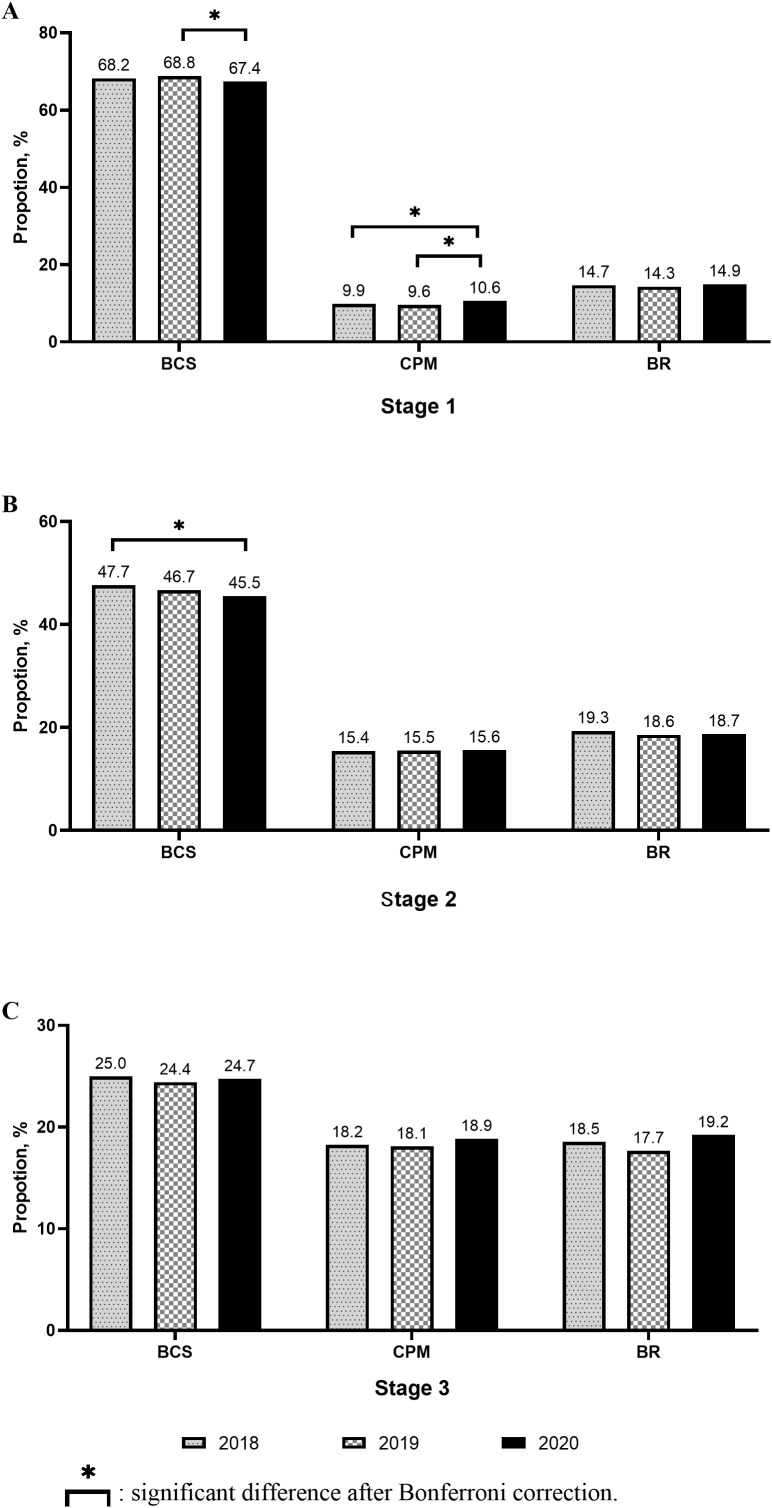
In Stage I breast cancer patients, the proportion of BCS cases decreased in 2020 compared to that in 2019, while that of CPM cases increased significantly compared to that in 2018 or 2019. The proportion of IBR cases did not change significantly ( Figure 4A ). In Stage II or III breast cancer patients, no significant change was observed in the proportion of BCS, CPM, and IBR cases among the three cohorts ( Figures 4B, C ).
Figure 4.
Changes of surgery received in different stages.
Although the proportion of chemotherapy cases decreased significantly in 2019, it was significantly higher in 2020 than in 2018 or 2019. The same was true among patients with HR+ breast cancer. However, among HR− patients, the proportion of chemotherapy cases did not differ significantly among these three cohorts. In HR+/HER2− Stage I breast cancer patients, the increased proportion of chemotherapy cases was statistically significant in 2020 ( Figure 2C ). The proportion of patients undergoing radiation decreased significantly every year, as observed in patients undergoing BCS or those with Stage III breast cancer ( Figure 2D ).
3.3. Treatment delay in early breast cancer
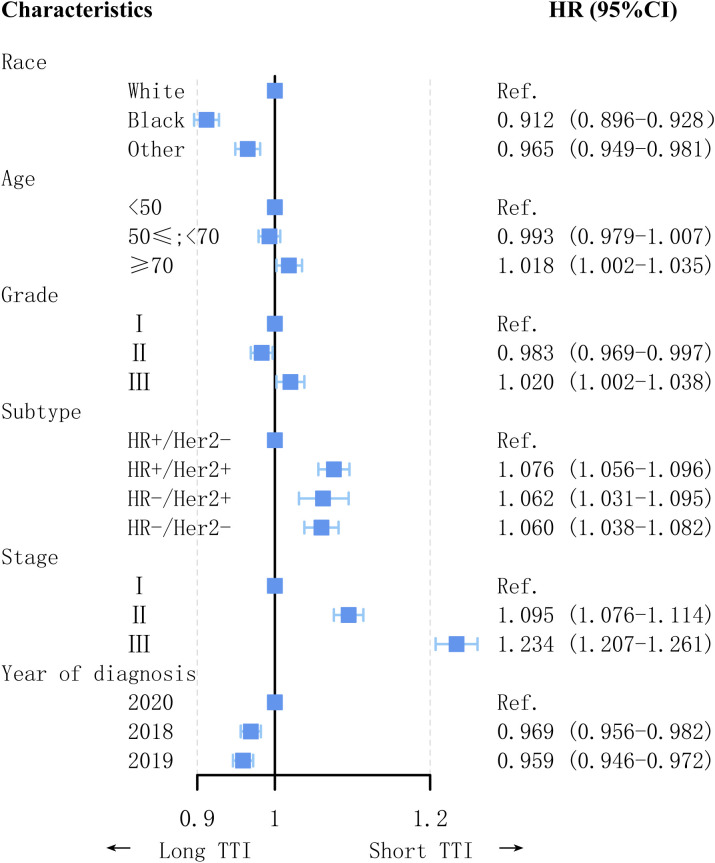
The proportion of patients with TTI within one month was the lowest in 2019 and highest in 2020. In all patient subgroups, the proportion of patients with TTI within one month was significantly higher in 2020 than in 2019 ( Figure 2E ). Factors associated with TTI are shown in Figure 5 . Patients diagnosed during the COVID-19 pandemic had a shorter TTI than those diagnosed in the pre-pandemic period (2018: HR = 0.969, 95% CI = 0.956–0.982, p < 0.001; 2019: HR = 0.959, 95% CI = 0.946–0.972, p < 0.001). Factors associated with short TTI included histological tumor Grade 3 (HR = 1.020, 95% CI = 1.002–1.038, p = 0.033), invasive subtype (HR+/HER2+: HR = 1.076, 95% CI = 1.056–1.096, p < 0.001; HR−/HER2+: HR = 1.062, 95% CI = 1.031– 1.095, p < 0.001; HR−/HER2−: HR = 1.060, 95% CI = 1.038–1.082, p < 0.001), higher cancer stage (Stage II: HR = 1.095, 95% CI = 1.076–1.114, p < 0.001; Stage III: HR = 1.234, 95% CI = 1.207–1.261, p < 0.001), and age >70 years (HR = 1.018, 95% CI = 1.002–1.035, p = 0.023). White patients had a significantly shorter TTI than non-White patients (Blacks: HR = 0.912, 95% CI = 0.896–0.928, p < 0.001; Other: HR = 0.965, 95% CI = 0.949–0.981, p < 0.001).
Figure 5.
Factors associated with time to treatment initiation (TTI).
3.4. Year of diagnosis and breast cancer survival in early breast cancer
There were 251 deaths in the 2020 cohort, of which 94 deaths were due to breast cancer. A multivariable Cox proportional hazards model was used to assess the factors associated with OS and BCSS ( Table 2 ). The OS (HR = 0.887, 95% CI = 0.766–1.026, p = 0.105) and BCSS (HR = 0.998, 95% CI = 0.789–1.262, p = 0.985) did not differ significantly between 2019 and 2020. However, compared to diagnosis in 2020, diagnosis in 2018 was an independent factor associated with better OS (HR = 0.861, 95% CI = 0.743–0.996, p = 0.045) but not better BCSS (HR = 1.001, 95% CI = 0.791–1.267, p = 0.994) ( Tables 2 , 3 ). Results for this modified Cox model were similar between 2018 and 2020 (OS: HR = 0.842, 95% CI = 0.723–0.981, p = 0.027; BCSS: HR = 0.945, 95% CI = 0.74–1.208, p = 0.652) ( Tables 2 , 4 ). In the population receiving chemotherapy or radiation, the year of diagnosis was not an independent factor associated with survival. However, in the patients who did not receive chemotherapy, the year of diagnosis significantly affected the OS (HR = 0.814, 95% CI = 0.675–0.982, p = 0.031) ( Table 2 ).
Table 2.
The impact of year of diagnosis on survival among patients with early breast cancer.
| Overall survival | Breast cancer-sepcific survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 95%CI | 95%CI | ||||||||||
| Events | HR | Low | High | P | Events | HR | Low | High | P | ||
| All patients | 2020 | 251 | Reference | 94 | Reference | ||||||
| 2018 | 2056 | 0.861 | 0.743 | 0.996 | 0.045 | 944 | 1.001 | 0.791 | 1.267 | 0.994 | |
| 2019 | 1117 | 0.887 | 0.766 | 1.026 | 0.105 | 503 | 0.998 | 0.789 | 1.262 | 0.985 | |
| All patients (modify) | 2020 | 251 | Reference | 94 | Reference | ||||||
| 2018 | 596 | 0.842 | 0.723 | 0.981 | 0.027 | 254 | 0.945 | 0.74 | 1.208 | 0.652 | |
| 2019 | 677 | 0.912 | 0.786 | 1.06 | 0.231 | 299 | 1.055 | 0.83 | 1.341 | 0.663 | |
| Subgroup | |||||||||||
| Patients with chemotherapy | 2020 | 97 | Reference | 40 | Reference | ||||||
| 2018 | 863 | 0.933 | 0.738 | 1.181 | 0.566 | 593 | 1.28 | 0.902 | 1.816 | 0.166 | |
| 2019 | 447 | 0.908 | 0.718 | 1.148 | 0.418 | 295 | 1.226 | 0.865 | 1.739 | 0.252 | |
| Patients without chemotherapy | 2020 | 154 | Reference | 54 | Reference | ||||||
| 2018 | 1193 | 0.814 | 0.675 | 0.982 | 0.031 | 351 | 0.757 | 0.548 | 1.045 | 0.09 | |
| 2019 | 670 | 0.873 | 0.725 | 1.052 | 0.153 | 208 | 0.805 | 0.584 | 1.109 | 0.185 | |
| Patients with radiation | 2020 | 35 | Reference | 12 | Reference | ||||||
| 2018 | 763 | 0.793 | 0.547 | 1.151 | 0.222 | 159 | 0.978 | 0.525 | 1.822 | 0.944 | |
| 2019 | 330 | 0.857 | 0.591 | 1.242 | 0.414 | 414 | 0.929 | 0.498 | 1.733 | 0.818 | |
| Patients without radiation | 2020 | 216 | Reference | 82 | Reference | ||||||
| 2018 | 1293 | 0.893 | 0.761 | 1.049 | 0.167 | 530 | 1.018 | 0.788 | 1.317 | 0.889 | |
| 2019 | 787 | 0.899 | 0.767 | 1.055 | 0.192 | 344 | 1.037 | 0.803 | 1.338 | 0.782 | |
Table 3.
Factors associated with OS and BCSS in a Cox proportional hazards model of patients diagnosed with early breast cancer in 2018,2019, and 2020.
| Characteristics | OS | BCSS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | ||||||||
| HR | Low | High | P | HR | Low | High | P | ||
| Race | |||||||||
| White | Reference | Reference | |||||||
| Black | 1.312 | 1.197 | 1.438 | <0.001 | 1.174 | 1.029 | 1.339 | 0.017 | |
| Other | 0.696 | 0.611 | 0.793 | <0.001 | 0.743 | 0.617 | 0.894 | 0.002 | |
| Age | |||||||||
| <50 | Reference | Reference | |||||||
| 50≤;<70 | 1.531 | 1.363 | 1.72 | <0.001 | 1.179 | 1.024 | 1.358 | 0.022 | |
| ≥70 | 4.048 | 3.606 | 4.544 | <0.001 | 2.37 | 2.045 | 2.748 | <0.001 | |
| Grade | |||||||||
| I | Reference | Reference | |||||||
| II | 1.161 | 1.042 | 1.293 | 0.007 | 1.768 | 1.385 | 2.257 | <0.001 | |
| III | 1.595 | 1.412 | 1.802 | <0.001 | 2.974 | 2.313 | 3.824 | <0.001 | |
| Subtype | |||||||||
| HR+/Her2- | Reference | Reference | |||||||
| HR+/Her2+ | 0.987 | 0.866 | 1.126 | 0.848 | 0.781 | 0.634 | 0.962 | 0.02 | |
| HR-/Her2+ | 1.152 | 0.984 | 1.348 | 0.078 | 1.013 | 0.816 | 1.256 | 0.91 | |
| HR-/Her2- | 1.444 | 1.312 | 1.59 | <0.001 | 1.555 | 1.371 | 1.764 | <0.001 | |
| Stage | |||||||||
| I | Reference | Reference | |||||||
| II | 2.472 | 2.241 | 2.727 | <0.001 | 4.366 | 3.714 | 5.133 | <0.001 | |
| III | 7.348 | 6.683 | 8.078 | <0.001 | 17.644 | 15.16 | 20.536 | <0.001 | |
| Radiation | |||||||||
| No | Reference | Reference | |||||||
| Yes | 0.341 | 0.317 | 0.367 | <0.001 | 0.366 | 0.329 | 0.407 | <0.001 | |
| Chemotherapy | |||||||||
| No | Reference | Reference | |||||||
| Yes | 0.773 | 0.708 | 0.844 | <0.001 | 0.935 | 0.822 | 1.063 | 0.305 | |
| Year of diagnosis | |||||||||
| 2020 | Reference | Reference | |||||||
| 2018 | 0.861 | 0.743 | 0.996 | 0.045 | 1.001 | 0.791 | 1.267 | 0.994 | |
| 2019 | 0.887 | 0.766 | 1.026 | 0.105 | 0.998 | 0.789 | 1.262 | 0.985 | |
Table 4.
Factors associated with OS and BCSS in a modified Cox proportional hazards model of patients diagnosed with early breast cancer in 2018, 2019, and 2020.
| Characteristics | OS | BCSS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | ||||||||
| HR | Low | High | P | HR | Low | High | P | ||
| Race | |||||||||
| White | Reference | Reference | |||||||
| Black | 1.381 | 1.207 | 1.58 | <0.001 | 1.33 | 1.093 | 1.618 | 0.004 | |
| Other | 0.683 | 0.561 | 0.833 | <0.001 | 0.719 | 0.535 | 0.966 | 0.028 | |
| Age | |||||||||
| <50 | Reference | Reference | |||||||
| 50≤;<70 | 2.032 | 1.675 | 2.464 | <0.001 | 1.443 | 1.13 | 1.842 | 0.003 | |
| ≥70 | 5.251 | 4.338 | 6.357 | <0.001 | 3.443 | 2.693 | 4.401 | <0.001 | |
| Grade | |||||||||
| I | Reference | Reference | |||||||
| II | 1.185 | 1.004 | 1.397 | 0.045 | 1.766 | 1.215 | 2.565 | 0.003 | |
| III | 1.557 | 1.294 | 1.875 | <0.001 | 2.666 | 1.814 | 3.919 | <0.001 | |
| Subtype | |||||||||
| HR+/Her2- | Reference | Reference | |||||||
| HR+/Her2+ | 1.326 | 1.105 | 1.592 | 0.002 | 0.987 | 0.729 | 1.337 | 0.933 | |
| HR-/Her2+ | 1.365 | 1.089 | 1.712 | 0.007 | 1.107 | 0.797 | 1.538 | 0.544 | |
| HR-/Her2- | 1.555 | 1.345 | 1.799 | <0.001 | 1.544 | 1.269 | 1.88 | <0.001 | |
| Stage | |||||||||
| I | Reference | Reference | |||||||
| II | 2.48 | 2.145 | 2.868 | <0.001 | 4.729 | 3.687 | 6.066 | <0.001 | |
| III | 6.789 | 5.894 | 7.819 | <0.001 | 18.161 | 14.376 | 22.942 | <0.001 | |
| Radiation | |||||||||
| No | Reference | Reference | |||||||
| Yes | 0.199 | 0.175 | 0.225 | <0.001 | 0.191 | 0.158 | 0.231 | <0.001 | |
| Chemotherapy | |||||||||
| No | Reference | Reference | |||||||
| Yes | 0.772 | 0.678 | 0.879 | <0.001 | 0.943 | 0.78 | 1.141 | 0.546 | |
| Year of diagnosis | |||||||||
| 2020 | Reference | Reference | |||||||
| 2018 | 0.842 | 0.723 | 0.981 | 0.027 | 0.945 | 0.74 | 1.208 | 0.652 | |
| 2019 | 0.912 | 0.786 | 1.06 | 0.231 | 1.055 | 0.83 | 1.341 | 0.663 | |
4. Discussion
This study found a decline in the number of breast cancer diagnoses during the pandemic in 2020 compared to that in 2018 or 2019. The proportion of Stage 0–I patients decreased in 2020 compared to 2019. Moreover, the proportion of patients undergoing radiation and BCS decreased in 2020, while that of patients undergoing chemotherapy increased. Patients diagnosed in 2020 experienced significantly fewer treatment delays compared to those diagnosed in 2018 or 2019. However, diagnosis of breast cancer in 2020 emerged as a significant independent risk factor for OS in breast cancer patients.
We observed that the number of patients diagnosed with and treated for breast cancer in 2020 was the lowest. Since the Dutch National Breast Screening Program was suspended by the COVID-19 pandemic starting in Week 12 of 2020, the incidence of screen-detected tumors nearly dropped to zero during weeks 14–25 (5). In the United States, a marked decrease of 54.9% in breast cancer incidence rates was observed during the pandemic (14). Some single-institution studies from different countries reported that patients presented with more advanced stages of breast cancer in 2020 (10, 11, 15). Similarly, we observed an increase in the proportion of stage III or IV breast cancers increased in 2020. However, the population-based study from the Netherlands Cancer Registry did not find a shift towards higher stage breast cancers (5). This discrepancy could be attributed to several reasons. Firstly, the time point of the Dutch study cut-off was August 2020. The breast cancer screening program restarted at a reduced capacity, and there were some delayed breast cancer diagnoses that have not yet been discovered. Secondly, the duration of disruptions to breast screening varied across countries. Poelhekken et al. used the simulation model on radiation risk and breast cancer screening and found no clinically significant changes in screening-detected tumor sizes after a 3-month interruption. However, interruptions of 6 or 12 months considerably influenced the tumor size (2).Our observations differed from the population study from Brazilian, wherein the HR negative or HER2 positive breast cancer, regarded as aggressive tumor subtypes, did not show higher percentage of patients with advanced-stage disease during the pandemic (16).
The Pandemic Breast Cancer Consortium provided expert guidance to categorize patients with breast cancer into priority levels to ensure timely and reasonable treatment during the COVID-19 pandemic (17). It recommended that patients eligible for breast conservation therapy were advised against undergoing mastectomy to minimize operative duration. Additionally, for those patients scheduled for mastectomy, contralateral prophylactic mastectomy and immediate reconstruction were discouraged to further reduce surgical time and the associated risk of complications. The COVID-19 pandemic affected surgical activities, but in contrast to these recommendations. In Italy, the mastectomy–BCS ratio was 39–61% in 2019 and 42–58% in 2020. Mastectomies with direct to implant reconstruction surgeries increased by 15%, whereas mastectomies with immediate expander reconstruction decreased by 20% (18). A study based on the Specific Health Checkups program in Japan showed that the proportion of BCS cases without axillary lymph node dissection decreased significantly, whereas no decrease was observed for other surgery types (19). All these studies, including ours, demonstrated that the proportion of patients not undergoing surgery increased significantly in 2020. The proportion of BCS cases decreased in 2020 compared to 2018 or 2019. This was also true for Stage I breast cancer patients. In light of the uncertainty caused by the pandemic, the possibility of missed or delayed radiotherapy, and frequent hospital visits for radiotherapy, some of these patients decided on mastectomy. We also found a significant increase in the proportion of CPM and IBR cases in the overall population. Patients refusing BCS were more likely to resort to IBR for esthetic breast appearance. Decreased surgical volume and low-priority surgery for non-tumor diseases led to more spare plastic surgeons than in the pre-pandemic period. Furthermore, poor communication between doctors and patients during the pandemic could have contributed to the change in surgical approach. Decisions for CPM appear to be patient-driven for reasons such as genetic testing, strong family cancer history, and increased concern about cancer recurrence. However, the knowledge of and recommendations for CPM among women with breast cancer in the United States are limited (20). Therefore, CPM is prevalent among patients without clinical indications. Physician–patient communication regarding CPM can effectively reduce potential overtreatment (21).
The proportion of radiation cases decreased significantly in patients with Stage III or those undergoing BCS, which are absolute indications for radiation as per the recommended guidelines. This could be attributed to several reasons. First, recently, some guidelines recommend considering the omission of post-lumpectomy radiation in older breast cancer patients (22). Second, some patients may refuse or delay radiation therapy to decrease the chance of COVID-19 infection. Third, delayed radiation therapy was widespread during the pandemic, which may have affected the data entry accuracy and timeliness. Finally, we cannot exclude the presence of undertreatment. In addition, practical patterns of radiotherapy to early breast cancer have changed. International guidelines recommended that ultra-hypofractionated radiotherapy was preferentially used in patients with lower risk disease in order to minimize patient time spent in the hospital (23). A population-based study from UK found the use of hypofractionated regimens rapidly increased by 60·4% in 2020 (24). MD Anderson Cancer Center reported the percentage of ultra-hypofractionated radiotherapy increased from 4.3% in March-April 2020 to 45.5% in July-August 2020 (25).
The application of genomic tests greatly reduced the chemotherapy application. 21-gene RS testing and 70-gene signature could aid to avoid adjuvant chemotherapy (26, 27). We found the proportion of chemotherapy cases decreased in 2019. However, it rose rapidly in 2020, even surpassing that in 2018. These results corroborate the findings of a population-based cohort study from the Netherlands and Canada (28, 29). In some studies from the United States, chemotherapy cases had increased in 2020 (30, 31). This was partly due to the advanced stages of breast cancer cases diagnosed in 2020. Additionally, the increase in the proportion of neoadjuvant chemotherapy cases could explain it. It is important to note that the proportion of patients treated with chemotherapy has significantly increased among those with Stage I HR+/HER2- tumors. However, this differs from the actual daily clinical practice. There may be overtreatment in these patients. Data obtained from the National Cancer Database (NCDB) indicated a statistically significant decrease in the proportion of patients undergoing BCS and radiotherapy, as reported in our study. However, no statistically significant change was observed in the proportion of patients receiving radiation or systemic therapy among those diagnosed with Stages II–IV breast cancer (32). The NCDB is a hospital-based and not a population-based cancer registry. Treatment modalities commonly provided in outpatient settings may be missed, such as chemotherapy.
Shorter TTI was associated with lower mortality and higher accessibility to cancer therapy (33, 34). Our study found that the proportion of patients with TTI less than one month increased significantly in 2020. The time-to-event analysis showed that patients received treatment faster in 2020. An NCDB study showed that the COVID-19 pandemic was not associated with delay in systemic therapy (35). The time between diagnosis and treatment initiation decreased in patients diagnosed with breast cancer even during lockdown in the Dutch population (29). Among the patients diagnosed with COVID-19, those with breast cancer received treatment sooner than those with other tumors. This could be attributed to a decrease in the incidence of breast cancer, application of neoadjuvant treatment (36), and prioritization for a different surgery (17). Neoadjuvant therapy was used for safe postponement of surgery in selected cases, which is recommended by several guidelines (17, 37–39). Particularly, an increased application of neoadjuvant endocrine therapy was observed (12, 40). Our study revealed that racial minorities, particularly black women, experienced prolonged TTI compared to white individuals. Existing literature consistently shows disparities in TTI for breast cancer patients (41). The COVID-19 pandemic has the potential to worsen existing delays in treatment initiation for black women with breast cancer (42, 43). Further research is necessary to examine the effects of public health emergencies on racial and ethnic disparities in cancer care.
A recent review showed evidence of an increased risk of COVID-19-related death in patients recently diagnosed with cancer, and the risk decreased with time since diagnosis (44). We found that the diagnosis of breast cancer in the year 2020 was a risk factor for OS compared to the diagnosis in 2018; however, the COVID-19 outbreak did not significantly affect the BCSS. This was because of worse OS due to non-breast cancer-related mortality. As mentioned in a study from the United States National Vital Statistics System, the incidence of cancer-related death increased slightly from 2018 to 2021 but was relatively less than the increase in the number of deaths from any cause, indicating that an excess number of persons with cancer died from COVID-19 and other diseases (45). Among patients receiving chemotherapy or radiation, OS did not change during pandemic. In a prospective cohort study, chemotherapy or other anticancer treatment did not increase risk of mortality from COVID-19 disease (46). Some studies on breast cancer showed no association between systemic therapy or loco-regional therapy and increased mortality risk (47, 48). In these studies, older age and pre-existing comorbidities were associated with adverse COVID-19 outcomes in breast cancer patients. These patients were likely to refuse chemotherapy and end up in the non-chemotherapy group during the pandemic. This could be the reason why the pandemic seemed to affect the OS in the non-chemotherapy population.
The pandemic’s impact on the duration of time between cancer diagnosis and initiation of treatment indicated a notable level of resilience in the US (49). Nevertheless, the present study also highlighted significant concerns, encompassing delayed diagnoses and potential deviations from treatment guidelines, such as overuse of chemotherapy, underuse of radiotherapy and underuse of BCS. Evidently, the pandemic greatly influenced the treatment approaches for early-stage breast cancer.
The pandemic has worsened pre-existing issues like poor doctor-patient communication and unequal healthcare access. Chen et al. showed that telehealth can improve communication and increase the effectiveness of cancer screening during this time (50). It would be helpful to ensure timely diagnosis and optimal treatment for patients with breast cancer in future public health emergencies.
This study has several limitations. First, it was a retrospective analysis with a short follow-up period, necessitating further studies with a longer follow-up duration. Second, the SEER database lacked detailed information regarding the specific month of diagnosis in 2020, and we had to consider the entire year of 2020 for comparison with 2018 or 2019. Third, we were unable to examine the status of comorbidities, COVID-19, and insurance as possible confounders in the survival analysis, as we lacked information on them.
5. Conclusion
To the best of our knowledge, this is the first nationwide cohort study to investigate the effect of the COVID-19 pandemic on the presentation, treatment, and survival of early breast cancer patients. We observed a shift towards advanced-stage breast cancers and a potential for over- or undertreatment in 2020. Patients with breast cancer had worse OS during the pandemic compared to that in the pre-pandemic period; however, their BCSS remained unaffected. Further research is necessary to investigate the reasons behind the divergence of clinical practice from established guidelines and evaluate the impact of such deviations on breast cancer outcomes.
Funding Statement
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
YL: Conceptualization, Data curation, Methodology, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. X-JL: Conceptualization, Data curation, Software, Writing – original draft, Writing – review & editing. BX: Software, Supervision, Validation, Writing – original draft, Writing – review & editing. W-WL: Data curation, Visualization, Writing – original draft, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Nokhostin F, Dargahi M, Tutunchi S, Rezaeeyan H. Evaluation of prognostic/diagnostic value of hematological markers in the detection of inflammation in coronavirus disease: A review study. J Adv Med Biomed Res. (2020) 28:171–4. doi: 10.30699/jambs.28.128.171 [DOI] [Google Scholar]
- 2. Poelhekken K, Greuter MJW, de Munck L, Siesling S, Brokken FB, de Bock GH. Long-term effects of the interruption of the Dutch breast cancer screening program due to COVID-19: A modelling study. Prev Med. (2023) 166:107376. doi: 10.1016/j.ypmed.2022.107376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee R, Xu W, Dozier M, McQuillan R, Theodoratou E, Figueroa J. A rapid review of COVID-19’s global impact on breast cancer screening participation rates and volumes from January to December 2020. Elife. (2023) 12. doi: 10.7554/eLife.85680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bakouny Z, Paciotti M, Schmidt AL, Lipsitz SR, Choueiri TK, Trinh QD. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol. (2021) 7:458–60. doi: 10.1001/jamaoncol.2020.7600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eijkelboom AH, de Munck L, Lobbes MBI, van Gils CH, Wesseling J, Westenend PJ, et al. Impact of the suspension and restart of the Dutch breast cancer screening program on breast cancer incidence and stage during the COVID-19 pandemic. Prev Med. (2021) 151:106602. doi: 10.1016/j.ypmed.2021.106602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gathani T, Dodwell D, Horgan K. The impact of the first 2 years of the COVID-19 pandemic on breast cancer diagnoses: a population-based study in England. Br J Cancer. (2023) 128:481–3. doi: 10.1038/s41416-022-02054-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA Netw Open. (2020) 3:e2017267. doi: 10.1001/jamanetworkopen.2020.17267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeGroff A, Miller J, Sharma K, Sun J, Helsel W, Kammerer W, et al. COVID-19 impact on screening test volume through the National Breast and Cervical Cancer early detection program, January-June 2020, in the United States. Prev Med. (2021) 151:106559. doi: 10.1016/j.ypmed.2021.106559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schommer L, Mikulski MF, Goodgame B, Brown KM. Racial disparities in breast cancer presentation and diagnosis in COVID-era central texas. J Surg Res. (2023) 288:79–86. doi: 10.1016/j.jss.2023.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Borsky K, Shah K, Cunnick G, Tsang-Wright F. Pattern of breast cancer presentation during the COVID-19 pandemic: results from a cohort study in the UK. Future Oncol. (2022) 18:437–43. doi: 10.2217/fon-2021-0970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bonadio RC, Messias AP, Moreira OA, Leis LV, Orsi BZ, Testa L, et al. Impact of the COVID-19 pandemic on breast and cervical cancer stage at diagnosis in Brazil. Ecancermedicalscience. (2021) 15:1299. doi: 10.3332/ecancer.2021.1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tonneson JE, Hoskin TL, Day CN, Durgan DM, Dilaveri CA, Boughey JC. Impact of the COVID-19 pandemic on breast cancer stage at diagnosis, presentation, and patient management. Ann Surg Oncol. (2022) 29:2231–9. doi: 10.1245/s10434-021-11088-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mathew G, Agha R, Albrecht J, Goel P, Mukherjee I, Pai P, et al. STROCSS 2021: Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg. (2021) 96:106165. doi: 10.1016/j.ijsu.2021.106165 [DOI] [PubMed] [Google Scholar]
- 14. Howlader N, Bhattacharya M, Scoppa S, Miller D, Noone AM, Negoita S, et al. Cancer and COVID-19: U.S. Cancer incidence rates during the first year of the pandemic. J Natl Cancer Inst. (2023). doi: 10.1093/jnci/djad205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adachi K, Kimura F, Takahashi H, Kaise H, Yamada K, Ueno E, et al. Delayed diagnosis and prognostic impact of breast cancer during the COVID-19 pandemic. Clin Breast Cancer. (2023) 23:265–71. doi: 10.1016/j.clbc.2023.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Resende CAA, Fernandes Cruz HM, Costa ESM, Paes RD, Dienstmann R, Barrios CHE, et al. Impact of the COVID-19 pandemic on cancer staging: an analysis of patients with breast cancer from a community practice in Brazil. JCO Glob Oncol. (2022) 8:e2200289. doi: 10.1200/GO.22.00289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dietz JR, Moran MS, Isakoff SJ, Kurtzman SH, Willey SC, Burstein HJ, et al. Recommendations for prioritization, treatment, and triage of breast cancer patients during the COVID-19 pandemic. the COVID-19 pandemic breast cancer consortium. Breast Cancer Res Treat. (2020) 181:487–97. doi: 10.1007/s10549-020-05644-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sgarzani R, Macrì G, Gurrado A, Curcio A, De Lorenzi F, Galimberti V, et al. The impact of COVID-19 pandemic on breast surgery in Italy: a multi-centric retrospective observational study. Updates Surg. (2023) 75:735–41. doi: 10.1007/s13304-023-01474-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fujita M, Hashimoto H, Nagashima K, Suzuki K, Kasai T, Yamaguchi K, et al. Impact of coronavirus disease 2019 pandemic on breast cancer surgery using the National Database of Japan. Sci Rep. (2023) 13:4977. doi: 10.1038/s41598-023-32317-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hawley ST, Jagsi R, Morrow M, Janz NK, Hamilton A, Graff JJ, et al. Social and clinical determinants of contralateral prophylactic mastectomy. JAMA Surg. (2014) 149:582–9. doi: 10.1001/jamasurg.2013.5689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jagsi R, Hawley ST, Griffith KA, Janz NK, Kurian AW, Ward KC, et al. Contralateral prophylactic mastectomy decisions in a population-based sample of patients with early-stage breast cancer. JAMA Surg. (2017) 152:274–82. doi: 10.1001/jamasurg.2016.4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Knowlton CA. Breast cancer management during the COVID-19 pandemic: the radiation oncology perspective. Curr Breast Cancer Rep. (2022) 14:8–16. doi: 10.1007/s12609-022-00441-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coles CE, Aristei C, Bliss J, Boersma L, Brunt AM, Chatterjee S, et al. International guidelines on radiation therapy for breast cancer during the COVID-19 pandemic. Clin Oncol (Royal Coll Radiol (Great Britain)). (2020) 32:279–81. doi: 10.1016/j.clon.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spencer K, Jones CM, Girdler R, Roe C, Sharpe M, Lawton S, et al. The impact of the COVID-19 pandemic on radiotherapy services in England, UK: a population-based study. Lancet Oncol. (2021) 22:309–20. doi: 10.1016/S1470-2045(20)30743-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Corrigan KL, Lei X, Ahmad N, Arzu I, Bloom E, Chun SG, et al. Adoption of ultrahypofractionated radiation therapy in patients with breast cancer. Adv Radiat Oncol. (2022) 7:100877. doi: 10.1016/j.adro.2021.100877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Piccart M, van ‘t Veer LJ, Poncet C, Lopes Cardozo JMN, Delaloge S, Pierga JY, et al. 70-gene signature as an aid for treatment decisions in early breast cancer: updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol. (2021) 22:476–88. doi: 10.1016/S1470-2045(21)00007-3 [DOI] [PubMed] [Google Scholar]
- 27. Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. New Engl J Med. (2018) 379:111–21. doi: 10.1056/NEJMoa1804710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Habbous S, Tai X, Beca JM, Arias J, Raphael MJ, Parmar A, et al. Comparison of use of neoadjuvant systemic treatment for breast cancer and short-term outcomes before vs during the COVID-19 era in ontario, Canada. JAMA Netw Open. (2022) 5:e2225118. doi: 10.1001/jamanetworkopen.2022.25118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eijkelboom AH, de Munck L, Menke-van der Houven van Oordt CW, Broeders MJM, van den Bongard D, Strobbe LJA, et al. Changes in breast cancer treatment during the COVID-19 pandemic: a Dutch population-based study. Breast Cancer Res Treat. (2023) 197:161–75. doi: 10.1007/s10549-022-06732-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Milgrom ZZ, Milgrom DP, Han Y, Hui SL, Haggstrom DA, Fisher CS, et al. Breast cancer screening, diagnosis, and surgery during the pre- and peri-pandemic: experience of patients in a statewide health information exchange. Ann Surg Oncol. (2023) 30:2883–94. doi: 10.1245/s10434-023-13183-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malmgren JA, Guo B, Atwood MK, Hallam P, Roberts LA, Kaplan HG. COVID-19 related change in breast cancer diagnosis, stage, treatment, and case volume: 2019-2021. Breast Cancer Res Treat. (2023) 202:105–15. doi: 10.1007/s10549-023-06962-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nogueira LM, Schafer EJ, Fan Q, Wagle NS, Zhao J, Shi KS, et al. Assessment of changes in cancer treatment during the first year of the COVID-19 pandemic in the US. JAMA Oncol. (2023). doi: 10.1001/jamaoncol.2023.4513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cone EB, Marchese M, Paciotti M, Nguyen DD, Nabi J, Cole AP, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA network Open. (2020) 3:e2030072. doi: 10.1001/jamanetworkopen.2020.30072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nguyen DD, Paciotti M, Marchese M, Cole AP, Cone EB, Kibel AS, et al. Effect of medicaid expansion on receipt of definitive treatment and time to treatment initiation by racial and ethnic minorities and at minority-serving hospitals: A patient-level and facility-level analysis of breast, colon, lung, and prostate cancer. JCO Oncol practice. (2021) 17:e654–65. doi: 10.1200/OP.21.00010 [DOI] [PubMed] [Google Scholar]
- 35. Chung SH, Romatoski KS, Rasic G, Beaulieu-Jones BR, Kenzik K, Merrill AL, et al. Impact of the COVID-19 pandemic on delays to breast cancer surgery: ripples or waves? Ann Surg Oncol. (2023) 30:6093–103. doi: 10.1245/s10434-023-13878-6 [DOI] [PubMed] [Google Scholar]
- 36. Sheng JY, Santa-Maria CA, Mangini N, Norman H, Couzi R, Nunes R, et al. Management of breast cancer during the COVID-19 pandemic: A stage- and subtype-specific approach. JCO Oncol Pract. (2020) 16:665–74. doi: 10.1200/OP.20.00364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Manoj Gowda S, Kabeer KK, Jafferbhoy S, Marla S, Soumian S, Misra V, et al. Breast cancer management guidelines during COVID-19 pandemic. Indian J Surg. (2020) 82:251–8. doi: 10.1007/s12262-020-02466-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martin M, Guerrero-Zotano A, Montero Á, Jara C, Filipovich E, Rojo F, et al. GEICAM guidelines for the management of patients with breast cancer during the COVID-19 pandemic in Spain. Oncologist. (2020) 25:e1339–45. doi: 10.1634/theoncologist.2020-0363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kawate T, Yoshida A, Sugae S, Asaga S, Kaise H, Saji S, et al. Recommendations for the management of breast cancer patients during the COVID-19 pandemic from the Japan Breast Cancer Society. Breast Cancer. (2021) 28:247–53. doi: 10.1007/s12282-020-01214-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilke LG, Nguyen TT, Yang Q, Hanlon BM, Wagner KA, Strickland P, et al. Analysis of the impact of the COVID-19 pandemic on the multidisciplinary management of breast cancer: review from the american society of breast surgeons COVID-19 and mastery registries. Ann Surg Oncol. (2021) 28:5535–43. doi: 10.1245/s10434-021-10639-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fasano GA, Bayard S, Gillot T, Hannibal Z, Pedreira M, Newman L. Disparities in time to treatment for breast cancer: existing knowledge and future directions in the COVID-19 era. Curr Breast Cancer Rep. (2022) 14:213–21. doi: 10.1007/s12609-022-00469-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Obeng-Gyasi S, Oppong B, Paskett ED, Lustberg M. Purposeful surgical delay and the coronavirus pandemic: how will black breast cancer patients fare? Breast Cancer Res Treat. (2020) 182:527–30. doi: 10.1007/s10549-020-05740-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Patel MI, Ferguson JM, Castro E, Pereira-Estremera C.D, Armaiz-Peña G.N, Duron Y, et al. Racial and ethnic disparities in cancer care during the COVID-19 pandemic. JAMA network Open. (2022) 5:e2222009. doi: 10.1001/jamanetworkopen.2022.22009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Steinberg J, Hughes S, Hui H, Allsop MJ, Egger S, David M, et al. Risk of COVID-19 death for people with a pre-existing cancer diagnosis prior to COVID-19-vaccination: A systematic review and meta-analysis. Int J Cancer. (2023). doi: 10.1002/ijc.34798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Henley SJ, Dowling NF, Ahmad FB, Ellington TD, Wu M, Richardson LC. COVID-19 and other underlying causes of cancer deaths - United States, january 2018-july 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:1583–8. doi: 10.15585/mmwr.mm7150a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee LY, Cazier JB, Angelis V, Arnold R, Bisht V, Campton NA, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. (2020) 395:1919–26. doi: 10.1016/S0140-6736(20)31173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marks DK, Budhathoki N, Kucharczyk J, Fa'ak F, D'Abreo N, Kwa M, et al. Outcomes of breast cancer patients treated with chemotherapy, biologic therapy, endocrine therapy, or active surveillance during the COVID-19 pandemic. Oncologist. (2022) 27:89–96. doi: 10.1093/oncolo/oyab042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nagaraj G, Vinayak S, Khaki AR, Sun T, Kuderer NM, Aboulafia DM, et al. Clinical characteristics, racial inequities, and outcomes in patients with breast cancer and COVID-19: A COVID-19 and cancer consortium (CCC19) cohort study. Elife. (2023), 12. doi: 10.7554/eLife.82618.sa2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Janczewski LM, Cotler J, Merkow RP, Palis B, Nelson H, Mullett T, et al. Alterations in cancer treatment during the first year of the COVID-19 pandemic in the US. JAMA Netw Open. (2023) 6:e2340148. doi: 10.1001/jamanetworkopen.2023.40148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of cancer screening deficit in the United States with the COVID-19 pandemic. JAMA Oncol. (2021) 7:878–84. doi: 10.1001/jamaoncol.2021.0884 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.