Abstract
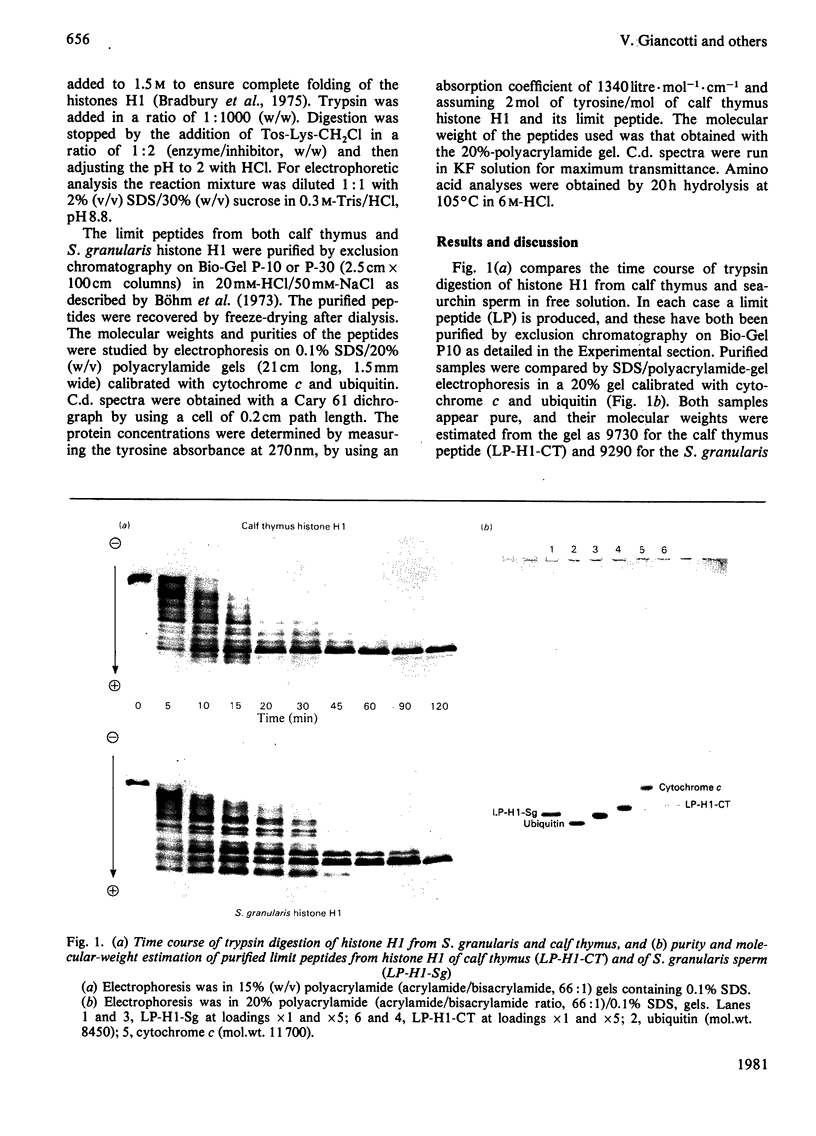
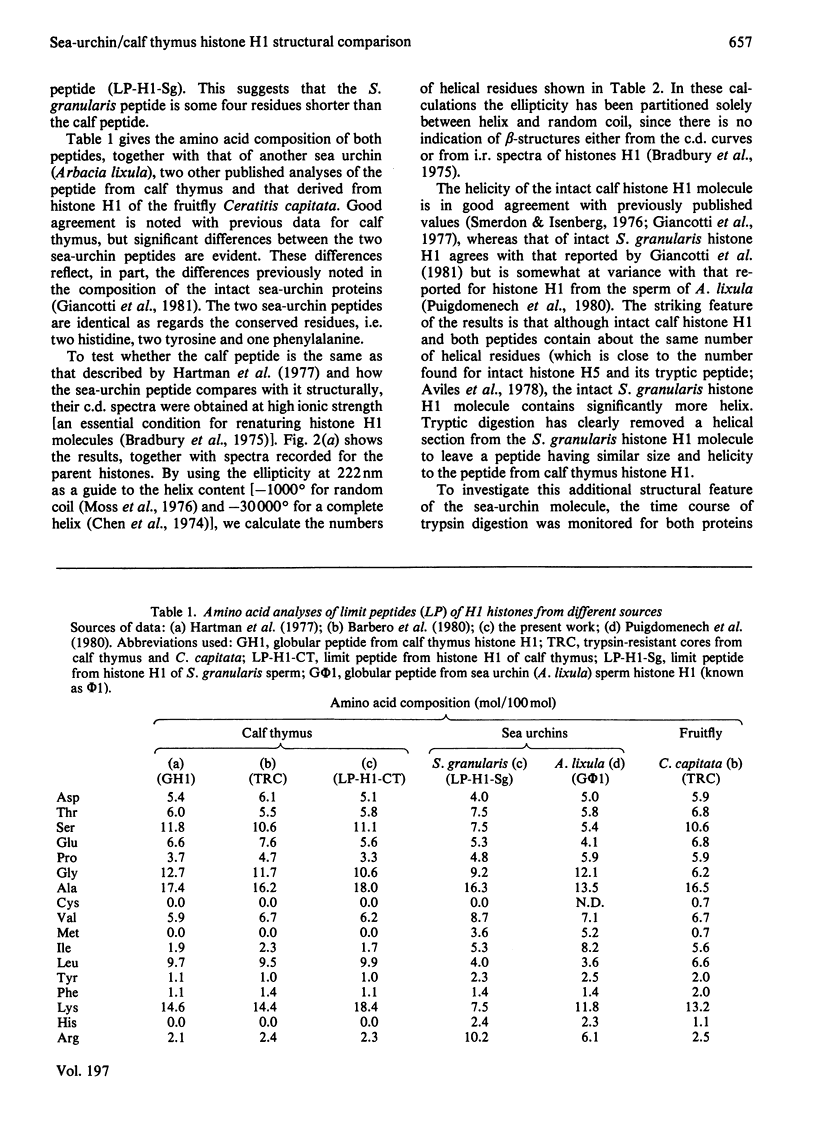
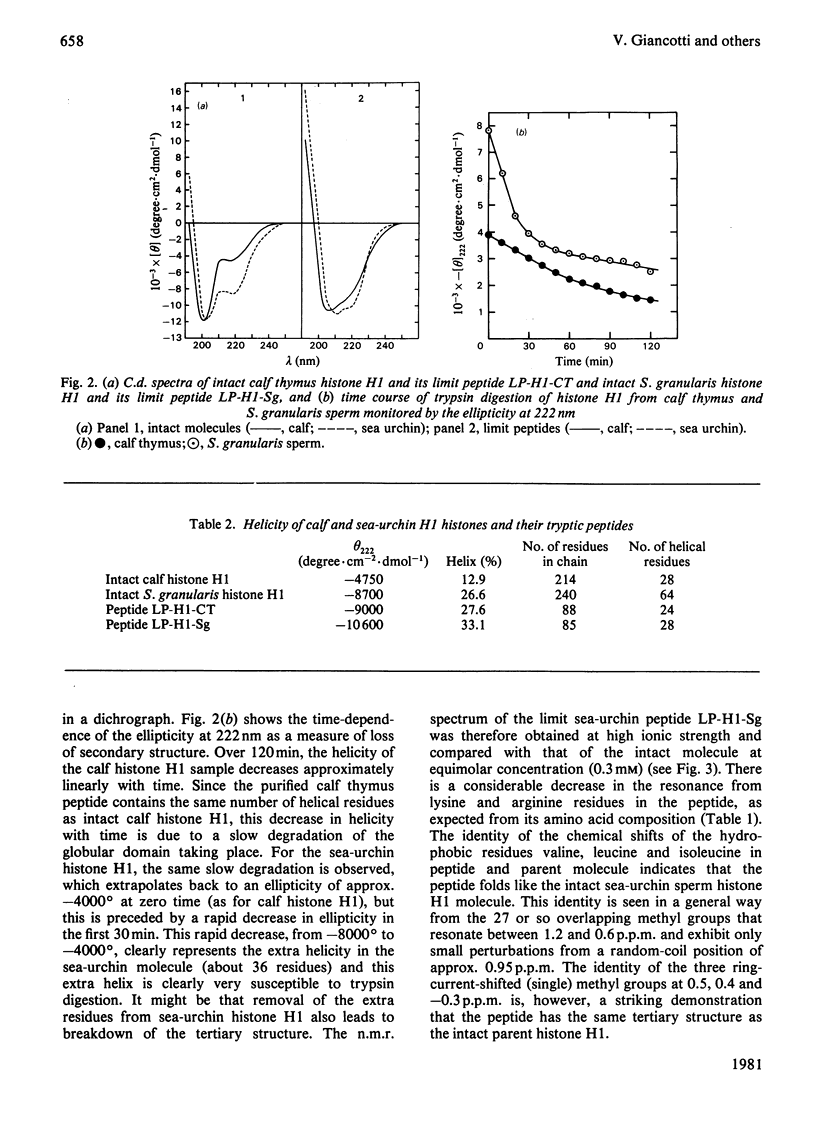
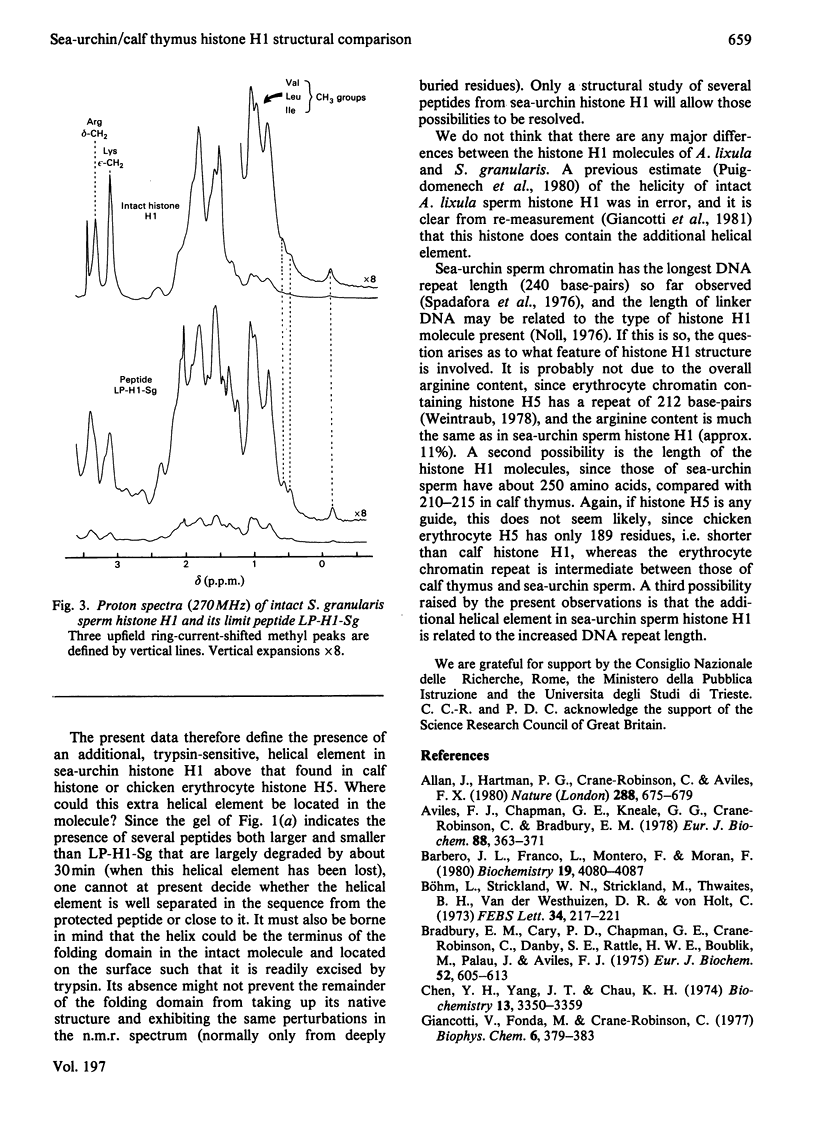
Tryptic digestion of histone H1 from the sperm of the sea urchin Sphaerechinus granularis leaves a limiting peptide of approx. 80 residues that is of similar size to the limit peptide from calf thymus H1 or chicken erythrocyte H5. The S. granularis limit peptide folds to form tertiary structure similar to that of the intact parent histone H1 (shown by n.m.r. spectra), but the helical content is decreased by the digestion from 64 residues to 28. In contrast, intact calf thymus H1 and chicken erythrocyte H5 histones have only about 28 helical residues, which are preserved in their limit peptides. The extra helix in S. granularis is shown to be rapidly digested away by trypsin, and its location in histone H1 is discussed. A possible relationship of this structural feature to the length of linker DNA is proposed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J., Hartman P. G., Crane-Robinson C., Aviles F. X. The structure of histone H1 and its location in chromatin. Nature. 1980 Dec 25;288(5792):675–679. doi: 10.1038/288675a0. [DOI] [PubMed] [Google Scholar]
- Aviles F. J., Chapman G. E., Kneale G. G., Crane-Robinson C., Bradbury E. M. The conformation of histone H5. Isolation and characterisation of the globular segment. Eur J Biochem. 1978 Aug 1;88(2):363–371. doi: 10.1111/j.1432-1033.1978.tb12457.x. [DOI] [PubMed] [Google Scholar]
- Barbero J. L., Franco L., Montero F., Morán F. Structural studies on histones H1. Circular dichroism and difference spectroscopy of the histones H1 and their trypsin-resistant cores from calf thymus and from the fruit fly Ceratitis capitata. Biochemistry. 1980 Aug 19;19(17):4080–4087. doi: 10.1021/bi00558a027. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Cary P. D., Chapman G. E., Crane-Robinson C., Danby S. E., Rattle H. W., Boublik M., Palau J., Aviles F. J. Studies on the role and mode of operation of the very-lysine-rich histone H1 (F1) in eukaryote chromatin. The conformation of histone H1. Eur J Biochem. 1975 Apr 1;52(3):605–613. doi: 10.1111/j.1432-1033.1975.tb04032.x. [DOI] [PubMed] [Google Scholar]
- Böhm E. L., Strickland W. N., Strickland M., Thwaits B. H., van der Westhuizen D. R., von Holt C. Purification of the five main calf thymus histone fractions by gel exclusion chromatography. FEBS Lett. 1973 Aug 15;34(2):217–221. doi: 10.1016/0014-5793(73)80797-5. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Giancotti V., Cosimi S., Cary P. D., Crane-Robinson C., Geraci G. Preparation and characterization of histone H1 from the sperm of the sea-urchin Sphaerechinus granularis. Biochem J. 1981 Apr 1;195(1):171–176. doi: 10.1042/bj1950171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti V., Fonda M., Crane-Robinson C. Tyrosine fluorescence of two tryptophan-free proteins: histones H1 and H5. Biophys Chem. 1977 Apr;6(3):379–383. doi: 10.1016/0301-4622(77)85019-9. [DOI] [PubMed] [Google Scholar]
- Hartman P. G., Chapman G. E., Moss T., Bradbury E. M. Studies on the role and mode of operation of the very-lysine-rich histone H1 in eukaryote chromatin. The three structural regions of the histone H1 molecule. Eur J Biochem. 1977 Jul 1;77(1):45–51. doi: 10.1111/j.1432-1033.1977.tb11639.x. [DOI] [PubMed] [Google Scholar]
- Johns E. W. Studies on histones. 7. Preparative methods for histone fractions from calf thymus. Biochem J. 1964 Jul;92(1):55–59. doi: 10.1042/bj0920055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss T., Cary P. D., Abercrombie B. D., Crane-Robinson C., Bradbury E. M. A pH-dependent interaction between histones H2A and H2B involving secondary and tertiary folding. Eur J Biochem. 1976 Dec 11;71(2):337–350. doi: 10.1111/j.1432-1033.1976.tb11120.x. [DOI] [PubMed] [Google Scholar]
- Noll M. Differences and similarities in chromatin structure of Neurospora crassa and higher eucaryotes. Cell. 1976 Jul;8(3):349–355. doi: 10.1016/0092-8674(76)90146-x. [DOI] [PubMed] [Google Scholar]
- Puigdomenech P., Palau J., Crane-Robinson C. The structure of sea-urchin-sperm histone phi 1 (H1) in chromatin and in free solution. Trypsin digestion and spectroscopic studies. Eur J Biochem. 1980 Feb;104(1):263–270. doi: 10.1111/j.1432-1033.1980.tb04424.x. [DOI] [PubMed] [Google Scholar]
- Smerdon M. J., Isenberg I. Interactions between the subfractons of calf thymus H1 and nonhistone chromosomal proteins HMG1 and HMG2. Biochemistry. 1976 Sep 21;15(19):4242–4247. doi: 10.1021/bi00664a017. [DOI] [PubMed] [Google Scholar]
- Spadafora C., Bellard M., Compton J. L., Chambon P. The DNA repeat lengths in chromatins from sea urchin sperm and gastrule cells are markedly different. FEBS Lett. 1976 Oct 15;69(1):281–285. doi: 10.1016/0014-5793(76)80704-1. [DOI] [PubMed] [Google Scholar]
- Weintraub H. The nucleosome repeat length increases during erythropoiesis in the chick. Nucleic Acids Res. 1978 Apr;5(4):1179–1188. doi: 10.1093/nar/5.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]



