Abstract
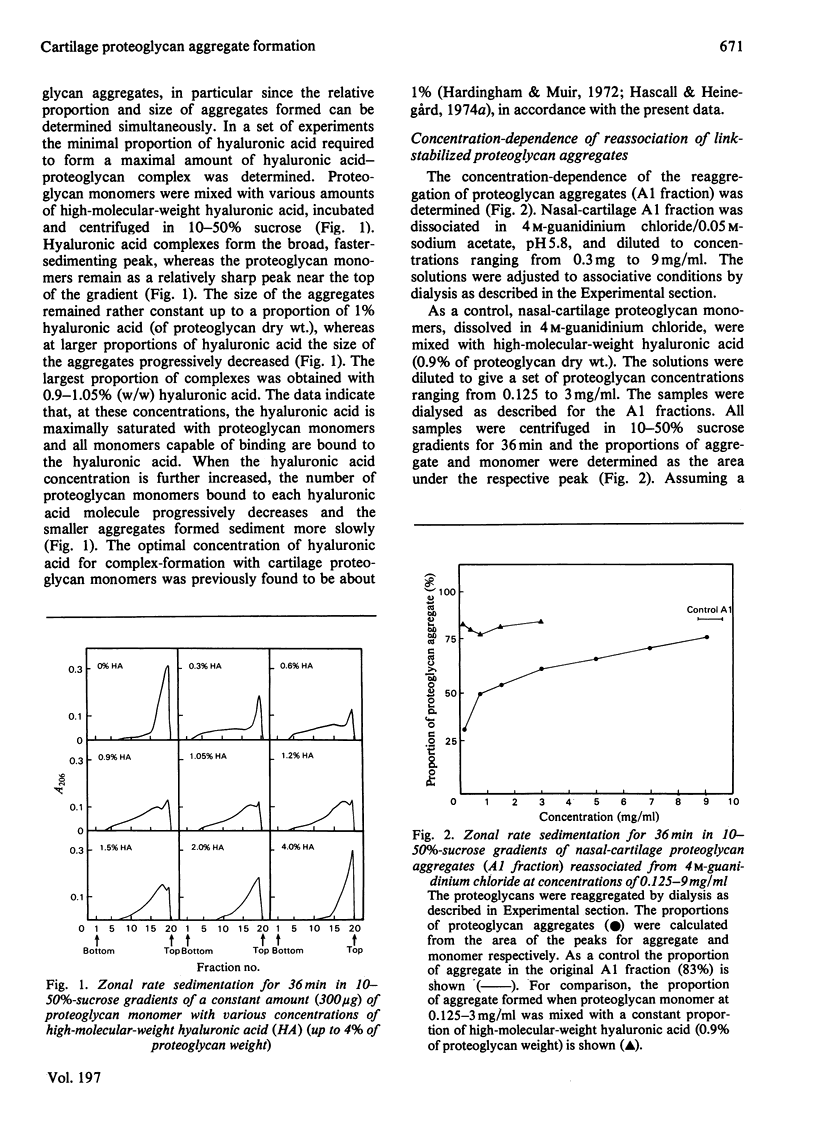
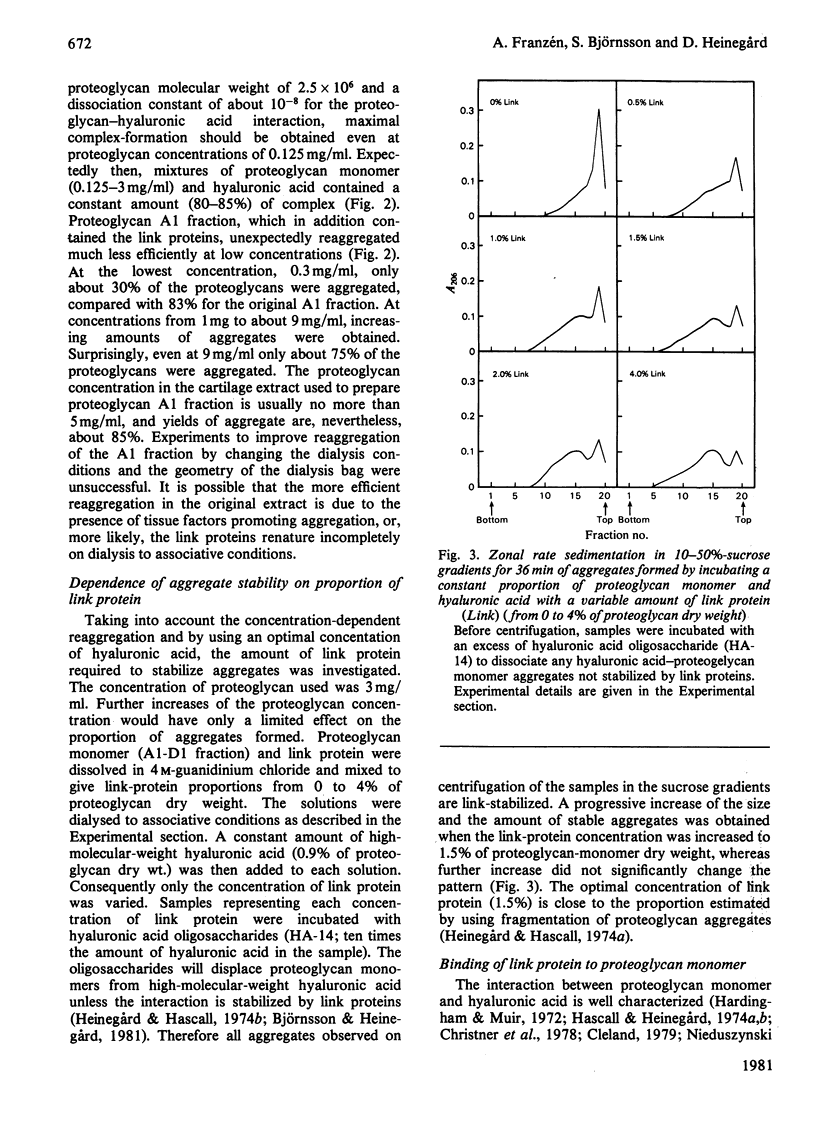
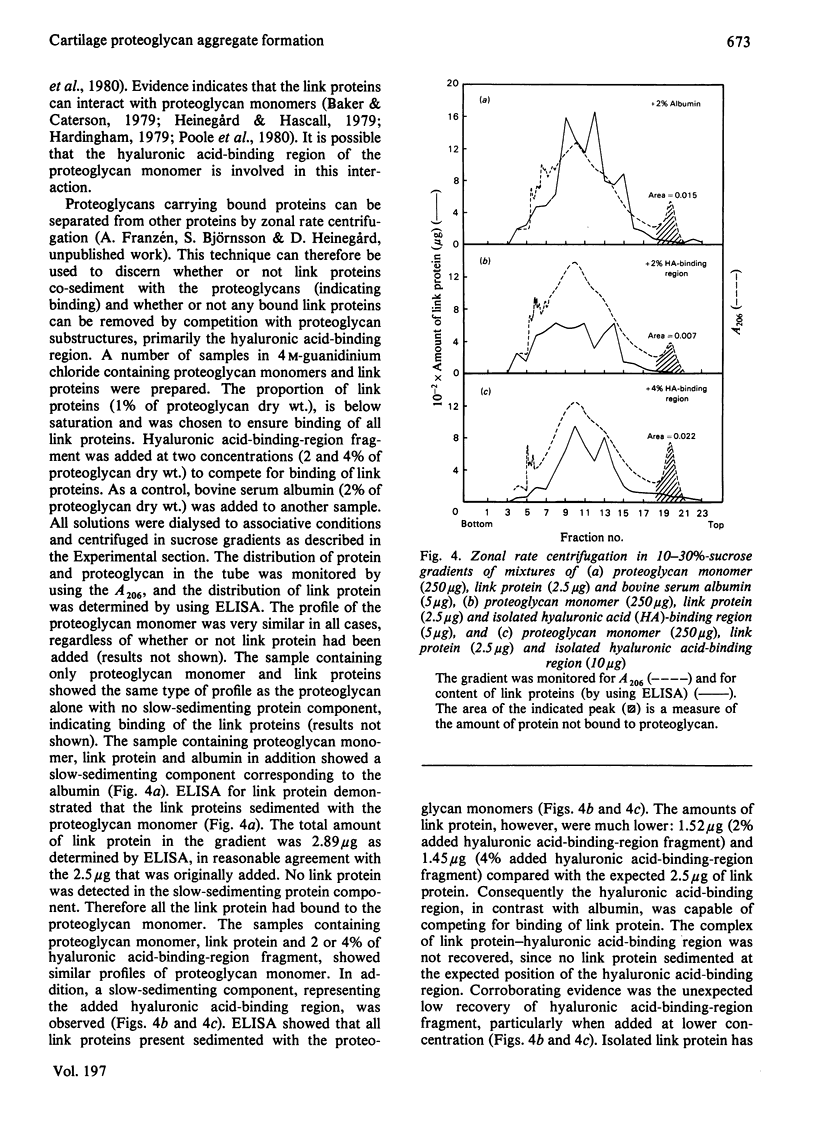
Cartilage proteoglycan aggregate formation was studied by zonal rate centrifugation in sucrose gradients. Proteoglycan aggregates, monomers and proteins could be resolved. It was shown that the optimal proportion of hyaluronic acid for proteoglycan aggregate formation was about 1% of proteoglycan dry weight. The reaggregation of dissociated proteoglycan aggregate A1 fraction was markedly concentration-dependent and even at 9 mg/ml only about 90% of the aggregates were reformed. The lowest proportion of link protein required for maximal formation of link-stabilized proteoglycan aggregates was 1.5% of proteoglycan dry weight. It was separately shown that link protein co-sedimented with the proteoglycan monomer. By competition with isolated hyaluronic acid-binding-region fragments, a proportion of the link proteins was removed from the proteoglycan monomers, indicating that the link protein binds to the hyaluronic acid-binding region of the proteoglycan monomer.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christner J. E., Brown M. L., Dziewiatkowski D. D. Affinity binding of the cartilage proteoglycan protein-keratan sulfate core to immobilized hyaluronic acid. Anal Biochem. 1978 Oct 1;90(1):22–32. doi: 10.1016/0003-2697(78)90004-0. [DOI] [PubMed] [Google Scholar]
- Chua N. H., Blomberg F. Immunochemical studies of thylakoid membrane polypeptides from spinach and Chlamydomonas reinhardtii. A modified procedure for crossed immunoelectrophoresis of dodecyl sulfate.protein complexes. J Biol Chem. 1979 Jan 10;254(1):215–223. [PubMed] [Google Scholar]
- Cleland R. L. Binding of hyaluronic acid oligosaccharides by cartilage proteoglycan. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1140–1145. doi: 10.1016/s0006-291x(79)80026-1. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay, Elisa. 3. Quantitation of specific antibodies by enzyme-labeled anti-immunoglobulin in antigen-coated tubes. J Immunol. 1972 Jul;109(1):129–135. [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem J. 1979 Jan 1;177(1):237–247. doi: 10.1042/bj1770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. I. The role of hyaluronic acid. J Biol Chem. 1974 Jul 10;249(13):4232–4241. [PubMed] [Google Scholar]
- Hascall V. C., Heinegård D. Aggregation of cartilage proteoglycans. II. Oligosaccharide competitors of the proteoglycan-hyaluronic acid interaction. J Biol Chem. 1974 Jul 10;249(13):4242–4249. [PubMed] [Google Scholar]
- Heinegård D. K., Hascall V. C. The effects of dansylation and acetylation on the interaction between hyaluronic acid and the hyaluronic acid-binding region of cartilage proteoglycans. J Biol Chem. 1979 Feb 10;254(3):921–926. [PubMed] [Google Scholar]
- Heinegård D., Axelsson I. Distribution of keratan sulfate in cartilage proteoglycans. J Biol Chem. 1977 Mar 25;252(6):1971–1979. [PubMed] [Google Scholar]
- Heinegård D. Extraction, fractionation and characterization of proteoglycans from bovine tracheal cartilage. Biochim Biophys Acta. 1972 Nov 28;285(1):181–192. doi: 10.1016/0005-2795(72)90190-0. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Characterization of chondroitin sulfate isolated from trypsin-chymotrypsin digests of cartilage proteoglycans. Arch Biochem Biophys. 1974 Nov;165(1):427–441. doi: 10.1016/0003-9861(74)90182-9. [DOI] [PubMed] [Google Scholar]
- Nieduszynski I. A., Sheehan J. K., Phelps C. F., Hardingham T. E., Muir H. Equilibrium-binding studies of pig laryngeal cartilage proteoglycans with hyaluronate oligosaccharide fractions. Biochem J. 1980 Jan 1;185(1):107–114. doi: 10.1042/bj1850107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R., Reiner A., Tang L. H., Rosenberg L. Proteoglycans from bovine nasal cartilage. Immunochemical studies of link protein. J Biol Chem. 1980 Oct 10;255(19):9295–9305. [PubMed] [Google Scholar]
- Tang L. H., Rosenberg L., Reiner A., Poole A. R. Proteoglycans from bovine nasal cartilage. Properties of a soluble form of link protein. J Biol Chem. 1979 Oct 25;254(20):10523–10531. [PubMed] [Google Scholar]
- Wieslander J., Heinegárd D. Immunochemical analysis of cartilage proteoglycans. Radioimmunoassay of the molecules and the substructures. Biochem J. 1980 Jun 1;187(3):687–694. doi: 10.1042/bj1870687. [DOI] [PMC free article] [PubMed] [Google Scholar]


