Abstract
Significance:
Potassium channels regulate the influx and efflux of K+ ions in various cell types that generate and propagate action potential associated with excitation, contraction, and relaxation of various cell types. Although redox active cysteines are critically important for channel activity, the redox regulation of K+ channels by thioredoxin (Trx) has not been systematically reviewed.
Recent Advances:
Redox regulation of K+ channel is now increasingly recognized as drug targets in the pathological condition of several cardiovascular disease processes. The role of Trx in regulation of these channels and its implication in pathological conditions have not been adequately reviewed. This review specifically focuses on the redox-regulatory role of Trx on K+ channel structure and function in physiological and pathophysiological conditions.
Critical Issues:
Ion channels, including K+ channel, have been implicated in the functioning of cardiomyocyte excitation–contraction coupling, vascular hyperpolarization, cellular proliferation, and neuronal stimulation in physiological and pathophysiological conditions. Although oxidation–reduction of ion channels is critically important in their function, the role of Trx, redox regulatory protein in regulation of these channels, and its implication in pathological conditions need to be studied to gain further insight into channel function.
Future Directions:
Future studies need to map all redox regulatory pathways in channel structure and function using novel mouse models and redox proteomic and signal transduction studies, which modulate various currents and altered excitability of relevant cells implicated in a pathological condition. We are yet at infancy of studies related to redox control of various K+ channels and structured and focused studies with novel animal models. Antioxid. Redox Signal. 41, 818–844.
Keywords: thioredoxin, redox, K+ channel, oxidative stress
Introduction
Reactive oxygen species (ROS) are produced and metabolized in all aerobic organisms, and play vital roles in signaling pathways required for normal cellular functions. However, excess ROS production is associated with cellular dysfunction and death (Nordberg and Arner, 2001; Sies et al., 2017; Thannickal and Fanburg, 2000). The steady-state ROS generation is balanced by interaction between ROS and their enzymatic or nonenzymatic antioxidants and scavengers. However, increased levels of ROS production due to altered pathophysiology overwhelm the endogenous defense systems resulting in the onset and propagation of various diseases. For example, increased ROS generation in the vascular cells is implicated in hypertension, ischemia–reperfusion, age-related arterial stiffness, atherosclerosis, cerebrovascular diseases, and other related diseases (Nordberg and Arner, 2001; Wolin, 2009). Cellular oxidants produced due to activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, mitochondria, and exposure to toxic chemicals or gases could cause overt oxidative stress resulting in impairment of normal physiological functions with onset of disease of various organ systems, such as cardiovascular, pulmonary, cerebral, kidney, and many other organs. However, physiological oxidants, such as hydrogen peroxide (H2O2), produced due to activation of NADPH oxidase or mitochondria could propagate various signaling resulting in protein function or dysfunction. Oxidants react with the thiol group of cysteine (Cys) and with the thioether group of methionine, leading to formation of reversible protein disulfides and methionine sulfoxides, respectively. The human proteome consists of 214,000 Cys residues (Go and Jones, 2013), many of them having the potential to be oxidized by oxidants such as H2O2 to form disulfides if accessible. These disulfides could be reverted to thiols by various enzymes and low-molecular-weight thiols, such as glutathione or thioredoxin (Trx) (Jerng and Pfaffinger, 2014; Lu and Holmgren, 2014; Nordberg and Arner, 2001; Sengupta et al., 2019; Whayne et al., 2015). About half of all proteins are sensitive to thiol modulation. Examples of Cys thiol modifications are S-glutathionylation, S-nitrosylation, S-palmitoylation, and S-sulfhydration. Recently, polysulfides, including hydrogen per- and poly-sulfides (H2Sn, n = 2 and n > 2), have been demonstrated as oxidants to S-sulfurate (S-sulfhydrate Cys residues of target proteins, including TRPA1 channels, PTEN, KATP channels, protein kinase G1α, and others (Kimura, 2020). One of the two responsible Cys residues can be S-sulfurated by H2Sn and react with the remaining Cys residues to produce the disulfide bridge. These posttranslational thiol modifications serve as redox-sensing molecular switches (Fig. 1) (Nagahara, 2011). Since oxidative stress could be transient or chronic, some of these modifications are reversible (disulfides, sulfenylation, persulfides, S-thiolations), whereas others are irreversible (sulfinylations, sulfonylations, etc.). For example, aging is a chronic oxidation process as we breathe oxygen and metabolize this using an oxidative process in the mitochondria. This chronic slow oxidation process over the life span of an organism promotes dysfunction of various proteins in the vascular system resulting in the onset of several cardiovascular diseases such as hypertension, chronic kidney disease, and various heart diseases. The transient oxidation followed by reduction propagates redox signaling resulting in transcription factor activation or inhibition that is linked to gene expression or suppression. Disruption of these reversible posttranslational modifications is the most central feature of oxidative stress that contributes to mechanisms of aging and age-related disease (Jones, 2008; Jones, 2006). In the cytosol, the redox state is predominantly in a reduced environment such that Cys residues exist mainly in the thiol form rather than in the oxidized disulfide form. Ion channels, specifically opening of potassium channels (K+), are regulated by the redox state of their Cys that is involved in various functions in the vasculature, heart, lung, brain, and other organs. Therefore, these channels are impacted by transient oxidations of their Cys and redox signaling. Although these channels are extensively studied, redox regulation of these channels is not adequately reviewed. In this study, we review the redox regulation of various K+ channels and their role in cardiovascular and pulmonary systems and diseases.
FIG. 1.
Examples of protein cysteine sulfhydryl modifications. Hydrogen peroxide (H2O2) can result in intra- or interprotein (P) cysteine disulfide bridge formation. In addition, it can cause cysteine residue oxidation reactions depending on the degree of H2O2 (e.g., S-sulfenation, S-sulfination, and S-sulfonation). Other reducing agents, such as glutathione (GSH), nitric oxide (NO), hydrogen sulfide (H2S), or palmitic acid, can result in posttranslational protein S-modifications.
Trx system
The cytosolic Trx system in mammals consists of Trx and Trx reductase-1 (TrxR1). Trx is a small (12 kDa) cell-permeable redox protein with antioxidant and disulfide reductase properties (Das and Das, 2000; Holmgren, 1985; Holmgren and Bjornstedt, 1995). Oxidized Trx (Trx-S2) is reduced by TrxR1 utilizing reducing equivalents from NADPH generating reduced Trx (Trx-(SH)2 (Fig. 2) (Holmgren, 2000). There are three isoforms of Trx; the most abundant form is the cytosolic Trx and is the focus of this review. In the remainder of this review, it is referred to as Trx. A mitochondrial Trx (Trx-2) (Spyrou et al., 1997) exists, as well as a Trx in spermatozoa (SpTrx) (Miranda-Vizuete et al., 2001). Trxs are evolutionarily highly conserved across the three domains of life, including bacteria, archaea, and eukaryotes (Eklund et al., 1991). The overall 3D structure of all Trxs has a central core of 5 β-strands surrounded by 4 α-helices and the conserved active-site sequence–Cys-Gly-Pro-Cys– (Weichsel et al., 1996). The two Cys residues in the catalytic active site are Cys32 and Cys35 and can form a disulfide bridge when Trx-S2, thereby inhibiting its activity (Jeng et al., 1994). Whereas Cys32 and Cys35 perform the direct transfer of electrons to a disulfide, Cys62, Cys69, and Cys73 are involved in regulatory functions in mammalian cells (Haendeler, 2006), and posttranslational modification of these residues such as nitrosylation could alter the activity of Trx (Haendeler, 2006). However, due to the low reduction potential of Trx (Escherichia coli, E. coli Trx = –270 mV) (Dyson et al., 1997), Trx is mainly in its reduced form Trx-(SH)2 under normal physiological conditions and functions as the major disulfide reductant in the cytosol. We have previously shown that Trx is not a superoxide anion (O2•−) scavenger (Das and Das, 2000).
FIG. 2.

Redox cycling of the thioredoxin (Trx) system. The reduced and redox active form of Trx [Trx-(SH)2] breaks disulfide bridges between two cysteine residues in oxidized proteins (P–S–S) to generate reduced sulfhydryl proteins [P-(SH)2] and oxidized Trx (Trx-S2). Trx reductase (TR) recycles Trx-S2 into Trx-(SH)2. The oxidized TR (TR-S–Se) uses NADPH as electron donors to form catalytically active TR (TR-Se−-SH).
Trx specifically converts protein disulfides to reduced thiol containing proteins using TrxR1 and NADPH. The studies by Dyson et al. and Foreman-Kay et al. support the model for Trx redox catalysis by initial attack of the thiolate anion of Cys-32. The Cys residues in the CxxC motif are the active-site Cys32 and the resolving Cys35. The experimentally observed pKa of Cys-32 is 6.3 ± 0.1, while that of Cys-35 is probably between 7.5 and 8.6. These values are in agreement with the previously measured pKa of active-site residues of E. coli Trx of 6.4–6.8 and 8.5–9.0 from chemical modification and fluorescence studies and 7.1–7.4 and 8.4 from Nuclear Magnetic Resonance (NMR) analysis (Dyson et al., 1997). The catalysis occurs in three stages as follows: (1) attack on the target disulfide by active-site nucleophilic CysN resulting in transient mixed disulfide between Trx and the target disulfide, (2) formation of intramolecular disulfide bond by attack of C-terminal buried Cys (CysC) residue with reduction of the substrate, (3) regeneration of the reduced state of Trx by TrxR1 and NADPH (Fig. 3).
FIG. 3.

Sequential catalysis by Cy32 and Cys35 to reduce protein disulfide with regeneration of Trx-SH by TrxR1. The catalysis occurs in three stages: (1) attack on the target disulfide by active-site nucleophilic cysteineN resulting in transient mixed disulfide between Trx and the target disulfide, (2) formation of intramolecular disulfide bond by attack C-terminal buried cysteine (CysC) residue with reduction of the substrate, and (3) regeneration of the reduced state of Trx by TrxR1 and NADPH (Fig. 3).
Thus, Trx regenerates oxidized proteins to native reduced state. In addition, we have shown that Trx deglutathionylates endothelial nitric oxide synthase (eNOS), which has been shown to be glutathionylated and uncoupled in aging (Kundumani-Sridharan et al., 2021). Trx is a multifunctional protein known to activate transcription factors, induces gene expression, and decreases oxidative stress and inflammation (Holmgren and Bjornstedt, 1995). Trx has been shown to induce its own expression by promoting activation of SP1 transcription factor (Bloomfield et al., 2003). Trx is also secreted in a leaderless pathway (Rubartelli et al., 1992), indicating that intracellular Trx could be secreted out of the cells and could impact various redox-sensitive cell surface receptors. In the cytosol, the redox state is kept in a reduced environment in that Cys residues exist mainly in thiol form rather than in the oxidized disulfide form. Trx is a major disulfide oxidoreductase responsible for maintaining proteins in their reduced state. Although Trx is frequently referred to as an antioxidant, its major function is disulfide reductase activity along with TrxR1 and NADPH, as shown in the above equation. Reduced Trx also provides electrons to Trx peroxidase to reduce H2O2 to water.
Trx activates proteins via an allosteric conformational change. This posttranslational reversible process is comparable with protein phosphorylation and has the potential to alter protein function and activity. Not surprisingly, the Trx system regulates a plethora of physiological processes that involve DNA replication and repair, embryogenesis, inflammation, growth, apoptosis, and angiogenesis that are involved in events ranging from pregnancy (Sahlin et al., 1997), embryonic development (Dong et al., 2016; Jakupoglu et al., 2005; Matsui et al., 1996; Nonn et al., 2003), birth (Das, 2005; Das, 2004; Tipple, 2014), to aging (Altschmied and Haendeler, 2009; Fierro-Gonzalez et al., 2011; Goy et al., 2014; Holmgren and Lu, 2010). During the course of life, it offers protection from cardiovascular diseases (for a review, see Whayne et al., 2015), cancer (Rubartelli et al., 1995), rheumatoid arthritis (Yoshida et al., 1999), AIDS (Nakamura et al., 1996), and more. In this review, we focus on the role of Trx in redox modulation of K+ channels. K+ channels are linked to many cellular processes, including apoptosis, cardiomyocyte excitation–contraction coupling, vascular hyperpolarization, cellular proliferation, and neuronal stimulation. We are aware that several K+ channel types are expressed in one specific cell type and that it is impossible to cover all K+ channels in this review. The function of one K+ channel may be different in an endothelial cell (EC) compared with a neuron. Hence, we tried to review K+ channel redox modulation according to their anatomical position by organizing them for vascular cells, cardiomyocytes, and central nervous cells. The human genome encodes for roughly 200 ion channels. This superfamily of ion channels is involved in many cellular processes, including apoptosis, cardiomyocyte excitation–contraction coupling, vascular hyperpolarization, cellular proliferation, and neuronal stimulation. In this study, we focus on the role of Trx in redox modulation of pore-forming K+ channels expressed in vascular cells and cardiomyocytes.
Small- and intermediate-conductance Ca2+-activated K+ channels
Nomenclature and function
Calcium-activated K+ (KCa) channels open in response to increases in intracellular [Ca2+] and contribute to the afterhyperpolarization in neurons and cardiomyocytes, and also in nonexcitable cells such as lymphocytes, erythrocytes, fibroblasts, secretory epithelial, and vascular ECs (Adelman et al., 2012; Grgic et al., 2009; King et al., 2015; Wulff and Castle, 2010; Zhang et al., 2015). In the vasculature, many K+ channels are involved in regulating vasomotor tone. KCa channels of different conductance have been identified, including small (10–15 pS), intermediate (20–60 pS), and large (200–300 pS) conductance channels. Three highly conserved small-conductance KCa (SK) channels have been cloned from the rat and human brain (SK1, SK2, and SK3), each containing 6 putative transmembrane segments (Kohler et al., 1996). A vascular apamin-sensitive SK3 channel has been cloned as well (Sokol et al., 1994), which has now been confirmed to be expressed in the endothelium (Burnham et al., 2002). This endothelial SK3 channel is also named KCa2.3. A fourth SK subtype (SK4) is now classified as the intermediate-conductance KCa (IKCa) channel, also referred to as IK1, but preferably as KCa3.1 (Ishii et al., 1997; Joiner et al., 1997). Endothelial KCa2.3 and KCa3.1 channels are voltage-insensitive and demonstrate a nonohmic current/voltage relationship with a conductance two to three times higher for inward than outward currents (Xia et al., 1998). Hence, they belong to the family of inward-rectifier K+ (KIR) channels (see the Inward-Rectifier K+ Channels section). Channel opening results in K+ efflux, which hyperpolarizes the EC membrane. This hyperpolarizing current is spread through adjacent ECs (via KCa2.3) (Lin et al., 2012) and to vascular smooth muscle cells (VSMCs) via myoendothelial junctions (via KCa3.1) (Dora et al., 2008; Sandow et al., 2006) or via activation of Na+/K+-ATPase and KIR channels (Edwards et al., 1998) resulting in VSMC hyperpolarization and subsequent relaxation (Fig. 4). This process is called endothelium-dependent hyperpolarization (EDH). Mice deficient in KCa2.3 and/or KCa3.1 display a disrupted EDH response and are hypertensive (Brahler et al., 2009; Si et al., 2006; Yap et al., 2016). Angiotensin II (Ang II)-induced hypertension has been shown to reduce the expression of these channels in rat mesenteric arteries (Hilgers et al., 2006). Besides its role in blood pressure regulation, the KCa3.1 channel modulates ion transport in epithelial cells (Singh et al., 2001), renal fibroblast proliferation and fibrogenesis (Grgic et al., 2009), and is a key cellular effector in several life-threatening diseases such as atherosclerosis, restenosis, and traumatic injury-induced edema (Kohler et al., 2003; Tharp and Bowles, 2009; Toyama et al., 2008). Knowledge in the molecular and functional understanding of KCa channel regulation is vital for the design of putative therapeutic KCa channel gating modulators applicable to a wide variety of health disorders (Christophersen and Wulff, 2015; Kohler et al., 2016).
FIG. 4.
Schematic overview of the role of vascular ion channels in mediating EC/VSMC communication and hereby regulating vasomotor tone. The dragging flow of the blood on the endothelial layer creates a shear stress that activates transient receptor potential vanilloid (TRPV) channel leading to an increase in intracellular Ca2+ [Ca2+]i. Agonists such as acetylcholine (ACh) and bradykinin (BK) can bind to G-protein-coupled receptors (Gq/11) on the EC membrane that couples to phospholipase C (PLC) to activate second messengers such inositol triphosphate (IP3) and DAG that result in store-operated Ca2+ release. The rise in [Ca2+]i activates endothelial Ca2+-activated K+ channels (KCa2.3 and KCa3.1) to trigger K+ efflux that hyperpolarizes the EC membrane. This hyperpolarization current can spread via other KCa2.3 channels to neighboring ECs. The current can spread through connexin channels located in myoendothelial gap junctions. The extracellular K+ activates inward-rectifier K+ channels (KIR) and Na+/K+ ATPase located on the VSMC membrane resulting in a decrease in membrane potential (↓ Vm) and subsequent VSMC hyperpolarization. Hyperpolarization inhibits L-VOCC, preventing VSMC Ca2+ increase suppressing contraction and causing vasorelaxation. In addition, a rise in EC [Ca2+]i activates endothelial nitric oxide synthase (eNOS) to generate NO, which diffuses to the VSMC to activate soluble guanylate cyclase (sGC). This generates cyclic guanidine monophosphate (cGMP), a second messenger known to stimulate protein kinase G (PKG1α). PKG1α can activate large-conductance Ca2+-activated K+ channels (BKCa), increase myosin light chain phosphatase (MLCP) activity, and decrease Rho kinase activity, reducing actin/myosin crosslinking and contraction. EC, endothelial cell; VSMC, vascular smooth muscle cell.
Structure
All K+ channels have essentially the same pore constitution, containing a critical amino acid sequence, the K+ channel signature sequence or regulator of conductance of K+ (RCK) domain (Heginbotham et al., 1994; Jiang et al., 2001; Kuang et al., 2015; Pau et al., 2011). This sequence is a part of the selectivity filter. The endothelial KCa channel is a tetrameric protein with each subunit compromising 427 amino acids organized in six transmembrane (S1-S6) segments. The first structural data for pore formation of K+ channels came with the solved crystal structure of the bacterial K+ channel KcsA (Doyle et al., 1998). The K+ channel resembles an inverted “teepee-like” structure in that the four inner helices pack against each other similar to a flower bundle near the cytosolic side of the membrane (Doyle et al., 1998). The pore helices are slotted in between and are pointed near the center of the channel. Herein lays the selectivity filter that binds and conducts K+ ions. This pore region in S5-S6 contains a conserved Glycine-Tyrosine-Glycine (GYG) pore motif (Lipkind et al., 1995).
Activity and redox modulation
Sensitivity to Ca2+ arises from binding of Ca2+ to calmodulin (CaM) (Khanna et al., 1999; Xia et al., 1998). This CaM binding domain (CaMBD) for the KCa3.1 channel is located at the C-terminal end of the S6 segment (residues Lys312-Thr329) (Morales et al., 2013), whereas in the KCa2.3 channel, the CaMBD resides deeper in the channel pore in or near the selectivity filter (Bruening-Wright et al., 2002). Upon binding of Ca2+ to CaM, the CaM/CaMBD complex undergoes a conformational change, which is coupled to the channel pore enabling the channel to transition from a nonconducting to a conducting configuration (Fig. 4) (Jiang et al., 2002; Keen et al., 1999; Li et al., 2009; Schumacher et al., 2001). The S6 segment proximal to the GYG motif extending from Val266 to Ala286 is preserved in KCa3.1, rat SK2, the bacterial K+ channel KcsA, and voltage-gated Shaker K+ channels (Simoes et al., 2002). The S5-pore-S6 region is most likely to play a crucial role in the KCa3.1 pore formation and channel gating. Simoes and colleagues used a technique called substituted cysteine accessibility mutagenesis (SCAM) (Akabas, 2015; Akabas et al., 1992). All residues between Val275 and Ala286 were mutated to Cys residues and these KCa3.1 mutants were expressed in Xenopus laevis oocytes (Simoes et al., 2002). Next the effects of the water-soluble methanethiosulfonate (MTS) derivates sodium (2-sulfonatoethyl) methanethiosulfonate (MTSES), 2-aminoethyl methanethiosulfonate hydrobromide (MTSEA), and [2-(trimethylammonium) ethyl] methanethiosulfonate bromide (MTSET) on the single-channel conductance, and gating properties of Cys KCa3.1 mutants were examined. These thiol-reactive agents reversibly block Cys residues and other sulfhydryl groups (–SH into –S-S-CH2-CH2-SO3– in the case of MTSES, –S-S-CH2-CH2-NH3+ in the case of MTSEA, and –S-CH2-CH-N-(CH3)3+ in the case of MTSET). Covalent addition of the side group to the S-atom of Cys may interfere with ion permeation if the modified side chain is positioned in or near the ion conduction pathway. Hence, these thiol-reactive agents provide important information on the location of Cys residues in the channel, since these water-soluble agents specifically bind to Cys residues that are accessible via the pore or are located near the cytoplasmic C-terminus. Simoes and colleagues observed that interaction of MTSET with these Cys residues leads to current inhibition, and closure by the gate occurs at a point between the luminal Thr278 and Val282 residues (Simoes et al., 2002). Further studies by this group showed that in closed conformation a narrow passage is created centered at Val282 that connects the channel cavity to the cytosol. However, this passage (∼10 Å) is not narrow enough to restrict passage of small reagents such as MTSEA but forms a tight seal permeable to smaller reagents and K+ ions (Garneau et al., 2009; Klein et al., 2007). Channel activation should occur distal to Val282 at the cytoplasmic COOH terminus (Ala283 and Ala286) (Garneau et al., 2009; Klein et al., 2007). However, in contrast to the previous investigations, which determined the role of Cys engineered along the luminal face of S6, the nonluminal face of S6 also participates in the activation mechanism. Interestingly, this nonluminal S6 region contains 2 adjacent Cys residues (Cys 276 and −277), which have the potential to undergo posttranslational modifications (Klein et al., 2007). Cys modulation with the thiol-reactive and membrane-impermeable agent parachloromercuribenze sulfonate (PCMBS) at Cys276 shifts the gating equilibrium toward the open conformation (Bailey et al., 2010). Substituting the hydrophobic Val282 with hydrophilic amino acids locks the channel in an open-like state, resulting in channels that are ion conducting in the absence of Ca2+ (Garneau et al., 2010). Hence, the nonluminal face of S6 forms a critical interaction surface, which shifts to the closed conformation. Thus, the luminal and nonluminal C-terminal portion of S6 could be allosterically coupled to the activation gate. H2O2 and other thiol reactive agents such as 5,5′-dithio-bis (2-nitrobenzoic acid) (DTNB or Ellman’s reagent) or [(O-carboxyphenyl)thio]ethyl mercury sodium salt (thimerosal) were shown to inhibit KCa3.1 channel activation in bovine aortic ECs only when applied on the intracellular side (Cai and Sauve, 1997). Channel activity could partly be restored by disulfide reducing agents dithiothreitol (DTT) or reduced glutathione. However, there are limited studies that determined Cys redox modulation of endothelial KCa channels in ex vivo models via EDH response in isolated ECs or contracted arteries. Hyperhomocysteinemia (HHCy) has been shown to inhibit the EDH response in murine small mesenteric arteries via oxidative and nitrosative stress, which was inhibited by catalase and the peroxynitrite inhibitor ebselen (Cheng et al., 2011). Auto-oxidation of HCy produces H2O2 resulting in conversion of HCy into its disulfides, which is mediated by thiol–disulfide exchange reactions (Sengupta et al., 2001). Upregulation of Trx suppresses HCy-induced reactive oxygen and nitrogen species (RONS) formation (Dai et al., 2008). In patients with combined coronary artery disease and HHCy, serum Trx levels are decreased (Wu et al., 2010). Taken together, a protective role for Trx in preventing and reducing disulfides and endothelial dysfunction associated with HHCy is likely, although more studies are needed to be performed in this area.
Recently, we have demonstrated that the KCa3.1 component of this EDH response was enhanced in ex vivo small mesenteric arteries from mice overexpressing human Trx (Trx-Tg) (Hilgers and Das, 2015) and that the oxidizing agent diamide inhibited the EDH, which was reversed by DTT, suggesting a redox regulation of the EDH response (Hilgers and Das, 2015). Furthermore, mice expressing a mutant redox inactive Trx (dnTrx-Tg) had severely blunted EDH response (Hilgers and Das, 2015). This suggests that the EDH response is redox regulated. Although our study demonstrated redox control of EDH by Trx, a direct mechanistic link between Trx and KCa3.1 channel Cys modulation needs to be further studied and confirmed (Fig. 5).
FIG. 5.
Mechanism of Ca2+-activated and Trx-activated KCa3.1 channel opening. Binding of Ca2+ to the calmodulin-binding domain located at the intracellular side of the KCa3.1 channel results in a conformational change opening the channel gate to allow passage of K+ ions (upper figure). A hypothetical model of KCa3.1 is presented in the lower figure in which active and reduced thioredoxin [Trx-(SH)2] breaks a disulfide cysteine bridge between Cys-276 and −277 resulting in a conformational change opening the channel gate. Cys, cysteine; Trx, thioredoxin.
Inward-rectifier K+ channels
Nomenclature and function
The inward-rectifier K+ channel (KIR) activation results in hyperpolarization and VSMC relaxation in a number of vascular beds, including coronary (Knot et al., 1996), cerebral (Knot et al., 1996; Quayle et al., 1993), cremaster (Loeb et al., 2000), mesenteric (Dora et al., 2000), and renal arteries (Chilton and Loutzenhiser, 2001). The KIR2.1 subtype is expressed in ECs and mediates EDH together with KCa2.3 and KCa3.1 channels (Sonkusare et al., 2016). KIR channels are unique among K+ channels in that their conductance is increased by a small rise (to <20 mM) in the extracellular K+ concentration. These channels differ from other K+ channels where small amounts of extracellular K+ (<20 mM) increase KIR current leading to hyperpolarization. KIR channels can be further separated on the basis of their functional characteristics: classical, G-protein gated, ATP-sensitive (KATP), and K+ transport channels (Hibino et al., 2010). KIR2.1 and KIR2.2 are expressed in VSMCs from cerebral arteries where they mediate parenchymal arteriolar vasodilation (Longden et al., 2014; Wu et al., 2007). ATP inhibits KATP channels at micromolar intracellular concentrations in pancreatic β cells resulting in insulin release, whereas endogenous substances such as adenosine, neuropeptides, and endothelial factors open KATP channels resulting in vasorelaxation (Babenko et al., 1998; Quayle et al., 1997).
Structure
The KIR channel family consists of seven subfamilies (KIR1–7) comprising a total of 15 subunit isoforms (KIR1.1, KIR2.1–4, KIR3.1–4, KIR4.1–2, KIR5.1, KIR6.1–2, and KIR7.1). Each subunit is a two-transmembrane protein connected by a pore-forming loop with intracellular C- and N-terminal domains. Functional KIR channels are tetramers formed from homo- or heteromeric assemblies of individual subunits. KATP channels consist of four KIR subunits (KIR6.1 and KIR6.2). Each subunit is associated with a larger sulfonylurea receptor (SUR) subunit assembled into an octameric protein (Brayden, 2002). The combination KIR6.2/SUR2B is likely the most prevalent in VSMCs. The primary structure of the human KIR2.1 channel contains a total of 13 Cys residues, most of which appear to be cytosolic, with two external Cys at positions 122 and 154 highly conserved among KIR channels. Cys122 and Cys154 in the KIR2.1 channel cause disulfide bond formation, which is required for correct folding of KIR2.1 (Cho et al., 2000; Leyland et al., 1999).
Activity and redox modulation
SCAM experiments have shown that Cys49 and Cys308 in KIR1.1, responsible for K+ secretion in the kidney, are susceptible to thiol oxidation by DTNB or MTSES, which results in irreversible channel closing (Schulte et al., 1998). Using a similar approach, Cys54 and Cys76 in KIR2.1 are modified by MTSET and it was proposed that these Cys residues could participate in the formation of an inner vestibule facing the channel central pore (Lu et al., 1999). Substitution of Cys76 by a polar residue (Ser) reduced channel activity and the open probability (Garneau et al., 2003). Mutation of the C-terminal Cys311 to Ser311 also decreased channel activity (Garneau et al., 2003). Interestingly NO activates cardiac KIR2.1 at Cys76 via S-nitrosylation (Gomez et al., 2009). Cys311 is important in drug-induced KIR2.1 channel opening (Gomez et al., 2014). Bychkov and colleagues have demonstrated a differential effect of H2O2 on endothelial K+ channel activation. Low concentrations of H2O2 (0.01–0.25 µM) inhibited KIR channel current resulting in EC depolarization, whereas high concentrations of H2O2 (>500 µM) activated the KCa current resulting in hyperpolarization (Bharadwaj and Prasad, 1995; Bychkov et al., 1999). The heteromeric KIR4.1/KIR5.1 channel is expressed in the kidney, brain stem, and retina. Exposure to H2O2 or diamide resulted in channel inactivation, whereas Cys158 in the KIR5.1 channel was S-glutathionylated by oxidized glutathione (GSSG) and inhibited the channel (Jin et al., 2012). The authors postulate that the GSH moiety occupies the ion-conducting pathway leading to channel inhibition. In a classical experiment, Bannister et al. used the Cys reactive agent, PCMBS, thimerosal, and H2O2 to inhibit Kir2.3 channel and compared the results with that of Kir1.1 channel (Fig. 6). They also made mutation of Cys to serine by site-directed mutagenesis (Bannister et al., 1999). Wild-type Kir 2.3 currents were significantly inhibited by PCMBS, thimerosal, and H2O2. Currents for mutant Kir2.3 C79S and C140S were also inhibited by PCMBS, thimerasol, and H2O2. These mutations affected the time-course of inhibition by all three agents (Bannister et al., 1999). The reducing agent DTT reversed the inhibition by both PCMBS and thimerosal of wild-type and mutant currents, but not the inhibition by H2O2. Thus, these results showed that inhibition of Kir2.3 channel is mediated by C79, a slow component, and by C140, a faster component, both residues are externally exposed (Bannister et al., 1999). Studies by Garneau et al. demonstrated that substitution of N-terminal Cys C76 or the C-terminal Cys C311 by polar residues strongly modifies the channels’ intrinsic kinetic properties with the mutation position 311, introducing long-lasting closed time intervals through a destabilization of the channel PIP2-Kir2.1 interaction (Garneau et al., 2003). Kir 2.1 model predicts that seven of the 13 Cys residues of the channel are distributed along the N- and C-terminus region with some of the residues comprised within highly conserved domains involved in channel gating (Garneau et al., 2003). Thus, these Cys contribute to the gating properties of the Kir2.1 channel (Garneau et al., 2003).
FIG. 6.
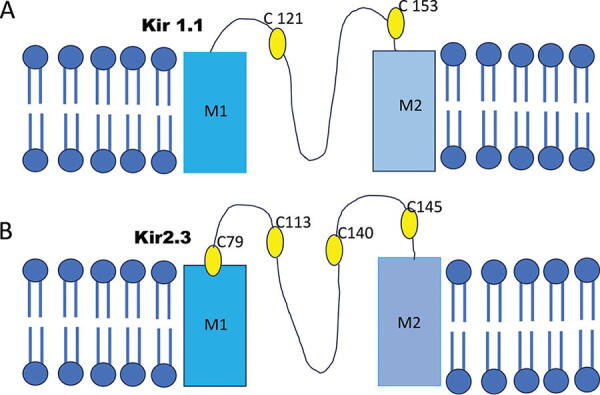
External cysteines in Kir1.1 and Kir2.3 channels modulate channels intrinsic kinetic properties and also gating properties. Wild-type Kir 2.3 currents were significantly inhibited by PCMBS, thimerosal, and H2O2. Currents for mutant Kir2.3 C79S and C140S were also inhibited by PCMBS, thimerasol, and H2O2. These mutations affected the time-course of inhibition by all three agents. The reducing agent DTT reversed the inhibition by both PCMBS and thimerosal of wild-type and mutant currents. These cysteines also contribute to the gating properties of the Kir2.1 channel. H2O2, hydrogen peroxide; DTT, dithithreitol; PCMBS, Parachoromercuribenzene sulphonate.
Previous studies have shown that KATP channels are inhibited by oxidative stress resulting in impairment in vasorelaxation (Armstead, 1999; Miura et al., 2003). However, ONOO− hyperpolarizes and results in relaxation of the rabbit internal carotid artery through activation of KATP channels (Ohashi et al., 2005). Sulfhydryl oxidizing agents such as diamide, GSSG, and micromolar concentrations of H2O2 have been shown to inhibit the KIR6.2/SUR2B channel (Yang et al., 2010). Oxidant screening of Kir6.1-Kir6.2 chimeras demonstrated that the N terminus and transmembrane domains of Kir6.1 were crucial (Yang et al., 2011). Systematic mutational analysis revealed three Cys residues in these domains: Cys43, Cys120, and Cys176 (Yang et al., 2011). This oxidant-mediated channel inhibition was rescued by DTT and the glutaredoxin, suggesting S-glutathionylation. In contrast to Cys176, Cys43 had only a modest contribution to S-glutathionylation, and Cys120 was modulated by extracellular oxidants but not intracellular GSSG. Using systematic Cys residue mutation and simulation modeling, the authors demonstrated that after binding to Cys176, the GSH moiety occupies a space between the slide helix and the transmembrane helices (Yang et al., 2011). Functional gating of the KATP channel requires a conformational change of these transmembrane helices for channel opening, which is hindered by the GSH moiety retaining the KIR6.1 channel in a closed state (Yang et al., 2011). Mutation of Cys166 of KIR6.2 situated at the cytosolic end of S2, increases the open probability consistent with the idea that Cys166 plays a role in the intrinsic gating of the channel, possibly by influencing a gate located at the intracellular end of the pore (Trapp et al., 1998). These reports evidently show that oxidative stress results in Cys sulfhydryl oxidation and KATP channel inhibition, which can lead to impairment of vasorelaxation in a variety of vascular complications. H2S causes Cys S-sulfhydration (Mustafa et al., 2009) and subsequent activation of KIR6.1/SUR2B channels in piglet cerebral arterioles (Jiang et al., 2010). Trx has recently been shown to cleave Cys S-sulfhydration resulting in H2S release (Mikami et al., 2011; Wedmann et al., 2016). Based on these reports, it is likely that Trx could play a critical role in channel activation or inhibition depending upon the Cys residue oxidation state and the accessibility of the Cys residues for reduction by Trx. However, the role of Trx is largely unexplored in these channel functions. We have recently shown that Trx deglutathionylates glutathionylated eNOS (SG-eNOS) (Subramani et al., 2021). Based on this study, Trx could also deglutathionylate K+ channel Cys glutathionylated in oxidative stress conditions, thereby increasing or decreasing channel function. The role of Trx is largely unexplored in the K+ channel function.
Large-conductance KCa channels
Nomenclature and function
Large-conductance Ca2+-activated K+ channels (BKCa), also known as Slo or Maxi-K channels, play important roles in oxygen sensing, VSMC relaxation, synaptic transmission, and hormone secretion (Hou et al., 2009; Nelson and Quayle, 1995). BKCa mediate the voltage-dependent and Ca2+-dependent K+ permeability involved in VSMC K+ efflux and hyperpolarization. One BKCa channel complex contains four pore-forming α subunits (BKα, KCNMA1, Slowpoke, Slo or Slo1) and up to four regulatory β subunits (BKβ, KCNMB1-4) (Brenner et al., 2000a; Knaus et al., 1994; Toro et al., 1998; Wang et al., 2002). Each α subunit includes a seven-helix transmembrane segment and a voltage-sensing domain (S1–S4) and contributes one-fourth of the ion conduction pore (S5–S6)(Adelman et al., 1992). β subunits consisting of 2 transmembrane units are involved in voltage sensitivity, BKCa channel inhibition via the “ball peptide” mechanism (see the Voltage-Dependent K+ Channels section) (Bentrop et al., 2001; Xia et al., 2003), and pharmacological inhibition by peptide blockers such as charybdotoxin and iberiotoxin (Meera et al., 2000). At the C-terminal end of the BKCa channel (past S6), there is a large cytoplasmic region, which contains two RCK domains (regulator of K+ conductance RCK1 and RCK2) essential for Ca2+ activation of the channel (Xia et al., 2002). RCK1 also serves as an H+ sensor (Hou et al., 2008). A segment linking both RCKs has been implicated in heme binding (Horrigan et al., 2005; Tang et al., 2003). A genetic disruption of the BK β1 subunit impairs vascular relaxation and causes chronic hypertension by inhibiting the Ca2+-dependent activation of BKCa channels (Brenner et al., 2000b; Pluger et al., 2000). During Ang II-induced hypertension, expression of the β1 subunit of the BKCa protein is downregulated in rat cerebral arteries (Amberg and Santana, 2003). The expression of the β1 subunit of BKCa is downregulated in cerebral artery VSMCs from diabetic rats resulting in decreased BKCa activity and hypertension (Wang et al., 2010). These observations highlight the importance of vascular BKCa channels in regulating vasomotor tone and blood pressure.
Activity and redox modulation
Physiologically, BKCa channels are activated, other than membrane potential (Vm) and Ca2+, by cGMP-dependent phosphorylation (Carrier et al., 1997), H2S (Jackson-Weaver et al., 2011), and RONS (Wang et al., 1997b). In the carotid body, the BKCa channel is sensitive to O2 concentration (Lewis et al., 2002; Liu et al., 1999). As described above, the BKCa channel contains a heme binding domain. Heme binding strongly inhibits the channel under hypoxic conditions, whereas under normoxic conditions, carbon monoxide (CO), which is generated by heme oxygenase-2 (HO-2) during heme degradation, activates the BKCa channel (Jaggar et al., 2005). This heme binding domain contains a Cys612-X-X-Cys615-His motif that serves as the heme ligand provided by His616. This dithiol binds to HO-2 with 14-fold more tightly than the oxidized disulfide state (Yi et al., 2010; Yi et al., 2009). Under hypoxic (reduced redox potential) conditions, the BKCa channel converts to the dithiol state having high affinity for Fe3+-heme, which inhibits BKCa channel leading to pulmonary vasoconstriction. Under normoxia, the situation is reversed: the BKCa channel is in its oxidized disulfide state having a high affinity for CO, which activates BKCa activity. In addition, Barnes et al. (2018) have shown that mice with deletion of Kcnmb1 gene (Kcnmb1−/−) that encodes for β1 subunit of BKCa channel showed increased right ventricular systolic pressure compared with Kcnmb1+/+ mice when exposed to chronic or acute hypoxia. β1 subunit expression was predominantly confined to pulmonary artery smooth muscle cells (PASMCs). Compromised PASMC β1 function may contribute to heightened microvascular vasoconstriction that characterizes pulmonary hypertension.
The mechanism of oxidative stress-induced BKCa channel modulation is complex and many studies have observed conflicting outcomes whether oxidation increases or decreases BKCa channel activity (for an extensive review, see Hermann et al., 2015). The inconsistency might arise from different experimental approaches (e.g., isolated cells using patch clamp versus intact precontracted vessels, intra-versus extracellular exposure, and concentration of oxidative agents applied), species/cell differences, or multiple Cys modulation sites having different gating effects (Sandow and Grayson, 2009). Numerous studies have observed BKCa channel activation by exogenously applied H2O2 (Barlow et al., 2000; Barlow and White, 1998; Bychkov et al., 1999; Fraile et al., 1994; Hayabuchi et al., 1998; Iida and Katusic, 2000; Matoba et al., 2002; Matoba et al., 2000; Thengchaisri and Kuo, 2003; Wei et al., 1996; Yang et al., 1998). Arachidonic acid (AA) triggers endogenous H2O2 release that activates BKCa channel causing in vivo cerebral arteriolar vasodilatation (Paravicini et al., 2004; Sobey et al., 1997; Sobey et al., 1998). Barlow and colleagues have shown that H2O2 relaxes coronary arteries by BKCa channel activation via the phospholipase A2/AA signaling cascade (Barlow et al., 2000). Furthermore, overexpression of glutathione peroxidase-1 and knockout of SOD1 blunted AA-induced cerebral arteriolar dilations (Modrick et al., 2009), indicating that endogenously produced H2O2 also activates BKCa channels. H2O2 activates soluble guanylate cyclase resulting in guanosine 3′−5′-cyclic monophosphate (cGMP) release in the pulmonary vasculature (Burke-Wolin et al., 1991). Protein kinase G (PKG) is activated by cGMP, and protein kinase G alpha (PKG1α) is an important regulator of various K+ channels, including BKCa channel (Schubert and Nelson, 2001). Opening of BKCs channel requires a signaling involving H2O2-mediated dimerization and translocation of PKG1α (Zhang et al., 2012). Zhang and colleagues provided compelling evidence that H2O2 relaxes human coronary arteries via PKG1α Cys oxidation and opening of BKCa channels (Zhang et al., 2012). In addition, PKG1α induces a decrease in VSMC Ca2+ and inhibits Rho kinase activity resulting in increased myosin light chain phosphatase activity via binding to its myosin phosphatase target subunit 1 (MYPT1) hereby reducing myosin actin crossbridge cycling and subsequent relaxation (Hofmann et al., 2000). It is now evident that PKG1α is redox sensitive at Cys42 because Cys thiol oxidation causes a disulfide bond formation between two adjacent Cys42 residues of PKG creating a catalytically active dimer (Burgoyne et al., 2007). This homodimer complex is independent of cGMP and has a higher affinity for substrate. cGMP binding to PKG1α results in an allosteric conformation that reorientates redox Cys residues and prevents or attenuates dimerization (Busch et al., 2002; Schnell et al., 2005). The importance of this Cys42 thiol oxidation in PKG has been confirmed by a single-atom mutation (oxygen instead of sulfur) in Cys42. Generation of a Cys42Ser PKG1α knockin mouse eliminated oxidant sensing (Prysyazhna et al., 2012). Hence, autoregulation of cGMP and the resulting reorientation move the Cys42 too far apart rendering PKG1α relatively more resistant to oxidant-induced disulfide formation (Burgoyne et al., 2012). A recent study has also shown that the constitutive activation of PKG Iα proceeds through oxidation of Cys-117 (Sheehe et al., 2018).
In contrast to these vasorelaxing effects of H2O2-induced BKCa channel stimulation, studies show that the activity of BKCa can be inhibited by H2O2 (Brakemeier et al., 2003; DiChiara and Reinhart, 1997; Lu et al., 2006; Soto et al., 2002; Tang et al., 2001). Equally conflicting observations have been reported studying the exogenous effects of ONOO−, which has been either shown to inhibit vascular BKCa in cerebral and coronary VSMCs (Brzezinska et al., 2000; DeWitt et al., 2001; Elliott et al., 1998; Liu et al., 2002; Tang et al., 2004) or activate K+ channels (Li et al., 2004; Ohashi et al., 2005). Using patch-clamp techniques in Xenopus laevis oocytes expressing BKCa channels, exposure of H2O2 to the intracellular side resulted in channel inactivation, whereas extracellular application had no effect (Soto et al., 2002). This suggests an intracellular inhibition site. Extracellularly applied H2O2 causes Ca2+ leakage due to membrane damage, resulting in a nonspecific rise in intracellular Ca2+, which activates BKCa channels (Gupta et al., 1998; Whyte et al., 2009). Taken together, Cys thiol modifying agents such as DTNB and MTSEA inhibit the BKCa current (DiChiara and Reinhart, 1997; Soh et al., 2001; Soto et al., 2002; Tang et al., 2001; Wang et al., 1997b), suggesting that oxidative modification of Cys residues in the BKCa channel promotes inhibition of BKCa channel function. This has been confirmed by using a mutant BKCa protein, in which Cys911 of the human slo1 (BKα) subunit is changed into Ala911 (Tang et al., 2004). Cys911 is in the Ca2+ bowl mediating the Ca2+-sensitivity of the slo1 channel (Niu and Magleby, 2002; Xia et al., 2002). Cys911 is elegantly shown to mediate the oxidation sensitization of the hslo1 channel during physiological Ca2+ concentrations. Absence of a free thiol group at Cys911 disrupts the Ca2+ bowl function by interfering with the Ca2+-binding step and/or with coupling of the Ca2+ sensor and the channel gate. In addition to Cys911, modification of Cys430 in the cytoplasmic regulator of potassium conductance domain alters the Ca2+-dependent activation of Slo1 (Zhang and Horrigan, 2005; Zhang et al., 2006). Hyperglycemia results in enhanced endogenous H2O2 production and BKCa channel inhibition via Cys911 oxidation (Lu et al., 2006). This implies that endogenously produced H2O2 can inhibit BKCa channel activity via Cys thiol oxidation. However, predicting whether oxidation will result in BKCa channel stimulation or inhibition is difficult as evidenced by the above studies. For instance, methionine oxidation by chloramine T increases BKCa channel activity, whereas Cys oxidation decreases BKCa channel activity (Tang et al., 2001). Even in the same cell type, pyramidal neurons from the hippocampal CA1 region, opposing effects of oxidizing agents (GSSG and DTNB) have been observed on BKCa channel activation (Gao and Fung, 2002; Gong et al., 2000; Soh et al., 2001). Nonetheless, it can be concluded that BKCa channel activity is highly sensitive to Cys thiol modulation. Modulation of different Cys residues in the BKCa channel may result in pore opening or gate closing depending on the location of that Cys residue. Recently, Hollywood et al. have shown that leucine-rich repeat and Ig domain containing 2 (LINGO2) is a regulator of BK channels, since its coexpression with BK channels yields rapid inactivating currents, the activation of which is shifted approximately −30 mV compared with that of BKα currents. Furthermore, they show that oxidation of BK: LINGO2 currents (by exposure to epifluorescence illumination or chloramine T) abolished inactivation. The effect of illumination was dependent on the presence of green fluorescent protein, suggesting that it released free radicals, which could have oxidized Cys or methionine residues. In addition, the oxidation effects were resistant to treatment with the Cys-specific reducing agent DTT, suggesting that methionine rather than Cys residues may be involved (Dudem et al., 2023). Therefore, we speculate that Trx may alter the redox state of LINGO2 or BK due to its reducing activity and could play a significant role in Cys disulfide reduction in these Cys residues present on BKCa channels or other regulators for these channels. Although the role of biological reductant such as Trx has not been studied in altered BKCa, we hypothesize a significant role of Trx in the redox control of BKCa. Although Trx can reduce oxidized Cys in general, accessibility of oxidized bonds is a critical issue for reduction by Trx, as it is a 12kD protein and needs to have physical contact with the oxidized protein to be able to exchange electrons. It cannot reduce buried Cys disulfides in the folded proteins.
Heterogeneity in vasorelaxation between conduit and resistance arteries
Based on the above differential effects of Cys modulation on KCa channels, it is not surprising that not all arteries function similarly in response to vasoactive compounds. For example, heterogeneity in the relaxation of different sized porcine coronary arteries to nitro-vasodilators has been observed. NO and nitro-vasodilators cause vasodilatation via phosphorylation of PKG- and cGMP-mediated BKCa activation (Alioua et al., 1998; Carrier et al., 1997; Fukao et al., 1999; Hayabuchi et al., 1998; Stockand and Sansom, 1996; Zhou et al., 2001). Large coronary arteries are more sensitive to Nitric Oxide-cyclic guanosine monophosphate (NO-cGMP) signaling than smaller coronary arteries, due to higher PKG and MYPT1 expressions in larger coronary vessels (Ying et al., 2012). As described above, cGMP-mediated PKG1α stimulation prevents oxidant-induced Cys42 disulfide formation within PKG1α rendering large arteries less sensitive to H2O2. Furthermore, larger vessels have higher peroxiredoxin (Prx) levels compared with smaller vessels, which could decrease the levels of H2O2 in contrast to smaller vessels. Therefore, it is likely that smaller vessels are more sensitive to H2O2-induced Cys disulfide bridge formation in PKG1α and the subsequent H2O2-induced vasorelaxation (Burgoyne et al., 2012; Prysyazhna et al., 2012). Santiago and colleagues confirmed that the larger proximal coronary arteries resulted in contractions in response to H2O2, whereas the smaller distal coronary arteries resulting in concentration-dependent relaxations (Santiago et al., 2013).
An important factor that modulates the outcome of vasoactive agents on vascular reactivity is the level of depolarization or Vm before relaxation. Under quiescent and noncontracted conditions, exogenous application of H2O2 constricts mouse abdominal aorta more than the thoracic aorta or carotid arteries but does not constrict the mesenteric artery (Ardanaz and Pagano, 2006). Raising Vm with 30 mM K+, enhanced contractile responses to H2O2 and unmasks a contractile response to H2O2 in the mesenteric artery (Santiago et al., 2013). This suggests that depolarization blocks an underlying hyperpolarization in smaller arteries. In smaller resistance arteries, the role of EDH is more important (Hilgers et al., 2006; Shimokawa et al., 1996) and the density of endothelial KCa3.1 and KCa2.3 channels and myo-endothelial gap junctions initiating and propagating the EDH (See Section Endothelial KCa channels) is more prominent compared with larger arteries (Hilgers et al., 2006; Sandow et al., 2009). H2O2 has been considered a contributor to EDH-mediated relaxations of coronary and small mesenteric arteries from mice, dogs, and humans (Matoba and Shimokawa, 2003; Matoba et al., 2003; Matoba et al., 2002; Matoba et al., 2000; Miura et al., 2003; Morikawa et al., 2003; Pomposiello et al., 1999; Rubanyi and Vanhoutte, 1986; Shimokawa and Morikawa, 2005; Yada et al., 2003). In most of these studies, oxidative stress is not enhanced and H2O2 is produced by eNOS and functions as a compensatory endothelium-derived relaxing factor (Fujiki et al., 2005; Kang et al., 2007; Matoba et al., 2000). These observations provide a mechanistic basis for the concept that large conduit artery vasodilation is mediated via NO, whereas EDH predominates in resistance vessels (Burgoyne et al., 2012). Intriguingly, in heterozygous GPx1+/− mice, metacholine-mediated dilatations are impaired in microvascular mesenteric arteries (Forgione et al., 2002). In homozygous GPx1−/− mice, responses to ACh were impaired in isolated carotid arteries and Gpx1 protects against Ang II-induced endothelial dysfunction, suggesting that elevated levels of H2O2 cause endothelial dysfunction (Chrissobolis et al., 2008). Apparently, a delicate threshold and a fine balance of intracellular H2O2 exist; normal H2O2 levels promote endothelium-dependent relaxations, whereas higher levels inhibit endothelium-dependent relaxations. Although the threshold of H2O2 remains unclear and unknown, Prxs play a major role in regulating the baseline levels of H2O2 in these vessels. Others dismiss a role for H2O2 in EDH, which may result from intra-artery differences or perhaps the source of catalase tested to scavenge H2O2 (Beny and von der Weid, 1991; Chaytor et al., 2003; Ellis et al., 2003; Hamilton et al., 2001). Intriguingly, we observed blunted H2O2-mediated relaxations in small mesenteric arteries from mice overexpressing human Trx (Trx-Tg) (Hilgers and Das, 2015).
Voltage-dependent K+ channels
Nomenclature and function
In resistance arteries, VSMC Vm is an important regulator of vasomotor tone. Voltage-dependent K+ channels (Neckář et al., 2019) are sensitive to changes in Vm since depolarization increases and hyperpolarization decreases KV channel activity. Vm is determined by the membrane permeability to K+, Ca2+, Na+, and Cl−, with K+ playing the most important role in regulating VSMC Vm. KV channels are also known as delayed rectifier or transient outward currents and are expressed throughout the vascular tree (Bonnet et al., 1991; Gelband and Hume, 1992; Okabe et al., 1987; Smirnov and Aaronson, 1992; Volk and Shibata, 1993). Following the discovery of Kv 1.3K+ channel in human T cells, several high-affinity inhibitors were developed to block Kv1.3 to inhibit autoimmune diseases (Varga et al., 2021).
Structure
The KV channel has a characteristic pore-forming unit composed of six transmembrane segments. The KV channel is assembled by four of these units. The pore region is formed by S5 and S6 and the voltage-sensor domain by S1 to S4. The S4 region of the KV channel containing positively charged residues has been implicated as the voltage sensor (Sigworth, 1994). Functional KV channels assemble as tetramers of pore-forming α subunits (Pongs et al., 1988).
Activity and redox modulation
Closure of the pore-forming α subunit depends on occlusion of a “ball like” peptide structure located in the amino terminus of the α subunit into the pore-forming cavity of the α subunit (Hoshi et al., 1990). A soluble peptide containing this ball sequence inhibited Shaker K+ channel in a concentration-dependent manner (Zagotta et al., 1990). This inactivation mechanism is redox-dependent such that Cys thiol oxidation causes a disulfide bridge formation between a critical Cys residue (Cys13) in the α subunit and the Cys residue of the ball peptide (Ruppersberg et al., 1991). Loss of this fast-acting inactivation is reversed by the reducing agents GSH and DTT (Ruppersberg et al., 1991). Diamide activates neuronal KV4 channels expressed in Xenopus laevis oocytes via Cys13 S-glutathionylation (Jerng and Pfaffinger, 2014). Apparently, the addition of glutathione to Cys13 results in a marked slowing of N-type inactivation and a concomitant increase in peak current, presumably via interfering with this ball-like inhibition. Also, in the mesenteric artery, exogenous application of H2O2 results in KV channel activation via Cys S-glutathionylation, although it was not discerned whether the site of S-glutathionylation occurred at Cys13 (Park et al., 2015). In addition, myocytes isolated from infarcted heart showed increased mRNA and protein expression of Kv4.2 and Kv4.3 channel alpha subunits. Pyruvate-induced increase in Kv4.x expression was blocked by auranofin, and inhibitor of Trx reductase. These data suggested that Kv4.x channel expression is redox regulated by the Trx system (Li et al., 2008b). In A-type KV channel subtypes expressed in neurons, a regulatory β subunit possesses a similar “ball-like” structure in the amino terminus, and its inactivation is dependent on a disulfide bridge formation between Cys residues (Heinemann et al., 1995; Heinemann et al., 1994; Wang et al., 1996). Indeed, SCAM analysis revealed important Cys residues in the S6 region involved in the pore gating mechanism, confirming an important modulatory role for Cys residues in KV channel gating (Liu et al., 1997). This mechanism of inactivation via an oxidoreductase activity of KV channels may be an important biological oxidative stress sensor allowing KV channels to switch rapidly between a closed and open state (Bahring et al., 2001a; Bahring et al., 2001b). Cys thiol oxidation by H2O2 and DTNP has been shown to suppress the KV current in mouse colonic SMCs (Prasad and Goyal, 2004), but to stabilize the open state of KV1.4 in Xenopus laevis oocytes (Stephens et al., 1996). The redox environment in the cell is more reduced compared with the more oxidized cell-free patch-clamp recording configuration (Schlief et al., 1996; Strupp et al., 1992). This may be a plausible explanation for many differential effects observed studying thiol oxidative agents and K+ channel activity. In the coronary circulation, H2O2 activates KV channels resulting in relaxations, whereas DTT can reverse this H2O2-mediated coronary vasodilation (Myers et al., 1996). It is believed that Cys thiol modification is a redox sensor in the control of coronary blood flow (Myers et al., 1996). In KV2.1 channels, all 15 Cys residues are located on the intracellular side, hence Cys modulation takes place at the cytosolic side, which means that H2O2 needs to cross the cell membrane when applied exogenously. This may be an explanation why such high concentrations of H2O2 are needed to promote Kv channel activation since H2O2 is rapidly degraded by cellular Prxs (Rhee et al., 2001; Wood et al., 2003). KV channels are oxygen-sensitive and play a major role in the pulmonary circulation where oxygen levels are high and cellular redox is in a more oxidized state. Kv 1.3K+ channel is a primary target of autoimmune disease (Panyi et al., 2004). Upon antigen presentation, activation of T cells requires Ca+ signal, which requires Ca+ entry to the cytosol through calcium release-activated Ca2+ channel (CRAC). Once CRACs are open, the level of Ca2+ signal is determined by an electrochemical driving force for Ca2+ entry (Feske et al., 2015). The driving force for Ca2+ is determined by an interplay between the Ca2+ influx via Kv1.3 and the intermediate conductance calcium-activated KCa3.1K+ channel (Cahalan and Chandy, 2009; Feske et al., 2015).
Trx system in the pulmonary circulation
Redox sensing in the pulmonary artery during normoxia
The pulmonary circulation is a low-pressure and highly compliant vasculature, quite different from the systemic circulation. The lungs are very sensitive to changes in O2 tension. In the developing fetus, the pulmonary arteries are exposed to hypoxia and constrict, while the ductus arteriosus (DA) is dilated and acts as a right-to-left shunt pathway to divert blood directly into the systemic circulation (Heymann and Rudolph, 1975). Immediately after birth, O2 induces Trx, TrxR, and Prx expression in the lungs of newborn primates, implying an important role for the Trx system in lung maturation and possibly pulmonary artery function (Das et al., 2001; Das et al., 1999). In response to the dramatic change in O2 tension, opposite effects on the pulmonary artery and DA occur, namely pulmonary artery vasorelaxation and closure of the DA (Michelakis et al., 2002; Olschewski et al., 2004). In contrast, systemic arteries, such as the renal, coronary, and mesenteric arteries, have opposing effects to the DA, namely a vasorelaxation upon K+ channel oxidation. What mediates this abrupt change in pulmonary vasoconstriction into vasodilation at birth? The O2 sensor and effector molecules mediating pulmonary artery contraction are resided in the mitochondria and PASMCs. During normoxia, the PASMCs convert glucose into ATP via the glycolysis in the mitochondria. NADPH donates electrons to O2 and Nox (complexes I and III) generate O2•– via the electron transfer chain (Chandel and Schumacker, 2000). Mitochondrial SOD converts O2•– into H2O2, which diffuses across the mitochondrial cell membrane into the cytosol. Here H2O2 causes PKG1α dimerization and thiol oxidation of KV and BKCa channels (Resnik et al., 2006; Zhang et al., 2012). However, it remains unknown whether upon birth, these dimerizations or oxidations occur, although in a uterine hypoxic environment to normoxic environment is an oxidative environment (3% pO2 vs. 21% pO2).
Redox sensing in the pulmonary artery during hypoxia
Pulmonary artery hypoxia has the opposing effects as described above. O2 deficiency shuts down mitochondrial electron transfer chain and oxidative phosphorylation. Hence, H2O2 production is decreased. There is still extensive debate whether an increase or decrease in ROS production occurs during hypoxia (Veit et al., 2015; Weir et al., 2005). It is possible that Nox enzymes in PASMC Noxs are still capable of generating ROS. Glycolysis is shifted toward the pentose phosphate pathway, which increases glucose transport via the glucose transporter and converts glucose into glucose-6-phospate (G6P). The enzyme G6P dehydrogenase converts G6P at the expense of NADP+ and the formation of NADPH. K+ channels are redox sensitive in that NAD(P)H inhibits BKCa and KV channel opening, whereas NAD(P)+ activates these channels (Lee et al., 1994; Park et al., 1995). For a more extensive review of the role of ion channels in hypoxia-induced RONS production in the pulmonary artery, the reader is referred to Veit and colleagues (Veit et al., 2015). Here we focus on Cys thiol redox modulation of K+ channels in the pulmonary vasculature. The molecular switch of KV channel gating is Cys thiol/disulfide redox modulation causing conformational changes in the gating machinery (Park et al., 1995; Reeve et al., 1995; Yuan et al., 1994). The Cys thiol oxidizer diamide is a potent inhibitor of pulmonary arterial constriction in response to hypoxia and prostaglandin F2α in intact dogs (Schach et al., 2007; Weir et al., 1983).
While the Trx system has been extensively investigated in lung diseases and epithelial cells (Das, 2004; Tipple, 2014), its role in modulating ion channel gating in the pulmonary vasculature is underexplored (Olschewski and Weir, 2015). Since mitochondrial Noxs utilize NADPH, and TrxR uses NADPH to reduce oxidized TrxR into reduced TrxR, the Trx system is expected to play an important role in controlling pulmonary vascular regulation. Interestingly, Burgoyne and colleagues observed that inhibition of TrxR with auranofin increased PKG1α dimerization in human embryonic kidney cells (Burgoyne et al., 2007). Treatment of bovine pulmonary arteries with Trx siRNA increased PKG1α dimerization during hypoxia (Neo et al., 2013). These data suggest that loss of Trx or oxidation of Trx promotes PKG1α disulfide formation. However, more conclusive studies are needed to establish these reasonings.
In the pulmonary artery, voltage-independent transient receptor potential cation (TRPC) channels are widely expressed. Especially the TRPC6 channel is highly expressed in PASMCs (Dietrich et al., 2005). Hypoxia triggers diacylglycerol (DAG) (Tang et al.)-induced TRPC6 channel opening leading to Na+ and Ca2+ influx, which inhibits KV channels and stimulates pulmonary contraction (Hofmann et al., 1999). Mice deficient in TRPC6 show no acute pulmonary vasoconstriction in response to hypoxia (Weissmann et al., 2006). The TRPC6 channel is inhibited by the NO/cGMP/PKG pathway (Takahashi et al., 2008). Cys thiol oxidation by N-ethylmaleimide or H2O2 activates the TRPC6 channel and sensitizes it even more to DAG. Cys sulfhydryl reducing agents such as DTT and GSH decrease the activity of TRPC6 (Graham et al., 2010). It is likely that Trx could regulate the activity of TRPC6 channel by modulating redox-active Cys of TRPC6 channel during hypoxic pulmonary vasoconstriction, where it may regulate the Cys disulfide–thiol exchange. Xu and colleagues have shown that Trx acts by breaking a disulfide bridge in the predicted extracellular loop adjacent to the ion-selectivity filter of TRPC5 expressed in human fibroblast-like synoviocytes, thereby opening the channel (Xu et al., 2008). The double-mutant C553A/C558A in TRPC5 was unresponsive to DTT. This modulation of Trx is different from the TRPC6 modulation where disulfide reduction favors channel closing.
Redox regulation of K+ channel and role of Trx in the heart
Cardiac K+ channels play an important role in repolarization of the action potential during a heartbeat. In fact, many channel-mediated currents are involved and are reviewed elsewhere (Li and Dong, 2010; Tamargo et al., 2004). All cardiac K+ channels are affected by RONS, most of them experience decreased repolarization currents (Matsuura and Shattock, 1991). Abnormal K+ channel activity has been linked to electrical remodeling, resulting in patients with heart failure (Armoundas et al., 2001; Rozanski and Xu, 2002a; Rozanski and Xu, 2002b). In ventricular myocytes, it has been shown that the inhibition of K+ currents is sufficient to delay repolarization, causing prolongation of the action potential duration, and thus affects myocyte contraction and may lead to cardiac arrhythmias (Pallandi et al., 1987; Tarr et al., 1994). Redox control of these cardiac K+ channels by ROS has been extensively reviewed by Aggarwal and Makielski (Aggarwal and Makielski, 2013). Many cardiac ion channels, such as L-type voltage-operated Ca2+ channels (L-VOCC) (Campbell et al., 1996; Chiamvimonvat et al., 1995; Lacampagne et al., 1995; Sims and Harvey, 2004), KIR (Gomez et al., 2014), KATP (Coetzee et al., 1995; Ichinari et al., 1996; Yan et al., 2009), and KV (Hool, 2004; Kolbe et al., 2010; Li et al., 2006b; Rozanski and Xu, 2002b) involved in myocyte contraction, apoptosis, and inflammation, are sensitive to thiol/disulfide protein modification by exogenous oxidoreductases. The abovementioned studies mainly use chemical sulfhydryl reducing or oxidizing agents, such DTT, the oxidizers DTNB and thymidine-5′-diphosphate (DTDP), or 1,3-bis-(2-chloroethyl)−1-nitrosourea (BCNU), to modify Cys thiol/disulfides on K+ channels. Atrial fibrillation (AF) is the most common cardiac arrhythmia where ultrarapid delayed rectifier current (Ikur) is the predominant repolarizing K+ current in the atria (Svoboda et al., 2012). This current is encoded by Kv 1.5 channel subunit, which in the human heart is selectively expressed in the atria and is considered an important therapeutic target for treatment of AF (Ford and Milnes, 2008; Svoboda et al., 2012; Wang et al., 1993). This Kv 1.5 channel is an important target for the treatment of AF. Multiple K+ channels, including Kv1.5 encoded Ikur, are regulated by oxidizing agents (Svoboda et al., 2012). Redox-sensitive posttranslational modifications of Kv 1.5 are a major mechanism of regulation of this channel (Poole et al., 2004; Svoboda et al., 2012). Reversible oxidative modification of Cys residue to Cys-sulfenic acid (Cys-SOH) is an important mechanism of protein function in response to modulation of cellular redox state (Poole et al., 2004). Kv 1.5 has 6 intracellular Cys within the NH2 and COOH termini (Svoboda et al., 2012). Svoboda et al. have shown a global increase in sulfenic acid-modified proteins in the atria of human patients with chronic AF (Svoboda et al., 2012). They have also shown that Kv 1.5 is a substrate for sulfenic acid modification, which functions as a switch for turning the channel from a recycling mode to a degradation pathway in myocytes. Furthermore, they showed that sulfenic acid modification of COOH terminal C581 alone is sufficient to trigger internalization and degradation of Kv1.5 (Svoboda et al., 2012).
Human Trx can be taken up by cardiomyocytes (Tao et al., 2004) and injection of recombinant human Trx in rats before subjection to myocardial ischemia/reperfusion injury reduced myocardial infarct size, myocyte apoptosis, and inflammation (Wu et al., 2008). We recently reported that vascular Trx overexpression protected against Ischemia-Reperfusion (I/R)-induced myocardial infarction and impairment in coronary artery relaxation via deglutathionylation of eNOS (Subramani et al., 2016). Trx interacts with apoptosis signal-regulated kinase-1 (ASK-1), a mitogen-activated protein kinase kinase kinase. Trx-(SH)2 binds to ASK-1 and inhibits its activity (Saitoh et al., 1998). Binding of ASK-1 prevents activation of c-Jun NH2-terminal kinase (JNK)/p38 MAPK-induced apoptosis pathway. Proapoptotic stimuli such as tumor necrosis factor-alpha and ROS can oxidize Trx-(SH)2 into Trx-S2 to dissociate Trx from ASK-1 (Liu and Min, 2002). JNK and p38 MAPKs are key regulators of cardiac hypertrophy and apoptosis via modulation of KV channels by Trx (Tang et al., 2011). During heart failure, decreased interaction of Trx with ASK-1 induces KV channel downregulation, which is the underlying mechanism of the decreased transient-outward K+ current (Ito) (Armoundas et al., 2001; Kaab et al., 1998; Liang et al., 2008). Downregulation of the Ito current is restored by Trx, which induces the expression of KV4.x channels (Li et al., 2008b). Furthermore, these studies suggest that the expression of Kv4.x is redox regulated by Trx although more work is needed to mechanistically establish the role of Trx at a molecular level. In diabetes, which is associated with elevated levels of Cys disulfide bridge formation in cellular proteins (Bidasee et al., 2003), Ito is reduced due to decreased TrxR activity (Li et al., 2005). K+ channels involved in Ito have one or more Cys sulfhydryl groups that directly control channel function (Rozanski and Xu, 2002b) and hence are susceptible to Trx redox modulation. Recently, H2S has been shown to target the Cys320/Cys529 motif in KV4.2 channels by breaking the disulfide bond to regulate the Ito current in cardiomyocytes (Ma et al., 2015).
KATP channels are highly expressed in the heart with the KIR6.2/SUR2A isoform being the predominant KATP channel type expressed in cardiac sarcolemma (Akrouh et al., 2009). As described above, ATP inactivates and hypoxia (↓ATP) activates IKATP current. Thus, KATP channels serve as metabolic sensors in the cell that play a major role in protecting the heart from ischemia/reperfusion-induced damage (Garlid et al., 2009). Not surprisingly, the KATP channel is modulated by Cys thiol modulators, since GSSG, p-chloromercuriphenyl sulfonate (pCMPS), DTNB, thimerosal, and H2O2 increase IKATP, which can be reversed by DTT and GSH (Coetzee et al., 1995; Ichinari et al., 1996; Yan et al., 2009).
Trx and K+ channel modulation in the central nervous system
Several neuronal ion channels and receptors are modulated by redox modulation that regulates excitability of neurons (Gamper et al., 2006). BK channels (Tang et al., 2004), heteromeric Iks-like K+ channels (Busch et al., 1995), ASIC channels (Andrey et al., 2005), and NMDA receptors (Aizenman et al., 1989) are inhibited by millimolar concentrations of oxidizing agents and GIRK K+ channels are activated by reducing agents (Zeidner et al., 2001). Effects of redox on the kinetics of several Kv channels and Human Ether-a-go-go-related Gene (HERG) channels have also been reported (Gamper et al., 2006). A major conceptual advance in this field was made by the demonstration of mechanism of neuroprotective silencing in hypoxia by showing that inwardly rectifying KATP channels assembled from the pore-forming Kir6.2 and auxiliary SUR subunits are augmented by endogenous H2O2 (Gamper et al., 2006). Another study has also shown a strong increase in M-channel activity by H2O2. Consistent with these, H2O2 was also shown to increase Kv7.2, 7.4, and 7.5 current (Gamper et al., 2006).
High levels of Trx and TrxR are present in neurons and their axons (Rozell et al., 1985). Trx mRNA has been observed in regions involved in neuroendocrine and/or cardiovascular control such as the paraventricular hypothalamic nucleus and the nucleus of the solitary tract (Lippoldt et al., 1995). Interestingly, Trx levels are higher in neurons compared with GSH levels (Patenaude et al., 2005). Furthermore, the Trx system has many neuroprotective effects in the brain where it plays a crucial role in combating increases in oxidative stress (Silva-Adaya et al., 2014). Another member of the Trx family is the macrophage migration inhibitory factor (MIF) (Bloom and Bennett, 1966). MIF is constitutively expressed in neurons within the brain (Bacher et al., 1998). The oxidoreductase activity of MIF is exerted by Cys57 and Cys60 (Kleemann et al., 1998), and one potential consequence of an increase in oxidoreductase activity is scavenging of ROS and blockade of oxidant-mediated intracellular actions (Nguyen et al., 2003; Sun et al., 2004). Acting via Ang II type 1 (AT1) receptors in hypothalamic paraventricular nucleus neurons, Ang II increases sympathetic activity and blood pressure (Ferguson et al., 2001; McKinley et al., 2003; Oparil et al., 1994). Increased rates of sympathetic nerve firing and reduced neuronal norepinephrine reuptake both contribute to sympathetic activation in hypertension (Schlaich et al., 2004). MIF intracellularly decreases basal neuronal firing and counteracts the AT1 receptor-mediated chronotropic effects of Ang II in hypothalamic neurons (Li et al., 2008a; Li et al., 2006a; Sun et al., 2004) via its oxidoreductase activity (Matsuura et al., 2006). This is an important observation with regard to the mechanism of neurogenic hypertension. Changes in neuronal outward K+ current contribute to changes in neuronal firing (Sun et al., 2005). Voltage-gated potassium (Neckář et al., 2019) channels, mediating this neuronal outward K+ current or delayed-rectifier KV currents (IKV), are widely expressed in the central and peripheral nervous system (Misonou et al., 2005). KV channels help generate action potentials (depolarization causes KV channel activation) as well as maintaining the resting Vm of neurons (hyperpolarization or repolarization closes the KV channel). Increased IKV currents inhibit the neuronal firing. Ang II has been shown to reduce the IKV current and to decrease the activity of a 4-AP-sensitive KV channel in neurons (Wang et al., 1997a). Intracellular application of MIF (80 nM) or Trx (0.8–80 nM) into rat hypothalamic/brain stem neurons in culture increased neuronal IKV, as measured by voltage-clamp recordings (Matsuura et al., 2007; Matsuura et al., 2006). It can be concluded from these studies that sulfhydryl modulation regulates basal and stimulated neuronal activity by increasing neuronal IKV within the hypothalamus and brain stem, since the mutant C32S/C35S Trx was ineffective (Matsuura et al., 2007).
Thiol oxidation of KV2.1 channels by DTDP induces an intracellular release of Zn2+ from metal-binding proteins, which is required for KV2.1 channel activation. This triggers a long-lasting enhancement of outward K+ current promoting neuronal apoptosis, contributing to widespread neuronal loss in neurodegenerative disorders such as Parkinson’s disease, Alzheimer’s disease, and stroke (Aizenman et al., 2000; McBean et al., 2015; McLaughlin et al., 2001; Shah and Aizenman, 2014; Yu et al., 1997). Neuronal apoptosis is dependent on ASK-1 (Saitoh et al., 1998). Trx is able to inhibit ASK-1 and halt the apoptotic current surge in neurons (Aras and Aizenman, 2005). However, whether Trx can reduce disulfides in Kv2.1 channels is unclear. The importance of Cys residues in KV channels has been well established (see the Voltage-Dependent K+ Channels section). Many KV channels contain two conserved Cys residues in putative transmembrane segments S2 and S6. It has been proposed that these Cys form an intrasubunit disulfide bond (Guy and Conti, 1990). Using a mutated KV2.1 channel or creating a Cys residue at 379 resulted in H2O2-induced disulfide bridge formation with the adjacent Cys394 locking the channel in a permanent nonconducting state (Zhang et al., 1996). The conserved Cys393 in S6 is positioned in a region of tight protein packing adjacent to the ion-conduction pathway (Zuhlke et al., 1994). This Cys residue participates in pore gating by stabilizing the open state (Liu and Joho, 1998). The sulfhydryl oxidizing agent thimerosal interacts with Cys214 in the KV7.1 or KCNQ1 channel resulting in a reduced current (Kerst et al., 2002). Neuronal Kv7 channels are important regulators of cell excitability, which are sensitive to ROS. The S2S3 linker of the voltage sensor was reported to be sensitive to redox regulation (Nunez et al., 2023). Cys residues in the S2S3 loop relieve Kv7 channel from constitutive inhibition by interaction between EF3 hand of CaM, which is crucial for redox regulation of Kv channel (Nunez et al., 2023). Another KV channel type, KV10.1, is widely expressed in the central nervous system. Interestingly, oxidative modification of KV10.1 channel is governed at the Cys pairs (Cys145/Cys214) situated at structural elements in the KV10.1 channel involved in channel gating (Sahoo et al., 2012). These observations suggest a potential modulatory role for Trx in reducing disulfide bridges in KV channels in the central nervous system.
Redox regulation of Kvβ channels
Kv pore-forming complex is composed of four distinct subunits (Kvα) that form the voltage-sensing, gating, and selectivity domains (Long et al., 2005; Matthies et al., 2018, Moran et al., 2015). Kvα pore proteins are linked to several types of cytoplasmic protein complexes, which regulate subcellular localization and gating kinetics (Long et al., 2005; Pongs and Schwarz, 2010). Kvβ proteins are associated with Kv1 and Kv4 families, which are aldo-keto reductases (AKRs) that catalyze the NADPH-dependent reduction carbonyl substrates to primary and secondary alcohols (Dwenger et al., 2022). Furthermore, Kvβ AKR activity has been shown to regulate channel trafficking and gating (Dwenger et al., 2022). In addition, it has been shown that Kvβ proteins catalyze reduction of carbonyl substrates via hydride transfer from NADPH (Mindnich and Penning, 2009). Thus, Kvβ proteins represent a molecular link between the cellular redox state and membrane potential regulation (Dwenger et al., 2022). Previous studies have suggested that Kvβ2 specifically uses NADPH as reducing equivalents for reduction (Tipparaju et al., 2008; Weng et al., 2006). However, the physiological carbonyl substrates for Kvβ and their impact on channel function have not been identified. Several in vitro studies show that purified Kv2 reduces a range of endogenous and exogenous carbonyls (Dwenger et al., 2022). Along with C-nitro compounds, Kvβ 2 readily reduces phenanthrenequinone, glycolytic by-product methylglyoxal, and oxidized phospholipids (1-palmitoyl-2-oxovaleroyl phosphatidylcholine and 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (PAPC) (Dwenger et al., 2022; Xie et al., 2011). The finding that Kvβ reduces the products of PAPC is consistent with the notion that Kvβ AKR activity may be responsible for membrane oxidative stress under physiological or pathological conditions (Dwenger et al., 2022).
K+ channels as therapeutic target
The discovery of the Kv1.3 voltage-gated K+ channel in human T cells in 1984 (Chandy et al., 1984), promoted the role of ion channels as important signal transducers in the immune system (Varga et al., 2021). Targeting the Kv1.3 potassium channel has been shown to decrease obesity and the severity of animal models of autoimmune disease. The voltage-gated Kv1.3 potassium channel has recently been targeted for obesity control, as mice lacking Kv1.3 or treated with a Kv1.3 blocker are protected from high-fat diet-induced obesity (Upadhyay et al., 2013; Xu et al., 2003). Kv1.3 is also a target for autoimmune diseases, as C-C chemokine 7 (CCR7)- effector memory T (Wedmann et al.) lymphocytes express high levels of Kv1.3 upon activation, and blocking of Kv1.3 inhibits their function (Chandy and Norton, 2017).
Cardiac K+ channels play an important role in maintaining the normal electrical activity of the heart by setting the cell resting membrane potential and by determining the shape and duration of the action potential (Walsh, 2015). Drugs that block the rapid (Steudel et al., 1999) and slow (Sahlin et al.) components of the delayed rectifier K+ current have been widely used as class III antiarrhythmic agents (Walsh, 2015). In addition, drugs that selectively target the ultrarapid delayed rectifier current (IKur) and the acetylcholine-gated inward rectifier current (IKAch) have shown efficacy in the treatment of patients with AF. To meet the future demand for new antiarrhythmic agents, novel approaches for cardiac K+ channel drug discovery will need to be developed. Furthermore, K+ channel screening assays utilizing primary and stem cell-derived cardiomyocytes will be essential for evaluating the cardiotoxicity of potential drug candidates. Small-conductance Ca2+-activated K+ (SK)-channel inhibitors have antiarrhythmic effects in animal models of AF, presenting a potential novel antiarrhythmic option. ISK is upregulated in patients with chronic AF due to enhanced channel function, mediated by phosphatase-2A-dependent CaM-Thr80 dephosphorylation and tachycardia-dependent enhanced trafficking and targeting of SK-channel subunits to the sarcolemma (Walsh, 2015). The observed AF-associated increases in ISK, which promote reentry-stabilizing action potential duration shortening, suggest an important role for SK-channels in AF auto-promotion and provide a rationale for pursuing the antiarrhythmic effects of SK-channel inhibition in humans (Walsh, 2015).
Dysfunctions of KCa2.3 and KCa3.1 that are associated with membrane hyperpolarization and EDH-mediated dilation are implicated in cardiovascular pathologies, including diabetes, atherosclerosis, inflammation, cancer, and fibrosis (Grgic et al., 2009; Kohler et al., 2016). In addition, KCa3.1 plays an important role in endothelial barrier dysfunction, edema formation in cardiac and pulmonary disease, and in stroke. Genome-wide association studies revealed an association of KCa2.3 channels with AF in humans. Accordingly, both channels are considered potential drug targets for cardiovascular diseases.
KATP channels are targets for antidiabetic sulfonylurea blockers, and for channel opening drugs that are used as antihypertensives (Rodrigo and Standen, 2005). In vascular smooth muscle, KATP channels are extensively regulated by signaling pathways and cause vasodilation, contributing both to resting blood flow and vasodilator-induced increases in flow (Rodrigo and Standen, 2005). Similarly, KATP channel activation relaxes smooth muscle of the bladder, gastrointestinal tract, and airways (Rodrigo and Standen, 2005). In cardiac muscle, sarcolemmal KATP channels open to protect cells under stress conditions such as ischemia or exercise, and appear central to the protection induced by ischemic preconditioning (IPC) (Rodrigo and Standen, 2005). Mitochondrial KATP channels are also strongly implicated in IPC (Rodrigo and Standen, 2005). Skeletal muscle KATP channels play roles in fatigue and recovery, K+ efflux, and glucose uptake. Current therapeutic considerations include the use of KATP openers to protect cardiac muscle (Rodrigo and Standen, 2005), attempts to develop openers selective for airway or bladder, and the question of whether block of extra-pancreatic KATP channels may cause adverse cardiovascular side effects of sulfonylureas (Rodrigo and Standen, 2005). Congenital hyperinsulinism (HI), is a beta cell disorder caused by inactivating mutations of beta cell KATP channels, results in dysregulated insulin secretion and persistent hypoglycemia (Juliana et al., 2023). Children with KATP-HI are unresponsive to diazoxide, the only FDA-approved drug for HI, and utility of octreotide (McMahon et al., 2017), the second-line therapy, is limited because of poor efficacy, desensitization, and somatostatin receptor type 2 (SST2)-mediated side effects. A recent study has shown that CRN02481, a highly selective nonpeptide SST5 agonist, significantly decreased basal and amino acid-stimulated insulin secretion in both Sur1−/− (a model for KATP-HI) and wild-type mouse islets. Oral administration of CRN02481 significantly increased fasting glucose and prevented fasting hypoglycemia compared with vehicle in an HI mouse model (Sur1−/− mice) (Juliana et al., 2023). During a glucose tolerance test, CRN02481 significantly increased glucose excursion in both WT and Sur1−/− mice compared with the control. CRN02481 also reduced glucose- and tolbutamide-stimulated insulin secretion from healthy, control human islets such as the effects observed with SS14 and peptide somatostatin analogs (Juliana et al., 2023). Moreover, CRN02481 significantly decreased glucose- and amino acid-stimulated insulin secretion in islets from two infants with KATP-HI and one with Beckwith–Wiedemann syndrome-HI (Juliana et al., 2023).
Selenium deficiency and disease
Selenium is a trace element and present in some natural foods and available as a dietary supplement. Selenium is in the active catalytic center of antioxidant enzymes such as TrxR and in glutathione peroxidase. These catalytic centers are responsible to regenerate thiols from Trx disulfide and neutralize various peroxides and function as antioxidants. However, clinical trials with selenium supplementation were not conclusive and provided contradictory results (Bleys et al., 2009; Hercberg et al., 2004). Serum concentrations decline with age (Akbaraly et al., 2007). Therefore, selenium deficiencies could promote age-related accumulation of oxidized products in the body and may cause accelerated aging and malfunction of antioxidant systems. Deficient selenium levels might be associated with age-related decline in brain function due to decrease in selenium’s antioxidant activities (Akbaraly et al., 2007). However, results of human observational studies are mixed (Loef et al., 2011). Taken together, more studies regarding the beneficial effects of supplemental selenium are needed. However, selenium is required to maintain some of the antioxidant enzyme function, but high levels of selenium may not be necessary as selenium in a minute quantity would perform its antioxidant function; therefore, selenium containing food should be adequate for enzymatic activity of antioxidants.
Future research directions
Almost all K+ channels contain reactive Cys in critical regions that regulate pore opening, closing, and regulating polarization and hyperpolarization, which not only impacts channel structure and function, but also allows for redox regulation of these channels in response to oxidative stress or drug-related activation/inhibition. Posttranslational Cys sulfhydryl modulations are physiologically reversible processes (such as phosphorylation/dephosphorylation) that are in perfect equilibrium in a healthy cellular environment. At advanced stages of oxidative stress where Trx, Grx, and Prx activities are oxidized to a certain extent, irreversible Cys thiol modifications could alter protein activities. Aging is an example of a progressive and chronic oxidative process where accumulation of oxidative proteins promotes cardiovascular dysfunction such as hypertension, cardiac dysfunction, atherosclerosis, and many others. We recently showed that overexpression of human Trx1 in mice protected against age-related hypertension via reversal of uncoupled eNOS that generates O2•– to functional eNOS that produces NO (Das et al., 2018; Hilgers and Das, 2015; Hilgers et al., 2017). We further showed that age-related hypertension is reversed by the treatment of aged mice with recombinant human Trx1, demonstrating a potential therapeutic efficacy of Trx in hypertension and potentially in many other cardiovascular diseases (Das et al., 2018; Hilgers et al., 2017). Given the abundance of Cys residues in proteins/enzymes, the potential for Cys oxidoreductase modulation by the Trx system is enormous and the Trx system is anticipated to play many more beneficial roles in maintaining normal physiology.
Abbreviations Used
- AA
arachidonic acid
- AF
atrial fibrillation
- AKRs
aldo-keto reductases
- ASK1
apoptosis signal-regulated kinase-1
- AT1
angiotensin II type I receptor
- BCNU
1,3-bis-(2-chloroethyl)-1-nitrosourea
- BKCa
large-conductance Ca2+-activated K+ channels
- CAM
calmodulin
- CaMBD
calmodulin binding protein
- CRAC
calcium-release activated Ca2+ channel
- cGMP
guanosine 3′-5′-cyclic monophosphate
- Cys
cysteine
- DAG
diacylglycerol
- DTDP
thymidine-5′-diphosphate
- DTNB
5,5′-dithio-bis (2-nitrobenzoic acid)
- DTT
dithiothreitol
- DA
ductus arteriosus
- E. Coli Trx
Escherichia coli Trx
- EC
endothelial cell
- EDH
endothelium-dependent hyperpolarization
- eNOS
endothelial nitric oxide synthase
- G6P
glucose-6-phospate
- Gly
glycine
- Gpx-1
glutathione peroxidase-1
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- GYG
Glycine-Tyrosine-Glycine
- H2O2
hydrogen peroxide
- H2S
hydrogen sulfide
- HCy
homocysteine
- HHCy
hyperhomocysteinemia
- HI
hyperinsulinism
- IKCa
intermediate conductance KCa
- IPC
ischemic preconditioning
- K+ channel
potassium channel
- KATP
ATP-sensitive and K+ channels
- KCa
calcium-activated potassium channel
- KCNMB1-4
potassium calcium-activated channel subfamily M regulatory beta subunit 1-4
- KcsA
bacterial K+ channel
- KIR
inward rectifier channel
- KV
voltage-dependent K+ channel
- L-VOCC
L-type voltage-operated calcium channels
- LINGO2
leucine-rich repeat and Ig domain containing 2
- MIF
migration inhibitory factor
- MTS
methanethiosulfonate
- MTSEA
2-aminoethyl methanethiosulfonate hydrobromide
- MTSES
sodium (2-sulfonatoethyl) methanethiosulfonate
- MTSET
[2-(trimethylammonium) ethyl] methanethiosulfonate bromide
- MYPT1
myosin phosphatase target subunit 1
- Na+/K+
ATPase-sodium/potassium adenosine triphosphatase
- NADP
nicotinamide adenine dinucleotide phosphate (oxidized form)
- NADPH
nicotinamide adenine dinucleotide phosphate (reduced form)
- O2.−
superoxide anion
- ONOO−
peroxynitrite
- PAPC
1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphocholine
- PASMC
pulmonary artery smooth muscle cell
- PCMBS
parachloromercuribenze sulfonate
- pCMPS
p-chloromercuriphenyl sulfonate
- PKG1α
protein kinase G alpha
- Pro
proline
- Prx
peroxiredoxin
- RCK1
regulator of K+ conductance 1
- RONS
reactive oxygen and nitrogen species
- ROS
reactive oxygen species
- SCAM
substituted cysteine accessibility mutagenesis
- SKCa
small conductance KCa
- SpTrx
spermatozoa Trx
- SUR
sulfonylurea receptor
- Thimerosal
[(O-carboxyphenyl)thio]ethyl mercury sodium salt
- TNFα
tumor necrosis factor-alpha
- TRPC
transient receptor potential cation
- Trx
thioredoxin (cytosolic)
- Trx-S2
oxidized form of Trx
- Trx-SH
reduced form of Trx
- TrxR1
thioredoxin reductase-1
- VSMC
vascular smooth muscle cell
Authors’ Contributions
R.H.P.H. and K.C.D. wrote the article and designed the figures. K.C.D. edited the article in the final form and provided funding.
Author Disclosure Statement
The authors declare no financial conflicts of interest.
Funding Information
Research reported in this publication is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number 1R01HL132953, 1R01HL144610, and 1R01HL157128. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
References
- Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: Form and function. Annu Rev Physiol 2012;74:245–269; doi: 10.1146/annurev-physiol-020911-153336 [DOI] [PubMed] [Google Scholar]
- Adelman JP, Shen KZ, Kavanaugh MP, et al. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron 1992;9(2):209–216. [DOI] [PubMed] [Google Scholar]
- Aggarwal NT, Makielski JC. Redox control of cardiac excitability. Antioxid Redox Signal 2013;18(4):432–468; doi: 10.1089/ars.2011.4234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenman E, Lipton SA, Loring RH. Selective modulation of NMDA responses by reduction and oxidation. Neuron 1989;2(3):1257–1263; doi: 10.1016/0896-6273(89)90310-3 [DOI] [PubMed] [Google Scholar]
- Aizenman E, Stout AK, Hartnett KA, et al. Induction of neuronal apoptosis by thiol oxidation: Putative role of intracellular zinc release. J Neurochem 2000;75(5):1878–1888. [DOI] [PubMed] [Google Scholar]
- Akabas MH, Stauffer DA, Xu M, et al. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science 1992;258(5080):307–310. [DOI] [PubMed] [Google Scholar]
- Akabas MH. Cysteine modification: Probing channel structure, function and conformational change. Adv Exp Med Biol 2015;869:25–54; doi: 10.1007/978-1-4939-2845-3_3 [DOI] [PubMed] [Google Scholar]
- Akbaraly TN, Hininger-Favier I, Carriere I, et al. Plasma selenium over time and cognitive decline in the elderly. Epidemiology 2007;18(1):52–58; doi: 10.1097/01.ede.0000248202.83695.4e [DOI] [PubMed] [Google Scholar]
- Akrouh A, Halcomb SE, Nichols CG, et al. Molecular biology of K(ATP) channels and implications for health and disease. IUBMB Life 2009;61(10):971–978; doi: 10.1002/iub.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alioua A, Tanaka Y, Wallner M, et al. The large conductance, voltage-dependent, and calcium-sensitive K+ channel, Hslo, is a target of cGMP-dependent protein kinase phosphorylation in vivo. J Biol Chem 1998;273(49):32950–32956. [DOI] [PubMed] [Google Scholar]
- Altschmied J, Haendeler J. Thioredoxin-1 and endothelial cell aging: Role in cardiovascular diseases. Antioxid Redox Signal 2009;11(7):1733–1740; doi: 10.1089/ARS.2008.2379 [DOI] [PubMed] [Google Scholar]
- Amberg GC, Santana LF. Downregulation of the BK channel beta1 subunit in genetic hypertension. Circ Res 2003;93(10):965–971; doi: 10.1161/01.RES.0000100068.43006.36 [DOI] [PubMed] [Google Scholar]
- Andrey F, Tsintsadze T, Volkova T, et al. Acid sensing ionic channels: Modulation by redox reagents. Biochim Biophys Acta 2005;1745(1):1–6; doi: 10.1016/j.bbamcr.2005.01.008 [DOI] [PubMed] [Google Scholar]
- Aras MA, Aizenman E. Obligatory role of ASK1 in the apoptotic surge of K+ currents. Neurosci Lett 2005;387(3):136–140; doi: 10.1016/j.neulet.2005.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardanaz N, Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: Regulation and signaling leading to dysfunction. Exp Biol Med (Maywood) 2006;231(3):237–251. [DOI] [PubMed] [Google Scholar]
- Armoundas AA, Wu R, Juang G, et al. Electrical and structural remodeling of the failing ventricle. Pharmacol Ther 2001;92(2–3):213–230. [DOI] [PubMed] [Google Scholar]
- Armstead WM. Superoxide generation links protein kinase C activation to impaired ATP-sensitive K+ channel function after brain injury. Stroke 1999;30(1):153–159. [DOI] [PubMed] [Google Scholar]
- Babenko AP, Aguilar-Bryan L, Bryan J. A view of sur/KIR6.X, KATP channels. Annu Rev Physiol 1998;60:667–687; doi: 10.1146/annurev.physiol.60.1.667 [DOI] [PubMed] [Google Scholar]
- Bacher M, Meinhardt A, Lan HY, et al. MIF expression in the rat brain: Implications for neuronal function. Mol Med 1998;4(4):217–230. [PMC free article] [PubMed] [Google Scholar]
- Bahring R, Boland LM, Varghese A, et al. Kinetic analysis of open- and closed-state inactivation transitions in human Kv4.2 A-type potassium channels. J Physiol 2001a;535(Pt 1):65–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahring R, Milligan CJ, Vardanyan V, et al. Coupling of voltage-dependent potassium channel inactivation and oxidoreductase active site of Kvbeta subunits. J Biol Chem 2001b;276(25):22923–22929; doi: 10.1074/jbc.M100483200 [DOI] [PubMed] [Google Scholar]
- Bailey MA, Grabe M, Devor DC. Characterization of the PCMBS-dependent modification of KCa3.1 channel gating. J Gen Physiol 2010;136(4):367–387; doi: 10.1085/jgp.201010430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister JP, Young BA, Main MJ, et al. The effects of oxidizing and cysteine-reactive reagents on the inward rectifier potassium channels Kir2.3 and Kir1.1. Pflugers Arch 1999;438(6):868–878; doi: 10.1007/s004249900126 [DOI] [PubMed] [Google Scholar]
- Barlow RS, El-Mowafy AM, White RE. H(2)O(2) opens BK(Ca) channels via the PLA(2)-arachidonic acid signaling cascade in coronary artery smooth muscle. Am J Physiol Heart Circ Physiol 2000;279(2):H475–H483. [DOI] [PubMed] [Google Scholar]
- Barlow RS, White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Physiol 1998;275(4):H1283–H1289. [DOI] [PubMed] [Google Scholar]
- Barnes EA, Lee L, Barnes SL, et al. beta1-Subunit of the calcium-sensitive potassium channel modulates the pulmonary vascular smooth muscle cell response to hypoxia. Am J Physiol Lung Cell Mol Physiol 2018;315(2):L265–L275; doi: 10.1152/ajplung.00060.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentrop D, Beyermann M, Wissmann R, et al. NMR structure of the “ball-and-chain” domain of KCNMB2, the beta 2-subunit of large conductance Ca2+- and voltage-activated potassium channels. J Biol Chem 2001;276(45):42116–42121; doi: 10.1074/jbc.M107118200 [DOI] [PubMed] [Google Scholar]
- Beny JL, von der Weid PY. Hydrogen peroxide: An endogenous smooth muscle cell hyperpolarizing factor. Biochem Biophys Res Commun 1991;176(1):378–384. [DOI] [PubMed] [Google Scholar]
- Bharadwaj L, Prasad K. Mediation of H2O2-induced vascular relaxation by endothelium-derived relaxing factor. Mol Cell Biochem 1995;149-150:267–270. [DOI] [PubMed] [Google Scholar]
- Bidasee KR, Nallani K, Besch HR,Jr, et al. Streptozotocin-induced diabetes increases disulfide bond formation on cardiac ryanodine receptor (RyR2). J Pharmacol Exp Ther 2003;305(3):989–998; doi: 10.1124/jpet.102.046201 [DOI] [PubMed] [Google Scholar]
- Bleys J, Navas-Acien A, Laclaustra M, et al. Serum selenium and peripheral arterial disease: Results from the national health and nutrition examination survey, 2003-2004. Am J Epidemiol 2009;169(8):996–1003; doi: 10.1093/aje/kwn414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom BR, Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science 1966;153(3731):80–82. [DOI] [PubMed] [Google Scholar]
- Bloomfield KL, Osborne SA, Kennedy DD, et al. Thioredoxin-mediated redox control of the transcription factor Sp1 and regulation of the thioredoxin gene promoter. Gene 2003;319:107–116; doi: 10.1016/s0378-1119(03)00799-6 [DOI] [PubMed] [Google Scholar]
- Bonnet P, Rusch NJ, Harder DR. Characterization of an outward K+ current in freshly dispersed cerebral arterial muscle cells. Pflugers Arch 1991;418(3):292–296. [DOI] [PubMed] [Google Scholar]
- Brahler S, Kaistha A, Schmidt VJ, et al. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation 2009;119(17):2323–2332; doi: 10.1161/CIRCULATIONAHA.108.846634 [DOI] [PubMed] [Google Scholar]
- Brakemeier S, Eichler I, Knorr A, et al. Modulation of Ca2+-activated K+ channel in renal artery endothelium in situ by nitric oxide and reactive oxygen species. Kidney Int 2003;64(1):199–207; doi: 10.1046/j.1523-1755.2003.00051.x [DOI] [PubMed] [Google Scholar]
- Brayden JE. Functional roles of KATP channels in vascular smooth muscle. Clin Exp Pharmacol Physiol 2002;29(4):312–316. [DOI] [PubMed] [Google Scholar]
- Brenner R, Jegla TJ, Wickenden A, et al. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J Biol Chem 2000a;275(9):6453–6461. [DOI] [PubMed] [Google Scholar]
- Brenner R, Perez GJ, Bonev AD, et al. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature 2000b;407(6806):870–876; doi: 10.1038/35038011 [DOI] [PubMed] [Google Scholar]
- Bruening-Wright A, Schumacher MA, Adelman JP, et al. Localization of the activation gate for small conductance Ca2+-activated K+ channels. J Neurosci 2002;22(15):6499–6506; doi: 10.1523/JNEUROSCI.22-15-06499.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzezinska AK, Gebremedhin D, Chilian WM, et al. Peroxynitrite reversibly inhibits Ca(2+)-activated K(+) channels in rat cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol 2000;278(6):H1883–H1890. [DOI] [PubMed] [Google Scholar]
- Burgoyne JR, Madhani M, Cuello F, et al. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science 2007;317(5843):1393–1397; doi: 10.1126/science.1144318 [DOI] [PubMed] [Google Scholar]
- Burgoyne JR, Prysyazhna O, Rudyk O, et al. cGMP-dependent activation of protein kinase G precludes disulfide activation: Implications for blood pressure control. Hypertension 2012;60(5):1301–1308; doi: 10.1161/HYPERTENSIONAHA.112.198754 [DOI] [PubMed] [Google Scholar]
- Burke-Wolin T, Abate CJ, Wolin MS, et al. Hydrogen peroxide-induced pulmonary vasodilation: Role of guanosine 3′,5′-cyclic monophosphate. Am J Physiol 1991;261(6 Pt 1):L393–L398. [DOI] [PubMed] [Google Scholar]
- Burnham MP, Bychkov R, Feletou M, et al. Characterization of an apamin-sensitive small-conductance Ca2+-activated K(+) channel in porcine coronary artery endothelium: Relevance to EDHF. Br J Pharmacol 2002;135(5):1133–1143; doi: 10.1038/sj.bjp.0704551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch AE, Waldegger S, Herzer T, et al. Molecular basis of IsK protein regulation by oxidation or chelation. J Biol Chem 1995;270(8):3638–3641; doi: 10.1074/jbc.270.8.3638 [DOI] [PubMed] [Google Scholar]
- Busch JL, Bessay EP, Francis SH, et al. A conserved serine juxtaposed to the pseudosubstrate site of type I cGMP-dependent protein kinase contributes strongly to autoinhibition and lower cGMP affinity. J Biol Chem 2002;277(37):34048–34054; doi: 10.1074/jbc.M202761200 [DOI] [PubMed] [Google Scholar]
- Bychkov R, Pieper K, Ried C, et al. Hydrogen peroxide, potassium currents, and membrane potential in human endothelial cells. Circulation 1999;99(13):1719–1725. [DOI] [PubMed] [Google Scholar]
- Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol Rev 2009;231(1):59–87; doi: 10.1111/j.1600-065X.2009.00816.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Sauve R. Effects of thiol-modifying agents on a K(Ca2+) channel of intermediate conductance in bovine aortic endothelial cells. J Membr Biol 1997;158(2):147–158. [DOI] [PubMed] [Google Scholar]
- Campbell DL, Stamler JS, Strauss HC. Redox modulation of L-type calcium channels in ferret ventricular myocytes. Dual mechanism regulation by nitric oxide and S-nitrosothiols. J Gen Physiol 1996;108(4):277–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier GO, Fuchs LC, Winecoff AP, et al. Nitrovasodilators relax mesenteric microvessels by cGMP-induced stimulation of Ca-activated K channels. Am J Physiol 1997;273(1 Pt 2):H76–H84. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Schumacker PT. Cellular oxygen sensing by mitochondria: Old questions, new insight. J Appl Physiol (1985) 2000;88(5):1880–1889. [DOI] [PubMed] [Google Scholar]
- Chandy KG, DeCoursey TE, Cahalan MD, et al. Voltage-gated potassium channels are required for human T lymphocyte activation. J Exp Med 1984;160(2):369–385; doi: 10.1084/jem.160.2.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandy KG, Norton RS. Peptide blockers of K(v)1.3 channels in T cells as therapeutics for autoimmune disease. Curr Opin Chem Biol 2017;38:97–107; doi: 10.1016/j.cbpa.2017.02.015 [DOI] [PubMed] [Google Scholar]
- Chaytor AT, Edwards DH, Bakker LM, et al. Distinct hyperpolarizing and relaxant roles for gap junctions and endothelium-derived H2O2 in NO-independent relaxations of rabbit arteries. Proc Natl Acad Sci USA 2003;100(25):15212–15217; doi: 10.1073/pnas.2435030100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Jiang X, Kruger WD, et al. Hyperhomocysteinemia impairs endothelium-derived hyperpolarizing factor-mediated vasorelaxation in transgenic cystathionine beta synthase-deficient mice. Blood 2011;118(7):1998–2006; doi: 10.1182/blood-2011-01-333310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamvimonvat N, O’Rourke B, Kamp TJ, et al. Functional consequences of sulfhydryl modification in the pore-forming subunits of cardiovascular Ca2+ and Na+ channels. Circ Res 1995;76(3):325–334. [DOI] [PubMed] [Google Scholar]
- Chilton L, Loutzenhiser R. Functional evidence for an inward rectifier potassium current in rat renal afferent arterioles. Circ Res 2001;88(2):152–158. [DOI] [PubMed] [Google Scholar]
- Cho HC, Tsushima RG, Nguyen TT, et al. Two critical cysteine residues implicated in disulfide bond formation and proper folding of Kir2.1. Biochemistry 2000;39(16):4649–4657. [DOI] [PubMed] [Google Scholar]
- Chrissobolis S, Didion SP, Kinzenbaw DA, et al. Glutathione peroxidase-1 plays a major role in protecting against angiotensin II-induced vascular dysfunction. Hypertension 2008;51(4):872–877; doi: 10.1161/HYPERTENSIONAHA.107.103572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophersen P, Wulff H. Pharmacological gating modulation of small- and intermediate-conductance Ca2+-activated K+ channels (KCa2.x and KCa3.1). Channels (Austin) 2015;9(6):336–343; doi: 10.1080/19336950.2015.1071748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee WA, Nakamura TY, Faivre JF. Effects of thiol-modifying agents on KATP channels in guinea pig ventricular cells. Am J Physiol 1995;269(5 Pt 2):H1625–H1633. [DOI] [PubMed] [Google Scholar]
- Dai J, Wang X, Feng J, et al. Regulatory role of thioredoxin in homocysteine-induced monocyte chemoattractant protein-1 secretion in monocytes/macrophages. FEBS Lett 2008;582(28):3893–3898; doi: 10.1016/j.febslet.2008.10.030 [DOI] [PubMed] [Google Scholar]
- Das KC, Das CK. Thioredoxin, a singlet oxygen quencher and hydroxyl radical scavenger: Redox independent functions. Biochem Biophys Res Commun 2000;277(2):443–447. [DOI] [PubMed] [Google Scholar]
- Das KC, Guo XL, White CW. Induction of thioredoxin and thioredoxin reductase gene expression in lungs of newborn primates by oxygen. Am J Physiol 1999;276(3):L530–L539. [DOI] [PubMed] [Google Scholar]
- Das KC, Kundumani-Sridharan V, Subramani J. Role of thioredoxin in age-related hypertension. Curr Hypertens Rep 2018;20(1):6; doi: 10.1007/s11906-018-0815-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das KC, Pahl PM, Guo XL, et al. Induction of peroxiredoxin gene expression by oxygen in lungs of newborn primates. Am J Respir Cell Mol Biol 2001;25(2):226–232; doi: 10.1165/ajrcmb.25.2.4314 [DOI] [PubMed] [Google Scholar]
- Das KC. Thioredoxin and its role in premature newborn biology. Antioxid Redox Signal 2005;7(11–12):1740–1743; doi: 10.1089/ars.2005.7.1740 [DOI] [PubMed] [Google Scholar]
- Das KC. Thioredoxin system in premature and newborn biology. Antioxid Redox Signal 2004;6(1):177–184; doi: 10.1089/152308604771978480 [DOI] [PubMed] [Google Scholar]
- DeWitt DS, Mathew BP, Chaisson JM, et al. Peroxynitrite reduces vasodilatory responses to reduced intravascular pressure, calcitonin gene-related peptide, and cromakalim in isolated middle cerebral arteries. J Cereb Blood Flow Metab 2001;21(3):253–261; doi: 10.1097/00004647-200103000-00009 [DOI] [PubMed] [Google Scholar]
- DiChiara TJ, Reinhart PH. Redox modulation of hslo Ca2+-activated K+ channels. J Neurosci 1997;17(13):4942–4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Mederos YSM, Gollasch M, et al. Increased vascular smooth muscle contractility in TRPC6−/− mice. Mol Cell Biol 2005;25(16):6980–6989; doi: 10.1128/MCB.25.16.6980-6989.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D, Reece EA, Lin X, et al. New development of the yolk sac theory in diabetic embryopathy: Molecular mechanism and link to structural birth defects. Am J Obstet Gynecol 2016;214(2):192–202; doi: 10.1016/j.ajog.2015.09.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dora KA, Damon DN, Duling BR. Microvascular dilation in response to occlusion: A coordinating role for conducted vasomotor responses. Am J Physiol Heart Circ Physiol 2000;279(1):H279–H284. [DOI] [PubMed] [Google Scholar]
- Dora KA, Gallagher NT, McNeish A, et al. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ Res 2008;102(10):1247–1255; doi: 10.1161/CIRCRESAHA.108.172379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, et al. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science 1998;280(5360):69–77. [DOI] [PubMed] [Google Scholar]
- Dudem S, Boon PX, Mullins N, et al. Oxidation modulates LINGO2-induced inactivation of large conductance, Ca(2+)-activated potassium channels. J Biol Chem 2023;299(3):102975; doi: 10.1016/j.jbc.2023.102975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwenger MM, Raph SM, Baba SP, et al. Diversification of potassium currents in excitable cells via kvbeta proteins. Cells 2022;11(14); doi: 10.3390/cells11142230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson HJ, Jeng MF, Tennant LL, et al. Effects of buried charged groups on cysteine thiol ionization and reactivity in Escherichia coli thioredoxin: Structural and functional characterization of mutants of Asp 26 and Lys 57. Biochemistry 1997;36(9):2622–2636; doi: 10.1021/bi961801a [DOI] [PubMed] [Google Scholar]
- Edwards G, Dora KA, Gardener MJ, et al. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 1998;396(6708):269–272; doi: 10.1038/24388 [DOI] [PubMed] [Google Scholar]
- Eklund H, Gleason FK, Holmgren A. Structural and functional relations among thioredoxins of different species. Proteins 1991;11(1):13–28; doi: 10.1002/prot.340110103 [DOI] [PubMed] [Google Scholar]
- Elliott SJ, Lacey DJ, Chilian WM, et al. Peroxynitrite is a contractile agonist of cerebral artery smooth muscle cells. Am J Physiol 1998;275(5):H1585–H1591. [DOI] [PubMed] [Google Scholar]
- Ellis A, Pannirselvam M, Anderson TJ, et al. Catalase has negligible inhibitory effects on endothelium-dependent relaxations in mouse isolated aorta and small mesenteric artery. Br J Pharmacol 2003;140(7):1193–1200; doi: 10.1038/sj.bjp.0705549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AV, Washburn DL, Latchford KJ. Hormonal and neurotransmitter roles for angiotensin in the regulation of central autonomic function. Exp Biol Med (Maywood) 2001;226(2):85–96. [DOI] [PubMed] [Google Scholar]
- Feske S, Wulff H, Skolnik EY. Ion channels in innate and adaptive immunity. Annu Rev Immunol 2015;33:291–353; doi: 10.1146/annurev-immunol-032414-112212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierro-Gonzalez JC, Gonzalez-Barrios M, Miranda-Vizuete A, et al. The thioredoxin TRX-1 regulates adult lifespan extension induced by dietary restriction in Caenorhabditis elegans. Biochem Biophys Res Commun 2011;406(3):478–482; doi: 10.1016/j.bbrc.2011.02.079 [DOI] [PubMed] [Google Scholar]
- Ford JW, Milnes JT. New drugs targeting the cardiac ultra-rapid delayed-rectifier current (I Kur): Rationale, pharmacology and evidence for potential therapeutic value. J Cardiovasc Pharmacol 2008;52(2):105–120; doi: 10.1097/FJC.0b013e3181719b0c [DOI] [PubMed] [Google Scholar]
- Forgione MA, Cap A, Liao R, et al. Heterozygous cellular glutathione peroxidase deficiency in the mouse: Abnormalities in vascular and cardiac function and structure. Circulation 2002;106(9):1154–1158. [DOI] [PubMed] [Google Scholar]
- Fraile ML, Conde MV, Sanz L, et al. Different influence of superoxide anions and hydrogen peroxide on endothelial function of isolated cat cerebral and pulmonary arteries. Gen Pharmacol 1994;25(6):1197–1205. [DOI] [PubMed] [Google Scholar]
- Fujiki T, Shimokawa H, Morikawa K, et al. Endothelium-derived hydrogen peroxide accounts for the enhancing effect of an angiotensin-converting enzyme inhibitor on endothelium-derived hyperpolarizing factor-mediated responses in mice. Arterioscler Thromb Vasc Biol 2005;25(4):766–771; doi: 10.1161/01.ATV.0000158498.19027.75 [DOI] [PubMed] [Google Scholar]
- Fukao M, Mason HS, Britton FC, et al. Cyclic GMP-dependent protein kinase activates cloned BKCa channels expressed in mammalian cells by direct phosphorylation at serine 1072. J Biol Chem 1999;274(16):10927–10935. [DOI] [PubMed] [Google Scholar]
- Gamper N, Zaika O, Li Y, et al. Oxidative modification of M-type K(+) channels as a mechanism of cytoprotective neuronal silencing. EMBO J 2006;25(20):4996–5004; doi: 10.1038/sj.emboj.7601374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao TM, Fung ML. Decreased large conductance Ca(2+)-activated K(+) channel activity in dissociated CA1 hippocampal neurons in rats exposed to perinatal and postnatal hypoxia. Neurosci Lett 2002;332(3):163–166. [DOI] [PubMed] [Google Scholar]
- Garlid KD, Costa AD, Quinlan CL, et al. Cardioprotective signaling to mitochondria. J Mol Cell Cardiol 2009;46(6):858–866; doi: 10.1016/j.yjmcc.2008.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garneau L, Klein H, Banderali U, et al. Hydrophobic interactions as key determinants to the KCa3.1 channel closed configuration. An analysis of KCa3.1 mutants constitutively active in zero Ca2+. J Biol Chem 2009;284(1):389–403; doi: 10.1074/jbc.M805700200 [DOI] [PubMed] [Google Scholar]
- Garneau L, Klein H, Parent L, et al. Contribution of cytosolic cysteine residues to the gating properties of the Kir2.1 inward rectifier. Biophys J 2003;84(6):3717–3729; doi: 10.1016/S0006-3495(03)75100-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garneau L, Klein H, Parent L, et al. Toward the rational design of constitutively active KCa3.1 mutant channels. Methods Enzymol 2010;485:437–457; doi: 10.1016/B978-0-12-381296-4.00024-5 [DOI] [PubMed] [Google Scholar]
- Gelband CH, Hume JR. Ionic currents in single smooth muscle cells of the canine renal artery. Circ Res 1992;71(4):745–758. [DOI] [PubMed] [Google Scholar]
- Go YM, Jones DP. The redox proteome. J Biol Chem 2013;288(37):26512–26520; doi: 10.1074/jbc.R113.464131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R, Caballero R, Barana A, et al. Nitric oxide increases cardiac IK1 by nitrosylation of cysteine 76 of Kir2.1 channels. Circ Res 2009;105(4):383–392; doi: 10.1161/CIRCRESAHA.109.197558 [DOI] [PubMed] [Google Scholar]
- Gomez R, Caballero R, Barana A, et al. Structural basis of drugs that increase cardiac inward rectifier Kir2.1 currents. Cardiovasc Res 2014;104(2):337–346; doi: 10.1093/cvr/cvu203 [DOI] [PubMed] [Google Scholar]
- Gong L, Gao TM, Huang H, et al. Redox modulation of large conductance calcium-activated potassium channels in CA1 pyramidal neurons from adult rat hippocampus. Neurosci Lett 2000;286(3):191–194. [DOI] [PubMed] [Google Scholar]
- Goy C, Czypiorski P, Altschmied J, et al. The imbalanced redox status in senescent endothelial cells is due to dysregulated Thioredoxin-1 and NADPH oxidase 4. Exp Gerontol 2014;56:45–52; doi: 10.1016/j.exger.2014.03.005 [DOI] [PubMed] [Google Scholar]
- Graham S, Ding M, Ding Y, et al. Canonical transient receptor potential 6 (TRPC6), a redox-regulated cation channel. J Biol Chem 2010;285(30):23466–23476; doi: 10.1074/jbc.M109.093500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grgic I, Kiss E, Kaistha BP, et al. Renal fibrosis is attenuated by targeted disruption of KCa3.1 potassium channels. Proc Natl Acad Sci U S A 2009;106(34):14518–14523; doi: 10.1073/pnas.0903458106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta MP, Steinberg HO, Hart CM. H2O2 causes endothelial barrier dysfunction without disrupting the arginine-nitric oxide pathway. Am J Physiol 1998;274(4):L508–L516. [DOI] [PubMed] [Google Scholar]
- Guy HR, Conti F. Pursuing the structure and function of voltage-gated channels. Trends Neurosci 1990;13(6):201–206. [DOI] [PubMed] [Google Scholar]
- Haendeler J. Thioredoxin-1 and posttranslational modifications. Antioxid Redox Signal 2006;8(9–10):1723–1728; doi: 10.1089/ars.2006.8.1723 [DOI] [PubMed] [Google Scholar]
- Hamilton CA, McPhaden AR, Berg G, et al. Is hydrogen peroxide an EDHF in human radial arteries? Am J Physiol Heart Circ Physiol 2001;280(6):H2451–H2455. [DOI] [PubMed] [Google Scholar]
- Hayabuchi Y, Nakaya Y, Matsuoka S, et al. Hydrogen peroxide-induced vascular relaxation in porcine coronary arteries is mediated by Ca2+-activated K+ channels. Heart Vessels 1998;13(1):9–17. [DOI] [PubMed] [Google Scholar]
- Heginbotham L, Lu Z, Abramson T, et al. Mutations in the K+ channel signature sequence. Biophys J 1994;66(4):1061–1067; doi: 10.1016/S0006-3495(94)80887-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann SH, Rettig J, Wunder F, et al. Molecular and functional characterization of a rat brain Kv beta 3 potassium channel subunit. FEBS Lett 1995;377(3):383–389; doi: 10.1016/0014-5793(95)01377-6 [DOI] [PubMed] [Google Scholar]
- Heinemann SH, Schlief T, Mori Y, et al. Molecular pore structure of voltage-gated sodium and calcium channels. Braz J Med Biol Res 1994;27(12):2781–2802. [PubMed] [Google Scholar]
- Hercberg S, Galan P, Preziosi P, et al. The SU.VI.MAX Study: A randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Intern Med 2004;164(21):2335–2342; doi: 10.1001/archinte.164.21.2335 [DOI] [PubMed] [Google Scholar]
- Hermann A, Sitdikova GF, Weiger TM. Oxidative stress and maxi calcium-activated potassium (BK) channels. Biomolecules 2015;5(3):1870–1911; doi: 10.3390/biom5031870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann MA, Rudolph AM. Control of the ductus arteriosus. Physiol Rev 1975;55(1):62–78. [DOI] [PubMed] [Google Scholar]
- Hibino H, Inanobe A, Furutani K, et al. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol Rev 2010;90(1):291–366; doi: 10.1152/physrev.00021.2009 [DOI] [PubMed] [Google Scholar]
- Hilgers RH, Das KC. Role of in vivo vascular redox in resistance arteries. Hypertension 2015;65(1):130–139; doi: 10.1161/HYPERTENSIONAHA.114.04473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgers RH, Kundumani-Sridharan V, Subramani J, et al. Thioredoxin reverses age-related hypertension by chronically improving vascular redox and restoring eNOS function. Sci Transl Med 2017;9(376); doi: 10.1126/scitranslmed.aaf6094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgers RH, Todd J,Jr, Webb RC. Regional heterogeneity in acetylcholine-induced relaxation in rat vascular bed: Role of calcium-activated K+ channels. Am J Physiol Heart Circ Physiol 2006;291(1):H216–H222; doi: 10.1152/ajpheart.01383.2005 [DOI] [PubMed] [Google Scholar]
- Hofmann F, Ammendola A, Schlossmann J. Rising behind NO: cGMP-dependent protein kinases. J Cell Sci 2000;113(Pt 10):1671–1676. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov AG, Schaefer M, et al. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 1999;397(6716):259–263; doi: 10.1038/16711 [DOI] [PubMed] [Google Scholar]
- Holmgren A, Bjornstedt M. Thioredoxin and thioredoxin reductase. Methods Enzymol 1995;252:199–208. [DOI] [PubMed] [Google Scholar]
- Holmgren A, Lu J. Thioredoxin and thioredoxin reductase: Current research with special reference to human disease. Biochem Biophys Res Commun 2010;396(1):120–124; doi: 10.1016/j.bbrc.2010.03.083 [DOI] [PubMed] [Google Scholar]
- Holmgren A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid Redox Signal 2000;2(4):811–820; doi: 10.1089/ars.2000.2.4-811 [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem 1985;54:237–271; doi: 10.1146/annurev.bi.54.070185.001321 [DOI] [PubMed] [Google Scholar]
- Hool LC. Differential regulation of the slow and rapid components of guinea-pig cardiac delayed rectifier K+ channels by hypoxia. J Physiol 2004;554(Pt 3):743–754; doi: 10.1113/jphysiol.2003.055442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan FT, Heinemann SH, Hoshi T. Heme regulates allosteric activation of the Slo1 BK channel. J Gen Physiol 2005;126(1):7–21; doi: 10.1085/jgp.200509262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RW. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science 1990;250(4980):533–538. [DOI] [PubMed] [Google Scholar]
- Hou S, Heinemann SH, Hoshi T. Modulation of BKCa channel gating by endogenous signaling molecules. Physiology (Bethesda) 2009;24:26–35; doi: 10.1152/physiol.00032.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S, Xu R, Heinemann SH, et al. Reciprocal regulation of the Ca2+ and H+ sensitivity in the SLO1 BK channel conferred by the RCK1 domain. Nat Struct Mol Biol 2008;15(4):403–410; doi: 10.1038/nsmb.1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinari K, Kakei M, Matsuoka T, et al. Direct activation of the ATP-sensitive potassium channel by oxygen free radicals in guinea-pig ventricular cells: Its potentiation by MgADP. J Mol Cell Cardiol 1996;28(9):1867–1877; doi: 10.1006/jmcc.1996.0179 [DOI] [PubMed] [Google Scholar]
- Iida Y, Katusic ZS. Mechanisms of cerebral arterial relaxations to hydrogen peroxide. Stroke 2000;31(9):2224–2230. [DOI] [PubMed] [Google Scholar]
- Ishii TM, Silvia C, Hirschberg B, et al. A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci U S A 1997;94(21):11651–11656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Weaver O, Paredes DA, Gonzalez Bosc LV, et al. Intermittent hypoxia in rats increases myogenic tone through loss of hydrogen sulfide activation of large-conductance Ca(2+)-activated potassium channels. Circ Res 2011;108(12):1439–1447; doi: 10.1161/CIRCRESAHA.110.228999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar JH, Li A, Parfenova H, et al. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res 2005;97(8):805–812; doi: 10.1161/01.RES.0000186180.47148.7b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakupoglu C, Przemeck GK, Schneider M, et al. Cytoplasmic thioredoxin reductase is essential for embryogenesis but dispensable for cardiac development. Mol Cell Biol 2005;25(5):1980–1988; doi: 10.1128/MCB.25.5.1980-1988.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng MF, Campbell AP, Begley T, et al. High-resolution solution structures of oxidized and reduced Escherichia coli thioredoxin. Structure 1994;2(9):853–868. [DOI] [PubMed] [Google Scholar]
- Jerng HH, Pfaffinger PJ. S-glutathionylation of an auxiliary subunit confers redox sensitivity to Kv4 channel inactivation. PLoS One 2014;9(3):e93315; doi: 10.1371/journal.pone.0093315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Tang G, Cao K, et al. Molecular mechanism for H(2)S-induced activation of K(ATP) channels. Antioxid Redox Signal 2010;12(10):1167–1178; doi: 10.1089/ars.2009.2894 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, et al. The open pore conformation of potassium channels. Nature 2002;417(6888):523–526; doi: 10.1038/417523a [DOI] [PubMed] [Google Scholar]
- Jiang Y, Pico A, Cadene M, et al. Structure of the RCK domain from the E. coli K+ channel and demonstration of its presence in the human BK channel. Neuron 2001;29(3):593–601. [DOI] [PubMed] [Google Scholar]
- Jin X, Yu L, Wu Y, et al. S-Glutathionylation underscores the modulation of the heteromeric Kir4.1-Kir5.1 channel in oxidative stress. J Physiol 2012;590(21):5335–5348; doi: 10.1113/jphysiol.2012.236885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WJ, Wang LY, Tang MD, et al. hSK4, a member of a novel subfamily of calcium-activated potassium channels. Proc Natl Acad Sci U S A 1997;94(20):11013–11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP. Redefining oxidative stress. Antioxid Redox Signal 2006;8(9–10):1865–1879; doi: 10.1089/ars.2006.8.1865 [DOI] [PubMed] [Google Scholar]
- Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 2008;295(4):C849–C868; doi: 10.1152/ajpcell.00283.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliana CA, Chai J, Arroyo P, et al. A selective nonpeptide somatostatin receptor 5 agonist effectively decreases insulin secretion in hyperinsulinism. J Biol Chem 2023;299(6):104816; doi: 10.1016/j.jbc.2023.104816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaab S, Dixon J, Duc J, et al. Molecular basis of transient outward potassium current downregulation in human heart failure: A decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation 1998;98(14):1383–1393. [DOI] [PubMed] [Google Scholar]
- Kang KT, Sullivan JC, Sasser JM, et al. Novel nitric oxide synthase–dependent mechanism of vasorelaxation in small arteries from hypertensive rats. Hypertension 2007;49(4):893–901; doi: 10.1161/01.HYP.0000259669.40991.1e [DOI] [PubMed] [Google Scholar]
- Keen JE, Khawaled R, Farrens DL, et al. Domains responsible for constitutive and Ca(2+)-dependent interactions between calmodulin and small conductance Ca(2+)-activated potassium channels. J Neurosci 1999;19(20):8830–8838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerst G, Brousos H, Schreiber R, et al. The oxidant thimerosal modulates gating behavior of KCNQ1 by interaction with the channel outer shell. J Membr Biol 2002;186(2):89–100; doi: 10.1007/s00232-001-0138-6 [DOI] [PubMed] [Google Scholar]
- Khanna R, Chang MC, Joiner WJ, et al. hSK4/hIK1, a calmodulin-binding KCa channel in human T lymphocytes. Roles in proliferation and volume regulation. J Biol Chem 1999;274(21):14838–14849. [DOI] [PubMed] [Google Scholar]
- Kimura H. Signalling by hydrogen sulfide and polysulfides via protein S-sulfuration. Br J Pharmacol 2020;177(4):720–733; doi: 10.1111/bph.14579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B, Rizwan AP, Asmara H, et al. IKCa channels are a critical determinant of the slow AHP in CA1 pyramidal neurons. Cell Rep 2015;11(2):175–182; doi: 10.1016/j.celrep.2015.03.026 [DOI] [PubMed] [Google Scholar]
- Kleemann R, Kapurniotu A, Frank RW, et al. Disulfide analysis reveals a role for macrophage migration inhibitory factor (MIF) as thiol-protein oxidoreductase. J Mol Biol 1998;280(1):85–102; doi: 10.1006/jmbi.1998.1864 [DOI] [PubMed] [Google Scholar]
- Klein H, Garneau L, Banderali U, et al. Structural determinants of the closed KCa3.1 channel pore in relation to channel gating: Results from a substituted cysteine accessibility analysis. J Gen Physiol 2007;129(4):299–315; doi: 10.1085/jgp.200609726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus HG, Garcia-Calvo M, Kaczorowski GJ, et al. Subunit composition of the high conductance calcium-activated potassium channel from smooth muscle, a representative of the mSlo and slowpoke family of potassium channels. J Biol Chem 1994;269(6):3921–3924. [PubMed] [Google Scholar]
- Knot HJ, Zimmermann PA, Nelson MT. Extracellular K(+)-induced hyperpolarizations and dilatations of rat coronary and cerebral arteries involve inward rectifier K(+) channels. J Physiol 1996;492(Pt 2):419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler M, Hirschberg B, Bond CT, et al. Small-conductance, calcium-activated potassium channels from mammalian brain. Science 1996;273(5282):1709–1714. [DOI] [PubMed] [Google Scholar]
- Kohler R, Olivan-Viguera A, Wulff H. Endothelial small- and intermediate-conductance K channels and endothelium-dependent hyperpolarization as drug targets in cardiovascular disease. Adv Pharmacol 2016;77:65–104; doi: 10.1016/bs.apha.2016.04.002 [DOI] [PubMed] [Google Scholar]
- Kohler R, Wulff H, Eichler I, et al. Blockade of the intermediate-conductance calcium-activated potassium channel as a new therapeutic strategy for restenosis. Circulation 2003;108(9):1119–1125; doi: 10.1161/01.CIR.0000086464.04719.DD [DOI] [PubMed] [Google Scholar]
- Kolbe K, Schonherr R, Gessner G, et al. Cysteine 723 in the C-linker segment confers oxidative inhibition of hERG1 potassium channels. J Physiol 2010;588(Pt 16):2999–3009; doi: 10.1113/jphysiol.2010.192468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang Q, Purhonen P, Jegerschold C, et al. Free RCK arrangement in Kch, a putative escherichia coli potassium channel, as suggested by electron crystallography. Structure 2015;23(1):199–205; doi: 10.1016/j.str.2014.10.018 [DOI] [PubMed] [Google Scholar]
- Kundumani-Sridharan V, Subramani J, Owens C, et al. Nrg1beta released in remote ischemic preconditioning improves myocardial perfusion and decreases ischemia/reperfusion injury via ErbB2-mediated rescue of endothelial nitric oxide synthase and abrogation of Trx2 autophagy. Arterioscler Thromb Vasc Biol 2021;41(8):2293–2314; doi: 10.1161/ATVBAHA.121.315957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacampagne A, Duittoz A, Bolanos P, et al. Effect of sulfhydryl oxidation on ionic and gating currents associated with L-type calcium channels in isolated guinea-pig ventricular myocytes. Cardiovasc Res 1995;30(5):799–806. [PubMed] [Google Scholar]
- Lee S, Park M, So I, et al. NADH and NAD modulates Ca2+-activated K+ channels in small pulmonary arterial smooth muscle cells of the rabbit. Pflugers Arch 1994;427(3–4):378–380. [DOI] [PubMed] [Google Scholar]
- Lewis A, Peers C, Ashford ML, et al. Hypoxia inhibits human recombinant large conductance, Ca(2+)-activated K(+) (maxi-K) channels by a mechanism which is membrane delimited and Ca(2+) sensitive. J Physiol 2002;540(3):771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyland ML, Dart C, Spencer PJ, et al. The possible role of a disulphide bond in forming functional Kir2.1 potassium channels. Pflugers Arch 1999;438(6):778–781. [DOI] [PubMed] [Google Scholar]
- Li GR, Dong MQ. Pharmacology of cardiac potassium channels. Adv Pharmacol 2010;59:93–134; doi: 10.1016/S1054-3589(10)59004-5 [DOI] [PubMed] [Google Scholar]
- Li H, Gao Y, Freire CD, et al. Macrophage migration inhibitory factor in the PVN attenuates the central pressor and dipsogenic actions of angiotensin II. FASEB J 2006a;20(10):1748–1750; doi: 10.1096/fj.06-5836fje [DOI] [PubMed] [Google Scholar]
- Li H, Gao Y, Qi Y, et al. Macrophage migration inhibitory factor in hypothalamic paraventricular nucleus neurons decreases blood pressure in spontaneously hypertensive rats. FASEB J 2008a;22(9):3175–3185; doi: 10.1096/fj.08-108662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Li W, Altura BT, et al. Peroxynitrite-induced relaxation in isolated canine cerebral arteries and mechanisms of action. Toxicol Appl Pharmacol 2004;196(1):176–182; doi: 10.1016/j.taap.2003.12.007 [DOI] [PubMed] [Google Scholar]
- Li W, Halling DB, Hall AW, et al. EF hands at the N-lobe of calmodulin are required for both SK channel gating and stable SK-calmodulin interaction. J Gen Physiol 2009;134(4):281–293; doi: 10.1085/jgp.200910295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li S, Xu Z, et al. Redox control of K+ channel remodeling in rat ventricle. J Mol Cell Cardiol 2006b;40(3):339–349; doi: 10.1016/j.yjmcc.2005.09.019 [DOI] [PubMed] [Google Scholar]
- Li X, Tang K, Xie B, et al. Regulation of Kv4 channel expression in failing rat heart by the thioredoxin system. Am J Physiol Heart Circ Physiol 2008b;295(1):H416–H424; doi: 10.1152/ajpheart.91446.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Xu Z, Li S, et al. Redox regulation of Ito remodeling in diabetic rat heart. Am J Physiol Heart Circ Physiol 2005;288(3):H1417–H1424; doi: 10.1152/ajpheart.00559.2004 [DOI] [PubMed] [Google Scholar]
- Liang H, Li X, Li S, et al. Oxidoreductase regulation of Kv currents in rat ventricle. J Mol Cell Cardiol 2008;44(6):1062–1071; doi: 10.1016/j.yjmcc.2008.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Adelman JP, Maylie J. Modulation of endothelial SK3 channel activity by Ca(2)+dependent caveolar trafficking. Am J Physiol Cell Physiol 2012;303(3):C318–C327; doi: 10.1152/ajpcell.00058.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkind GM, Hanck DA, Fozzard HA. A structural motif for the voltage-gated potassium channel pore. Proc Natl Acad Sci U S A 1995;92(20):9215–9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippoldt A, Padilla CA, Gerst H, et al. Localization of thioredoxin in the rat brain and functional implications. J Neurosci 1995;15(10):6747–6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Moczydlowski E, Haddad GG. O(2) deprivation inhibits Ca(2+)-activated K(+) channels via cytosolic factors in mice neocortical neurons. J Clin Invest 1999;104(5):577–588; doi: 10.1172/JCI7291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Holmgren M, Jurman ME, et al. Gated access to the pore of a voltage-dependent K+ channel. Neuron 1997;19(1):175–184. [DOI] [PubMed] [Google Scholar]
- Liu Y, Joho RH. A side chain in S6 influences both open-state stability and ion permeation in a voltage-gated K+ channel. Pflugers Arch 1998;435(5):654–661; doi: 10.1007/s004240050566 [DOI] [PubMed] [Google Scholar]
- Liu Y, Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ Res 2002;90(12):1259–1266. [DOI] [PubMed] [Google Scholar]
- Liu Y, Terata K, Chai Q, et al. Peroxynitrite inhibits Ca2+-activated K+ channel activity in smooth muscle of human coronary arterioles. Circ Res 2002;91(11):1070–1076. [DOI] [PubMed] [Google Scholar]
- Loeb AL, Godeny I, Longnecker DE. Functional evidence for inward-rectifier potassium channels in rat cremaster muscle arterioles. Microvasc Res 2000;59(1):1–6; doi: 10.1006/mvre.1999.2187 [DOI] [PubMed] [Google Scholar]
- Loef M, Schrauzer GN, Walach H. Selenium and Alzheimer’s disease: A systematic review. J Alzheimers Dis 2011;26(1):81–104; doi: 10.3233/JAD-2011-110414 [DOI] [PubMed] [Google Scholar]
- Long SB, Campbell EB, Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 2005;309(5736):897–903; doi: 10.1126/science.1116269 [DOI] [PubMed] [Google Scholar]
- Longden TA, Dabertrand F, Hill-Eubanks DC, et al. Stress-induced glucocorticoid signaling remodels neurovascular coupling through impairment of cerebrovascular inwardly rectifying K+ channel function. Proc Natl Acad Sci U S A 2014;111(20):7462–7467; doi: 10.1073/pnas.1401811111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Holmgren A. The thioredoxin antioxidant system. Free Radic Biol Med 2014;66:75–87; doi: 10.1016/j.freeradbiomed.2013.07.036 [DOI] [PubMed] [Google Scholar]
- Lu T, He T, Katusic ZS, et al. Molecular mechanisms mediating inhibition of human large conductance Ca2+-activated K+ channels by high glucose. Circ Res 2006;99(6):607–616; doi: 10.1161/01.RES.0000243147.41792.93 [DOI] [PubMed] [Google Scholar]
- Lu T, Zhu YG, Yang J. Cytoplasmic amino and carboxyl domains form a wide intracellular vestibule in an inwardly rectifying potassium channel. Proc Natl Acad Sci USA 1999;96(17):9926–9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma SF, Luo Y, Ding YJ, et al. Hydrogen sulfide targets the Cys320/Cys529 Motif in Kv4.2 to inhibit the Ito potassium channels in cardiomyocytes and regularizes fatal arrhythmia in myocardial infarction. Antioxid Redox Signal 2015;23(2):129–147; doi: 10.1089/ars.2014.6094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Kubota H, et al. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem Biophys Res Commun 2002;290(3):909–913; doi: 10.1006/bbrc.2001.6278 [DOI] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Morikawa K, et al. Electron spin resonance detection of hydrogen peroxide as an endothelium-derived hyperpolarizing factor in porcine coronary microvessels. Arterioscler Thromb Vasc Biol 2003;23(7):1224–1230; doi: 10.1161/01.ATV.0000078601.79536.6C [DOI] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Nakashima M, et al. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 2000;106(12):1521–1530; doi: 10.1172/JCI10506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J Pharmacol Sci 2003;92(1):1–6. [DOI] [PubMed] [Google Scholar]
- Matsui M, Oshima M, Oshima H, et al. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev Biol 1996;178(1):179–185; doi: 10.1006/dbio.1996.0208 [DOI] [PubMed] [Google Scholar]
- Matsuura H, Shattock MJ. Effects of oxidant stress on steady-state background currents in isolated ventricular myocytes. Am J Physiol 1991;261(5 Pt 2):H1358–H1365. [DOI] [PubMed] [Google Scholar]
- Matsuura T, Harrison RA, Westwell AD, et al. Basal and angiotensin II-inhibited neuronal delayed-rectifier K+ current are regulated by thioredoxin. Am J Physiol Cell Physiol 2007;293(1):C211–C217; doi: 10.1152/ajpcell.00615.2006 [DOI] [PubMed] [Google Scholar]
- Matsuura T, Sun C, Leng L, et al. Macrophage migration inhibitory factor increases neuronal delayed rectifier K+ current. J Neurophysiol 2006;95(2):1042–1048; doi: 10.1152/jn.00499.2005 [DOI] [PubMed] [Google Scholar]
- Matthies D, Bae C, Toombes GE, et al. Single-particle cryo-EM structure of a voltage-activated potassium channel in lipid nanodiscs. Elife 2018;7; doi: 10.7554/eLife.37558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBean GJ, Aslan M, Griffiths HR, et al. Thiol redox homeostasis in neurodegenerative disease. Redox Biol 2015;5:186–194; doi: 10.1016/j.redox.2015.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley MJ, Albiston AL, Allen AM, et al. The brain renin-angiotensin system: Location and physiological roles. Int J Biochem Cell Biol 2003;35(6):901–918. [DOI] [PubMed] [Google Scholar]
- McLaughlin B, Pal S, Tran MP, et al. p38 activation is required upstream of potassium current enhancement and caspase cleavage in thiol oxidant-induced neuronal apoptosis. J Neurosci 2001;21(10):3303–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon AW, Wharton GT, Thornton P, et al. Octreotide use and safety in infants with hyperinsulinism. Pharmacoepidemiol Drug Saf 2017;26(1):26–31; doi: 10.1002/pds.4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci U S A 2000;97(10):5562–5567; doi: 10.1073/pnas.100118597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelakis ED, Rebeyka I, Wu X, et al. O2 sensing in the human ductus arteriosus: Regulation of voltage-gated K+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ Res 2002;91(6):478–486. [DOI] [PubMed] [Google Scholar]
- Mikami Y, Shibuya N, Kimura Y, et al. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem J 2011;439(3):479–485; doi: 10.1042/BJ20110841 [DOI] [PubMed] [Google Scholar]
- Mindnich RD, Penning TM. Aldo-keto reductase (AKR) superfamily: Genomics and annotation. Hum Genomics 2009;3(4):362–370; doi: 10.1186/1479-7364-3-4-362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Vizuete A, Ljung J, Damdimopoulos AE, et al. Characterization of Sptrx, a novel member of the thioredoxin family specifically expressed in human spermatozoa. J Biol Chem 2001;276(34):31567–31574; doi: 10.1074/jbc.M101760200 [DOI] [PubMed] [Google Scholar]
- Misonou H, Mohapatra DP, Trimmer JS. Kv2.1: A voltage-gated K+ channel critical to dynamic control of neuronal excitability. Neurotoxicology 2005;26(5):743–752; doi: 10.1016/j.neuro.2005.02.003 [DOI] [PubMed] [Google Scholar]
- Miura H, Bosnjak JJ, Ning G, et al. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res 2003;92(2):e31–e40. [DOI] [PubMed] [Google Scholar]
- Modrick ML, Didion SP, Lynch CM, et al. Role of hydrogen peroxide and the impact of glutathione peroxidase-1 in regulation of cerebral vascular tone. J Cereb Blood Flow Metab 2009;29(6):1130–1137; doi: 10.1038/jcbfm.2009.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales P, Garneau L, Klein H, et al. Contribution of the KCa3.1 channel-calmodulin interactions to the regulation of the KCa3.1 gating process. J Gen Physiol 2013;142(1):37–60; doi: 10.1085/jgp.201210933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran Y, Barzilai MG, Liebeskind BJ, et al. Evolution of voltage-gated ion channels at the emergence of Metazoa. J Exp Biol 2015;218(Pt 4):515–525; doi: 10.1242/jeb.110270 [DOI] [PubMed] [Google Scholar]
- Morikawa K, Shimokawa H, Matoba T, et al. Pivotal role of Cu, Zn-superoxide dismutase in endothelium-dependent hyperpolarization. J Clin Invest 2003;112(12):1871–1879; doi: 10.1172/JCI19351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AK, Gadalla MM, Sen N, et al. H2S signals through protein S-sulfhydration. Sci Signal 2009;2(96):ra72; doi: 10.1126/scisignal.2000464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers PR, Gupta M, Rogers S, et al. Chronic endotoxemia and endothelium-dependent vasodilation in coronary arteries. Shock 1996;6(4):267–273. [DOI] [PubMed] [Google Scholar]
- Nagahara N. Intermolecular disulfide bond to modulate protein function as a redox-sensing switch. Amino Acids 2011;41(1):59–72; doi: 10.1007/s00726-010-0508-4 [DOI] [PubMed] [Google Scholar]
- Nakamura H, De Rosa S, Roederer M, et al. Elevation of plasma thioredoxin levels in HIV-infected individuals. Int Immunol 1996;8(4):603–611. [DOI] [PubMed] [Google Scholar]
- Neckář J, Hye Khan MA, Gross GJ, et al. Epoxyeicosatrienoic acid analog EET-B attenuates post-myocardial infarction remodeling in spontaneously hypertensive rats. Clin Sci (Lond) 2019;133(8):939–951; doi: 10.1042/CS20180728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol 1995;268(4 Pt 1):C799–C822. [DOI] [PubMed] [Google Scholar]
- Neo BH, Patel D, Kandhi S, et al. Roles for cytosolic NADPH redox in regulating pulmonary artery relaxation by thiol oxidation-elicited subunit dimerization of protein kinase G1alpha. Am J Physiol Heart Circ Physiol 2013;305(3):H330–H343; doi: 10.1152/ajpheart.01010.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MT, Lue H, Kleemann R, et al. The cytokine macrophage migration inhibitory factor reduces pro-oxidative stress-induced apoptosis. J Immunol 2003;170(6):3337–3347. [DOI] [PubMed] [Google Scholar]
- Niu X, Magleby KL. Stepwise contribution of each subunit to the cooperative activation of BK channels by Ca2+. Proc Natl Acad Sci U S A 2002;99(17):11441–11446; doi: 10.1073/pnas.172254699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonn L, Williams RR, Erickson RP, et al. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol 2003;23(3):916–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med 2001;31(11):1287–1312. [DOI] [PubMed] [Google Scholar]
- Nunez E, Jones F, Muguruza-Montero A, et al. Redox regulation of K(V)7 channels through EF3 hand of calmodulin. Elife 2023;12; doi: 10.7554/eLife.81961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi M, Faraci F, Heistad D. Peroxynitrite hyperpolarizes smooth muscle and relaxes internal carotid artery in rabbit via ATP-sensitive K+ channels. Am J Physiol Heart Circ Physiol 2005;289(5):H2244–H2250; doi: 10.1152/ajpheart.00254.2005 [DOI] [PubMed] [Google Scholar]
- Okabe K, Kitamura K, Kuriyama H. Features of 4-aminopyridine sensitive outward current observed in single smooth muscle cells from the rabbit pulmonary artery. Pflugers Arch 1987;409(6):561–568. [DOI] [PubMed] [Google Scholar]
- Olschewski A, Hong Z, Peterson DA, et al. Opposite effects of redox status on membrane potential, cytosolic calcium, and tone in pulmonary arteries and ductus arteriosus. Am J Physiol Lung Cell Mol Physiol 2004;286(1):L15–L22; doi: 10.1152/ajplung.00372.2002 [DOI] [PubMed] [Google Scholar]
- Olschewski A, Weir EK. Redox regulation of ion channels in the pulmonary circulation. Antioxid Redox Signal 2015;22(6):465–485; doi: 10.1089/ars.2014.5899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oparil S, Yang RH, Jin HG, et al. Role of anterior hypothalamic angiotensin II in the pathogenesis of salt sensitive hypertension in the spontaneously hypertensive rat. Am J Med Sci 1994;307(Suppl 1):S26–S37. [PubMed] [Google Scholar]
- Pallandi RT, Perry MA, Campbell TJ. Proarrhythmic effects of an oxygen-derived free radical generating system on action potentials recorded from guinea pig ventricular myocardium: A possible cause of reperfusion-induced arrhythmias. Circ Res 1987;61(1):50–54. [DOI] [PubMed] [Google Scholar]
- Panyi G, Vamosi G, Bodnar A, et al. Looking through ion channels: Recharged concepts in T-cell signaling. Trends Immunol 2004;25(11):565–569; doi: 10.1016/j.it.2004.09.002 [DOI] [PubMed] [Google Scholar]
- Paravicini TM, Chrissobolis S, Drummond GR, et al. Increased NADPH-oxidase activity and Nox4 expression during chronic hypertension is associated with enhanced cerebral vasodilatation to NADPH in vivo. Stroke 2004;35(2):584–589; doi: 10.1161/01.STR.0000112974.37028.58 [DOI] [PubMed] [Google Scholar]
- Park MK, Lee SH, Ho WK, et al. Redox agents as a link between hypoxia and the responses of ionic channels in rabbit pulmonary vascular smooth muscle. Exp Physiol 1995;80(5):835–842. [DOI] [PubMed] [Google Scholar]
- Park SW, Noh HJ, Sung DJ, et al. Hydrogen peroxide induces vasorelaxation by enhancing 4-aminopyridine-sensitive Kv currents through S-glutathionylation. Pflugers Arch 2015;467(2):285–297; doi: 10.1007/s00424-014-1513-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude A, Murthy MR, Mirault ME. Emerging roles of thioredoxin cycle enzymes in the central nervous system. Cell Mol Life Sci 2005;62(10):1063–1080; doi: 10.1007/s00018-005-4541-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau VP, Smith FJ, Taylor AB, et al. Structure and function of multiple Ca2+-binding sites in a K+ channel regulator of K+ conductance (RCK) domain. Proc Natl Acad Sci U S A 2011;108(43):17684–17689; doi: 10.1073/pnas.1107229108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluger S, Faulhaber J, Furstenau M, et al. Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca(2+) spark/STOC coupling and elevated blood pressure. Circ Res 2000;87(11):E53–E60. [DOI] [PubMed] [Google Scholar]
- Pomposiello S, Rhaleb NE, Alva M, et al. Reactive oxygen species: Role in the relaxation induced by bradykinin or arachidonic acid via EDHF in isolated porcine coronary arteries. J Cardiovasc Pharmacol 1999;34(4):567–574. [DOI] [PubMed] [Google Scholar]
- Pongs O, Kecskemethy N, Muller R, et al. Shaker encodes a family of putative potassium channel proteins in the nervous system of Drosophila. EMBO J 1988;7(4):1087–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongs O, Schwarz JR. Ancillary subunits associated with voltage-dependent K+ channels. Physiol Rev 2010;90(2):755–796; doi: 10.1152/physrev.00020.2009 [DOI] [PubMed] [Google Scholar]
- Poole LB, Karplus PA, Claiborne A. Protein sulfenic acids in redox signaling. Annu Rev Pharmacol Toxicol 2004;44:325–347; doi: 10.1146/annurev.pharmtox.44.101802.121735 [DOI] [PubMed] [Google Scholar]
- Prasad M, Goyal RK. Differential modulation of voltage-dependent K+ currents in colonic smooth muscle by oxidants. Am J Physiol Cell Physiol 2004;286(3):C671–C682; doi: 10.1152/ajpcell.00137.2003 [DOI] [PubMed] [Google Scholar]
- Prysyazhna O, Rudyk O, Eaton P. Single atom substitution in mouse protein kinase G eliminates oxidant sensing to cause hypertension. Nat Med 2012;18(2):286–290; doi: 10.1038/nm.2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle JM, McCarron JG, Brayden JE, et al. Inward rectifier K+ currents in smooth muscle cells from rat resistance-sized cerebral arteries. Am J Physiol 1993;265(5 Pt 1):C1363–C1370. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev 1997;77(4):1165–1232. [DOI] [PubMed] [Google Scholar]
- Reeve HL, Weir EK, Nelson DP, et al. Opposing effects of oxidants and antioxidants on K+ channel activity and tone in rat vascular tissue. Exp Physiol 1995;80(5):825–834. [DOI] [PubMed] [Google Scholar]
- Resnik E, Herron J, Keck M, et al. Chronic intrauterine pulmonary hypertension selectively modifies pulmonary artery smooth muscle cell gene expression. Am J Physiol Lung Cell Mol Physiol 2006;290(3):L426–L433; doi: 10.1152/ajplung.00281.2005 [DOI] [PubMed] [Google Scholar]
- Rhee SG, Kang SW, Chang TS, et al. Peroxiredoxin, a novel family of peroxidases. IUBMB Life 2001;52(1–2):35–41; doi: 10.1080/15216540252774748 [DOI] [PubMed] [Google Scholar]
- Rodrigo GC, Standen NB. ATP-sensitive potassium channels. Curr Pharm Des 2005;11(15):1915–1940; doi: 10.2174/1381612054021015 [DOI] [PubMed] [Google Scholar]
- Rozanski GJ, Xu Z. Glutathione and K(+) channel remodeling in postinfarction rat heart. Am J Physiol Heart Circ Physiol 2002a;282(6):H2346–H2355; doi: 10.1152/ajpheart.00894.2001 [DOI] [PubMed] [Google Scholar]
- Rozanski GJ, Xu Z. Sulfhydryl modulation of K+ channels in rat ventricular myocytes. J Mol Cell Cardiol 2002b;34(12):1623–1632. [DOI] [PubMed] [Google Scholar]
- Rozell B, Hansson HA, Luthman M, et al. Immunohistochemical localization of thioredoxin and thioredoxin reductase in adult rats. Eur J Cell Biol 1985;38(1):79–86. [PubMed] [Google Scholar]
- Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol 1986;250(5 Pt 2):H822–H827. [DOI] [PubMed] [Google Scholar]
- Rubartelli A, Bajetto A, Allavena G, et al. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J Biol Chem 1992;267(34):24161–24164. [PubMed] [Google Scholar]
- Rubartelli A, Bonifaci N, Sitia R. High rates of thioredoxin secretion correlate with growth arrest in hepatoma cells. Cancer Res 1995;55(3):675–680. [PubMed] [Google Scholar]
- Ruppersberg JP, Stocker M, Pongs O, et al. Regulation of fast inactivation of cloned mammalian IK(A) channels by cysteine oxidation. Nature 1991;352(6337):711–714; doi: 10.1038/352711a0 [DOI] [PubMed] [Google Scholar]
- Sahlin L, Stjernholm Y, Holmgren A, et al. The expression of thioredoxin mRNA is increased in the human cervix during pregnancy. Mol Hum Reprod 1997;3(12):1113–1117. [DOI] [PubMed] [Google Scholar]
- Sahoo N, Schonherr R, Hoshi T, et al. Cysteines control the N- and C-linker-dependent gating of KCNH1 potassium channels. Biochim Biophys Acta 2012;1818(5):1187–1195; doi: 10.1016/j.bbamem.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J 1998;17(9):2596–2606; doi: 10.1093/emboj/17.9.2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow SL, Grayson TH. Limits of isolation and culture: Intact vascular endothelium and BKCa. Am J Physiol Heart Circ Physiol 2009;297(1):H1–H7; doi: 10.1152/ajpheart.00042.2009 [DOI] [PubMed] [Google Scholar]
- Sandow SL, Gzik DJ, Lee RM. Arterial internal elastic lamina holes: Relationship to function? J Anat 2009;214(2):258–266; doi: 10.1111/j.1469-7580.2008.01020.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow SL, Neylon CB, Chen MX, et al. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels [K(Ca)] and connexins: Possible relationship to vasodilator function? J Anat 2006;209(5):689–698; doi: 10.1111/j.1469-7580.2006.00647.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago E, Contreras C, Garcia-Sacristan A, et al. Signaling pathways involved in the H2O2-induced vasoconstriction of rat coronary arteries. Free Radic Biol Med 2013;60:136–146; doi: 10.1016/j.freeradbiomed.2013.02.014 [DOI] [PubMed] [Google Scholar]
- Schach C, Xu M, Platoshyn O, et al. Thiol oxidation causes pulmonary vasodilation by activating K+ channels and inhibiting store-operated Ca2+ channels. Am J Physiol Lung Cell Mol Physiol 2007;292(3):L685–L698; doi: 10.1152/ajplung.00276.2006 [DOI] [PubMed] [Google Scholar]
- Schlaich MP, Lambert E, Kaye DM, et al. Sympathetic augmentation in hypertension: Role of nerve firing, norepinephrine reuptake, and Angiotensin neuromodulation. Hypertension 2004;43(2):169–175; doi: 10.1161/01.HYP.0000103160.35395.9E [DOI] [PubMed] [Google Scholar]
- Schlief T, Schonherr R, Heinemann SH. Modification of C-type inactivating Shaker potassium channels by chloramine-T. Pflugers Arch 1996;431(4):483–493. [DOI] [PubMed] [Google Scholar]
- Schnell JR, Zhou GP, Zweckstetter M, et al. Rapid and accurate structure determination of coiled-coil domains using NMR dipolar couplings: Application to cGMP-dependent protein kinase Ialpha. Protein Sci 2005;14(9):2421–2428; doi: 10.1110/ps.051528905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert R, Nelson MT. Protein kinases: Tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci 2001;22(10):505–512. [DOI] [PubMed] [Google Scholar]
- Schulte U, Hahn H, Wiesinger H, et al. pH-dependent gating of ROMK (Kir1.1) channels involves conformational changes in both N and C termini. J Biol Chem 1998;273(51):34575–34579. [DOI] [PubMed] [Google Scholar]
- Schumacher MA, Rivard AF, Bachinger HP, et al. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature 2001;410(6832):1120–1124; doi: 10.1038/35074145 [DOI] [PubMed] [Google Scholar]
- Sengupta R, Coppo L, Mishra P, et al. Glutathione-glutaredoxin is an efficient electron donor system for mammalian p53R2-R1-dependent ribonucleotide reductase. J Biol Chem 2019;294(34):12708–12716; doi: 10.1074/jbc.RA119.008752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Chen H, Togawa T, et al. Albumin thiolate anion is an intermediate in the formation of albumin-S-S-homocysteine. J Biol Chem 2001;276(32):30111–30117; doi: 10.1074/jbc.M104324200 [DOI] [PubMed] [Google Scholar]
- Shah NH, Aizenman E. Voltage-gated potassium channels at the crossroads of neuronal function, ischemic tolerance, and neurodegeneration. Transl Stroke Res 2014;5(1):38–58; doi: 10.1007/s12975-013-0297-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehe JL, Bonev AD, Schmoker AM, et al. Oxidation of cysteine 117 stimulates constitutive activation of the type Ialpha cGMP-dependent protein kinase. J Biol Chem 2018;293(43):16791–16802; doi: 10.1074/jbc.RA118.004363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa H, Morikawa K. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J Mol Cell Cardiol 2005;39(5):725–732; doi: 10.1016/j.yjmcc.2005.07.007 [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Yasutake H, Fujii K, et al. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol 1996;28(5):703–711. [DOI] [PubMed] [Google Scholar]
- Si H, Heyken WT, Wolfle SE, et al. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res 2006;99(5):537–544; doi: 10.1161/01.RES.0000238377.08219.0c [DOI] [PubMed] [Google Scholar]
- Sies H, Berndt C, Jones DP. Oxidative Stress. Annu Rev Biochem 2017;86:715–748; doi: 10.1146/annurev-biochem-061516-045037 [DOI] [PubMed] [Google Scholar]
- Sigworth FJ. Voltage gating of ion channels. Q Rev Biophys 1994;27(1):1–40. [DOI] [PubMed] [Google Scholar]
- Silva-Adaya D, Gonsebatt ME, Guevara J. Thioredoxin system regulation in the central nervous system: Experimental models and clinical evidence. Oxid Med Cell Longev 2014;2014:590808; doi: 10.1155/2014/590808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes M, Garneau L, Klein H, et al. Cysteine mutagenesis and computer modeling of the S6 region of an intermediate conductance IKCa channel. J Gen Physiol 2002;120(1):99–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims C, Harvey RD. Redox modulation of basal and beta-adrenergically stimulated cardiac L-type Ca(2+) channel activity by phenylarsine oxide. Br J Pharmacol 2004;142(4):797–807; doi: 10.1038/sj.bjp.0705845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Mennone A, Gigliozzi A, et al. Cl(-)-dependent secretory mechanisms in isolated rat bile duct epithelial units. Am J Physiol Gastrointest Liver Physiol 2001;281(2):G438–G446. [DOI] [PubMed] [Google Scholar]
- Smirnov SV, Aaronson PI. Ca(2+)-activated and voltage-gated K+ currents in smooth muscle cells isolated from human mesenteric arteries. J Physiol 1992;457:431–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobey CG, Heistad DD, Faraci FM. Mechanisms of bradykinin-induced cerebral vasodilatation in rats. Evidence that reactive oxygen species activate K+ channels. Stroke 1997;28(11):2290–2294; discussion 2295. [DOI] [PubMed] [Google Scholar]
- Sobey CG, Heistad DD, Faraci FM. Potassium channels mediate dilatation of cerebral arterioles in response to arachidonate. Am J Physiol 1998;275(5):H1606–1612. [DOI] [PubMed] [Google Scholar]
- Soh H, Jung W, Uhm DY, et al. Modulation of large conductance calcium-activated potassium channels from rat hippocampal neurons by glutathione. Neurosci Lett 2001;298(2):115–118. [DOI] [PubMed] [Google Scholar]
- Sokol PT, Hu W, Yi L, et al. Cloning of an apamin binding protein of vascular smooth muscle. J Protein Chem 1994;13(1):117–128. [DOI] [PubMed] [Google Scholar]
- Sonkusare SK, Dalsgaard T, Bonev AD, et al. Inward rectifier potassium (Kir2.1) channels as end-stage boosters of endothelium-dependent vasodilators. J Physiol 2016;594(12):3271–3285; doi: 10.1113/JP271652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto MA, Gonzalez C, Lissi E, et al. Ca(2+)-activated K+ channel inhibition by reactive oxygen species. Am J Physiol Cell Physiol 2002;282(3):C461–C471; doi: 10.1152/ajpcell.00167.2001 [DOI] [PubMed] [Google Scholar]
- Spyrou G, Enmark E, Miranda-Vizuete A, et al. Cloning and expression of a novel mammalian thioredoxin. J Biol Chem 1997;272(5):2936–2941. [DOI] [PubMed] [Google Scholar]
- Stephens GJ, Owen DG, Robertson B. Cysteine-modifying reagents alter the gating of the rat cloned potassium channel Kv1.4. Pflugers Arch 1996;431(3):435–442. [DOI] [PubMed] [Google Scholar]
- Steudel W, Watanabe M, Dikranian K, et al. Expression of nitric oxide synthase isoforms (NOS II and NOS III) in adult rat lung in hyperoxic pulmonary hypertension. Cell Tissue Res 1999;295(2):317–329. [DOI] [PubMed] [Google Scholar]
- Stockand JD, Sansom SC. Role of large Ca(2+)-activated K+ channels in regulation of mesangial contraction by nitroprusside and ANP. Am J Physiol 1996;270(6 Pt 1):C1773–C1779. [DOI] [PubMed] [Google Scholar]
- Strupp M, Quasthoff S, Mitrovic N, et al. Glutathione accelerates sodium channel inactivation in excised rat axonal membrane patches. Pflugers Arch 1992;421(2–3):283–285. [DOI] [PubMed] [Google Scholar]
- Subramani J, Kundumani-Sridharan V, Das KC. Chaperone-mediated autophagy of eNOS in myocardial ischemia-reperfusion injury. Circ Res 2021;129(10):930–945; doi: 10.1161/CIRCRESAHA.120.317921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramani J, Kundumani-Sridharan V, Hilgers RH, et al. Thioredoxin uses a GSH-independent route to deglutathionylate endothelial nitric-oxide synthase and protect against myocardial infarction. J Biol Chem 2016;291(45):23374–23389; doi: 10.1074/jbc.M116.745034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Li H, Leng L, et al. Macrophage migration inhibitory factor: An intracellular inhibitor of angiotensin II-induced increases in neuronal activity. J Neurosci 2004;24(44):9944–9952; doi: 10.1523/JNEUROSCI.2856-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Sellers KW, Sumners C, et al. NAD(P)H oxidase inhibition attenuates neuronal chronotropic actions of angiotensin II. Circ Res 2005;96(6):659–666; doi: 10.1161/01.RES.0000161257.02571.4b [DOI] [PubMed] [Google Scholar]
- Svoboda LK, Reddie KG, Zhang L, et al. Redox-sensitive sulfenic acid modification regulates surface expression of the cardiovascular voltage-gated potassium channel Kv1.5. Circ Res 2012;111(7):842–853; doi: 10.1161/CIRCRESAHA.111.263525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Lin H, Geshi N, et al. Nitric oxide-cGMP-protein kinase G pathway negatively regulates vascular transient receptor potential channel TRPC6. J Physiol 2008;586(17):4209–4223; doi: 10.1113/jphysiol.2008.156083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamargo J, Caballero R, Gomez R, et al. Pharmacology of cardiac potassium channels. Cardiovasc Res 2004;62(1):9–33; doi: 10.1016/j.cardiores.2003.12.026 [DOI] [PubMed] [Google Scholar]
- Tang K, Li X, Zheng MQ, et al. Role of apoptosis signal-regulating kinase-1-c-Jun NH2-terminal kinase-p38 signaling in voltage-gated K+ channel remodeling of the failing heart: Regulation by thioredoxin. Antioxid Redox Signal 2011;14(1):25–35; doi: 10.1089/ars.2010.3095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XD, Daggett H, Hanner M, et al. Oxidative regulation of large conductance calcium-activated potassium channels. J Gen Physiol 2001;117(3):253–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XD, Garcia ML, Heinemann SH, et al. Reactive oxygen species impair Slo1 BK channel function by altering cysteine-mediated calcium sensing. Nat Struct Mol Biol 2004;11(2):171–178; doi: 10.1038/nsmb725 [DOI] [PubMed] [Google Scholar]
- Tang XD, Xu R, Reynolds MF, et al. Haem can bind to and inhibit mammalian calcium-dependent Slo1 BK channels. Nature 2003;425(6957):531–535; doi: 10.1038/nature02003 [DOI] [PubMed] [Google Scholar]
- Tao L, Gao E, Bryan NS, et al. Cardioprotective effects of thioredoxin in myocardial ischemia and reperfusion: Role of S-nitrosation [corrected]. Proc Natl Acad Sci U S A 2004;101(31):11471–11476; doi: 10.1073/pnas.0402941101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr M, Arriaga E, Goertz KK, et al. Properties of cardiac I(leak) induced by photosensitizer-generated reactive oxygen. Free Radic Biol Med 1994;16(4):477–484. [DOI] [PubMed] [Google Scholar]
- Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 2000;279(6):L1005–L1028. [DOI] [PubMed] [Google Scholar]
- Tharp DL, Bowles DK. The intermediate-conductance Ca2+-activated K+ channel (KCa3.1) in vascular disease. Cardiovasc Hematol Agents Med Chem 2009;7(1):1–11. [DOI] [PubMed] [Google Scholar]
- Thengchaisri N, Kuo L. Hydrogen peroxide induces endothelium-dependent and-independent coronary arteriolar dilation: Role of cyclooxygenase and potassium channels. Am J Physiol Heart Circ Physiol 2003;285(6):H2255–H2263; doi: 10.1152/ajpheart.00487.2003 [DOI] [PubMed] [Google Scholar]
- Tipparaju SM, Barski OA, Srivastava S, et al. Catalytic mechanism and substrate specificity of the beta-subunit of the voltage-gated potassium channel. Biochemistry 2008;47(34):8840–8854; doi: 10.1021/bi800301b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipple TE. The thioredoxin system in neonatal lung disease. Antioxid Redox Signal 2014;21(13):1916–1925; doi: 10.1089/ars.2013.5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro L, Wallner M, Meera P, et al. Maxi-K(Ca), a unique member of the voltage-gated k channel superfamily. News Physiol Sci 1998;13:112–117. [DOI] [PubMed] [Google Scholar]
- Toyama K, Wulff H, Chandy KG, et al. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J Clin Invest 2008;118(9):3025–3037; doi: 10.1172/JCI30836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Tucker SJ, Ashcroft FM. Mechanism of ATP-sensitive K channel inhibition by sulfhydryl modification. J Gen Physiol 1998;112(3):325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay SK, Eckel-Mahan KL, Mirbolooki MR, et al. Selective Kv1.3 channel blocker as therapeutic for obesity and insulin resistance. Proc Natl Acad Sci U S A 2013;110(24):E2239–E2248; doi: 10.1073/pnas.1221206110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z, Tajti G, Panyi G. The Kv1.3 K(+) channel in the immune system and its “precision pharmacology” using peptide toxins. Biol Futur 2021;72(1):75–83; doi: 10.1007/s42977-021-00071-7 [DOI] [PubMed] [Google Scholar]
- Veit F, Pak O, Brandes RP, et al. Hypoxia-dependent reactive oxygen species signaling in the pulmonary circulation: Focus on ion channels. Antioxid Redox Signal 2015;22(6):537–552; doi: 10.1089/ars.2014.6234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk KA, Shibata EF. Single delayed rectifier potassium channels from rabbit coronary artery myocytes. Am J Physiol 1993;264(4 Pt 2):H1146–H1153. [DOI] [PubMed] [Google Scholar]
- Walsh KB. Targeting cardiac potassium channels for state-of-the-art drug discovery. Expert Opin Drug Discov 2015;10(2):157–169; doi: 10.1517/17460441.2015.983471 [DOI] [PubMed] [Google Scholar]
- Wang D, Sumners C, Posner P, et al. A-type K+ current in neurons cultured from neonatal rat hypothalamus and brain stem: Modulation by angiotensin II. J Neurophysiol 1997a;78(2):1021–1029. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang HT, Su XL, et al. Experimental diabetes mellitus down-regulates large-conductance Ca2+-activated K+ channels in cerebral artery smooth muscle and alters functional conductance. Curr Neurovasc Res 2010;7(2):75–84. [DOI] [PubMed] [Google Scholar]
- Wang YW, Ding JP, Xia XM, et al. Consequences of the stoichiometry of Slo1 alpha and auxiliary beta subunits on functional properties of large-conductance Ca2+-activated K+ channels. J Neurosci 2002;22(5):1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Fermini B, Nattel S. Delayed rectifier outward current and repolarization in human atrial myocytes. Circ Res 1993;73(2):276–285; doi: 10.1161/01.res.73.2.276 [DOI] [PubMed] [Google Scholar]
- Wang Z, Kiehn J, Yang Q, et al. Comparison of binding and block produced by alternatively spliced Kvbeta1 subunits. J Biol Chem 1996;271(45):28311–28317. [DOI] [PubMed] [Google Scholar]
- Wang ZW, Nara M, Wang YX, et al. Redox regulation of large conductance Ca(2+)-activated K+ channels in smooth muscle cells. J Gen Physiol 1997b;110(1):35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedmann R, Onderka C, Wei S, et al. Improved tag-switch method reveals that thioredoxin acts as depersulfidase and controls the intracellular levels of protein persulfidation. Chem Sci 2016;7(5):3414–3426; doi: 10.1039/c5sc04818d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei EP, Kontos HA, Beckman JS. Mechanisms of cerebral vasodilation by superoxide, hydrogen peroxide, and peroxynitrite. Am J Physiol 1996;271(3 Pt 2):H1262–H1266. [DOI] [PubMed] [Google Scholar]
- Weichsel A, Gasdaska JR, Powis G, et al. Crystal structures of reduced, oxidized, and mutated human thioredoxins: Evidence for a regulatory homodimer. Structure 1996;4(6):735–751. [DOI] [PubMed] [Google Scholar]
- Weir EK, Lopez-Barneo J, Buckler KJ, et al. Acute oxygen-sensing mechanisms. N Engl J Med 2005;353(19):2042–2055; doi: 10.1056/NEJMra050002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir EK, Will JA, Lundquist LJ, et al. Diamide inhibits pulmonary vasoconstriction induced by hypoxia or prostaglandin F2 alpha. Proc Soc Exp Biol Med 1983;173(1):96–103. [DOI] [PubMed] [Google Scholar]
- Weissmann N, Dietrich A, Fuchs B, et al. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci U S A 2006;103(50):19093–19098; doi: 10.1073/pnas.0606728103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J, Cao Y, Moss N, et al. Modulation of voltage-dependent Shaker family potassium channels by an aldo-keto reductase. J Biol Chem 2006;281(22):15194–15200; doi: 10.1074/jbc.M513809200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whayne TF,Jr, Parinandi N, Maulik N. Thioredoxins in cardiovascular disease. Can J Physiol Pharmacol 2015;93(11):903–911; doi: 10.1139/cjpp-2015-0105 [DOI] [PubMed] [Google Scholar]
- Whyte KA, Hogg RC, Dyavanapalli J, et al. Reactive oxygen species modulate neuronal excitability in rat intrinsic cardiac ganglia. Auton Neurosci 2009;150(1–2):45–52; doi: 10.1016/j.autneu.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin MS. Reactive oxygen species and the control of vascular function. Am J Physiol Heart Circ Physiol 2009;296(3):H539–H549; doi: 10.1152/ajpheart.01167.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science 2003;300(5619):650–653; doi: 10.1126/science.1080405 [DOI] [PubMed] [Google Scholar]
- Wu BN, Luykenaar KD, Brayden JE, et al. Hyposmotic challenge inhibits inward rectifying K+ channels in cerebral arterial smooth muscle cells. Am J Physiol Heart Circ Physiol 2007;292(2):H1085–H1094; doi: 10.1152/ajpheart.00926.2006 [DOI] [PubMed] [Google Scholar]
- Wu XW, Teng ZY, Jiang LH, et al. Human thioredoxin exerts cardioprotective effect and attenuates reperfusion injury in rats partially via inhibiting apoptosis. Chin Med J (Engl) 2008;121(9):819–826. [PubMed] [Google Scholar]
- Wu Y, Yang L, Zhong L. Decreased serum levels of thioredoxin in patients with coronary artery disease plus hyperhomocysteinemia is strongly associated with the disease severity. Atherosclerosis 2010;212(1):351–355; doi: 10.1016/j.atherosclerosis.2010.06.002 [DOI] [PubMed] [Google Scholar]
- Wulff H, Castle NA. Therapeutic potential of KCa3.1 blockers: Recent advances and promising trends. Expert Rev Clin Pharmacol 2010;3(3):385–396; doi: 10.1586/ecp.10.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Ding JP, Lingle CJ. Inactivation of BK channels by the NH2 terminus of the beta2 auxiliary subunit: An essential role of a terminal peptide segment of three hydrophobic residues. J Gen Physiol 2003;121(2):125–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Fakler B, Rivard A, et al. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 1998;395(6701):503–507; doi: 10.1038/26758 [DOI] [PubMed] [Google Scholar]
- Xia XM, Zeng X, Lingle CJ. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature 2002;418(6900):880–884; doi: 10.1038/nature00956 [DOI] [PubMed] [Google Scholar]
- Xie Z, Barski OA, Cai J, et al. Catalytic reduction of carbonyl groups in oxidized PAPC by Kvbeta2 (AKR6). Chem Biol Interact 2011;191(1–3):255–260; doi: 10.1016/j.cbi.2011.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Koni PA, Wang P, et al. The voltage-gated potassium channel Kv1.3 regulates energy homeostasis and body weight. Hum Mol Genet 2003;12(5):551–559; doi: 10.1093/hmg/ddg049 [DOI] [PubMed] [Google Scholar]
- Xu SZ, Sukumar P, Zeng F, et al. TRPC channel activation by extracellular thioredoxin. Nature 2008;451(7174):69–72; doi: 10.1038/nature06414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yada T, Shimokawa H, Hiramatsu O, et al. Hydrogen peroxide, an endogenous endothelium-derived hyperpolarizing factor, plays an important role in coronary autoregulation in vivo. Circulation 2003;107(7):1040–1045. [DOI] [PubMed] [Google Scholar]
- Yan XS, Ma JH, Zhang PH. Modulation of K(ATP) currents in rat ventricular myocytes by hypoxia and a redox reaction. Acta Pharmacol Sin 2009;30(10):1399–1414; doi: 10.1038/aps.2009.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shi W, Chen X, et al. Molecular basis and structural insight of vascular K(ATP) channel gating by S-glutathionylation. J Biol Chem 2011;286(11):9298–9307; doi: 10.1074/jbc.M110.195123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shi W, Cui N, et al. Oxidative stress inhibits vascular K(ATP) channels by S-glutathionylation. J Biol Chem 2010;285(49):38641–38648; doi: 10.1074/jbc.M110.162578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZW, Zhang A, Altura BT, et al. Endothelium-dependent relaxation to hydrogen peroxide in canine basilar artery: A potential new cerebral dilator mechanism. Brain Res Bull 1998;47(3):257–263. [DOI] [PubMed] [Google Scholar]
- Yap FC, Weber DS, Taylor MS, et al. Endothelial SK3 channel-associated Ca2+ microdomains modulate blood pressure. Am J Physiol Heart Circ Physiol 2016;310(9):H1151–H1163; doi: 10.1152/ajpheart.00787.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L, Jenkins PM, Leichert LI, et al. Heme regulatory motifs in heme oxygenase-2 form a thiol/disulfide redox switch that responds to the cellular redox state. J Biol Chem 2009;284(31):20556–20561; doi: 10.1074/jbc.M109.015651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi L, Morgan JT, Ragsdale SW. Identification of a thiol/disulfide redox switch in the human BK channel that controls its affinity for heme and CO. J Biol Chem 2010;285(26):20117–20127; doi: 10.1074/jbc.M110.116483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying L, Xu X, Liu J, et al. Heterogeneity in relaxation of different sized porcine coronary arteries to nitrovasodilators: Role of PKG and MYPT1. Pflugers Arch 2012;463(2):257–268; doi: 10.1007/s00424-011-1040-4 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Katoh T, Tetsuka T, et al. Involvement of thioredoxin in rheumatoid arthritis: Its costimulatory roles in the TNF-alpha-induced production of IL-6 and IL-8 from cultured synovial fibroblasts. J Immunol 1999;163(1):351–358. [PubMed] [Google Scholar]
- Yu SP, Yeh CH, Sensi SL, et al. Mediation of neuronal apoptosis by enhancement of outward potassium current. Science 1997;278(5335):114–117. [DOI] [PubMed] [Google Scholar]
- Yuan XJ, Tod ML, Rubin LJ, et al. Deoxyglucose and reduced glutathione mimic effects of hypoxia on K+ and Ca2+ conductances in pulmonary artery cells. Am J Physiol 1994;267(1 Pt 1):L52–L63. [DOI] [PubMed] [Google Scholar]
- Zagotta WN, Hoshi T, Aldrich RW. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science 1990;250(4980):568–571. [DOI] [PubMed] [Google Scholar]
- Zeidner G, Sadja R, Reuveny E. Redox-dependent gating of G protein-coupled inwardly rectifying K+ channels. J Biol Chem 2001;276(38):35564–35570; doi: 10.1074/jbc.M105189200 [DOI] [PubMed] [Google Scholar]
- Zhang DX, Borbouse L, Gebremedhin D, et al. H2O2-induced dilation in human coronary arterioles: role of protein kinase G dimerization and large-conductance Ca2+-activated K+ channel activation. Circ Res 2012;110(3):471–480; doi: 10.1161/CIRCRESAHA.111.258871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Horrigan FT. Cysteine modification alters voltage- and Ca(2+)-dependent gating of large conductance (BK) potassium channels. J Gen Physiol 2005;125(2):213–236; doi: 10.1085/jgp.200409149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Xu R, Heinemann SH, et al. Cysteine oxidation and rundown of large-conductance Ca2+-dependent K+ channels. Biochem Biophys Res Commun 2006;342(4):1389–1395; doi: 10.1016/j.bbrc.2006.02.079 [DOI] [PubMed] [Google Scholar]
- Zhang HJ, Liu Y, Zuhlke RD, et al. Oxidation of an engineered pore cysteine locks a voltage-gated K+ channel in a nonconducting state. Biophys J 1996;71(6):3083–3090; doi: 10.1016/S0006-3495(96)79502-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XD, Lieu DK, Chiamvimonvat N. Small-conductance Ca2+-activated K+ channels and cardiac arrhythmias. Heart Rhythm 2015;12(8):1845–1851; doi: 10.1016/j.hrthm.2015.04.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XB, Arntz C, Kamm S, et al. A molecular switch for specific stimulation of the BKCa channel by cGMP and cAMP kinase. J Biol Chem 2001;276(46):43239–43245; doi: 10.1074/jbc.M104202200 [DOI] [PubMed] [Google Scholar]
- Zuhlke RD, Zhang HJ, Joho RH. Role of an invariant cysteine in gating and ion permeation of the voltage-sensitive K+ channel Kv2.1. Recept Channels 1994;2(3):237–248. [PubMed] [Google Scholar]





