Abstract
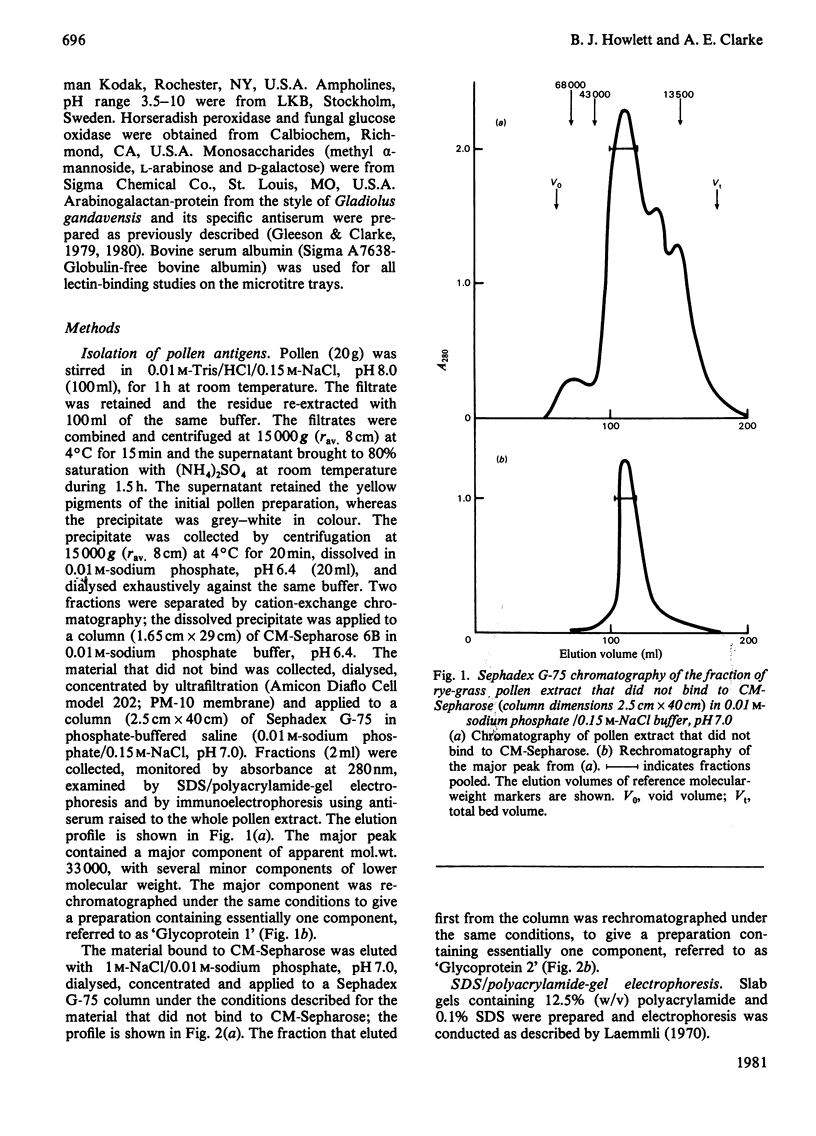
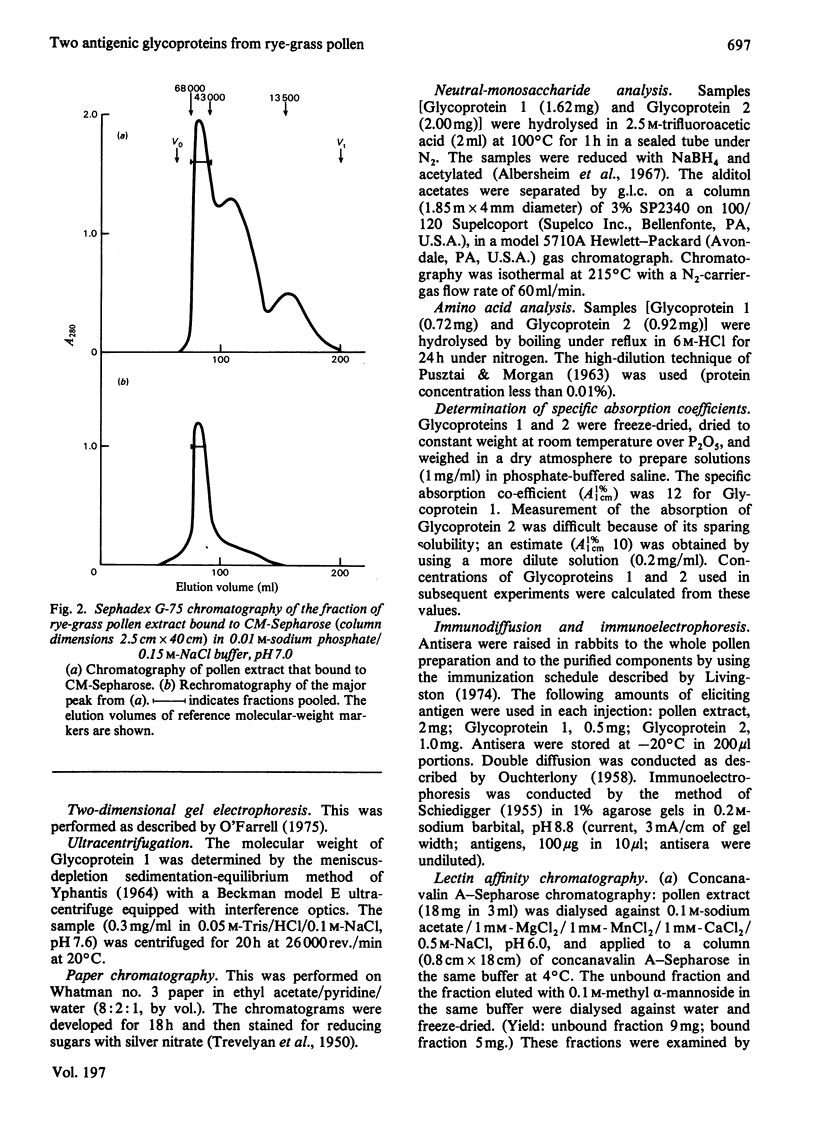
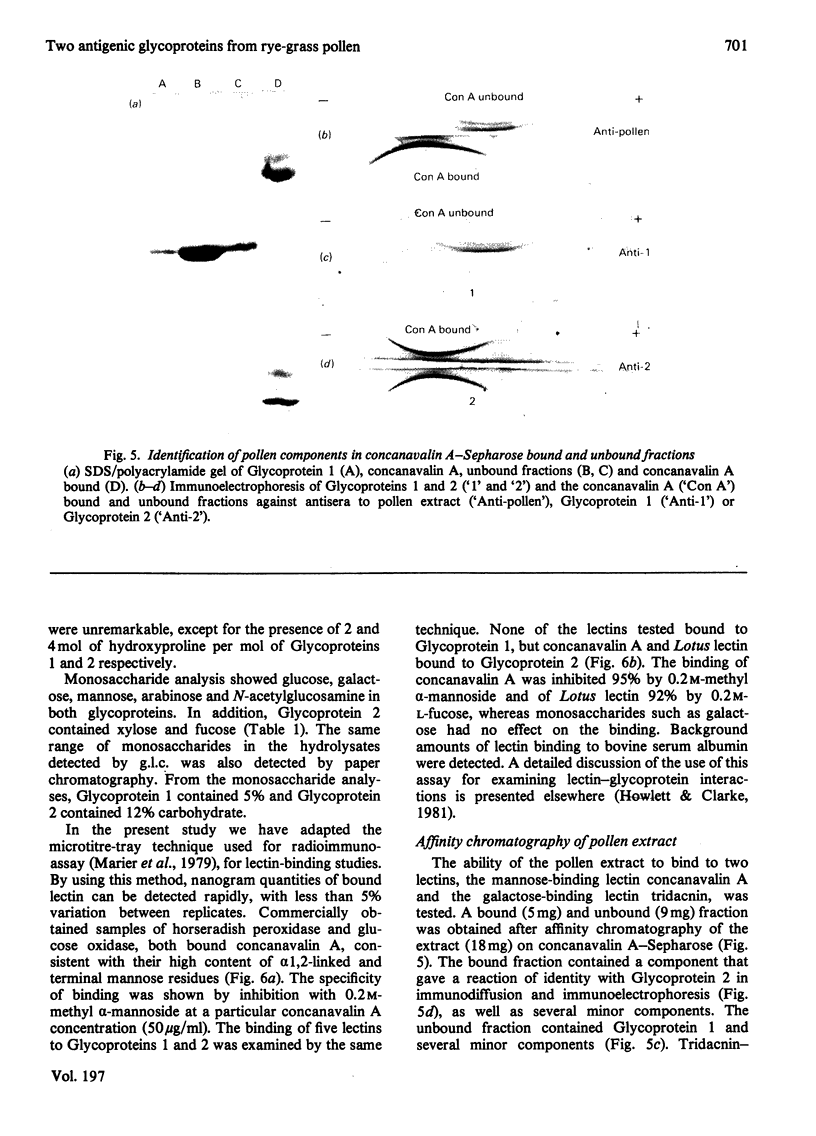
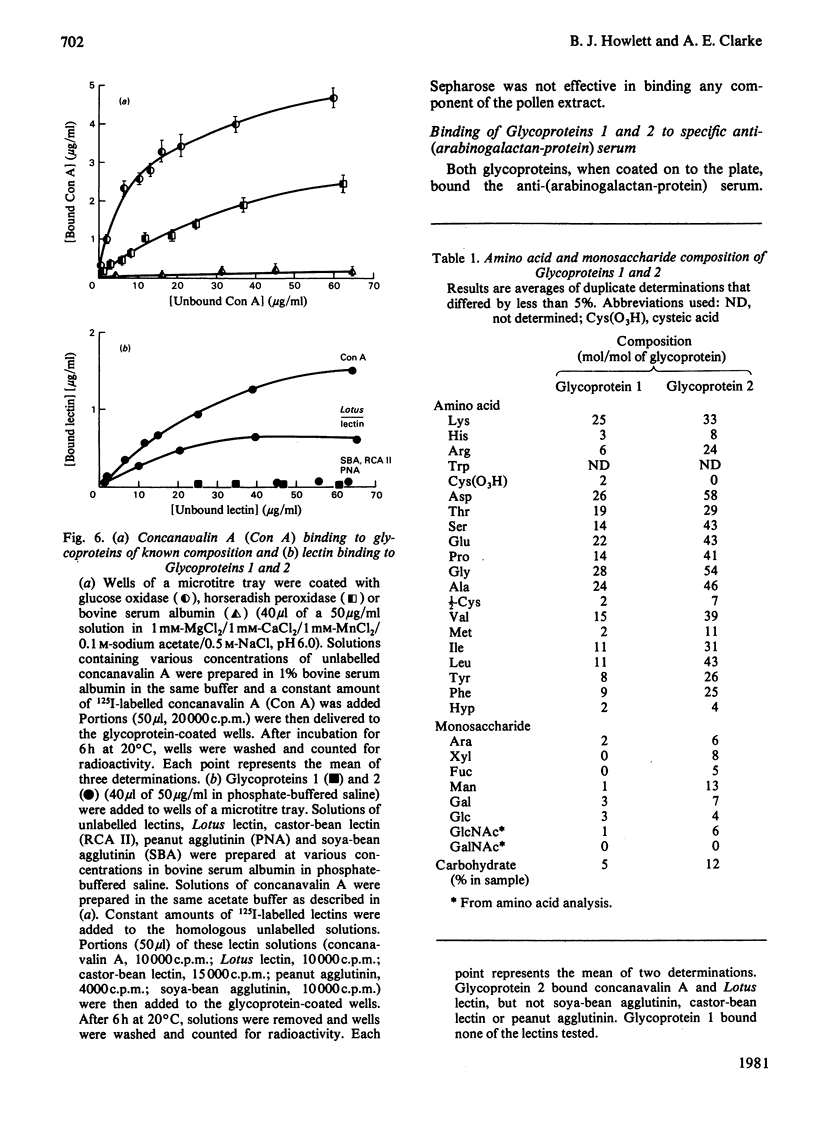
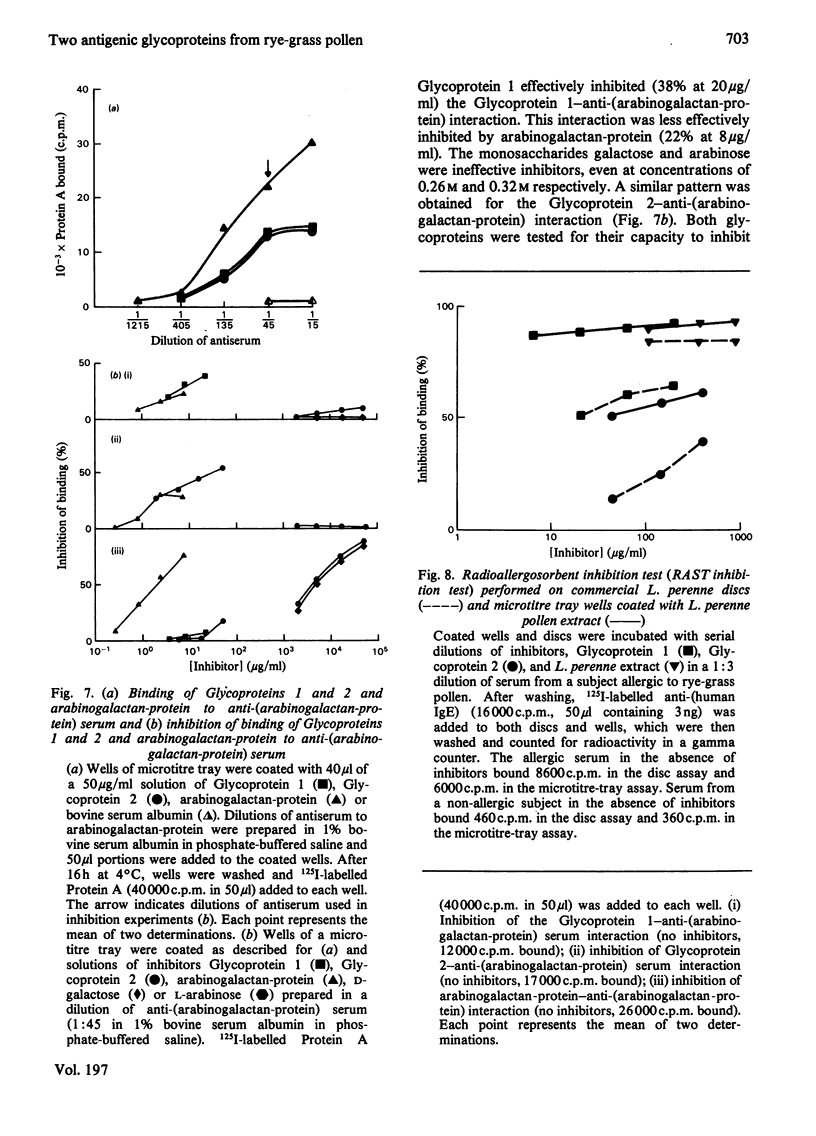
Two glycoproteins have been purified from a buffer extract of rye-grass (Lolium perenne) pollen. Both migrated as single bands on sodium dodecyl sulphate/polyacrylamide gels. Glycoprotein 1 (0.8 mg/g of pollen) had a apparent mol.wt. of 33 000 and contained 95% protein and 5% carbohydrate. The monosaccharides glucose, galactose, mannose, arabinose and N-acetylglucosamine were present in the proportions 3:3:1:2:1. Glycoprotein 2 (0.4 mg/g of pollen) had an apparent mol. wt. of 68000 and contained 88% protein and 12% carbohydrate. The monosaccharides glucose, galactose, mannose, fucose, xylose, arabinose and N-acetylglucosamine were present in the proportions 4:7:13:5:8:6:6. This glycoprotein bound concanavalin A and Lotus tetragonolobus (asparagus pea) lectin. Radioallergosorbent (RAST) inhibition tests showed that Glycoprotein 1 is an effective allergen, whereas Glycoprotein 2 has less allergenic activity. A method for performing both lectin-binding assays and RAST inhibition tests using microtitre trays is described.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustin R., O'Sullivan S., Davies I ab I. Isolation of grass pollen antigens failing to induce IgE reagin formation although capable of inducing IgG antibody formation. Int Arch Allergy Appl Immunol. 1971;41(1):144–147. doi: 10.1159/000230506. [DOI] [PubMed] [Google Scholar]
- Burke D., Kaufman P., McNeil M., Albersheim P. The Structure of Plant Cell Walls: VI. A Survey of the Walls of Suspension-cultured Monocots. Plant Physiol. 1974 Jul;54(1):109–115. doi: 10.1104/pp.54.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gleeson P. A., Clarke A. E. Antigenic determinants of a plant proteoglycan, the Gladiolus style arabinogalactan-protein. Biochem J. 1980 Nov 1;191(2):437–447. doi: 10.1042/bj1910437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson P. A., Clarke A. E. Structural studies on the major component of Gladiolus style mucilage, an arabinogalactan-protein. Biochem J. 1979 Sep 1;181(3):607–621. doi: 10.1042/bj1810607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson P. A., Jermyn M. A., Clarke A. E. Isolation of an arabinogalactan protein by lectin affinity chromatography on tridacnin-sepharose 4B. Anal Biochem. 1979 Jan 1;92(1):41–45. doi: 10.1016/0003-2697(79)90622-5. [DOI] [PubMed] [Google Scholar]
- Gleich G. J., Jones R. T. Measurement of IgE antibodies by the radioallergosorbent test. I. Technical considerations in the performance of the test. J Allergy Clin Immunol. 1975 May;55(5):334–345. doi: 10.1016/0091-6749(75)90005-6. [DOI] [PubMed] [Google Scholar]
- Ishihara H., Takahashi N., Oguri S., Tejima S. Complete structure of the carbohydrate moiety of stem bromelain. An application of the almond glycopeptidase for structural studies of glycopeptides. J Biol Chem. 1979 Nov 10;254(21):10715–10719. [PubMed] [Google Scholar]
- Johnson P., Marsh D. G. Allergens from common rye grass pollen (Lolium perenne). I. Chemical composition and structure. Immunochemistry. 1966 Mar;3(2):91–100. doi: 10.1016/0019-2791(66)90290-4. [DOI] [PubMed] [Google Scholar]
- Johnson P., Marsh D. G. Allergens from common rye grass pollen (Lolium perenne). II. The allergenic determinants and carbohydrate moiety. Immunochemistry. 1966 Mar;3(2):101–110. doi: 10.1016/0019-2791(66)90291-6. [DOI] [PubMed] [Google Scholar]
- Knox R. B., Vithanage H. I., Howlett B. J. Botanical immunocytochemistry: a review with special reference to pollen antigens and allergens. Histochem J. 1980 May;12(3):247–272. doi: 10.1007/BF01006951. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lis H., Sharon N. Soybean agglutinin--a plant glycoprotein. Structure of the carboxydrate unit. J Biol Chem. 1978 May 25;253(10):3468–3476. [PubMed] [Google Scholar]
- Livingston D. M. Immunoaffinity chromatography of proteins. Methods Enzymol. 1974;34:723–731. doi: 10.1016/s0076-6879(74)34094-3. [DOI] [PubMed] [Google Scholar]
- Løwenstein H. Quantitative immunoelectrophoretic methods as a tool for the analysis and isolation of allergens. Prog Allergy. 1978;25:1–62. doi: 10.1159/000314432. [DOI] [PubMed] [Google Scholar]
- Marier R., Jansen M., Andriole V. T. A new method for measuring antibody using radiolabeled protein A1 in a solid-phase radioimmunoassay. J Immunol Methods. 1979;28(1-2):41–49. doi: 10.1016/0022-1759(79)90326-0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- PUSZTAI A., MORGAN W. T. STUDIES IN IMMUNOCHEMISTRY. 22. THE AMINO ACID COMPOSITION OF THE HUMAN BLOOD-GROUP A, B, H AND LE-A SPECIFIC SUBSTANCES. Biochem J. 1963 Sep;88:546–555. doi: 10.1042/bj0880546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- Smart I. J., Knox R. B. Rapid batch fractionation of ryegrass pollen allergens. Int Arch Allergy Appl Immunol. 1980;62(2):179–187. doi: 10.1159/000232510. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]