Abstract
Pituitary apoplexy (PA) is a clinical syndrome caused by acute hemorrhage and/or infarction of the pituitary gland, most commonly in the setting of a pituitary macroadenoma. PA generally presents with severe headache, nausea, vomiting, visual disturbance, and, in more severe cases, altered mental status. Many factors have been attributed to the risk of developing PA, including most recently, numerous reports showcasing an association with COVID-19 infection or vaccination. Initial management of PA includes evaluation and correction of deficient hormones and electrolytes and an assessment if surgical decompression to relieve pressure on optic nerves and other brain structures is needed. While prompt recognition and treatment are crucial to avoid morbidity and mortality, in the modern era, PA is less commonly considered a true neurosurgical emergency requiring immediate (< 24 h) surgical decompression. Traditionally, surgical decompression has been the standard of care for significant mass effects. However, several studies have shown similar outcomes in visual and hormonal recovery with either surgical decompression or conservative medical management. Unfortunately, most evidence on optimal management strategies is limited to retrospective case series, small prospective studies, and one multi-center observational study. This review aims to provide the most up-to-date evidence on the role of COVID-19 in PA and best management strategies.
Keywords: Pituitary apoplexy, Transsphenoidal surgery, Medical management, Outcomes, Review
Introduction and historical overview
Pituitary apoplexy (PA) is a clinical syndrome caused by acute hemorrhage and/or infarction of a tumor in the pituitary fossa. The first formal use of the term PA was adopted by Brougham et al. in 1950 when authors used it to reference a case of necrosis and hemorrhage that occurred in patients with pituitary tumors [1]. Since then, there have been numerous reports and case series describing the clinical presentation, treatment, and outcome of PA patients. PA, although rare, has traditionally been considered a life-threatening condition that requires urgent medical and surgical intervention to prevent morbidity and mortality. However, with improved early recognition, understanding of the underlying disease process, and effective therapies, the treatment strategies for PA have evolved considerably. Consequently, there is considerable debate in the literature regarding the management of acute PA, with discussions often centered on the merits of medical versus surgical interventions. This review will provide a comprehensive assessment of PA, outlining the most evidence-based approach to managing this condition.
Epidemiology
PA is most common in the 5th decade of life [2–4], with most patients between 37 and 58 years old [3]. Some studies suggest that PA is more common in men, although female predominance has also been observed in others [5, 6]. Prior reports have highlighted varying incidence rates. Among patients with known pituitary adenomas, the rate of PA ranges from 1.5 to nearly 28% [5, 7, 8]. This wide range has been attributed to the consideration of symptomatic versus subclinical or non-symptomatic PA cases, with the latter being diagnosed following neuroimaging studies and is more frequent [7]. For purposes of this review, we consider PA an acute clinical syndrome with clear symptoms related to acute hemorrhagic and/or infection and do not consider asymptomatic bleeding found on routine imaging as part of this clinical spectrum.
Pathophysiology
Most PA occurs in previously undetected or clinically asymptomatic non-functioning pituitary macroadenomas [9]. After non-functioning adenomas, prolactinomas, and growth hormone-secreting tumors comprise the second and third most common pituitary neoplasms seen in the setting of PA (Table 1) [10–12]. Macroadenomas have fragile, relatively immature blood vessels [13, 14]. Turner et al. compared the expression of CD31, an endothelial cell marker, revealing that pituitary adenomas typically exhibit lower vascular density than normal, reducing bleeding risk, although invasive macroprolactinomas demonstrate increased vascularity, which could influence observed bleeding risks [14].
Table 1.
Hypersecreting adenomas reported amongst PA cases in select studies
| Author | Year | No. of PA cases | Cohort | Hypersecreting Adenomas, (%) | ||
|---|---|---|---|---|---|---|
| Prolactin | Growth hormone | Cortisol | ||||
| Randeva et al. [15] | 1999 | 35 | All | 5.7 | 8.6 | 5.7 |
| Sibal et al. [16] | 2004 | 45 | All | 4.4 | 2.2 | 6.7 |
| Grzywotz et al. [2] | 2017 | 60 | All | 10 | 6.7 | 6.7 |
| Rutkowski et al. [17] | 2017 | 32 | All | 9.4 | 3.1 | 0 |
| Marx et al. [18] | 2021 | 19 | Surgery | 15.8 | 0 | 0 |
| 27 | Conservative | 33 | 3.7 | 3.7 | ||
| Shepard et al. [19] | 2021 | 64 | All | 6.3 | 0 | 0 |
| Mamelak et al. [4] | 2024 | 67 | Surgery | 4.5 | 0 | 1.5 |
| 30 | Conservative | 13 | 3.3 | 0 | ||
Sudden and rapid bleeding in a tumor and/or infarction of that same tumor (either from ischemia or mass effect) results in an acute rise in intrasellar pressure. Furthermore, infarction of pituitary tissue may cause swelling on its own. This expanding mass within the bony limits of the sella and skull results in rapid compression of surrounding tissues. Superior extension of an expanding pituitary mass is the primary mechanism leading to visual field loss, most commonly bitemporal hemianopsia resulting from optic chiasm compression. Alternatively, unilateral hemianopsia, asymmetric loss, or even blindness may develop depending on the severity of nerve compression. Expansion laterally may exert a mass effect on the cavernous sinus, leading to diplopia caused by oculomotor palsies of cranial nerves III, IV, or VI, with incidence rates ranging from 25 to 39% [16, 20]. Although less common in the modern era, superior extension can also compress other brain structures, resulting in diminished consciousness, coma, hydrocephalus, and even death [21]. Furthermore, there is a risk of hemorrhage extending into the basal cisterns, potentially leading to vasospasm and meningeal irritation, worsening headaches, and nausea [22].
Precipitating factors
While most cases of PA occur sporadically, several reports, often limited to small retrospective studies and case reports, have postulated potential factors. One of the better-recognized precipitating risk factors for PA is cerebral hypoperfusion or hemodynamic disturbances due to major surgery, most notably cardiovascular procedures [23]. Other medical conditions and medications such as hypertension, hemorrhagic pregnancy, anticoagulation use, and endocrine function tests have been reported as potential risk factors for PA onset [15, 24]. Sphenoid sinus mucosal thickening on MRI scans in patients with PA is now a well-recognized co-occurrence [25, 26]. It is unclear if this mucosal thickening is a precipitating factor or if PA may induce inflammatory changes in the sphenoid mucosa.
There is also a small risk of hemorrhage following subtotal adenoma resection, which can produce symptomatic PA [27–29]. Furthermore, alternative treatments for adenomas, such as radiation therapy, have also been implicated in post-procedure hemorrhage. Fu et al. retrospectively reviewed 751 consecutive pituitary adenoma patients treated with gamma knife radiosurgery and found that 55 (7.3%) patients developed new or worsened pituitary hemorrhage within a median time of 18.9 months [30].
SARS-CoV-2
Several cases of PA in the setting of a confirmed SARS-CoV-2 infection or vaccination have been reported (Table 2). In most cases, the presentation is like that of other cases of PA, with similar co-morbidities and outcomes. Early in the pandemic, we reported a case of PA in pregnant women with COVID-19 [31], raising the possibility that the COVID-19 infection may have contributed to PA in this setting. Since then, several other cases have been reported. Not surprisingly, most patients had a clinically silent pre-existing adenoma, with two reported cases presenting with a hypersecreting prolactinoma [32, 33]. Given the high incidence and prevalence of COVID-19 in the general population, it is unclear if there is a causal relationship between infection and/or vaccination and subsequent development of PA.
Table 2.
Cases of PA in the setting of confirmed COVID-19 infection or vaccination reported in the literature
| Author | Year | Age | Sex | Co-morbidities | PA onset after vaccination | Symptoms | Time to PA onset from infection or vaccine | Treatment | Hormonal Defects |
Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Chan [31] | 2020 | 28 | F | Pregnant | N |
HA blurry vision |
Same | C + S | NR | NR |
| dos Santos e Santos [34] | 2020 | 47 | M | None | N | HA | 3 weeks | S | ACTH | NR |
| Solorio-Pineda [35] | 2020 | 27 | M | None | N |
HA ↓visual acuity |
4 days | C | FSH/LH | Death due to Resp. failure |
| LaRoy [36] | 2021 | 35 | M | None | N | HA | 3 days | C | NR | |
| Martinez-Perez [37] | 2021 | 54 | F | None | N |
HA, blurry vision |
1 week | S |
ACTH TSH |
1 month: Improved vision |
| 56 | M | HTN | N |
HA diplopia |
10 days | S |
ACTH TSH |
6 weeks: Resolution of diplopia | ||
| 52 | M | HTN | N |
HA, bitemporal hemianopsia |
NR | S |
Pan hypopituitarism |
NR | ||
| Katti [38] | 2021 | 46 | M | None | N | Sudden vision loss | Same | C | NR | NR |
| Ghosh [39] | 2021 | 44 | F | None | N |
HA blurry vision |
6 days | C | ACTH | |
| Bordes [40] | 2021 | 65 | F | HTN, Fibromyalgia | N |
HA nausea/emesis |
1 month | C |
ACTH TSH |
6 months: Stable |
| Liew [41] | 2021 | 75 | F | Irritable bowel syndrome | N | HA | 6 weeks | C |
Pan hypopituitarism |
Y |
| Kamel [42] | 2021 | 55 | M |
HTN, DM Pituitary adenoma |
N |
HA ptosis ↓ vision L eye |
6 days | S | HRT* |
1 week Death due to COVID pneumonia |
| Murvelashvili [43] | 2021 | 51 | F | None |
Y After 2nd vaccination (Moderna) |
Nausea/emesis, epigastric pain, ↓ libido |
2 days | C |
ACTH FSH/LH TSH |
1 month: MRI with reduction in PA volume |
| Balmain [32] | 2022 | 79 | M | None | N |
HA visual field deficit |
8 weeks | C |
ACTH TSH |
5 months: Hypopituitarism VF defect stable |
| 69 | F | HTN, Pulmonary embolism | N | Abnormal visual fields | 16 weeks | C |
ACTH TSH |
6 months: Stable clinical exam |
||
| 64 | F | DM | N |
HA abnormal visual fields |
Same | C | ACTH | Lost to follow up | ||
| 63 | F | None | N |
HA ↓visual acuity |
Same | C |
ACTH TSH |
3 months: Complete visual recovery |
||
| 71 | F | HTN, HLD, Hypothyroidism | N |
HA complete blindness |
Same | S |
ACTH TSH |
2 months: Visual acuity improved |
||
| 75 | F | HTN, DM | N | HA | 12 weeks | C | ACTH |
3 months: No HA, hormonal replacement |
||
| 47 | F | None | N |
HA bitemporal hemianopsia |
4 weeks | C | ACTH |
6 weeks: No HA, hormonal replacement |
||
| 72 | M | None | N |
HA bilateral blindness |
7 weeks | S | ACTH |
7 months: Partial recovery of vision |
||
| Aliberti [44] | 2022 | 50 | M | None |
Y, following 3rd vaccination (Moderna) |
Nausea/emesis | 1 day | S |
ACTH FSH/LH |
4 months: Resolution of symptoms, Full recovery of hormones |
| Pinar-Gutierrez [45] | 2022 | 37 | F | None |
Y, following 1st vaccination (ChAdO) |
HA | 4 days | C | NR |
3 weeks: Symptoms resolved |
| Zainordin [46] | 2022 | 24 | F | None |
Y, following 2nd vaccination (AstraZeneca) |
HA | 24 h | C | GH |
3 weeks: HA improved |
| Hazzi [47] | 2023 | 65 | M | None | N |
HA, CN III, IV, VI paresis |
13 days | S |
Pan hypopituarism |
6 months: Stable, improvement in visual field |
| 61 | F | None | N | Right CN III paresis | 6 days | C + S |
ACTH TSH |
2 months: CN III paresis improved |
||
| 89 | M | HTN, HLD | N | HA | 2 months | C |
Pan hypopituitarism |
6 months: Reduction in lesion |
||
| Roncati [33] | 2023 | 28 | F | None |
Y, Following 2nd vaccination (AstraZeneca) |
HA amenorrhea |
24 h | C | ↑PRL |
3.5 months Improving, Menses resumed |
Legend: HA = headache; CN = cranial nerve; Y = yes; N = no; C = conservative management; S = Surgery; F = female; M = Male; DM = diabetes mellitus; HTN = hypertension; HLD = Hyperlipidemia; NR = not reported; ACTH = adrenocorticotrophic hormone (pituitary related cortisol deficiency); TSH = thyroid stimulating hormone (pituitary related thyroid hormone deficiency); PRL = prolactin
There have been several proposed mechanisms by which SARS-CoV-2 infection might induce PA onset. Coronavirus uses ACE-2 receptors to enter cells, including those in the central nervous system (CNS) [48, 49]. This infection might trigger an immune response, causing damage to the gland. Alberti et al. described a case of PA in which SARS-CoV-2 proteins were located next to pituitary blood vessels on immunohistochemical analysis, suggesting some element of antigen cross-reactivity leading to hypophysitis-induced PA [44]. The investigators also observed lymphocytic cell infiltrate. SARS-CoV-2 infection leads to an overall system immune response, which can indirectly lead to PA onset. The inflammation associated with a severe viral infection can activate the hypothalamic-pituitary-adrenal (HPA) axis. This activation could lead to hypophysitis.
The association between COVID-19 infection and coagulation disorders has also been well-documented in the literature. von Willebrand factor (vWF) is upregulated in COVID-19, highlighting the role of endothelial involvement. ADAMTS13 is a metalloprotease that cleaves high-molecular-weight vWF. Downregulation of its expression has been observed in COVID-19. This results in an aberrant ratio of vWF to ADAMTS13, which is fundamental to the pathogenesis of COVID-19-associated coagulopathy [50]. This disturbed ratio contributes to the hypercoagulable state observed in severe COVID-19 cases, which can lead to thrombosis in the blood vessels supplying the pituitary gland, causing infarction and subsequent hemorrhage. Moreover, common comorbidities in COVID-19 and PA cases include hypertension and diabetes mellitus, both linked to endothelial dysfunction [51].
Far fewer cases of PA following vaccination for COVID-19 have been reported in the literature, with three cases [33, 45, 46] occurring following a dose of the AstraZeneca (Cambridge, UK) vaccine [33, 45, 46] and two cases following the Moderna (Cambridge, MA, USA) vaccine [43, 44]. These patients had no prior medical history or comorbidities commonly associated with PA. Vaccination for coronavirus may induce thrombosis and bleeding due to Vaccine-Induced Thrombotic Thrombocytopenia syndrome (VITT) [52]. Although an extremely rare syndrome, VITT is most commonly observed following administration of viral-vector-based vaccines [53]. However, it is likely that this phenomenon is more complex, given that Zainordin et al. reported a case of PA following vaccination that presented with a normal coagulation profile [46].
Taken as a whole, these limited data suggest that COVID-19 infection and/or vaccination may be a predisposing risk factor for the development of PA and should be added to that list for future investigation. However, at present, no definitive statement can be made regarding the contribution of COVID-19 to the development of PA.
Clinical assessment and diagnosis
The most commonly reported symptoms of PA, in descending order of frequency, include headache (86%), visual disturbances (62%), nausea/vomiting (40%), and extra-ocular palsies (25%) [54]. Most of these symptoms have a rapid onset, much like aneurysmal subarachnoid hemorrhage. Importantly, the spectrum of PA encompasses severe symptoms such as blindness, coma, and death.
The constellation of these symptoms often leads to differential diagnoses, which include acute subarachnoid hemorrhage (SAH) and meningitis. Cerebrospinal fluid (CSF) analysis may not distinguish between SAH, bacterial meningitis, and pituitary apoplexy (PA). Based on the aforementioned clinical features, prompt evaluation of suspected PA should include a comprehensive hypothalamic-pituitary axis endocrine assessment, visual field and acuity testing, oculomotor examination for cranial nerve deficits, and consciousness evaluation. MRI should be obtained as soon as feasible, though a CT scan, often more readily available, can demonstrate acute sellar hemorrhage.
Computed tomography (CT) is often used to assess neurological anatomy in patients with sudden severe headaches, identifying an intrasellar lesion in 80% of cases. However, magnetic resonance imaging (MRI) remains the preferred imaging study. Within the first 7 days post-PA, lesions typically appear iso- to hypointense on T1-weighted images and hypointense on T2-weighted images. During the subacute phase, from 7 to 21 days, lesions may become hypointense on both T1 and T2 images due to methemoglobin accumulation. Intravenous gadolinium administration can reveal a contrast enhancement around the pituitary gland, known as the “pituitary ring sign.” [55] Beyond 21 days, signal intensities may become more heterogeneous as the gland undergoes blood product degradation and fibrosis.
Assessment of endocrine abnormalities is a crucial step in clinical assessment. Acute cortisol deficiency is a frequent and critical complication observed in patients with PA, occurring in 50–80% of cases [4, 16, 56, 57]. This condition can lead to severe hemodynamic instability and hyponatremia, necessitating prompt identification and management. Immediate administration of glucocorticoid replacement is essential for patients exhibiting signs of PA to avert an adrenal crisis. Beyond cortisol, supplementation of deficient hormones in the relatively early stages of management is of critical importance to avoiding other endocrinopathies such as hyper- or hyponatremia.
To categorize the clinical manifestations of PA and define which groups of patients were most likely to undergo surgery, the United Kingdom Pituitary Apoplexy Guidelines Development Group introduced the Pituitary Apoplexy Score (PAS) [58]. The PAS quantifies severity using consciousness level, visual acuity, field deficits, and ocular paresis. Developed through a retrospective review to standardize treatment, the score remains unvalidated for outcome prediction. In the modern era, most patients present with a PAS of 4 or less, so it is unclear if this scoring impacts advising best treatment approaches.
Management strategies and outcomes
Surgical versus conservative management
The debate over the optimal treatment strategy for patients with PA has evolved significantly since the initial view by many that acute neurosurgical intervention was almost always needed [59]. Numerous retrospective and a few prospective studies have tried to establish a more pragmatic approach to treatment [4, 18–61] in which non-surgical treatment plays an increasingly important role. Currently, there is a consensus that surgical decompression of the PA mass, most commonly via the transsphenoidal route, should be considered for any patient presenting with severe symptoms, which include severe visual field deficits and/or impaired consciousness [4, 62, 63]. However, whether surgery offers a superior long-term therapeutic benefit to conservative management remains unclear [4, 15–17, 62, 64]. Sibal et al. retrospectively reviewed 45 cases of PA and found that most patients experienced complete or near complete resolution of ophthalmologic deficits with no reported deaths following conservative management or surgery [16]. These findings were further supported in a recent multicenter, prospective study reported by Mamelak et al., in which investigators found no significant difference in outcomes in patients who underwent surgery compared to conservative management [4]. Many studies have documented little difference in hormonal outcomes between surgical and non-surgical patients, with only about 10% of patients recovering hormonal defects in either group (Table 3) [2, 4, 18, 19]. The resolution of cranial nerve palsies is comparable and often more favorable in conservatively managed patients at three to six-month follow-up [61].
Table 3.
Comparison of initial endocrine deficiency compared to last follow-up in select studies
| Author | Year | No. of cases | Cohort | Endocrine Deficiency (%) | Last follow-up (3 months or 1 year) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Corticotropic | Thyrotropic | Gonadotropic | Somatotropic | Hyponatremia | Corticotropic | Thyrotropic | Gonadotropic | Somatotropic | ||||
| Grzywotz et al. [2] | 2017 | 60 | All | 55 | 42.6 | 68.9 | 32 | 21.4 | 56.5 | 56.8 | 58.7 | 53.3 |
| Marx et al. [18] | 2021 | 19 | Surgery | 53.8 | 50 | 72.7 | 30.8 | NR | 64.7 | 70.6 | 80 | 60 |
| 27 | Conservative | 57.1 | 54.2 | 66.7 | 8.7 | NR | 28.6 | 54.5 | 44.4 | 31.3 | ||
| Shepard et al. [19] | 2021 | 64 | All | 21.9 | 18.8 | 25 | 9.4 | 3.1 | NR | NR | NR | NR |
| Mamelak et al. [4]. | 2024 | 67 | Surgery | 67 | 52 | 52 | 21 | 7.6 | 11.6* | 8.9* | 6.9* | NR |
| 30 | Conservative | 63 | 43 | 53 | 14 | 20 | 10.5* | 8.3* | 6.3* | NR | ||
*Indicates % of patients who recovered from their endocrine deficiency at initial presentation. NR, not reported
In the largest observational study performed to date, symptoms, co-morbidities, demographics, and hormonal defects were almost identical between surgical and medical management cohorts, except that surgical patients had slightly larger tumors, but this difference was not significant [4]. The only statistically significant difference between these groups was the rate of bitemporal hemianopsia. Hospital stays were similar, though surgical patients had more complications. Patients with severe visual field defects and greater optic nerve compression were more likely to undergo surgery. At 3 to 6 months post-apoplexy, visual field, hormonal, oculomotor, and quality of life metrics were similar in both groups. The study did not show that surgery led to better visual outcomes, only that these criteria were commonly used to recommend surgery. This lack of outcome difference does not necessarily mean surgery is preferred for visual field defects, just that it was more often chosen.
An important but often overlooked aspect of PA is that the resulting mass effect is largely due to blood and edematous, necrotic tissue, which regress over time. Prior reports have noted regression in 70–95% of patients 3 months post apoplexy (see Fig. 1) [4, 19, 61]. Thus, symptom resolution due to diminished mass effect may be noted in most patients with PA, regardless of whether they undergo surgery. Whether these studies, often performed at centers with expert pituitary teams, represent general outcomes remains to be seen, particularly since growing evidence suggests that the degree of an institution’s experience in pituitary surgery is associated with overall outcomes [4, 65–67]. Despite the uncertainty in current literature, immediate decompression is generally safe and commonly used as the primary treatment modality in cases of severe visual deficits [68].
Fig. 1.
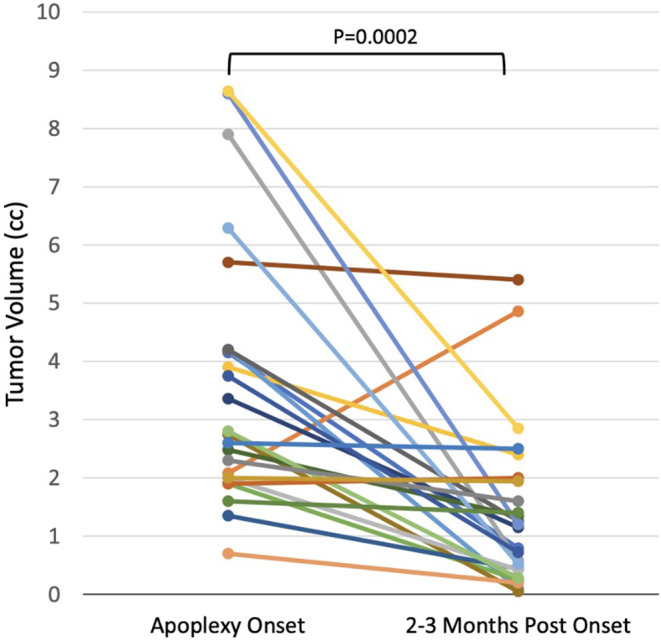
Change in Volume of Apoplectic Tissue in Medically Managed Patients by 2 to 3 Months After Symptom Onset. Volumes were measured using the (A × B × C)/2 approximation method. Each line represents one patient (n = 24). There was a significant 61% median reduction in volume over time (P = .0002), with only one patient showing volume enlargement. Reproduced with permission from: Mamelak, A. et al. (2023). A Prospective, Multicenter, Observational Study of Surgical vs. Nonsurgical Management for Pituitary Apoplexy. Journal of Clinical Endocrinology & Metabolism, 109(2), e711–e725. 10.1210/clinem/dgad541
Surgical timing
The optimal timing of surgical intervention when indicated is also not well defined. Patients presenting with severe cognitive impairment and visual deficits such as blindness should be promptly identified as urgent surgical candidates and likely undergo rapid decompression. However, such cases are relatively infrequent. Jho et al. found that patients with PA associated with more severe symptoms were more likely to undergo earlier surgery, similar to other reported studies [18, 62]. Establishing guidelines for intervention becomes more nuanced when addressing patients whose symptoms are stable or are showing signs of improvement. The challenge stems from the lack of evidence-based criteria guiding clinical decision-making in these scenarios.
The existing literature offers varied conclusions on the influence of early surgical intervention, and a consistent definition of “early surgery” is lacking. Woo et al. found that patients who underwent transsphenoidal surgery within three days of symptom onset experienced complete recovery of visual acuity, compared to 83% who had surgery later [69]. Furthermore, this study indicated that 66% of patients who underwent early surgery showed complete or partial recovery, compared to only 40% who received later interventions. These findings parallel those of Rutkowski et al., who ultimately found no differences in the rate of neurological and endocrine deficits following either early, defined as resection within three days of symptom onset, or delayed surgery. The authors also observed that earlier surgery was associated with greater gross total resection rates than delayed treatment (100% versus 44%, p = .003) despite similar size at the initial presentation [17]. Ultimately, no differences were found in the rates of neurological and endocrine deficits following either early or delayed surgery. Similarly, Bill et al. reported that patients who had surgery within seven days of symptom onset achieved complete recovery of visual functions [70]. Meanwhile, Randeva et al. reported that all patients who underwent surgery within eight days of symptom onset experienced complete recoveries [15].
A more comprehensive multi-center analysis compared outcomes by defining early surgery as either two, three, or four days post-symptom onset [4]. Investigators also considered 3-, 4-, and 5 days post-admission to the center where surgery was performed to exclude care delays. Their analysis indicated no advantage to any time-point cut-off for early surgery, with identical outcomes in the visual field, hormonal recovery, and oculomotor palsy regardless of the timing of surgical intervention. While patients who underwent surgery within these time frames exhibited more adverse visual field defects initially, their overall outcomes were comparable to those of patients who had surgery at 7 to 30 days post PA onset as well as those managed medically at the three-month evaluation mark.
Management strategy based on best available data
In the acute clinical setting, once a presumptive diagnosis of PA has been made, pituitary axis hormones should be measured, and corticosteroids should be initiated immediately, even if lab results are unavailable, as this can always be discontinued if no longer indicated. Meticulous monitoring of vital signs and neurological status is crucial to detect any signs of clinical deterioration. Laboratory evaluations should include assessments of all pituitary axis hormones, electrolytes, renal function, liver function, coagulation parameters, and sodium levels. Monitoring urinary output and fluid balance is essential for detecting hyper or hyponatremia, which should be corrected rapidly. Depending on the institution’s protocols, adrenal insufficiency may be initially managed with a hydrocortisone bolus of 100 mg or equivalent, followed by a maintenance dose ranging from 10 to 40 mg in twice-daily doses. Thyroid-related deficiencies can be replaced slowly, and acute replacement of gonadotroph or somatotroph hormones is rarely indicated. If a patient is found to have a hormonally active prolactinoma, prompt initiation of medical therapy with dopamine agonists is likely indicated.
Once stabilized, an initial decision regarding acute neurosurgical intervention is appropriate. Patients with altered consciousness after corticosteroid replacement and correction of hyponatremia or with severe visual acuity diminishment should undergo surgery promptly, preferably within 48–72 h. Other patients can be observed for several days to monitor visual field stability or improvement, with surgery indicated only if deterioration occurs. This approach allows for transferring patients to specialized centers, improving outcomes. This approach also allows the transfer of patients to centers with greater expertise in pituitary surgery if needed, thereby reducing sup-optimal surgical outcomes. Discussion between the surgery team, endocrinologists, and the patient or family will determine the preferred treatment choice, with many patients opting for surgery, especially as this can often relieve headaches. In stable patients, the timing of surgery does not seem to impact outcomes greatly and can be done in an elective fashion within 2 to 10 days after onset. For patients with surgical risks, observation, and medical management may result in similar outcomes at 3 months due to regression of the apoplectic tissue and blood.
Postoperative care and monitoring
Regardless of the management approach, all patients with PA require subsequent monitoring. MRI of the pituitary is important to evaluate for residual tumors, as these tumors can continue to grow. This is especially important in patients treated with medical management alone, as residual tumors have been reported to grow in 11–12% [20, 71]. Some of these patients may eventually undergo elective surgery to remove residual tumors even after the apoplectic event is resolved. Imaging studies should be conducted approximately three to six months after the apoplexy incident. Subsequently, annual MRI scans are recommended for a minimum of five years.
Hormone levels should be evaluated approximately four to eight weeks after an apoplexy event. Approximately 10 to 20% of patients achieve partial or complete recovery of pituitary function; however, about 80% will require ongoing hormonal supplementation or replacement therapy [2, 4, 18, 19]. GH deficiency is the most commonly observed endocrine deficiency in these patients, though it is infrequently replaced due to various factors, including clinical guidelines and patient-specific considerations. Long-term follow-up with endocrinology is recommended in most cases, especially if hormone supplementation or suppression (for functioning adenomas) is required. This protocol ensures comprehensive monitoring and management, maintaining appropriate neuroendocrine functions and quality of life.
Conclusion
In the modern era, treatment strategies for PA have evolved considerably. Truly urgent cases of PA that require immediate and early (< 48 h) surgery for decompression are generally limited to patients with diminished levels of consciousness, severe visual defects, or progressively worsening exams. Fortunately, with prompt recognition of PA, these cases are relatively rare and, as such, are not commonly a true neurosurgical emergency. The reported association between COVID-19 infection or vaccination and PA should be kept in mind when patients present to medical attention, as many of the symptoms of PA can mimic those seen with acute COVID-19 infection. In most patients, correcting endocrine deficiencies, especially cortisol and hypo- or hypernatremia, will stabilize the patient. A decision for surgical intervention within the first 7–10 days after presentation can be made by the endocrine and neurosurgical services, as there is no clear evidence that earlier intervention results in better outcomes. Medical management without surgery is a highly effective strategy in many cases, partly due to the regression of apoplectic tissue and subsequent mass effect in most patients over time. Conducting a randomized trial to compare surgical and medical management appears warranted to define treatment strategies better, though designing and executing such a study would be challenging.
Author contributions
A.M. developed the overall concept and design of the study. A.E.B. played a central role in writing the main manuscript, conducting the literature review, and structuring the article. M.M. co-authored sections of the manuscript, performed critical revisions, and created and formatted Tables 1 and 2, and 3. All authors reviewed and approved the final manuscript, providing valuable feedback and ensuring high academic standards.
Funding
declaration.
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Open access funding provided by SCELC, Statewide California Electronic Library Consortium
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
The authors declare that they have no conflicting interests related to the subject matter or materials discussed in this manuscript. All authors have approved the manuscript and agree with its submission to the journal “Pituitary.” There are no financial or personal relationships that could inappropriately influence the content or outcome of this work. The research was conducted ethically and in compliance with all relevant guidelines and regulations.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Brougham M, Heusner AP, Adams RD (1950) Acute degenerative changes in adenomas of the pituitary body–with special reference to pituitary apoplexy. J Neurosurg 7:421–439 [DOI] [PubMed] [Google Scholar]
- 2.Grzywotz A, Kleist B, Möller LC et al (2017) Pituitary apoplexy - A single center retrospective study from the neurosurgical perspective and review of the literature. Clin Neurol Neurosurg 163:39–45 [DOI] [PubMed] [Google Scholar]
- 3.Ricciuti R, Nocchi N, Arnaldi G et al (2018) Pituitary Adenoma Apoplexy: review of Personal Series. Asian J Neurosurg 13:560–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mamelak AN, Little AS, Gardner PA et al (2024) A prospective, Multicenter, Observational Study of Surgical vs Nonsurgical Management for Pituitary Apoplexy. J Clin Endocrinol Metab 109:e711–e725 [DOI] [PubMed] [Google Scholar]
- 5.Murad-Kejbou S, Eggenberger E (2009) Pituitary apoplexy: evaluation, management, and prognosis. Curr Opin Ophthalmol 20:456–461 [DOI] [PubMed] [Google Scholar]
- 6.Lubina A, Olchovsky D, Berezin M et al (2005) Management of pituitary apoplexy: clinical experience with 40 patients. Acta Neurochir 147:151–157 discussion 157 [DOI] [PubMed] [Google Scholar]
- 7.Briet C, Salenave S, Bonneville J-F et al (2015) Pituitary Apoplexy. Endocr Rev 36:622–645 [DOI] [PubMed] [Google Scholar]
- 8.Mohr G, Hardy J (1982) Hemorrhage, necrosis, and apoplexy in pituitary adenomas. Surg Neurol 18:181–189 [DOI] [PubMed] [Google Scholar]
- 9.Falhammar H, Tornvall S, Höybye C (2021) Pituitary apoplexy: a retrospective study of 33 cases from a single Center. Front Endocrinol 12:656950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinoshita Y, Tominaga A, Usui S et al (2014) Impact of subclinical haemorrhage on the pituitary gland in patients with pituitary adenomas. Clin Endocrinol 80:720–725 [DOI] [PubMed] [Google Scholar]
- 11.Sarwar KN, Huda MSB, Van de Velde V et al (2013) The prevalence and natural history of pituitary hemorrhage in prolactinoma. J Clin Endocrinol Metab 98:2362–2367 [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto T, Yano S, Kuroda J-I et al (2012) Pituitary apoplexy associated with endocrine stimulation test: endocrine stimulation test, treatment, and outcome. Case Rep Endocrinol 2012:826901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou J, Hu Y, Zhu W et al (2022) Sprouting angiogenesis in human pituitary adenomas. Front Oncol 12:875219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner HE, Nagy Z, Gatter KC et al (2000) Angiogenesis in pituitary adenomas and the normal pituitary gland. J Clin Endocrinol Metab 85:1159–1162 [DOI] [PubMed] [Google Scholar]
- 15.Randeva HS, Schoebel J, Byrne J et al (1999) Classical pituitary apoplexy: clinical features, management and outcome. Clin Endocrinol 51:181–188 [DOI] [PubMed] [Google Scholar]
- 16.Sibal L, Ball SG, Connolly V et al (2004) Pituitary apoplexy: a review of clinical presentation, management and outcome in 45 cases. Pituitary 7:157–163 [DOI] [PubMed] [Google Scholar]
- 17.Rutkowski MJ, Kunwar S, Blevins L, Aghi MK (2018) Surgical intervention for pituitary apoplexy: an analysis of functional outcomes. J Neurosurg 129:417–424 [DOI] [PubMed] [Google Scholar]
- 18.Marx C, Rabilloud M, Borson Chazot F et al (2021) A key role for conservative treatment in the management of pituitary apoplexy. Endocrine 71:168–177 [DOI] [PubMed] [Google Scholar]
- 19.Shepard MJ, Snyder MH, Soldozy S et al (2021) Radiological and clinical outcomes of pituitary apoplexy: comparison of conservative management versus early surgical intervention. J Neurosurg 135:1310–1318 [DOI] [PubMed] [Google Scholar]
- 20.Pal A, Capatina C, Tenreiro AP et al (2011) Pituitary apoplexy in non-functioning pituitary adenomas: long term follow up is important because of significant numbers of tumour recurrences. Clin Endocrinol 75:501–504 [DOI] [PubMed] [Google Scholar]
- 21.Chang CV, Felicio AC, Toscanini AC et al (2009) Pituitary tumor apoplexy. Arq Neuropsiquiatr 67:328–333 [DOI] [PubMed] [Google Scholar]
- 22.Skljarevski V, Khoshyomn S, Fries TJ (2003) Pituitary apoplexy in the setting of coronary angiography. J Neuroimaging 13:276–279 [PubMed] [Google Scholar]
- 23.Semple PL, Jane JA Jr, Laws ER Jr (2007) Clinical relevance of precipitating factors in pituitary apoplexy. Neurosurgery 61:956–961 discussion 961–2 [DOI] [PubMed] [Google Scholar]
- 24.Szabolcs I, Késmárki N, Bor K et al (1997) Apoplexy of a pituitary macroadenoma as a severe complication of preoperative thyrotropin-releasing hormone (TRH) testing. Exp Clin Endocrinol Diabetes 105:234–236 [DOI] [PubMed] [Google Scholar]
- 25.Waqar M, McCreary R, Kearney T et al (2017) Sphenoid sinus mucosal thickening in the acute phase of pituitary apoplexy. Pituitary 20:441–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu JK, Couldwell WT (2006) Pituitary apoplexy in the magnetic resonance imaging era: clinical significance of sphenoid sinus mucosal thickening. J Neurosurg 104:892–898 [DOI] [PubMed] [Google Scholar]
- 27.Patel SK, Christiano LD, Eloy JA, Liu JK (2012) Delayed postoperative pituitary apoplexy after endoscopic transsphenoidal resection of a giant pituitary macroadenoma. J Clin Neurosci 19:1296–1298 [DOI] [PubMed] [Google Scholar]
- 28.Ahmad FU, Pandey P, Mahapatra AK (2005) Post operative pituitary apoplexy in giant pituitary adenomas: a series of cases. Neurol India 53:326–328 [DOI] [PubMed] [Google Scholar]
- 29.Pezzutti DL, Magill ST, Albonette-Felicio T et al (2021) Endoscopic endonasal Transtubercular Approach for Resection of Giant Pituitary Adenomas with Subarachnoid extension: the second floor strategy to avoid postoperative apoplexy. World Neurosurg 153:e464–e472 [DOI] [PubMed] [Google Scholar]
- 30.Fu J, Li Y, Wu L et al (2021) Pituitary hemorrhage in pituitary adenomas treated with gamma knife radiosurgery: incidence, risk factors and prognosis. J Cancer 12:1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan JL, Gregory KD, Smithson SS et al (2020) Pituitary apoplexy associated with acute COVID-19 infection and pregnancy. Pituitary 23:716–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balmain J, Jarebi M, Al-Salameh A et al (2022) Pituitary apoplexy in the aftermath of a SARS-CoV-2 infection: a case series from Amiens University Hospital. Eur J Endocrinol 187:K19–K25 [DOI] [PubMed] [Google Scholar]
- 33.Roncati L, Manenti A (2023) Pituitary apoplexy following adenoviral vector-based COVID-19 vaccination. Brain Hemorrhages 4:27–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santos CDSE, Filho LM, da Santos CL CAT, et al (2020) Pituitary tumor resection in a patient with SARS-CoV-2 (COVID-19) infection. A case report and suggested airway management guidelines. Braz J Anesthesiol 70:165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solorio-Pineda S, Almendárez-Sánchez CA, Tafur-Grandett AA et al (2020) Pituitary macroadenoma apoplexy in a severe acute respiratory syndrome-coronavirus-2-positive testing: causal or casual? Surg Neurol Int 11:304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.LaRoy M, McGuire M (2021) Pituitary apoplexy in the setting of COVID-19 infection. Am J Emerg Med 47:329e1–329e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez-Perez R, Kortz MW, Carroll BW et al (2021) Coronavirus Disease 2019 and Pituitary Apoplexy: a single-Center Case Series and Review of the literature. World Neurosurg 152:e678–e687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katti V, Ramamurthy LB, Kanakpur S et al (2021) Neuro-ophthalmic presentation of COVID-19 disease: a case report. Indian J Ophthalmol 69:992–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh R, Roy D, Roy D et al (2021) A rare case of SARS-CoV-2 infection Associated with Pituitary Apoplexy without comorbidities. J Endocr Soc 5:bvaa203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bordes SJ, Phang-Lyn S, Najera E et al (2021) Pituitary Apoplexy attributed to COVID-19 infection in the absence of an underlying Macroadenoma or Other Identifiable cause. Cureus 13:e13315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liew S-Y, Seese R, Shames A, Majumdar K (2021) Apoplexy in a previously undiagnosed pituitary macroadenoma in the setting of recent COVID-19 infection. BMJ Case Rep 14. 10.1136/bcr-2021-243607 [DOI] [PMC free article] [PubMed]
- 42.Kamel WA, Najibullah M, Saleh MS, Azab WA (2021) Coronavirus disease 2019 infection and pituitary apoplexy: a causal relation or just a coincidence? A case report and review of the literature. Surg Neurol Int 12:317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murvelashvili N, Tessnow A (2021) A case of Hypophysitis following immunization with the mRNA-1273 SARS-CoV-2 vaccine. J Investig Med High Impact Case Rep 9:23247096211043386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aliberti L, Gagliardi I, Rizzo R et al (2022) Pituitary apoplexy and COVID-19 vaccination: a case report and literature review. Front Endocrinol 13:1035482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piñar-Gutiérrez A, Remón-Ruiz P, Soto-Moreno A (2022) Case report: pituitary apoplexy after COVID-19 vaccination. Med Clin 158:498–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zainordin NA, Hatta SFWM, Ab Mumin N et al (2022) Pituitary apoplexy after COVID-19 vaccination: a case report. J Clin Transl Endocrinol Case Rep 25:100123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hazzi C, Villemure-Poliquin N, Nadeau S, Champagne P-O (2024) SARS-CoV-2 infection, a risk factor for Pituitary Apoplexy? A Case Series and Literature Review. Ear Nose Throat J 103:153S–161S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tachibana K, Nakamura Y, Do TL et al (2024) Mutations in the SARS-CoV-2 spike proteins affected the ACE2-binding affinity during the development of Omicron pandemic variants. Biochem Biophys Res Commun 719:150120 [DOI] [PubMed] [Google Scholar]
- 49.Gu WT, Zhou F, Xie WQ et al (2021) A potential impact of SARS-CoV-2 on pituitary glands and pituitary neuroendocrine tumors. Endocrine 72:340–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ward SE, Fogarty H, Karampini E et al (2021) ADAMTS13 regulation of VWF multimer distribution in severe COVID-19. J Thromb Haemost 19:1914–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pelle MC, Zaffina I, Lucà S et al (2022) Endothelial dysfunction in COVID-19: potential mechanisms and possible therapeutic options. 10.3390/life12101605. Life 12. [DOI] [PMC free article] [PubMed]
- 52.Greinacher A, Langer F, Makris M et al (2022) Vaccine-induced immune thrombotic thrombocytopenia (VITT): update on diagnosis and management considering different resources. J Thromb Haemost 20:149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Suhaimi SNAA, Zaki IAH, Noordin ZM et al (2023) COVID-19 vaccine-induced immune thrombotic thrombocytopenia: a review. Clin Exp Vaccine Res 12:265–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu X, Wang Y, Zhao X et al (2015) Incidence of Pituitary Apoplexy and its risk factors in Chinese people: a database study of patients with Pituitary Adenoma. PLoS ONE 10:e0139088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaphiades MS (2017) Pituitary Ring sign plus sphenoid sinus Mucosal Thickening: Neuroimaging signs of Pituitary Apoplexy. Neuroophthalmology 41:306–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ayuk J, McGregor EJ, Mitchell RD, Gittoes NJL (2004) Acute management of pituitary apoplexy–surgery or conservative management? Clin Endocrinol 61:747–752 [DOI] [PubMed] [Google Scholar]
- 57.Gruber A, Clayton J, Kumar S et al (2006) Pituitary apoplexy: retrospective review of 30 patients—is surgical intervention always necessary? Br J Neurosurg 20:379–385 [DOI] [PubMed] [Google Scholar]
- 58.Rajasekaran S, Vanderpump M, Baldeweg S et al (2011) UK guidelines for the management of pituitary apoplexy. Clin Endocrinol 74:9–20 [DOI] [PubMed] [Google Scholar]
- 59.Laws ER Jr, Ebersold MJ (1982) Pituitary apoplexy–an endocrine emergency. World J Surg 6:686–688 [DOI] [PubMed] [Google Scholar]
- 60.Seo Y, Kim YH, Dho Y-S et al (2018) The outcomes of Pituitary Apoplexy with Conservative Treatment: experiences at a single Institution. World Neurosurg 115:e703–e710 [DOI] [PubMed] [Google Scholar]
- 61.Almeida JP, Sanchez MM, Karekezi C et al (2019) Pituitary Apoplexy: results of Surgical and Conservative Management Clinical Series and Review of the literature. World Neurosurg 130:e988–e999 [DOI] [PubMed] [Google Scholar]
- 62.Jho DH, Biller BMK, Agarwalla PK, Swearingen B (2014) Pituitary apoplexy: large surgical series with grading system. World Neurosurg 82:781–790 [DOI] [PubMed] [Google Scholar]
- 63.Glezer A, Bronstein MD (2015) Pituitary apoplexy: pathophysiology, diagnosis and management. Arch Endocrinol Metab 59:259–264 [DOI] [PubMed] [Google Scholar]
- 64.Bujawansa S, Thondam SK, Steele C et al (2014) Presentation, management and outcomes in acute pituitary apoplexy: a large single-centre experience from the United Kingdom. Clin Endocrinol 80:419–424 [DOI] [PubMed] [Google Scholar]
- 65.Casanueva FF, Barkan AL, Buchfelder M et al (2017) Criteria for the definition of Pituitary Tumor Centers of Excellence (PTCOE): a Pituitary Society Statement. Pituitary 20:489–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barker FG 2nd, Klibanski A, Swearingen B (2003) Transsphenoidal surgery for pituitary tumors in the United States, 1996–2000: mortality, morbidity, and the effects of hospital and surgeon volume. J Clin Endocrinol Metab 88:4709–4719 [DOI] [PubMed] [Google Scholar]
- 67.Bates PR, Carson MN, Trainer PJ et al (2008) Wide variation in surgical outcomes for acromegaly in the UK. Clin Endocrinol 68:136–142 [DOI] [PubMed] [Google Scholar]
- 68.Capatina C, Inder W, Karavitaki N, Wass JAH (2015) Management of endocrine disease: pituitary tumour apoplexy. Eur J Endocrinol 172:R179–R190 [DOI] [PubMed] [Google Scholar]
- 69.Woo H-J, Hwang J-H, Hwang S-K, Park Y-M (2010) Clinical outcome of cranial neuropathy in patients with pituitary apoplexy. J Korean Neurosurg Soc 48:213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bills DC, Meyer FB, Laws ER Jr et al (1993) A retrospective analysis of pituitary apoplexy. Neurosurgery 33:602–608 discussion 608–9 [DOI] [PubMed] [Google Scholar]
- 71.Zhang F, Chen J, Lu Y, Ding X (2009) Manifestation, management and outcome of subclinical pituitary adenoma apoplexy. J Clin Neurosci 16:1273–1275 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.


