Abstract
Objective
To assess the methodological quality of radiomics-based models in endometrial cancer using the radiomics quality score (RQS) and METhodological radiomICs score (METRICS).
Methods
We systematically reviewed studies published by October 30th, 2023. Inclusion criteria were original radiomics studies on endometrial cancer using CT, MRI, PET, or ultrasound. Articles underwent a quality assessment by novice and expert radiologists using RQS and METRICS. The inter-rater reliability for RQS and METRICS among radiologists with varying expertise was determined. Subgroup analyses were performed to assess whether scores varied according to study topic, imaging technique, publication year, and journal quartile.
Results
Sixty-eight studies were analysed, with a median RQS of 11 (IQR, 9–14) and METRICS score of 67.6% (IQR, 58.8–76.0); two different articles reached maximum RQS of 19 and METRICS of 90.7%, respectively. Most studies utilised MRI (82.3%) and machine learning methods (88.2%). Characterisation and recurrence risk stratification were the most explored outcomes, featured in 35.3% and 19.1% of articles, respectively. High inter-rater reliability was observed for both RQS (ICC: 0.897; 95% CI: 0.821, 0.946) and METRICS (ICC: 0.959; 95% CI: 0.928, 0.979). Methodological limitations such as lack of external validation suggest areas for improvement. At subgroup analyses, no statistically significant difference was noted.
Conclusions
Whilst using RQS, the quality of endometrial cancer radiomics research was apparently unsatisfactory, METRICS depicts a good overall quality. Our study highlights the need for strict compliance with quality metrics. Adhering to these quality measures can increase the consistency of radiomics towards clinical application in the pre-operative management of endometrial cancer.
Clinical relevance statement
Both the RQS and METRICS can function as instrumental tools for identifying different methodological deficiencies in endometrial cancer radiomics research. However, METRICS also reflected a focus on the practical applicability and clarity of documentation.
Key Points
The topic of radiomics currently lacks standardisation, limiting clinical implementation.
METRICS scores were generally higher than the RQS, reflecting differences in the development process and methodological content.
A positive trend in METRICS score may suggest growing attention to methodological aspects in radiomics research.
Keywords: Endometrial neoplasms, Radiomics, Machine learning, Deep learning, Quality indicators
Introduction
Radiomics is the extraction of quantitative features—invisible to the human eye—from radiological images. These data, utilized independently or in conjunction with other clinical parameters, contribute to developing prediction models and support clinical decision-making [1, 2]. In the last decade, the number of studies investigating the role of radiomics in oncological imaging has exponentially grown, but its translation into clinical practice remains an unsolved issue [3–7]. Indeed, the lack of standardised methodology, reproducibility and interpretability represents the main limitations [8–12].
To address these concerns, many guidelines, checklists and scoring systems have been developed [13–17]. In 2017, the radiomics quality score (RQS) was proposed to improve the methodological rigour of the radiomic studies. Despite being originally conceived as a guide for designing appropriate radiomics studies it has since then become the “standard” evaluation tool of radiomics papers [18]. However, RQS suffers from some limitations regarding its interpretability and applicability in the assessment of deep learning studies [19]. Furthermore, it lacks an accurate description of its development process and the criteria used for assigning scores to individual items [20]. Following this initiative, several checklists have been successfully proposed to help authors and reviewers evaluate the quality of papers on the application of artificial intelligence in medicine, such as the checklist for artificial intelligence in medical imaging [15], the Must AI Criteria-10 [21] and the checklist for evaluation of radiomics research [14]. More recently, the European Society of Medical Imaging Informatics proposed the METhodological RadiomICs Score (METRICS) as an easy-to-use quality assessment tool to evaluate and improve the methodology and reproducibility of radiomics studies [20]. It includes 30 items (+ 5 conditional items) divided into nine categories and is available as a user-friendly online automated calculation tool.
Endometrial cancer is the most common gynaecological cancer in developed countries [22]. Lately, many advances have been made in understanding the complex biological behaviour of endometrial carcinoma, including the importance of molecular phenotypes in determining the prognosis [23, 24]. Furthermore, the recently revised International Federation of Gynaecologist and Obstetrics (FIGO) staging system of endometrial carcinoma now incorporates parameters exclusively accessible through biopsy and post-surgical pathological analysis, including histology, grading, lymphovascular space invasion, and molecular features [25]. Given the limitation of conventional imaging techniques in assessing these factors preoperatively, there is a growing emphasis on the role of radiomics for predictive purposes [26, 27].
In this context, we aim to critically assess the methodological quality of radiomics-based models for endometrial cancer, by means of RQS and METRICS in radiologists with different expertise.
Methods
Protocol
This research was conducted according to the preferred reporting items for systematic reviews and meta-analyses checklist [28].
Search strategy
Two investigators (L.R. and S.B.) conducted a comprehensive search of PubMed and Scopus databases to identify papers published by 30th October 2023. The search string included the following terms and their variations: (“radiomics” OR “radiogenomics” OR “machine learning” OR “deep learning”) AND “endometrial cancer” AND (“computed tomography” OR “magnetic resonance” OR “positron emission tomography” OR “ultrasound”). Duplicates, non-original articles (e.g. reviews, commentaries, editorials), studies on different topics, and studies in languages other than English were removed.
Data collection and analysis
The evaluation was carried out by four researchers, divided into pairs based on their expertise in radiomics and familiarity with the scores: two novices (L.R. and S.B.) and two expert readers (B.K. and A.P.). As an introduction to radiomics scoring systems, a preliminary training session was carried on, analysing and discussing the items of both scores. Inter-rater reliability (IRR) between reviewers was preliminarily assessed. For this task, 30 articles were randomly selected and independently reviewed by each reader [29]. Discrepancies were solved in consensus among each pair of readers and these results were considered for further analysis. For the remaining articles, each pair independently examined the components of both the RQS, made of 16 elements and the METRICS score, which includes 30 elements, with scores expressed as a percentage out of 100%. Additionally, a comprehensive analysis of the full manuscripts was conducted to extract specific data: the topic of the study (classification, characterisation, prediction of lymph node metastasis, prediction of prognosis, segmentation or treatment planning), the imaging technique (MRI, CT, US or PET/CT), the year of publication, and journal quartile.
Statistical analysis
Qualitative variables were summarised with absolute values and percentage frequencies. The normality of the distribution of quantitative data was assessed using the Shapiro–Wilk test, and they were presented as the median and interquartile range (IQR) if not normally distributed. The intraclass correlation coefficient (ICC) was assessed with a two-way random effect, single rater, absolute agreement model based on the scores assigned by all four readers and reader pairs based on experience level on a random sample of 30 papers, based on commonly employed guidelines [29]. The resulting values were interpreted as follows: ICC < 0.5 indicated poor reliability, ICC between 0.5 and 0.75 indicated moderate reliability, ICC between 0.75 and 0.9 indicated good reliability and ICC greater than 0.90 indicated excellent reliability. A two one-sided t-tests (TOST) procedure was employed to compare equivalence and/or differences in the distribution of total RQS and METRICS scores, both overall and by experience group. TOST was performed with an alpha level of 0.05, assuming equivalence bounds to be between − 5% and + 5% of the total score. To determine if the overall RQS and METRICS scores differed based on the study’s objective, the type of imaging technique, the year of publication and journal quartile subgroup analyses were conducted employing the Kruskal–Wallis rank test. The statistical analysis was carried out with the use of “TOSTER” (version 0.8), “irr” (version 0.84.1), and “R” (version 4.3.2). A p value < 0.05 was considered statistically significant.
Results
Literature search
The flowchart of the study selection is presented in Fig. 1. One hundred eighty-seven articles were initially identified. Of these, 85 were duplicated. Afterwards, the reviewers removed 34/102 records for the following reasons: non-original articles (24), different topics (6) and not English (4). Finally, 68 articles were included in the systematic review.
Fig. 1.
Flow diagram of literature search and selection process
Study characteristics
The median study population number was 171 (IQR, 138–333). Among the included papers, 24/68 (35.3%) focused on characterisation, 13/68 (19.1%) on risk stratification, 10/63 (14.7%) on classification, 9/68 (13.2%) on prediction of lymph-node metastasis, 8/68 (11.8%) on prognosis, 4/68 (5.9%) on segmentation and treatment planning (two papers each). Two out of 68 articles (2.9%) were published in 2018 and 2019 (1 each year), 8/68 (11.8%) in 2020, 19/68 (27.9%) in 2021, 18/68 (26.5%) in 2022 and 21/68 (30.9%) in 2023. MRI was the most adopted imaging modality (56/68, 82.3%), followed by CT (5/63, 7.4%), PET/CT (5/63, 7.4%) and US (2/68, 2.9%). The majority of studies used machine-learning methods (60/68, 88.2%), while deep learning was employed in 8/68 (11.8%) only.
IRR
ICC values indicated excellent agreement across all raters for both the RQS and METRICS scores (Fig. 2). In detail, ICC for METRICS evaluations among all raters was 0.959 (95% CI: 0.928, 0.979). For RQS evaluations, the agreement was also strong, with an ICC of 0.897 (95% CI: 0.821, 0.946), indicating a high degree of reliability among the reviewers. ICC across different pairs of readers showed excellent agreement for both METRICS (0.980, 95% CI: 0.959, 0.990) and RQS (0.940, 95% CI: 0.874, 0.971) in expert readers. For novice readers, the ICC resembled that of experts for METRICS (0.980, 95% CI: 0.958, 0.990), while it was slightly lower for RQS (0.913, 95% CI: 0.825, 0.957).
Fig. 2.
Boxplot comparison of METRICS and RQS by reviewer expertise. Scores are presented in percentage, with red boxes representing expert raters and blue boxes novice raters
Study evaluation—RQS
Median RQS was 11 (30.6%) for both pairs, with IQR between 9–14 (24.3–38.9%) for experts and 8–14 (22.2–38.9%) for novices. Detailed results for expert readers are reported in Table 1. The imaging protocol was well documented in 63/68 papers (92.6%), but a public protocol was used only once. Multiple segmentations were performed in 35/68 cases (51.5%). No study was conducted on phantoms or used imaging at multiple time points. In 61/68 (89.7%) studies, feature selection methods were employed. Non-radiomic variables were included in the analysis in 36/68 (52.9%), while biological correlates were detected and discussed in 12/68 (17.6%) studies. No study had a prospective design or included a cost-effectiveness analysis. A comparison with the current gold standard was performed in 40/68 cases (58.8%), whilst the potential clinical utility was analysed in 24/68 studies (35.3%). Finally, the code and/or data were available in 8/68 cases (11.8%). Code was available in 7/8 papers: for preprocessing the images and network implementation in 1 paper, for rerunning the segmentation experiments in 1 paper, for image preprocessing and data analysis in 1 paper, for data analysis in 2 papers, for radiomic data preprocessing in 1 paper, and for rerunning whole experiments in 1 paper.
Table 1.
RQS of expert readers for all the included studies
| Author (year) | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | Item 9 | Item 10 | Item 11 | Item 12 | Item 13 | Item 14 | Item 15 | Item 16 | RQS, (total) | RQS (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bereby-Kahane M (2020) | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 2 | 0 | 0 | − 5 | 0 | 0 | 0 | 0 | 2 | 5.6% |
| Bi Q (2022) | 1 | 0 | 0 | 0 | 3 | 1 | 1 | 0 | 2 | 2 | 0 | 3 | 2 | 2 | 0 | 0 | 17 | 47.2% |
| Bo J (2022) | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 1 | 2 | 2 | 0 | 2 | 2 | 2 | 0 | 0 | 17 | 47.2% |
| Celli V (2022) | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 7 | 19.4% |
| Chen J (2021) | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 1 | 1 | 1 | 0 | 2 | 2 | 2 | 0 | 0 | 14 | 38.9% |
| Chen J (2023) | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 4 | 2 | 2 | 0 | 0 | 15 | 41.7% |
| Chen X (2022) | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 2 | 0 | 0 | 4 | 2 | 0 | 0 | 0 | 12 | 33.3% |
| K. E. Fasmer (2021) | 2 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 1 | 0 | 2 | 2 | 0 | 0 | 0 | 12 | 33.3% |
| Han Y (2020) | 1 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 2 | 0 | 0 | − 5 | 2 | 0 | 0 | 0 | 4 | 11.1% |
| Hodneland E (2021) | 1 | 1 | 0 | 0 | − 3 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 1 | 5 | 13.9% |
| Hoivik EA (2021) | 1 | 0 | 0 | 0 | − 3 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 3 | 8.3% |
| Jacob H (2021) | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 8 | 22.2% |
| Jiang X (2023) | 1 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 10 | 27.8% |
| Kurata Y (2021) | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 9 | 25.0% |
| Lefebvre TL (2023) | 1 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 2 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 11 | 30.6% |
| Lefebvre TL (2022) | 1 | 1 | 0 | 0 | 3 | 0 | 1 | 0 | 1 | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 12 | 33.3% |
| Li X (2023) | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 9 | 25.0% |
| Li X (2023) | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 1 | 2 | 0 | 0 | 3 | 2 | 2 | 0 | 0 | 15 | 41.7% |
| Lin Z (2023) | 1 | 1 | 0 | 0 | 3 | 0 | 0 | 1 | 1 | 1 | 0 | 2 | 0 | 2 | 0 | 0 | 12 | 33.3% |
| Lin Z (2023) | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 2 | 0 | 2 | 2 | 2 | 0 | 0 | 15 | 41.7% |
| Lin Z (2023) | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 1 | 2 | 1 | 0 | 5 | 2 | 2 | 0 | 0 | 19 | 52.8% |
| Liu D (2022) | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 2 | 0 | 2 | 2 | 2 | 0 | 0 | 15 | 41.7% |
| Liu J (2023) | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 1 | 1 | 1 | 0 | 3 | 2 | 2 | 0 | 0 | 16 | 44.4% |
| Liu XF (2022) | 1 | 1 | 0 | 0 | 3 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 2 | 2 | 0 | 0 | 14 | 38.9% |
| Liu XF (2022) | 1 | 1 | 0 | 0 | 3 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 2 | 2 | 0 | 0 | 14 | 38.9% |
| Liu XF (2023) | 1 | 1 | 0 | 0 | 3 | 1 | 1 | 0 | 1 | 2 | 0 | 2 | 2 | 2 | 0 | 0 | 16 | 44.4% |
| Long L (2021) | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 13 | 36.1% |
| Luo Y (2020) | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 9 | 25.0% |
| Mainenti PP (2022) | 1 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 1 | 0 | 3 | 0 | 0 | 0 | 0 | 10 | 27.8% |
| Miccò M (2022) | 1 | 1 | 0 | 0 | 3 | 0 | 0 | 1 | 2 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 11 | 30.6% |
| Otani S (2022) | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 9 | 25.0% |
| Rodríguez-Ortega A (2021) | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 7 | 19.4% |
| Song XL (2023) | 1 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 2 | 0 | 0 | 11 | 30.6% |
| Stanzione A (2021) | 1 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 10 | 27.8% |
| Tan Q (2023) | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 9 | 25.0% |
| Wang Y (2023) | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 11 | 30.6% |
| Xu X (2019) | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 2 | 0 | 0 | 0 | 12 | 33.3% |
| Xu Y (2021) | 0 | 0 | 0 | 0 | − 3 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0.0% |
| Yan B (2023) | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 2 | 0 | 0 | 12 | 33.3% |
| Yan BC (2020) | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 1 | 1 | 1 | 0 | 5 | 0 | 2 | 0 | 0 | 16 | 44.4% |
| Yan B (2023) | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 9 | 25.0% |
| Coada CA (2023) | 1 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | − 5 | 0 | 0 | 0 | 0 | 1 | 2.8% |
| Crivellaro C (2020) | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 7 | 19.4% |
| De Bernardi E (2018) | 1 | 0 | 0 | 0 | − 3 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 3 | 8.0% |
| Huang XW (2023) | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 1 | 1 | 1 | 0 | 2 | 2 | 0 | 0 | 0 | 12 | 33.3% |
| Le Z (2023) | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 9 | 25.0% |
| Yan BC (2021) | 1 | 1 | 0 | 0 | 3 | 0 | 1 | 0 | 1 | 0 | 0 | 4 | 2 | 0 | 0 | 0 | 13 | 36.1% |
| Yan BC (2021) | 1 | 1 | 0 | 0 | 3 | 1 | 1 | 0 | 1 | 1 | 0 | 2 | 2 | 0 | 0 | 0 | 13 | 36.1% |
| Yang L (2023) | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 1 | 1 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 12 | 33.3% |
| Yang LY (2021) | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 1 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 11 | 30.6% |
| Yue X (2023) | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 2 | 0 | 2 | 2 | 2 | 0 | 0 | 15 | 41.7% |
| Zhang J (2022) | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 2 | 2 | 0 | 2 | 2 | 2 | 0 | 0 | 16 | 44.4% |
| Zhang K (2021) | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 2 | 2 | 0 | 0 | 14 | 38.9% |
| Zhang Y (2021) | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 7 | 19.4% |
| Zhao M (2022) | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 1 | 0 | 0 | 3 | 2 | 2 | 0 | 0 | 14 | 38.9% |
| Zhang K (2021) | 1 | 1 | 0 | 0 | 3 | 1 | 1 | 1 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 12 | 33.3% |
| Shen L (2023) | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 9 | 25.0% |
| Li D (2021) | 1 | 1 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 4 | 2 | 0 | 0 | 1 | 13 | 36.1% |
| Moro F (2022) | 0 | 0 | 0 | 0 | 3 | 1 | 0 | 1 | 2 | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 12 | 33.3% |
| Nakajo M (2021) | 1 | 0 | 0 | 0 | 3 | 1 | 0 | 1 | 0 | 0 | 0 | − 5 | 0 | 0 | 0 | 0 | 1 | 2.8% |
| Veeraraghavan H (2020) | 1 | 0 | 0 | 0 | 3 | 1 | 1 | 0 | 1 | 0 | 0 | 2 | 0 | 0 | 0 | 2 | 11 | 30.6% |
| Wang X (2021) | 1 | 0 | 0 | 0 | 3 | 0 | 1 | 1 | 0 | 1 | 0 | 3 | 2 | 2 | 0 | 1 | 15 | 41.7% |
| Soydal C (2022) | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | − 5 | 0 | 0 | 0 | 0 | − 1 | 0.0% |
| Chen X (2020) | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 10 | 27.8% |
| Dong H (2020) | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 9 | 25.0% |
| Tao J (2022) | 1 | 0 | 0 | 0 | − 3 | 0 | 0 | 0 | 1 | 0 | 0 | − 5 | 0 | 0 | 0 | 0 | − 6 | 0.0% |
| Mao W (2022) | 1 | 0 | 0 | 0 | − 3 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 4 | 7 | 19.4% |
| Urushibara (2022) | 1 | 0 | 0 | 0 | − 3 | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 3 | 8.3% |
Study evaluation—METRICS
The overall median METRICS score was 67.6% (IQR, 58.8–76.0) and 68.7% (IQR, 60.4–76.5) for expert and novice readers, respectively. Figure 3 shows the results of the METRICS score for expert readers. Only 3/68 studies (4.4%) adhered to specific radiomics guidelines or checklists. The eligibility criteria of the study population and reference standard were clearly defined in 66/68 (97.1%) and 61/68 (89.7%), respectively. Clinical translatability was applicable in 65/68 studies (95.6%), and the interval between imaging used and the gold standard was clear only in 34/68 cases (50.0%). Fully automatic segmentations were performed in 4/68 studies (5.9%), with formal evaluation in 3 of them. Segmentation methods were clearly described in 57/68 (83.8%) and a single reader or automated tool produced test set segmentation in 38/68 (55.9%). Appropriate image pre-processing techniques and standardised feature extraction software were used in 36/68 (52.9%) and 43/68 (63.2%), respectively. Regarding feature selection, redundant features were removed in 57/68 (83.8%), while non-robust features were removed in 37/68 cases (54.4%). The dimensionality was appropriate to data size in 42/68 studies (61.8%). The robustness of the end-to-end pipeline was assessed in only 1/8 of studies (12.5%) employing deep learning methods. Then, metrics for evaluation of the model performance were appropriate in 66/68 studies (97.1%), including uncertainty consideration in 54/68 cases (79.4%). Uni-parametric imaging was used or proved to be inferior in 33/68 studies (48.5%). Radiomics was compared to non-radiomics approaches in 46/68 studies (67.6%), but only one compared a radiomics-based model with a simple baseline reference model. Model validation was internal in 43/68 studies (63.2%), external in 10/68 (14.7%) and both internal and external in 9/68 (13.2%), while absent in 6/68 studies (8.8%).
Fig. 3.
METRICS of expert readers for all the included studies
Statistical analysis
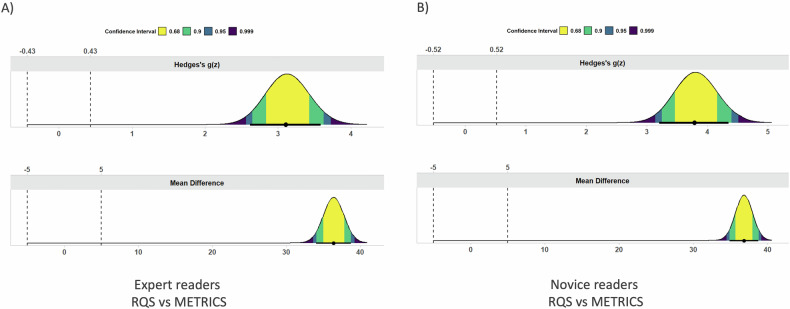
The TOST procedure showed equivalence within the − 5% to 5% bounds for both RQS and METRICS in the comparison between experienced and novice readers (both p < 0.01). On the other hand, for both experience levels, no equivalence was found when comparing RQS, as well as METRICS total score (both p = 1). Figures 4 and 5 present the mean difference and effect size (i.e. Hedges’s g), as obtained by TOST. No significant differences in RQS and METRICS scores across different years of publication, imaging modalities, study topics or journal quartile. Detailed results are reported in Table 2.
Fig. 4.
Mean difference (lower row) and effect size (upper row) plots depicting the results of the equivalence test for both RQS (A) and METRICS (B) in expert vs novice readers
Fig. 5.
Mean difference (lower row) and effect size (upper row) plots depicting the results of the equivalence test for both expert (A) and novice (B) readers in RQS vs METRICS
Table 2.
Subgroup analysis of endometrial cancer radiomics studies assessed by RQS and METRICS
| Subgroup analysis | Number of studies | % | Median RQS, (%) | IQR, (%) | p value | Median METRICS, (%) | IQR, (%) | p value | |
|---|---|---|---|---|---|---|---|---|---|
| Modality | 0.13 | 0.13 | |||||||
| MRI | 56 | 82.3 | 33.3 | 25.0–38.9 | 67.6 | 60.9–77.4 | |||
| CT | 5 | 7.4 | 25.0 | 13.9–33.3 | 70.5 | 36.1–79.6 | |||
| PET/CT | 5 | 7.4 | 8.0 | 1.4–30.6 | 53.8 | 30.9–65.4 | |||
| US | 2 | 2.9 | 33.3 | 33.3–33.3 | 78.4 | 69.6–NA | |||
| Year of publication | 0.14 | 0.17 | |||||||
| 2023 | 21 | 30.9 | 33.3 | 25.0–41.7 | 69.9 | 67.3–77.8 | |||
| 2022 | 18 | 26.5 | 33.3 | 19.3–39.6 | 66.8 | 59.4–83.6 | |||
| 2021 | 19 | 27.9 | 30.6 | 19.4–36.1 | 63.4 | 56.1–72.2 | |||
| 2018–2020 | 10 | 14.7 | 25.0 | 10.3–31.3 | 63.3 | 48.9–68.9 | |||
| Topic | 0.59 | 0.32 | |||||||
| Characterisation | 24 | 35.3 | 30.6 | 25.0–41.0 | 69.9 | 63.0–74.7 | |||
| Classification | 10 | 14.7 | 29.2 | 16.3–44.4 | 69.5 | 48.8–78.3 | |||
| Risk stratification | 13 | 19.1 | 33.3 | 27.8–38.9 | 67.5 | 63.0–84.3 | |||
| Prognosis and survival | 8 | 11.8 | 29.2 | 11.8–39.6 | 60.8 | 54.4–68.2 | |||
| Lymph-node involvement | 9 | 13.2 | 31.9 | 10.9–38.2 | 60.8 | 40.6–67.5 | |||
| Others | 4 | 5.9 | 25.0 | 6.9–30.6 | 67.6 | 37.3–83.2 | |||
| Journal quartile | 0.19 | 0.09 | |||||||
| First | 31 | 45.6 | 28.0 | 25.0–36.1 | 71.1 | 60.5–82.8 | |||
| Others | 37 | 54.6 | 33.3 | 19.4–40.3 | 67.5 | 54.9–72.3 |
The studies are divided by imaging modality, year of publication, research topic and journal quartile
Discussion
The majority of radiomics applications were for characterisation (35.3%) [30–53], recurrence risk stratification (19.1%) [54–66] and classification [67–76]. Both RQS and METRICS scores have demonstrated excellent inter-rater reproducibility among both novice and expert readers, underscoring their reliability. This high degree of agreement can be partly ascribed to the introductory training session designed to familiarise all readers with METRICS, which is a relatively new tool compared to the more established RQS. The RQS assessment revealed an overall unsatisfactory quality, highlighting significant defects, such as the absence of phantom studies, prospective studies and cost-effectiveness analyses. Furthermore, the lack of detection and discussion of biological correlates, cut-off analysis and open-source data were noted as flaws. Interestingly, METRICS depicted an overall better quality of methodology, while still identifying weaknesses in adherence to specific radiomics and machine learning guidelines, the inclusion of fully automated segmentation processes, and limited external testing, among others.
Our findings concerning common study limitations align with previous literature, where the methodological quality of radiomics studies across various medical imaging fields has been reported as unsatisfactory by RQS application. Specifically, for endometrial cancer, Huang et al analysed MRI-based radiomics studies and reported a median total RQS percentage of 38.2%, highlighting the need for methodological improvement [77]. In other fields, the results are even less satisfactory: median total RQS percentage of 16.7%, 11.8%, 19% and 9.4% for ovarian cancer, breast cancer, meningioma and renal cell carcinoma, respectively [78–80]. To date, METRICS has just begun to be applied to radiomics studies, given its recent development.
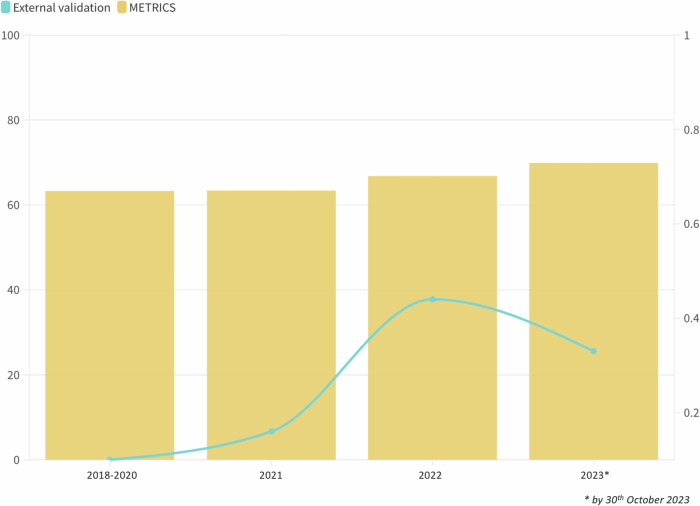
Although the scores cannot be directly compared, when applied to deep-learning-based studies [71, 74, 76, 81–83], RQS scores these articles lower than the median compared to METRICS, due to the lack of feature selection or multiple segmentations. Furthermore, the relative weight of some items of the RQS (e.g. ± 7 points for prospective design) might be unbalanced and penalise those preliminary, exploratory studies needing retrospective design as a first ground on which stronger evidence must be built. Conversely, METRICS facilitates a step-by-step quality assessment, allowing to depict specific methodological shortcomings and, thanks to its conditional format, it permits more nuanced assessment according to methodology. One of these is the scarcity of automatic segmentation process. Despite several deep-learning-based models that have shown similar accuracy to that of experienced radiologists in segmenting endometrial cancers, automatic segmentations are far from being routinely used for radiomics studies [84]. They have shown similar accuracy to that of experienced radiologists in segmenting endometrial cancers, reducing the time needed and improving the robustness of the segmentations [82, 83]. Another lack specifically raised by METRICS is the comparison between radiomics and simple or classical statistical methods, found in one study only. This may reflect a trend towards more complex models, possibly due to the appeal of advanced machine learning for potentially superior predictive power [85]. However, comparing radiomics to simpler models is crucial [86]. It assists in establishing whether the complexity of radiomics offers significant advantages over more traditional approaches. Furthermore, cost-effectiveness and interpretability should be considered [87]. External validation has been also found to be infrequent (30.2% of studies). Although there has been a positive trend towards the years, the frequency of external validation is suboptimal, limiting the generalizability of the proposed models (Fig. 6). The discussion of biological correlates is essential for interpreting radiomics results, but infrequently depicted (17.6% of studies). In endometrial cancer, it is even more critical than in some other types of cancer because of the well-established roles of histopathological and molecular characteristics, which are now included in the FIGO staging [88, 89]. Since preoperative staging is challenging in current clinical practice, radiomics research aimed at bridging this gap must detect and discuss these biological correlates to improve its clinical translatability [90].
Fig. 6.
Comparative analysis of METRICS scores and external validation frequency in endometrial cancer radiomics studies from 2018 to 2023. The graph displays the annual METRICS scores (yellow bars) and the proportion of studies that included external validation (blue line)
Overall, based on our experience, the RQS appears to skew negatively in the overall study score, reflecting an item weight distribution that is highly concentrated in a few key items. METRICS presents a generally more homogeneous item weight distribution and clearer item definitions, as well as increased flexibility in handling varying study designs. These points, partly due to the collegial, systematic development process of METRICS which may have mitigated biases by a single or a few experts, may help explain the differences in final score distribution between the two tools. In the direct comparison, as seen in Fig. 2, it appears more reasonable that the bulk of studies would hover around a moderate to good score as portrayed by METRICS rather than the low results obtained using RQS. These differences are further accentuated by the non-linear nature of the conversion from absolute to percentage score in the RQS, compared to METRICS [91].
Our review presents certain limitations. First, the rapidly evolving field of radiomics, together with variations in nomenclature, poses a risk of overlooking potential eligible studies. However, it is expected that the involvement of two reviewers has minimised this risk. Second, the chronological framework, wherein certain studies precede the adoption of the RQS and all are prior to the METRICS guidelines, could affect our evaluation [92]. Last, the necessity of conducting a training session might have affected the ICC outcomes.
To conclude, both RQS and METRICS may serve as instrumental tools in highlighting different methodological shortcomings in endometrial cancer radiomics research, that limit the interpretability and generalizability of the models. The accurate prediction of histopathological and molecular factors would preoperatively reveal the definitive FIGO stage, stratifying patients’ treatments accordingly. Notably, although being quite far from perfect, METRICS has revealed a generally satisfactory methodological quality in these studies and high reproducibility, with its scores being more accommodating, particularly for retrospective and exploratory research, reflecting its emphasis on comprehensive documentation and practical applicability. Adopting these quality scoring tools, especially METRICS, can also be used as a step-by-step guide to design radiomics studies, facilitating translatability into clinical practice.
Supplementary information
Abbreviations
- FIGO
International Federation of Gynaecologist and Obstetrics
- ICC
Intraclass correlation coefficient
- IQR
Interquartile range
- METRICS
METhodological RadiomICs Score
- RQS
Radiomics quality score
- TOST
Two one-sided t-tests
Funding
Open access funding provided by Università degli Studi di Salerno within the CRUI-CARE Agreement.
Compliance with ethical standards
Guarantor
The scientific guarantor of this publication is Prof. Renato Cuocolo.
Conflict of interest
R.C., B.K. and A.S. are members of the Scientific Editorial Board of European Radiology; they did not take part in the review or selection processes of this article. A.P. holds the position of Junior Deputy Editor at European Radiology, but was not involved in the manuscript handling process. E.S. is a member of the Scientific Editorial Board of European Radiology Experimental, and is a co-founder and shareholder of Lucida Medical. The remaining authors declare no conflicts of interest.
Statistics and biometry
One of the authors (R.C.) has significant statistical expertise.
Informed consent
Not applicable.
Ethical approval
Institutional Review Board approval was not required for systematic review.
Study subjects or cohorts overlap
Not applicable.
Methodology
Systematic review
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1007/s00330-024-10947-6.
References
- 1.Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts H (2018) Artificial intelligence in radiology. Nat Rev Cancer 18:500–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russo L, Bottazzi S, Sala E (2023) Artificial intelligence in female pelvic oncology: tailoring applications to clinical needs. Eur Radiol. 10.1007/s00330-023-10455-z [DOI] [PubMed]
- 4.Stanzione A, Cuocolo R, Ugga L et al (2022) Oncologic imaging and radiomics: a walkthrough review of methodological challenges. Cancers 14:4871 [DOI] [PMC free article] [PubMed]
- 5.Fournier L, Costaridou L, Bidaut L et al (2021) Incorporating radiomics into clinical trials: expert consensus endorsed by the European Society of Radiology on considerations for data-driven compared to biologically driven quantitative biomarkers. Eur Radiol 31:6001–6012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang EP, O’Connor JPB, McShane LM et al (2023) Criteria for the translation of radiomics into clinically useful tests. Nat Rev Clin Oncol 20:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cannella R, Vernuccio F, Klontzas ME et al (2023) Systematic review with radiomics quality score of cholangiocarcinoma: an EuSoMII radiomics auditing group initiative. Insights Imaging 14:21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Recht MP, Dewey M, Dreyer K et al (2020) Integrating artificial intelligence into the clinical practice of radiology: challenges and recommendations. Eur Radiol 30:3576–3584 [DOI] [PubMed] [Google Scholar]
- 9.Ponsiglione A, Gambardella M, Stanzione A et al (2023) Radiomics for the identification of extraprostatic extension with prostate MRI: a systematic review and meta-analysis. Eur Radiol. 10.1007/s00330-023-10427-3 [DOI] [PMC free article] [PubMed]
- 10.Shrestha P, Poudyal B, Yadollahi S et al (2022) A systematic review on the use of artificial intelligence in gynecologic imaging—background, state of the art, and future directions. Gynecol Oncol 166:596–605 [DOI] [PubMed] [Google Scholar]
- 11.van der Velden BHM (2024) Explainable AI: current status and future potential. Eur Radiol 34:1187–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang ML, Ren J, Jin ZY et al (2023) A systematic review and meta-analysis of CT and MRI radiomics in ovarian cancer: methodological issues and clinical utility. Insights Imaging 14:117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klontzas ME, Gatti AA, Tejani AS, Kahn CE Jr (2023) AI reporting guidelines: how to select the best one for your research. Radiol Artif Intell 5:e230055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kocak B, Baessler B, Bakas S et al (2023) CheckList for evaluation of radiomics research (CLEAR): a step-by-step reporting guideline for authors and reviewers endorsed by ESR and EuSoMII. Insights Imaging 14:75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mongan J, Moy L, Kahn CE Jr (2020) Checklist for artificial intelligence in medical imaging (CLAIM): a guide for authors and reviewers. Radiol Artif Intell 2:e200029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zwanenburg A, Vallieres M, Abdalah MA et al (2020) The image biomarker standardisation initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 295:328–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collins GS, Reitsma JB, Altman DG, Moons KG (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med 162:55–63 [DOI] [PubMed] [Google Scholar]
- 18.Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14:749–762 [DOI] [PubMed] [Google Scholar]
- 19.Spadarella G, Stanzione A, Akinci D’Antonoli T et al (2023) Systematic review of the radiomics quality score applications: an EuSoMII radiomics auditing group initiative. Eur Radiol 33:1884–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kocak B, Akinci D’Antonoli T, Mercaldo N et al (2024) METhodological RadiomICs score (METRICS): a quality scoring tool for radiomics research endorsed by EuSoMII. Insights Imaging 15:8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerda-Alberich L, Solana J, Mallol P et al (2023) MAIC-10 brief quality checklist for publications using artificial intelligence and medical images. Insights Imaging 14:11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249 [DOI] [PubMed] [Google Scholar]
- 23.Arciuolo D, Travaglino A, Raffone A et al (2022) TCGA molecular prognostic groups of endometrial carcinoma: current knowledge and future perspectives. Int J Mol Sci 23:11684 [DOI] [PMC free article] [PubMed]
- 24.Concin N, Matias-Guiu X, Vergote I et al (2021) ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer 31:12–39 [DOI] [PubMed] [Google Scholar]
- 25.Berek JS, Matias-Guiu X, Creutzberg C et al (2023) FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet 162:383–394 [DOI] [PubMed] [Google Scholar]
- 26.Di Donato V, Kontopantelis E, Cuccu I et al (2023) Magnetic resonance imaging-radiomics in endometrial cancer: a systematic review and meta-analysis. Int J Gynecol Cancer 33:1070–1076 [DOI] [PubMed] [Google Scholar]
- 27.Manganaro L, Nicolino GM, Dolciami M et al (2021) Radiomics in cervical and endometrial cancer. Br J Radiol 94:20201314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bereby-Kahane M, Dautry R, Matzner-Lober E et al (2020) Prediction of tumor grade and lymphovascular space invasion in endometrial adenocarcinoma with MR imaging-based radiomic analysis. Diagn Interv Imaging 101:401–411 [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Wang X, Lv H et al (2023) Development and external validation of a clinical-radiomics nomogram for preoperative prediction of LVSI status in patients with endometrial carcinoma. J Cancer Res Clin Oncol 149:13943–13953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han Y, Xu H, Ming Y et al (2020) Predicting myometrial invasion in endometrial cancer based on whole-uterine magnetic resonance radiomics. J Cancer Res Ther 16:1648–1655 [DOI] [PubMed] [Google Scholar]
- 33.Lefebvre TL, Ciga O, Bhatnagar SR et al (2023) Predicting histopathology markers of endometrial carcinoma with a quantitative image analysis approach based on spherical harmonics in multiparametric MRI. Diagn Interv Imaging 104:142–152 [DOI] [PubMed] [Google Scholar]
- 34.Li X, Dessi M, Marcus D et al (2023) Prediction of deep myometrial infiltration, clinical risk category, histological type, and lymphovascular space invasion in women with endometrial cancer based on clinical and T2-weighted MRI radiomic features. Cancers 15:2209 [DOI] [PMC free article] [PubMed]
- 35.Lin Z, Gu W, Guo Q et al (2023) Multisequence MRI-based radiomics model for predicting POLE mutation status in patients with endometrial cancer. Br J Radiol 96:20221063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin Z, Wang T, Li H et al (2023) Magnetic resonance-based radiomics nomogram for predicting microsatellite instability status in endometrial cancer. Quant Imaging Med Surg 13:108–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu D, Yang L, Du D et al (2022) Multi-parameter MR radiomics based model to predict 5-year progression-free survival in endometrial cancer. Front Oncol 12:813069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu XF, Yan BC, Li Y, Ma FH, Qiang JW (2023) Radiomics nomogram in aiding preoperatively dilatation and curettage in differentiating type II and type I endometrial cancer. Clin Radiol 78:e29–e36 [DOI] [PubMed] [Google Scholar]
- 39.Long L, Sun J, Jiang L et al (2021) MRI-based traditional radiomics and computer-vision nomogram for predicting lymphovascular space invasion in endometrial carcinoma. Diagn Interv Imaging 102:455–462 [DOI] [PubMed] [Google Scholar]
- 40.Luo Y, Mei D, Gong J, Zuo M, Guo X (2020) Multiparametric MRI-based radiomics nomogram for predicting lymphovascular space invasion in endometrial carcinoma. J Magn Reson Imaging 52:1257–1262 [DOI] [PubMed] [Google Scholar]
- 41.Rodríguez-Ortega A, Alegre A, Lago V et al (2021) Machine learning-based integration of prognostic magnetic resonance imaging biomarkers for myometrial invasion stratification in endometrial cancer. J Magn Reson Imaging 54:987–995 [DOI] [PubMed] [Google Scholar]
- 42.Song XL, Luo HJ, Ren JL et al (2023) Multisequence magnetic resonance imaging-based radiomics models for the prediction of microsatellite instability in endometrial cancer. Radiol Med 128:242–251 [DOI] [PubMed] [Google Scholar]
- 43.Stanzione A, Cuocolo R, Del Grosso R et al (2021) Deep myometrial infiltration of endometrial cancer on MRI: a radiomics-powered machine learning pilot study. Acad Radiol 28:737–744 [DOI] [PubMed] [Google Scholar]
- 44.Tan Q, Wang Q, Jin S, Zhou F, Zou X (2023) Network evolution model-based prediction of tumor mutation burden from radiomic-clinical features in endometrial cancers. BMC Cancer 23:712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Bi Q, Deng Y et al (2023) Development and validation of an MRI-based radiomics nomogram for assessing deep myometrial invasion in early stage endometrial adenocarcinoma. Acad Radiol 30:668–679 [DOI] [PubMed] [Google Scholar]
- 46.Yue X, He X, He S et al (2023) Multiparametric magnetic resonance imaging-based radiomics nomogram for predicting tumor grade in endometrial cancer. Front Oncol 13:1081134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Veeraraghavan H, Friedman CF, DeLair DF et al (2020) Machine learning-based prediction of microsatellite instability and high tumor mutation burden from contrast-enhanced computed tomography in endometrial cancers. Sci Rep 10:17769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang X, Wu K, Li X, Jin J, Yu Y, Sun H (2021) Additional value of PET/CT-based radiomics to metabolic parameters in diagnosing lynch syndrome and predicting PD1 expression in endometrial carcinoma. Front Oncol 11:595430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan B, Jia Y, Li Z et al (2023) Preoperative prediction of lymphovascular space invasion in endometrioid adenocarcinoma: an MRI-based radiomics nomogram with consideration of the peritumoral region. Acta Radiol 64:2636–2645 [DOI] [PubMed] [Google Scholar]
- 50.Yan B, Zhao T, Li Z, Ren J, Zhang Y (2023) An MR-based radiomics nomogram including information from the peritumoral region to predict deep myometrial invasion in stage I endometrioid adenocarcinoma: a preliminary study. Br J Radiol 96:20230026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao M, Wen F, Shi J et al (2022) MRI-based radiomics nomogram for the preoperative prediction of deep myometrial invasion of FIGO stage I endometrial carcinoma. Med Phys 49:6505–6516 [DOI] [PubMed] [Google Scholar]
- 52.Chen X, Wang Y, Shen M et al (2020) Deep learning for the determination of myometrial invasion depth and automatic lesion identification in endometrial cancer MR imaging: a preliminary study in a single institution. Eur Radiol 30:4985–4994 [DOI] [PubMed] [Google Scholar]
- 53.Dong HC, Dong HK, Yu MH, Lin YH, Chang CC (2020) Using deep learning with convolutional neural network approach to identify the invasion depth of endometrial cancer in myometrium using MR images: a pilot study. Int J Environ Res Public Health 17:5993 [DOI] [PMC free article] [PubMed]
- 54.Celli V, Guerreri M, Pernazza A et al (2022) MRI- and histologic-molecular-based radio-genomics nomogram for preoperative assessment of risk classes in endometrial cancer. Cancers 14:5881 [DOI] [PMC free article] [PubMed]
- 55.Chen J, Gu H, Fan W et al (2021) MRI-based radiomic model for preoperative risk stratification in stage I endometrial cancer. J Cancer 12:726–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang X, Song J, Zhang A et al (2023) Preoperative assessment of MRI-invisible early-stage endometrial cancer with MRI-based radiomics analysis. J Magn Reson Imaging 58:247–255 [DOI] [PubMed] [Google Scholar]
- 57.Lefebvre TL, Ueno Y, Dohan A et al (2022) Development and validation of multiparametric MRI-based radiomics models for preoperative risk stratification of endometrial cancer. Radiology 305:375–386 [DOI] [PubMed] [Google Scholar]
- 58.Lin Z, Wang T, Li Q et al (2023) Development and validation of MRI-based radiomics model to predict recurrence risk in patients with endometrial cancer: a multicenter study. Eur Radiol 33:5814–5824 [DOI] [PubMed] [Google Scholar]
- 59.Mainenti PP, Stanzione A, Cuocolo R et al (2022) MRI radiomics: a machine learning approach for the risk stratification of endometrial cancer patients. Eur J Radiol 149:110226 [DOI] [PubMed] [Google Scholar]
- 60.Miccò M, Gui B, Russo L et al (2022) Preoperative tumor texture analysis on MRI for high-risk disease prediction in endometrial cancer: a hypothesis-generating study. J Pers Med 12:1854 [DOI] [PMC free article] [PubMed]
- 61.Coada CA, Santoro M, Zybin V et al (2023) A radiomic-based machine learning model predicts endometrial cancer recurrence using preoperative CT radiomic features: a pilot study. Cancers 15:4534 [DOI] [PMC free article] [PubMed]
- 62.Moro F, Albanese M, Boldrini L et al (2022) Developing and validating ultrasound-based radiomics models for predicting high-risk endometrial cancer. Ultrasound Obstet Gynecol 60:256–268 [DOI] [PubMed] [Google Scholar]
- 63.Yan BC, Li Y, Ma FH et al (2020) Preoperative assessment for high-risk endometrial cancer by developing an MRI- and clinical-based radiomics nomogram: a multicenter study. J Magn Reson Imaging 52:1872–1882 [DOI] [PubMed] [Google Scholar]
- 64.Yang J, Cao Y, Zhou F, Li C, Lv J, Li P (2023) Combined deep-learning MRI-based radiomic models for preoperative risk classification of endometrial endometrioid adenocarcinoma. Front Oncol 13:1231497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang K, Zhang Y, Fang X, Dong J, Qian L (2021) MRI-based radiomics and ADC values are related to recurrence of endometrial carcinoma: a preliminary analysis. BMC Cancer 21:1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang K, Zhang Y, Fang X et al (2021) Nomograms of combining apparent diffusion coefficient value and radiomics for preoperative risk evaluation in endometrial carcinoma. Front Oncol 11:705456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bi Q, Wang Y, Deng Y et al (2022) Different multiparametric MRI-based radiomics models for differentiating stage IA endometrial cancer from benign endometrial lesions: a multicenter study. Front Oncol 12:939930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen X, Wang X, Gan M et al (2022) MRI-based radiomics model for distinguishing endometrial carcinoma from benign mimics: a multicenter study. Eur J Radiol 146:110072 [DOI] [PubMed] [Google Scholar]
- 69.Liu J, Li S, Lin H et al (2023) Development of MRI-based radiomics predictive model for classifying endometrial lesions. Sci Rep 13:1590 [DOI] [PMC free article] [PubMed]
- 70.Zhang J, Zhang Q, Wang T et al (2022) Multimodal MRI-based radiomics-clinical model for preoperatively differentiating concurrent endometrial carcinoma from atypical endometrial hyperplasia. Front Oncol 12:887546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y, Gong C, Zheng L, Li X, Yang X (2021) Deep learning for intelligent recognition and prediction of endometrial cancer. J Healthc Eng 2021:1148309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shen L, Du L, Hu Y et al (2023) MRI-based radiomics model for distinguishing Stage I endometrial carcinoma from endometrial polyp: a multicenter study. Acta Radiol 64:2651–2658 [DOI] [PubMed] [Google Scholar]
- 73.Li D, Hu R, Li H et al (2021) Performance of automatic machine learning versus radiologists in the evaluation of endometrium on computed tomography. Abdom Radiol (NY) 46:5316–5324 [DOI] [PubMed] [Google Scholar]
- 74.Mao W, Chen C, Gao H, Xiong L, Lin Y (2022) A deep learning-based automatic staging method for early endometrial cancer on MRI images. Front Physiol 13:974245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tao J, Wang Y, Liang Y, Zhang A (2022) Evaluation and monitoring of endometrial cancer based on magnetic resonance imaging features of deep learning. Contrast Media Mol Imaging 2022:5198592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Urushibara A, Saida T, Mori K et al (2022) The efficacy of deep learning models in the diagnosis of endometrial cancer using MRI: a comparison with radiologists. BMC Med Imaging 22:80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huang ML, Ren J, Jin ZY et al (2024) Application of magnetic resonance imaging radiomics in endometrial cancer: a systematic review and meta-analysis. Radiol Med. 10.1007/s11547-024-01765-3 [DOI] [PubMed]
- 78.Granzier RWY, van Nijnatten TJA, Woodruff HC, Smidt ML, Lobbes MBI (2019) Exploring breast cancer response prediction to neoadjuvant systemic therapy using MRI-based radiomics: a systematic review. Eur J Radiol 121:108736 [DOI] [PubMed] [Google Scholar]
- 79.Ponsiglione A, Stanzione A, Spadarella G et al (2023) Ovarian imaging radiomics quality score assessment: an EuSoMII radiomics auditing group initiative. Eur Radiol 33:2239–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ursprung S, Beer L, Bruining A et al (2020) Radiomics of computed tomography and magnetic resonance imaging in renal cell carcinoma-a systematic review and meta-analysis. Eur Radiol 30:3558–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoivik EA, Hodneland E, Dybvik JA et al (2021) A radiogenomics application for prognostic profiling of endometrial cancer. Commun Biol 4:1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kurata Y, Nishio M, Moribata Y et al (2021) Automatic segmentation of uterine endometrial cancer on multi-sequence MRI using a convolutional neural network. Sci Rep 11:14440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hodneland E, Dybvik JA, Wagner-Larsen KS et al (2021) Automated segmentation of endometrial cancer on MR images using deep learning. Sci Rep 11:179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Timmeren JE, Cester D, Tanadini-Lang S, Alkadhi H, Baessler B (2020) Radiomics in medical imaging-“how-to” guide and critical reflection. Insights Imaging 11:91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miotto R, Wang F, Wang S, Jiang X, Dudley JT (2018) Deep learning for healthcare: review, opportunities and challenges. Brief Bioinform 19:1236–1246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shur JD, Doran SJ, Kumar S et al (2021) Radiomics in oncology: a practical guide. Radiographics 41:1717–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McCague C, Ramlee S, Reinius M et al (2023) Introduction to radiomics for a clinical audience. Clin Radiol 78:83–98 [DOI] [PubMed] [Google Scholar]
- 88.Jamieson A, Bosse T, McAlpine JN (2021) The emerging role of molecular pathology in directing the systemic treatment of endometrial cancer. Ther Adv Med Oncol 13:17588359211035959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gaffney D, Matias-Guiu X, Mutch D et al (2024) 2023 FIGO staging system for endometrial cancer: the evolution of the revolution. Gynecol Oncol 184:245–253 [DOI] [PubMed] [Google Scholar]
- 90.Tomaszewski MR, Gillies RJ (2021) The biological meaning of radiomic features. Radiology 299:E256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kocak B, Akinci D’Antonoli T, Cuocolo R (2024) Exploring radiomics research quality scoring tools: a comparative analysis of METRICS and RQS. Diagn Interv Radiol. 10.4274/dir.2024.242793 [DOI] [PMC free article] [PubMed]
- 92.Kocak B, Akinci D’Antonoli T, Ates Kus E et al (2024) Self-reported checklists and quality scoring tools in radiomics: a meta-research. Eur Radiol. 10.1007/s00330-023-10487-5 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.