Abstract
Background
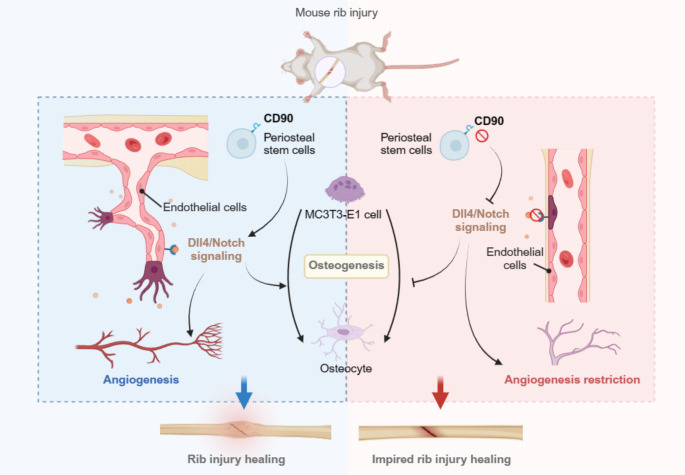
Vascularization after rib fracture is a crucial physiological process that is essential for the repair and healing of the rib. Studies have shown that CD90 plays a critical role in regulating rib fracture healing, but the underlying mechanism of its role has not been fully elucidated.
Methods
CD90 adenovirus knockout mice were used to construct a rib injury model. The bone healing was observed by micro-CT. CD31/EMCN immunofluorescence staining was performed on bone tissue to observe the density of H-shaped and L-shaped blood vessels at the site of bone injury. CD31 and EMCN dual-stained single cells from the rib fracture sites were detected by flow cytometry. The periosteal stem cells transfected with CD90 or Notch1 overexpression and silencing vector were co-cultured with osteoblast MC3T3-E1 in osteogenic induction medium. Moreover, bone microvascular endothelial cells were extracted from the rib injury and co-cultured with the periosteal stem cells transfected with CD90. CCK-8 was used to detect cell viability, RT-qPCR and Western blot were used to detect Notch1, Notch2, Notch3, Notch4, CD31, HIF-1α, CD90, RUNX2, OCN and OPN expression. Alkaline phosphatase (ALP) staining and alizarin red staining were used to observe mineralized nodules. Immunofluorescence staining was used to detect the expression of Dll4, Notch, and CD90 in each group of cells. The angiogenesis experiment was conducted to observe cellular vascular formation.
Results
Compared with the Adsh-NC group, the bone healing in the Adsh-CD90 group was significantly impaired, with a marked reduction in the number and volume of blood vessels at the rib fracture site, as evidenced by CD31/EMCN immunofluorescence staining, which showed a reduction in the number of H type vessels at the site of bone injury. It was found that CD90 depletion can inhibit the signaling of Dll4/Notch in the rib fracture site. Furthermore, we found that overexpression of Notch1 reverses the impairment of tubule formation in bone microvascular endothelial cells caused by CD90 suppression.r.Dll4 protein reverses the inhibitory effect of CD90 deletion on periosteal stem cells and MC3T3-E1 cell viability and osteogenesis. In the end, we found that overexpression of Notch1 and CD90 can promote angiogenesis of bone microvascular endothelial cells and Notch pathway activation.
Conclusion
CD90 can affect vascular formation in mouse rib fractures, and CD90 may be regulated by Dll4/Notch.
Keywords: Rib fracture, CD90, Angiogenesis, Dll4/Notch, Periosteal stem cell
Introduction
Ribs are the bony structure of the chest in the human body. When the ribs suffer from external force or other reasons leading to injury or damage, it can cause various diseases or complications [1]. The most common complication of rib injury is rib fracture, which can lead to difficulty breathing, chest pain, and other discomfort. Additionally, rib fractures can cause damage to the pleura, leading to inflammation in the chest cavity and causing pleuritis to develop [2]. At present, the mechanism of regulating rib injury has not been fully elucidated. In-depth study of the mechanism of rib injury can provide a theoretical basis for improving the treatment and rehabilitation of patients.
The periosteum is a thin membrane that covers the surface of bones and provides protection for the bone. It is also one of the sources of nutrition for the bone. When the periosteum is damaged or injured, the body initiates a repair process, which includes angiogenesis [3, 4]. New blood vessels will form at the site of damage, providing oxygen and nutrients to the damaged area, promoting healing and repair. In cases of rib fractures, if the periosteum is damaged, the body will also generate new blood vessels to meet the needs of the injured area. These new blood vessels will provide oxygen and nutrients to the injured rib area, promoting healing and repair. Bone perimembranous deposits refer to the biologically active substances that are rich in the bone perimembrane, which usually include cells, cytokines, growth factors, and extracellular matrix components [5]. These deposits play an important role in the structure and function of the bone perimembrane, promoting bone growth, repair, and regeneration. CD90, also known as Thy-1 antigen [6], is a cell surface glycoprotein widely expressed in various cell types, including osteoblasts, chondrocytes, and mesenchymal stem cells. In the periosteum, CD90 is typically utilized as a marker enriched in the periosteum due to its higher expression levels there. CD90 plays a significant role in skeletal development, bone repair, and regeneration processes, and is considered one of the typical surface markers of periosteal stem cells. Picke AK and colleagues found that CD90 can promote bone formation [7]. Saalbach A and other researchers reported that CD90 can promote osteogenic differentiation and bone formation in mesenchymal stromal cells [8]. Additionally, Lee WS and colleagues found that CD90 is a vascularization marker associated with upregulation of inflammatory factors [9]. Pérez LA also discovered that CD90 is associated with angiogenesis, and silencing the CD90 gene can delay the wound healing process [10]. These studies indicate that CD90 plays a role in both angiogenesis and bone formation processes. However, the mechanism by which CD90 regulates vascularization in rib injury remains unclear.
The DLL4/Notch pathway is an important cellular signaling pathway that plays a crucial role in the growth, differentiation, and tissue development of various cells [11–13]. Research has found that the DLL4/Notch pathway is involved in determining cell fate, angiogenesis, immune response, nervous system development, as well as the occurrence and progression of tumors in multiple biological processes [14, 15]. Additionally, an increasing body of research also highlights the association between the DLL4/Notch pathway and bone healing, as well as the development of bone diseases. Zhao Y et al. found that Notch pathway is related to osteogenic differentiation [16]. Hultgren NW et al. have shown that Slug regulates the DLL4-notching-VEGFR2 axis to control endothelial cell activation and angiogenesis [17]. Moreover, in angiogenesis, the DLL4/Notch pathway regulates the differentiation of endothelial cells and the formation of vascular networks. By modulating the proliferation, migration, and luminal formation of endothelial cells, this pathway is crucial for the formation and maintenance of a functional vascular system. Therefore, a thorough investigation into the role of the DLL4/Notch pathway in rib injury healing processes is highly critical.
Therefore, in this study, we first investigated the effects of CD90 depletion on angiogenesis and bone healing in rib injury mice. We further explored the key mechanisms of its action in mice, periosteal stem cells, and bone microvascular endothelial cells. Our findings provide new research directions for guiding further studies on skeletal injury healing.
Materials and methods
Construction of CD90 adeno-associated virus (AAV) knockdown mice
Twelve female BALB/C mice (100 ± 20) g aged 6–8 weeks were purchased from Beijing VitalRiver Laboratory Animal Technology Co., LTD. All mice were kept in a shielded environment at constant temperature of 22–24℃, constant humidity of 55–60%, and light/dark condition of 12h/12h, After purchasing AAV-shCD90-GFP and AAV-GFP, transfect mice with adeno-associated virus (AAV-shCD90-GFP) to knock down CD90, with AAV-GFP mice as controls. Specifically, 100 μL of adeno-associated virus was injected into the mouse via the tail vein.
Establishment of mouse rib injury model
Mice anesthetized with 3% isoflurane were placed in a supine position, stratified incision of the skin, subcutaneous tissue and muscle separation, stripping of the periosteum to expose the middle rib of the chest, transverse incision of the middle rib, and then stratified counterposition suture.
Observation of bone regeneration in mice by micro-CT
In this study, mice were divided into the following two groups: Adsh-NC and Adsh-CD90. After four weeks of mouse model preparation and culture, we used X-ray technology to observe bone healing.
Cell culture
Both bone endothelial cells, perivascular cells and MC3T3-E1 cell were cultured in MEM-α culture medium(Gibco, USA) containing 10% FBS, and cells were passaged every 2–3 days to avoid cell contamination. Subsequently, cells in the logarithmic phase were selected for the experiment.
Cell grouping
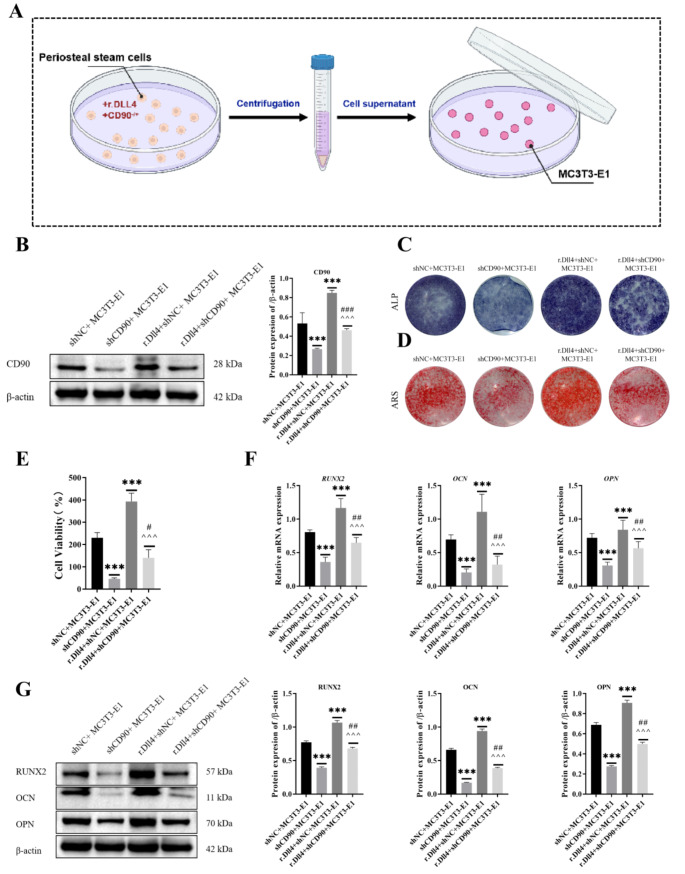
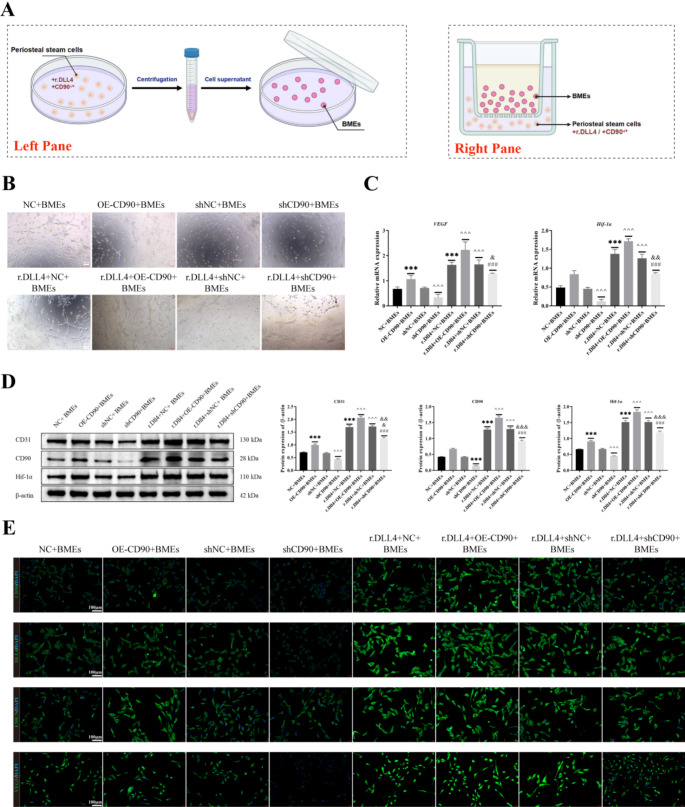
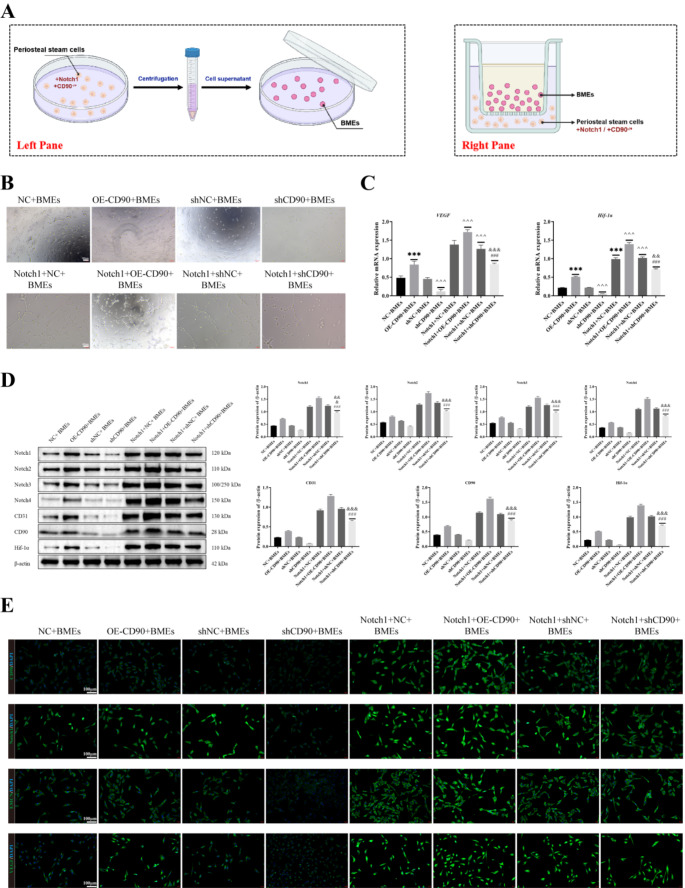
The periosteal stem cells treated with r.DLL4 and transfected with shCD90 were incubated for 24 h, centrifuged at 3000×g for 5 min, and filtered through a 0.3μm filter to obtain the cell supernatant, which was then co-cultured with MC3T3-E1 cells. The periosteal stem cells were co-cultured with MC3T3-E1 cells in four groups: shNC + MC3T3-E1, shCD90 + MC3T3-E1, rdll4 + shNC + MC3T3-E1, and rdll4 + shCD90 + MC3T3-E1. In addition, bone microvascular endothelial cells (BMEs) were co-cultured with periosteal stem cells using cell culture dishes and Transwell culture dishes, respectively. The cells cultured with bone microvascular endothelial cells and transfected CD90 bone membrane stem cells were divided into the following eight groups: NC + BMEs, OE-CD90 + BMEs, shNC + BMEs, shCD90 + BMEs, r.Dll4 + NC + BMEs, r.Dll4 + OE-CD90 + BMEs, r.Dll4 + shNC + BMEs, r.Dll4 + shCD90 + BMEs. Transfection of CD90 and Notch1 overexpression vectors into bone microvascular endothelial cells and co-culture with CD90 periosteal stem cells was divided into 8 groups: NC + BMEs, OE-CD90 + BMEs, shNC + BMEs, shCD90 + BMEs, Notch1 + NC + BMEs, Notch1 + OE-CD90 + BMEs, Notch1 + shNC + BMEs, and Notch1 + shCD90 + BMEs. The mechanism of this study was shown in Fig. 7.
Fig. 7.
Experimental global research mechanism display
Cell transfection
First, periosteal stem cells were selected based on the markers PDPN + CD146-CD73 + CD164 + , and then transfected with NC, OE-CD90, shNC, or shCD90. The periosteum stem cells transfected with plasmid were mixed with MC3T3-E1 cells, and the co-cultured cells were incubated with R.DELL4 protein, and transfected with shNC and shCD90. In addition, after the periosteal stem cells transfected with CD90 were transfected with the Notch1 overexpression vector, the follow-up experiment was performed. The transfection procedure was as follows: the recombinant plasmid and liposome were mixed by Turbofect (Thermo Scientific, R0533), then gently added into the cells, and the cells were incubated in the incubator after standing at room temperature for 20 min.
RT-qPCR
Rib injury tissues of mice in each group and periosteum stem cells in each transfection group were collected, and Trizol reagent was added to extract total RNA, the concentration of total RNA was detected by ultraviolet spectrophotometer, and template cDNA was synthesized according to reverse transcription kit, using GAPDH as the internal reference. The transcriptional levels of RUNX2, OCN, VEGF, HIF-1α, OPN, Dll4, Notch1, Notch2, Notch3, Notch4, Hes1, Hes5, Hey1, and Efnb2 were detected with 3 parallel pores in each group. The amplification conditions for PCR are as follows: pre-denaturation at 94 °C for 5 min; denaturation at 94 °C for 15 s, annealing at 62 °C for 30 s, extension at 72°C for 30 s, and 35 cycles in total, with a final extension at 72 °C for 5 min. The relative expression levels were detected by the 2−ΔΔCt method. The sequence and length of primers were shown in Table 1.
Immunofluorescence
The damaged rib tissues of mice and periosteum stem cells of each transfection group were collected and inoculated on the crawling plate, soaked with PBS 3 times, fixed with paraformaldehyde for 15min, soaked with PBS and added with 0.5% Triton X-100 at room temperature for 15min, cleaned with PBS, and sealed with goat serum for 30 min. The primary antibody [Dll4 (GB11322-100, Servicebio, 1:200), Notch1 (ab52627, 1:500), CD90 (ab92574, 1:500), VEGF (ab32152, 1:500), HIF-1α (Catalog# ER1802-41, Huabio, 1:100), CD31 (ab9498, 1:500), and Endomucin (GB112648-100, Servicebio, 1:600)] was added and incubated overnight, the fluorescent secondary antibody was added to avoid light, incubated for 1.5 h, soaked with PBS, and then DAPI was added to avoid light and incubated for 5 min. The tablets were sealed with a sealing solution containing fluorescence quencher, and the acquired images were observed under a fluorescence microscope.
Flow cytometry
CD31 and EMCN dual staining in single cell suspensions from rib fracture sites were detected by Flow cytometry. The specific operation should be carried out according to the instructions of Annexin V-FITC apoptosis detection kit (Shanghai Beyotime Biotechnology Co., Ltd). Collect the supernatant of the rib fracture from each group of mice stained with CD31 and EMCN, and mix with an appropriate amount of PBS. Then, centrifuge for 5 min, aspirate the supernatant, and resuspend in serum-free basal culture medium to prepare cell suspension. Add 1μL of flow cytometry antibody (1 × 106 cell/100μl) to each tube and incubate at room temperature in the dark for 20 min. Subsequently, wash with PBS, centrifuge, resuspend in 200 μL of PBS, and finally detect using flow cytometry.
Western blot
Periosteal stem cells of each transfection group were collected, cleaned with PBS for 3 times, and total protein was obtained by adding cell lysate containing protease inhibitor, the protein content was determined by BCA kit (Shanghai Beyotime Biotechnology Co., Ltd), and then denatured at 100℃ for 5 min. After separation by SDS-PAGE gel electrophoresis, protein transfer was performed. The protein membrane was enclosed in 5% BSA at room temperature for 2 h, and the corresponding primary antibodies [RUNX2 (ab76956, 1:1000), OCN (ab133612, 1:1000), CD31 (ab9498, 1:5000), HIF-1α (ab308637, 1:2000), OPN (ab75285, 1:1000), CD90 (ab92574, 1:2000), Notch1 (ab52627, 1:1000), Notch2 (ab307700, 1:1000), Notch3 (ab300527, 1:1000), Notch4 (ab184742, 1:1000), Hes1 (ab119776, 1:1000), Hes5 (ab194111, 1:500), Hey1 (ab154077, 1:500), Hey2 (ab167280, 1:500), and Efnb2 (ab131536, 1:500)] were added. The cells were incubated at 4℃ overnight, and the secondary antibodies were incubated for 1 h. Finally, the luminescent solution was added for gel imaging for exposure and photography. ImageJ software counted the gray values of proteins in each transfected histone cell.
ALP staining
After co-culturing the transfected plasmid bone marrow mesenchymal stem cells with MC3T3-E1, wash three times with PBS, fix with 4% paraformaldehyde for 20 min, and wash three times with PBS again. The alkaline phosphatase staining working solution was prepared according to the instructions of the staining kit. Add an appropriate amount of staining working solution to the cells, then incubate at room temperature in the dark for 20 min. The staining solution was removed and washed with distilled water to stop the color reaction, and finally observed the staining and capture relevant images.
Alizarin red staining
The culture medium of periosteal stem cells in each transfection group was discarded, 400 μL PBS was added to each well, the cell surface was cleaned 3 times, and 300 μL fixing solution was added to each well for 10 min. The fixing solution was discarded, and 500 μL PBS was added to each well to clean the cell surface, and 300 μL staining solution was added to each well to stain the cells at room temperature for 15 min, and PBS was added to clean the cells for 3 times. The formation of mineralized nodules was observed under optical microscope and images were collected.
CCK-8
CCK8 cell viability assay kit came from Shanghai Beyotime Biotechnology Co., Ltd. The cell suspension was prepared by co-culture of periosteal stem cells and MC3T3-E1 cells in logarithmic growth stage. A total of 4 × 103 cells per well were inoculated on 96-well plates, and after 48 h of culture, 10 μL of CCK-8 solution was added to each well, and incubated in a 5% CO2 cell incubator at 37℃ for 4 h. The light absorption value was detected at 450 nm by enzyme-labeled instrument, and cell viability was calculated.
Angiogenesis formation assay
Matrige was diluted and placed it in a 24-well plate. A total of 150μL diluted Matrige was added to each well and incubated at room temperature to solidify into a gel. Bone microvascular endothelial cells transfected with CD90 and Notch1 overexpression vector were co-cultured with CD90 periosteal stem cells, and cells were seeded at a density of 5 × 104 cells per well in 24-well plates coated with Matrigel. After 24 h of culture, the tube formation was observed by optical microscope, and 5 fields were randomly selected to count the number of tubes formed under the field.
Statistical analysis
Data analysis was performed using SPSS23.0 software, and statistical graphs were drawn using GraphPad Prism 8.30 software. All experimental data were presented in the form of mean ± standard deviation (x ± s). Data comparisons were made using one-way analysis of variance (ANOVA), and LSD-t test was used to compare two-by-two data within each group. P < 0.05 was considered statistically significant.
Result
CD90 depletion inhibited angiogenesis at the rib fracture site in mice
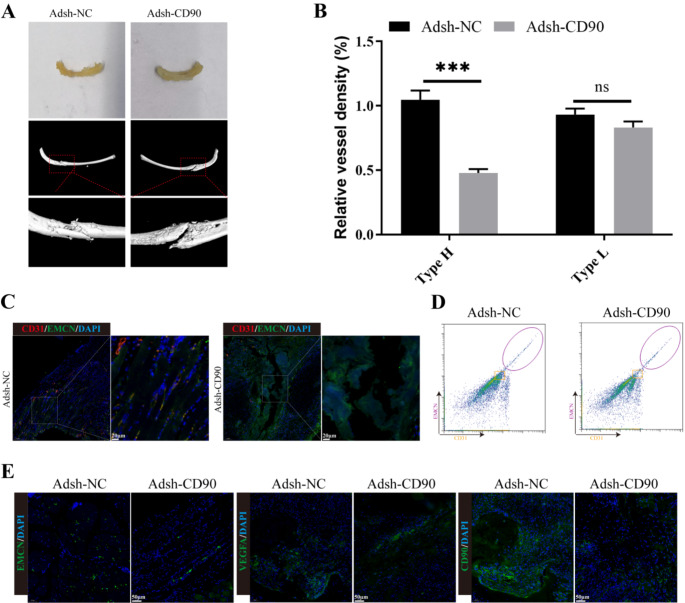
We first analyzed the bone healing conditions of Adsh-NC and Adsh-CD90 mice using X-ray imaging, and found that the bone healing conditions of Adsh-NC mice were significantly higher than those of Adsh-CD90 mice (Fig. 1A). Through immunofluorescence, we also found that compared to the control group, the expression of CD31 and EMCN in H type collagen fibers at the site of bone damage in Adsh-CD90 mice was significantly lower, and the vessel density was significantly reduced (Fig. 1B-C). Additionally, flow cytometry analysis further indicated that the expression of CD31 and EMCN in the H-shaped lesions of ribs in CD90-depleted group was reduced (Fig. 1D). Finally, immunofluorescence staining revealed that compared to the Adsh-NC group, the expression of VEGFA, EMCN, and CD90 was significantly lower in the Adsh-CD90 group (Fig. 1E). The above results indicated that CD90 depletion can significantly inhibit mouse rib fracture healing and angiogenesis.
Fig. 1.
CD90 depletion inhibited angiogenesis at the rib fracture site in mice. (A) The bone healing conditions of Adsh-NC and Adsh-CD90 mice were observed using X-ray imaging. (B-C) The expression of CD31 and EMCN and blood vessel type of Adsh-NC and Adsh-CD90 mice were observed by immunofluorescence staining. (D) The expressions of CD31, EMCN in the rib H-shaped injury site of Adsh-NC and Adsh-CD90 mice were detected by flow cytometry. (E) The expressions of VEGFA, EMCN and CD90 of Adsh-NC and Adsh-CD90 mice were detected by immunofluorescence staining. n = 3, ***P < 0.001, vs. Adsh-NC. Vessel density in Adsh-NC and Adsh-CD90 groups was compared by Student’s t test
CD90 depletion inhibited the Dll4/Notch signaling in rib fractures
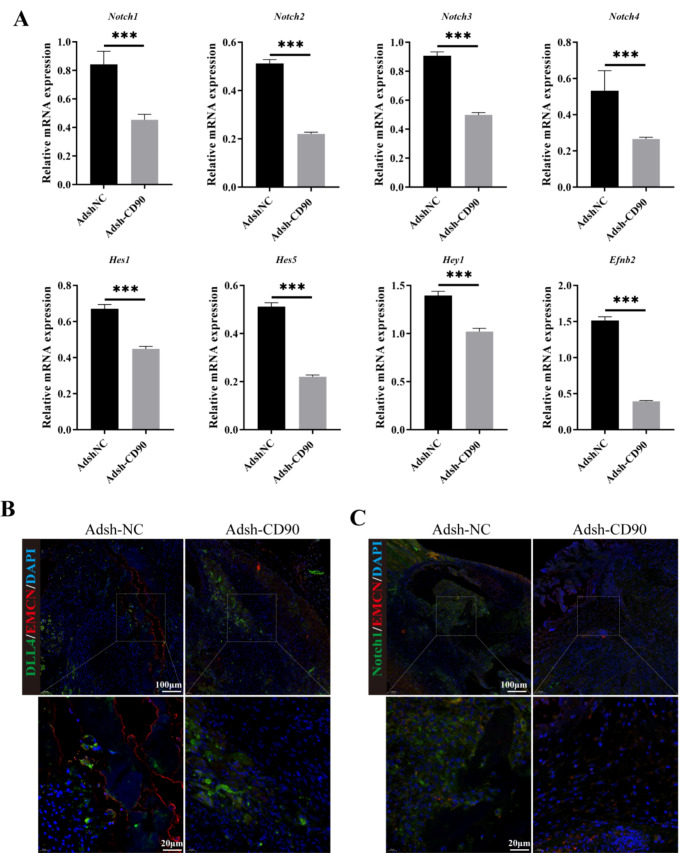
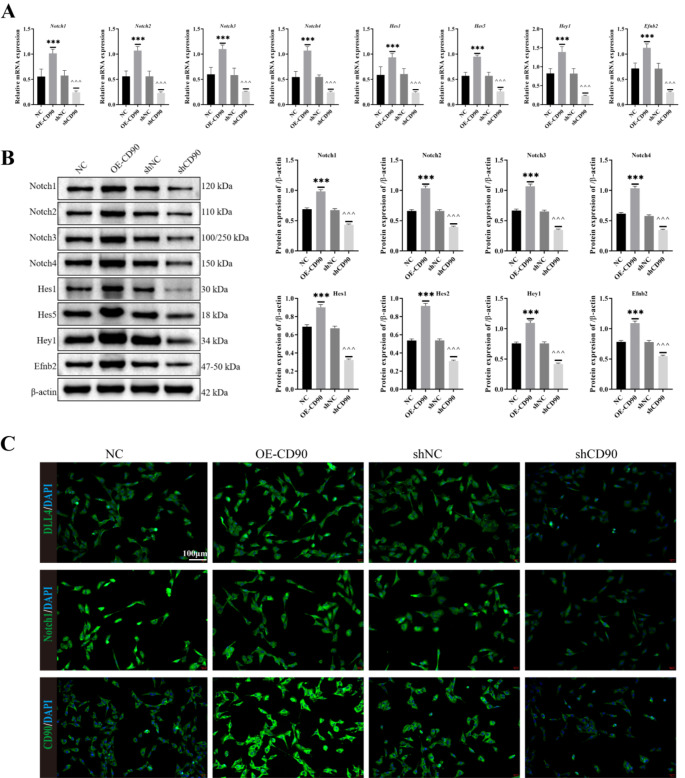
First, we performed RT-qPCR to detect the transcription levels of Dll4, Notch1, Notch2, Notch3, Notch4, Hes1, Hes5, Hey1, Heyl, and Efnb2 in each group of mice. The result was shown in Fig. 2A, we found that transcription levels of these genes were significantly reduced in Adsh-CD90 mice compared to Adsh-NC mice. Subsequently, we performed immunofluorescence labeling of Dll4/EMCN, Notch/EMCN and observed the Dll4/Notch content in the tissue at the rib fracture site. We found that in the Adsh-CD90 group of mice, the Dll4/Notch content was lower than that in the Adsh-NC group (Fig. 2B-C). From the RT-qPCR and Western blot results in Fig. 3A-B, it was evident that compared to the NC or shNC group, the OE-CD90 group showed a significant increase in the expression of Notch1, Notch2, Notch3, Notch4, Hes1, Hes5, Hey1, Heyl, and Efnb2 at both the gene and protein levels, while the shCD90 group exhibited a significant decrease. Finally, based on the results of immunofluorescence staining, it was found that the expression of Dll4, Notch, and CD90 in the NC or shNC groups was lower than that in the OE-CD90 group, but higher than that in the shCD90 group (Fig. 3C). Based on these analysis results, we can further hypothesize that CD90 depletion can inhibit the signaling of Dll4/Notch in the rib fracture site.
Fig. 2.
Detection of Dll4/EMCN and Notch/EMCN related genes and proteins. (A) RT-qPCR to detect the transcription levels of Dll4, Notch1, Notch2, Notch3, Notch4, Hes1, Hes5, Hey1, Heyl, and Efnb2 in each group of mice. (B-C) The content of Dll4/Notch in the fracture site was observed by immunofluorescence double staining. n = 3, ***P < 0.001, vs. Adsh-NC. The measurement data of Adsh-NC and Adsh-CD90 groups were compared by Student’s t test
Fig. 3.
Effect of knockdown CD90 on expression of DLL4 and Notch in periosteal stem cells. (A) The genes expression of Notch1, Notch2, Notch3, Notch4, Hes1, Hes5, Hey1, Heyl and Efnb2 were detected by RT-qPCR. (B) The protein expression of Notch1, Notch2, Notch3, Notch4, Hes1, Hes5, Hey1, Heyl and Efnb2 were detected by Western blot. (C) The expression of DLL4, Notch and CD90 in periosteal stem cells with CD90 knockdown was stained by immunofluorescence staining. n = 3, ***P < 0.001, vs. NC. ^^^P < 0.001, vs. shNC. One-Way ANOVA was used to compare measurement data among multiple groups
Effect of r.Dll4 protein on the viability and osteogenesis of CD90-depleted periosteal stem cells and MC3T3-E1 cells
In order to observe the effect of CD90 derived from periosteal stem cells on the proliferation and osteogenesis of osteoblasts during the process of rib injury healing, we used cell co-culture mode to extract the supernatant of shCD90 transfected periosteal stem cells to incubation MC3T3-E1 (Fig. 4A), and then the biological function of MC3T3-E1 cells was detected by molecular experiments. Firstly, we detected the expression of the CD90 protein in cells transfected with different constructs using Western blotting. As shown in Fig. 4B, compared to the shNC + MC3T3-E1 group, the r.Dll4 + shNC + MC3T3-E1 group exhibited a significant increase in CD90 protein expression, while the r.Dll4 + shCD90 + MC3T3-E1 group showed a decrease. Additionally, the shCD90 + MC3T3-E1 group had lower CD90 protein expression than the r.Dll4 + shCD90 + MC3T3-E1 group. Next, MC3T3 E1 cells after the osteogenesis medium induction, we observed mineralized nodules through ALP staining and alizarin red staining (Fig. 4C-D). We found that after r.Dll4 treatment, the positive staining area of cells increased significantly, cell viability significantly improved (Fig. 4E), and the expression of RUNX2, OCN, OPN genes and proteins significantly increased. However, after CD90 treatment, these indicators showed the opposite trend (Fig. 4F and G).
Fig. 4.
Effect of r.Dll4 protein on the viability and osteogenesis of CD90-depleted periosteal stem cells and MC3T3-E1 cells. (A) Co-culture system of periosteal stem cells and MC3T3-E1 cells. (B) The expression of CD90 protein was detected by Western blot. (C-D) The mineralized nodules were observed through ALP staining and alizarin red staining. (E) Cell viability was measured by CCK-8. (F) The expressions of RUNX2, OCN and OPN were detected by Western blot. (G) The expressions of RUNX2, OCN and OPN were detected by RT-qPCR. n = 3, ***P < 0.001, vs. shNC + MC3T3-E1. ^^^P < 0.001, vs. r.Dll4 + shNC + MC3T3-E1. ###P < 0.001, ##P < 0.01, vs. shCD90 + MC3T3-E1. One-Way ANOVA was used to compare measurement data among multiple groups
Effects of r.Dll4 and CD90 on angiogenesis in bone microvascular endothelial cells
We isolated bone microvascular endothelial cells from the rib injury site and co-cultured them with transfected CD90 bone marrow stem cells (Fig. 5A). We observed cell vascular formation through a tube formation assay. As shown in Fig. 5B, compared to the r.Dll4 + NC + BMEs or r.Dll4 + shNC + BMEs groups, there was a significant increase in cell vascular formation in the r.Dll4 + OE-CD90 + BMEs group, while cell vascular formation was significantly reduced in the r.Dll4 + shCD90 + BMEs group. Furthermore, compared to the NC + BMEs or shNC + BMEs groups, there was a significant increase in cell vascular formation in the OE-CD90 + BMEs group, while cell vascular formation was significantly reduced in the shCD90 + BMEs group. Additionally, cell vascular formation was significantly higher in the r.Dll4 + shCD90 + BMEs group than in the OE-CD90 + BMEs group. Subsequently, we assessed the expression of VEGF and HIF-1α through RT-qPCR (Fig. 5C) and examined the expression of CD31, CD90, and HIF-1α using Western Blotting (Fig. 5D). The trends were consistent with those observed in Fig. 5C-D. Finally, we utilized cell fluorescence to detect the expression of CD90, VEGF, HIF-1α, CD31, and Endomucin in different transfection groups. Their expression trends were consistent with VEGF and HIF-1α expression, with the highest expression levels observed in r.Dll4 + OE-CD90 + BMEs cells and the lowest in shCD90 + BMEs cells (Fig. 5E).
Fig. 5.
Effects of r.Dll4 and CD90 on angiogenesis in bone microvascular endothelial cells. (A) Co-culture system of periosteal stem cells and BMEs. The left pane of the co-culture cells used for pipe experiment and immunofluorescence analysis, right pane for cell co-culture after Western Blotting and qRT-PCR analysis. (B) Cell vascular formation was detected through a tube formation assay. (C) The expressions of VEGF and HIF-1α were detected by RT-qPCR. (D) The expressions of CD31, CD90 and HIF-1α were detected by Western blot. (E) The expressions of CD90, VEGF, HIF-1α, CD31 and Endomucin were detected by cell fluorescence staining. n = 3, ***P < 0.001, vs. NC + BMEs. ^^^P < 0.001, vs. shNC + BMEs. ###P < 0.001, vs. r.Dll4 + OE-CD90 + BMEs. &&&P < 0.001, &&P < 0.01, &P < 0.05, vs. r.Dll4 + shNC + BMEs. One-Way ANOVA was used to compare measurement data among multiple groups
Overexpression of Notch1 and CD90 affects angiogenesis and wound healing in bone microvascular endothelial cells
In this study, we transfected CD90 bone marrow stem cells with a Notch1 overexpression vector and extracted bone microvascular endothelial cells (Fig. 6A). After co-culturing with transfected CD90 bone marrow stem cells, we first observed cell vascular formation based on a tube formation assay. The results, as shown in Fig. 6, revealed that compared to the Notch1 + NC + BMEs or Notch1 + shNC + BMEs groups, there was a significant increase in cell vascular formation in the Notch1 + OE-CD90 + BMEs group. Conversely, cell vascular formation was significantly reduced in the r.Dll4 + shCD90 + BMEs group but remained higher than in the OE-CD90 + BMEs group. Compared to the NC + BMEs or shNC + BMEs groups, the cell vascular formation was significantly reduced in the shCD90 + BMEs group, while it was significantly increased in the OE-CD90 + BMEs group (Fig. 6B). We also utilized RT-qPCR (Fig. 6C) to assess the expression of VEGF and HIF-1α. Additionally, we employed cell fluorescence to detect the expression of CD90, VEGF, HIF-1α, CD31, and Endomucin. Subsequently, we performed Western blotting to detect the expression of Notch1, Notch2, Notch3, Notch4, CD31, CD90, and HIF-1α in cells from different treatment groups (Fig. 6D). The results revealed that the expression trends of these genes were consistent with those observed in Fig. 6E.
Fig. 6.
Overexpression of Notch1 and CD90 affects angiogenesis and wound healing in bone microvascular endothelial cells. (A) Co-culture system of periosteal stem cells and BMEs. The left pane of the co-culture cells used for pipe experiment and immunofluorescence analysis, right pane for cell co-culture after Western Blotting and qRT-PCR analysis. (B) Cell vascular formation was detected through a tube formation assay. (C) The expressions of VEGF and HIF-1α were detected by RT-qPCR. (D) The expressions of Notch1, Notch2, Notch3, Notch4, CD31, CD90 and HIF-1α were detected by Western blot. (E) The expressions of CD90, VEGF, HIF-1α, CD31 and Endomucin were detected by cell fluorescence staining. n = 3, ***P < 0.001, vs. NC + BMEs. ^^^P < 0.001, vs. shNC + BMEs. ###P < 0.001, vs. Notch1 + OE-CD90 + BMEs. &&&P < 0.001, &&P < 0.01, vs. Notch1 + shNC + BMEs. One-Way ANOVA was used to compare measurement data among multiple groups
Discussion
As is well known, the presence or absence of the periosteum, as well as the extent of soft tissue injury, will impact the quality of fracture healing. The integrity of the periosteum is crucial for fracture healing and bone tissue regeneration [18]. The process of skeletal healing involves a series of complex mechanisms that enable damaged bone tissue to restore its original cellular structure and biomechanical properties. Cells with osteogenic potential and substances such as osteogenic growth factors present in the periosteum are involved in the formation and reconstruction of bone tissue, making them crucial for skeletal injury healing [19]. CD90 is a crucial periosteal-enriched marker that plays a key role in bone injury repair. In this study, we selected CD90, a periosteal-enriched molecule, for analysis. The results revealed that depletion of CD90 significantly inhibited both mouse rib fracture healing and vascular formation. Furthermore, we also observed that CD90 depletion inhibited Dll4/Notch signaling transduction at the site of rib injury in both periosteal stem cells and model mice. Lastly, when periosteal stem cells transfected with plasmids were co-cultured with MC3T3-E1 cells, we found that downregulation or upregulation of CD90 and Notch1 expression affected the vascular formation, mineralization function of osteoblasts, and cell viability (Fig. 7).
Angiogenesis plays a crucial role in the process of bone healing. Blood vessels provide essential support and nourishment to cells at the healing site by supplying oxygen, nutrients, and growth factors. During fracture healing, the formation of new blood vessels accelerates the regeneration and repair of bone tissue, aids in clearing metabolic waste, and promotes the migration and proliferation of bone cells. The regulation of angiogenesis has also become an important aspect of studying fracture healing [20]. Researchers like Schmidt-Bleek et al. have found that timely termination of inflammation and vascularization are crucial for the process of bone regeneration and healing [21]. Jiang et al. found that PTH1-34 promotes fracture healing by stimulating angiogenesis and osteogenesis, further facilitating bone repair [22]. Furthermore, Xu et al. discovered that H-type vessels play a crucial role in angiogenesis and in bone tissue engineering [23]. The studies mentioned above all reveal the crucial role of angiogenesis in the healing process following bone injury. Some studies have found that CFU-F of CD90 + cells is highly enriched in bone marrow and endobone [24]. Kaigler et al. found that the higher the CD90 composition of transplanted cells, the higher the bone volume score of regenerated bone [25]. Furthermore, Kim YK et al. found CD90 to serve as a marker for mesenchymal stem cells, bone marrow-derived cells sorted by CD90 displayed a higher osteogenic potential compared to unsorted bone marrow-derived cells [26]. Yang et al. further discovered that KGE can promote bone repair during the process of vascular regeneration by recruiting CD90 stem cells [27]. These studies all found a close association between the bone marrow-enriched marker CD90 and bone repair and vascular regeneration. Therefore, in this study, we further analyzed the effect of CD90 on vascular regeneration in rib injury by using specific CD90 knockout mice. The results revealed that CD90 depletion significantly inhibited vascular regeneration at the site of rib injury, and reduced the expression of VEGFA, EMCN, CD31, and EMCN, indicating the crucial role of CD90 in vascular regeneration at the site of rib injury. Subsequently, we further investigated the role of CD90 in regulating vascular regeneration at the site of rib injury. We found that CD90 depletion inhibited Dll4/Notch signaling at the site of rib injury. Bone marrow-derived stromal cells marked with PDPN + CD146-CD73 + CD164 + were selected, and after upregulating or downregulating CD90 expression, we observed that downregulating CD90 inhibited Dll4/Notch signaling, while upregulating CD90 had the opposite effect. In addition, we also found that the r.Dll4 protein and the overexpression vector of Notch1 both affect the regulatory role of CD90 in vascular regeneration and cell growth of periosteal stem cells and skeletal vascular endothelial cells.
Many studies have demonstrated the presence of multiple signaling pathways during angiogenesis, among which the Dll4/Notch signaling pathway is particularly crucial. The Notch pathway consists of Notch receptors and their ligands, with Dll4 being one of the ligands in the Notch signaling pathway. It interacts with Notch receptors and participates in regulating cell fate and biological processes such as vascular development. In the Dll4/Notch signaling pathway, Dll4 is a transmembrane protein ligand that primarily interacts with Notch receptors such as Notch1 and Notch4. The Dll4/Notch signaling pathway plays a crucial role in vascular development. In vascular endothelial cells, Dll4 regulates endothelial cell growth and vascular branching by binding to Notch receptors [28]. This signaling pathway is crucial for maintaining vascular structure and function and plays a significant role in angiogenesis. Research by Yang et al. found that neural proteins can promote angiogenesis by regulating the Dll4/Notch signaling pathway [29]. Wang et al. also discovered that TRIM28 regulates angiogenesis through the VEGFR-DLL4-Notch signaling pathway [30]. Additionally, research by Hu et al. indicated that blocking DLL4/Notch disrupts condyle ossification by inhibiting H-type vessel formation [31]. These reports and our findings highlight the indispensable role of signaling pathways in Dll4/Notch angiogenesis. Furthermore, our study revealed the critical role of this pathway in rib injury healing. In the future, targeting Dll4/Notch for angiogenesis intervention could serve as an adjunctive therapeutic approach for bone injuries.
In summary, our study reveals the crucial role of CD90-enriched perichondrium in angiogenesis of rib fracture, and uncovers the importance of the new Dll4/Notch signaling pathway in rib fracture healing. However, we need to acknowledge that the CD90 and its signaling pathway discovered in our study lack subsequent clinical validation. Nevertheless, our study contributes to the development of new therapeutic strategies.
Acknowledgements
Not applicable.
Author contributions
Lei Wang, Liya Dong and Fengqing Hu designed the study and wrote the main manuscript; Lei Wang, Rui Hu, Pei Xu, Liya Dong and Fengqing Hu conducted the data analysis; Lei Wang, Rui Hu, Pei Xu, Pengkai Gao, Liya Dong and Fengqing Hu performed the experiments; Lei Wang, Bin Mo, Liya Dong and Fengqing Hu edited the data; Lei Wang, Rui Hu, Pei Xu, Pengkai Gao, Liya Dong and Fengqing Hu verified the results; Liya Dong and Fengqing Hu reviewed and finalized the manuscript. All authors critically reviewed the article and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (81602418).
Data availability
All data are provided in this study, and raw data can be requested to the corresponding author.
Declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Institutional review board
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All the animal works were approved by the Ethics Committee of Xinhua Hospital Affiliated to Shanghai Jiao Tong University School of Medicine (XHEC-NSFC-2022-418).
Informed consent
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Liya Dong, Email: dongliya@xinhuamed.com.cn.
Fengqing Hu, Email: hufengqing@xinhuamed.com.cn.
References
- 1.Kurihara Y, Yakushiji YK, Matsumoto J, Ishikawa T, Hirata K. The ribs: anatomic and radiologic considerations. Radiographics. 1999;19(1):105–19; quiz 151–2. 10.1148/radiographics.19.1.g99ja02105. [DOI] [PubMed]
- 2.Park J, Kim SJ, Kim H, Jung H, Shin HY. Ultrasound diagnosis and treatment of intractable anterior chest pain from golf - A case report. Anesth Pain Med (Seoul). 2023;18(1):65–9. 10.17085/apm.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Driscoll SW, Fitzsimmons JS. The role of periosteum in cartilage repair. Clin Orthop Relat Res. 2001;391(Suppl):S190-207. 10.1097/00003086-200110001-00019. [DOI] [PubMed] [Google Scholar]
- 4.Li Q, Liu W, Hou W, Wu X, Wei W, Liu J, Hu Y, Dai H. Micropatterned photothermal double-layer periosteum with angiogenesis-neurogenesis coupling effect for bone regeneration. Mater Today Bio. 2022;18: 100536. 10.1016/j.mtbio.2022.100536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alcorta-Sevillano N, Macías I, Infante A, Rodríguez CI. Deciphering the Relevance of Bone ECM Signaling. Cells. 2020;9(12):2630. 10.3390/cells9122630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marangoni RG, Datta P, Paine A, Duemmel S, Nuzzo M, Sherwood L, Varga J, Ritchlin C, Korman BD. Thy-1 plays a pathogenic role and is a potential biomarker for skin fibrosis in scleroderma. JCI Insight. 2022;7(19): e149426. 10.1172/jci.insight.149426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Picke AK, Campbell GM, Blüher M, Krügel U, Schmidt FN, Tsourdi E, Winzer M, Rauner M, Vukicevic V, Busse B, Salbach-Hirsch J, Tuckermann JP, Simon JC, Anderegg U, Hofbauer LC, Saalbach A. Thy-1 (CD90) promotes bone formation and protects against obesity. Sci Transl Med. 2018;10(453):eaao6806. 10.1126/scitranslmed.aao6806. [DOI] [PubMed]
- 8.Saalbach A, Anderegg U. Thy-1: more than a marker for mesenchymal stromal cells. FASEB J. 2019;33(6):6689–96. 10.1096/fj.201802224R. [DOI] [PubMed] [Google Scholar]
- 9.Lee WS, Jain MK, Arkonac BM, Zhang D, Shaw SY, Kashiki S, Maemura K, Lee SL, Hollenberg NK, Lee ME, Haber E. Thy-1, a novel marker for angiogenesis upregulated by inflammatory cytokines. Circ Res. 1998;82(8):845–51. 10.1161/01.res.82.8.845. [DOI] [PubMed] [Google Scholar]
- 10.Pérez LA, León J, López J, Rojas D, Reyes M, Contreras P, Quest AFG, Escudero C, Leyton L. The GPI-anchored protein Thy-1/CD90 promotes wound healing upon injury to the skin by enhancing skin perfusion. Int J Mol Sci. 2022;23(20):12539. 10.3390/ijms232012539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lobov I, Mikhailova N. The role of Dll4/notch signaling in normal and pathological ocular angiogenesis: Dll4 controls blood vessel sprouting and vessel remodeling in normal and pathological conditions. J Ophthalmol. 2018;2018:3565292. 10.1155/2018/3565292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu S, Sun J, Zhang J, Xu X, Li H, Shan B, Tian T, Wang H, Ma D, Ji C. Aberrant expression and association of VEGF and Dll4/Notch pathway molecules under hypoxia in patients with lung cancer. Histol Histopathol. 2013;28(2):277–84. 10.14670/HH-28.277. [DOI] [PubMed] [Google Scholar]
- 13.Gao N, Xiao L, Tao Z, Zheng Y, Wang W, Huang H. Preliminary research of main components of Dll4/ notch-VEGF signaling pathway under high-glucose stimulation in vitro. Diabetes Metab Syndr Obes. 2022;15:1165–71. 10.2147/DMSO.S355004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z, Wang Y, Zhu H, Qiu C, Guan G, Wang J, Guo Y. Matrine blocks AGEs- induced HCSMCs phenotypic conversion via suppressing Dll4-Notch pathway. Eur J Pharmacol. 2018;835:126–31. 10.1016/j.ejphar.2018.07.051. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Huang X, Zhang J, Shao N, Chen LO, Ma D, Ji C. The expression of VEGF and Dll4/Notch pathway molecules in ovarian cancer. Clin Chim Acta. 2014;436:243–8. 10.1016/j.cca.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Richardson K, Yang R, Bousraou Z, Lee YK, Fasciano S, Wang S. Notch signaling and fluid shear stress in regulating osteogenic differentiation. Front Bioeng Biotechnol. 2022;10:1007430. 10.3389/fbioe.2022.1007430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hultgren NW, Fang JS, Ziegler ME, Ramirez RN, Phan DTT, Hatch MMS, Welch-Reardon KM, Paniagua AE, Kim LS, Shon NN, Williams DS, Mortazavi A, Hughes CCW. Slug regulates the Dll4-Notch-VEGFR2 axis to control endothelial cell activation and angiogenesis. Nat Commun. 2020;11(1):5400. 10.1038/s41467-020-18633-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lan KC, Wei KT, Lin PW, Lin CC, Won PL, Liu YF, Chen YJ, Cheng BH, Chu TG, Chen JF, Huang KE, Chang C, Kang HY. Targeted activation of androgen receptor signaling in the periosteum improves bone fracture repair. Cell Death Dis. 2022;13(2):123. 10.1038/s41419-022-04595-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Safari B, Davaran S, Aghanejad A. Osteogenic potential of the growth factors and bioactive molecules in bone regeneration. Int J Biol Macromol. 2021;175:544–57. 10.1016/j.ijbiomac.2021.02.052. [DOI] [PubMed] [Google Scholar]
- 20.Peng Y, Wu S, Li Y, Crane JL. Type H blood vessels in bone modeling and remodeling. Theranostics. 2020;10(1):426–36. 10.7150/thno.34126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt-Bleek K, Schell H, Lienau J, Schulz N, Hoff P, Pfaff M, Schmidt G, Martin C, Perka C, Buttgereit F, Volk HD, Duda G. Initial immune reaction and angiogenesis in bone healing. J Tissue Eng Regen Med. 2014;8(2):120–30. 10.1002/term.1505. [DOI] [PubMed] [Google Scholar]
- 22.Jiang X, Xu C, Shi H, Cheng Q. PTH1-34 improves bone healing by promoting angiogenesis and facilitating MSCs migration and differentiation in a stabilized fracture mouse model. PLoS ONE. 2019;14(12): e0226163. 10.1371/journal.pone.0226163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z, Kusumbe AP, Cai H, Wan Q, Chen J. Type H blood vessels in coupling angiogenesis-osteogenesis and its application in bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2023;111(7):1434–46. 10.1002/jbm.b.35243. [DOI] [PubMed] [Google Scholar]
- 24.Cao Y, Bolam SM, Boss AL, Murray HC, Munro JT, Poulsen RC, Dalbeth N, Brooks AES, Matthews BG. Characterization of adult human skeletal cells in different tissues reveals a CD90+CD34+ periosteal stem/progenitor population. Bone. 2024;178: 116926. 10.1016/j.bone.2023.116926. [DOI] [PubMed] [Google Scholar]
- 25.Kaigler D, Avila-Ortiz G, Travan S, Taut AD, Padial-Molina M, Rudek I, Wang F, Lanis A, Giannobile WV. Bone engineering of maxillary sinus bone deficiencies using enriched CD90+ stem cell therapy: a randomized clinical trial. J Bone Miner Res. 2015;30(7):1206–16. 10.1002/jbmr.2464. [DOI] [PubMed] [Google Scholar]
- 26.Kim YK, Nakata H, Yamamoto M, Miyasaka M, Kasugai S, Kuroda S. Osteogenic potential of mouse periosteum-derived cells sorted for CD90 in vitro and in vivo. Stem Cells Transl Med. 2016;5(2):227–34. 10.5966/sctm.2015-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Y, Zheng W, Tan W, Wu X, Dai Z, Li Z, Yan Z, Ji Y, Wang Y, Su W, Zhong S, Li Y, Sun Y, Li S, Huang W. Injectable MMP1-sensitive microspheres with spatiotemporally controlled exosome release promote neovascularized bone healing. Acta Biomater. 2023;157:321–36. 10.1016/j.actbio.2022.11.065. [DOI] [PubMed] [Google Scholar]
- 28.Pitulescu ME, Schmidt I, Giaimo BD, Antoine T, Berkenfeld F, Ferrante F, Park H, Ehling M, Biljes D, Rocha SF, Langen UH, Stehling M, Nagasawa T, Ferrara N, Borggrefe T, Adams RH. Dll4 and Notch signalling couples sprouting angiogenesis and artery formation. Nat Cell Biol. 2017;19(8):915–27. 10.1038/ncb3555. [DOI] [PubMed] [Google Scholar]
- 29.Yang L, Wang X, Sun J, Liu C, Li G, Zhu J, Huang J. Neuritin promotes angiogenesis through inhibition of DLL4/Notch signaling pathway. Acta Biochim Biophys Sin (Shanghai). 2021;53(6):663–72. 10.1093/abbs/gmab039. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Singh AR, Zhao Y, Du T, Huang Y, Wan X, Mukhopadhyay D, Wang Y, Wang N, Zhang P. TRIM28 regulates sprouting angiogenesis through VEGFR-DLL4-Notch signaling circuit. FASEB J. 2020;34(11):14710–24. 10.1096/fj.202000186RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu Y, Li H. DLL4/Notch blockade disrupts mandibular advancement-induced condylar osteogenesis by inhibiting H-type angiogenesis. J Oral Rehabil. 2024;51(4):754–61. 10.1111/joor.13642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are provided in this study, and raw data can be requested to the corresponding author.