Abstract
Background
Increased diameters of the aorta are associated with increased mortality risk. In the present analyses, we assessed whether aortic diameters are associated with cardiovascular and all-cause mortality in community-dwelling individuals free of known cardiovascular disease (CVD).
Methods
MRI-derived vascular parameters of the thoracic and abdominal aorta from 2668 participants (median age = 53 years; 51.1% women) of the population-based SHIP-START-2 and SHIP-TREND-0 cohorts without CVD were analyzed. Age- and sex-adjusted, as well as multivariable-adjusted Cox-proportional hazard models, were used to estimate associations of diameters of six different aortic segments to mortality.
Results
Over a median follow-up time of 10.6 years (IQR: 8.7; 12.4), a total of 188 participants (126 men and 62 women) died, of which 38 deaths were due to CVD. In unadjusted models, mortality rates were higher in participants with aortic diameters above the median compared to below the median for all investigated aortic sections (all log-rank p < 0.001). In multivariable-adjusted models, the diameters of the ascending thoracic aorta (HR = 1.34 95% CI: 1.04; 1.72, p = 0.022) and of the infrarenal aorta (HR = 3.75 95% CI: 1.06; 13.3, p = 0.040), modeled continuously, were associated with greater cardiovascular mortality. The diameter of the subphrenic aorta was associated with higher cardiovascular mortality only in the age and sex-adjusted model (HR = 3.65 95% CI: 1.01; 13.3, p = 0.049). None of the investigated aortic segments were associated with all-cause mortality.
Conclusion
Non-indexed diameters of the ascending thoracic and infrarenal aorta were associated with higher cardiovascular mortality but not with all-cause mortality in a population sample free of clinically overt CVD at baseline.
Clinical relevance statement
Increased aortic diameter is associated with cardiovascular mortality and can help to identify high-risk patients.
Key Points
Increased aortic diameter is associated with mortality.
Non-indexed diameters of the ascending and infrarenal aorta are associated with cardiovascular mortality but not all-cause mortality.
Aortic diameter measurements support the estimate of cardiovascular mortality.
Keywords: Thoracic aorta, Abdominal aorta, Magnetic resonance imaging, Cardiovascular diseases
Introduction
The aortic diameter is associated beside sex, age, and body surface area (BSA) with traditional risk factors of cardiovascular disease (CVD) such as smoking, blood pressure, and dyslipidemia [1, 2]. It is well known that increased aortic diameter is correlated with the risk of life-threatening aortic wall diseases such as dissection and aneurysm formation [3, 4]. However, there is also some evidence that even a subclinical increase in aortic diameter at various anatomic locations is associated with adverse CVD events and mortality. A recent study showed that larger BMI-indexed ascending and descending aortic diameters are associated with a greater risk of cardiovascular mortality in women and all-cause mortality in both sexes [5]. However, a previous study found, that increased infrarenal abdominal aorta and lower abdominal aorta were associated with an increased risk of CVD events in women and men (including cardiovascular death). In contrast, increased diameters of the ascending and descending thoracic aorta were not significantly associated with CVD events [6].
The pathophysiology leading to aortic dilation is complex and not fully understood, but the final common path of all mechanisms is aortic wall degeneration with a subsequent increase in diameter [7, 8]. The aortic wall at various anatomic locations is affected to a different extent by aortic wall stressors, leading to different degrees of diameter increase [6, 9].
Therefore, the aim of this study was to assess if thoracic and abdominal aortic segments were associated with cardiovascular and all-cause mortality in a large population free of CVD at baseline.
Methods
Sample
The studied population was part of the Study of Health in Pomerania (SHIP). The study was approved by the Ethics Committee of the University of Greifswald and complies with the Declaration of Helsinki. All participants provided written informed consent.
SHIP is a prospective population-based project consisting of several independent cohorts established in the northeast area of Germany [10]. Between 2008 and 2012, the second examination cycle of the SHIP-START cohort (N = 2332) and the first examination cycle of the SHIP-TREND cohort (N = 4420) were conducted.
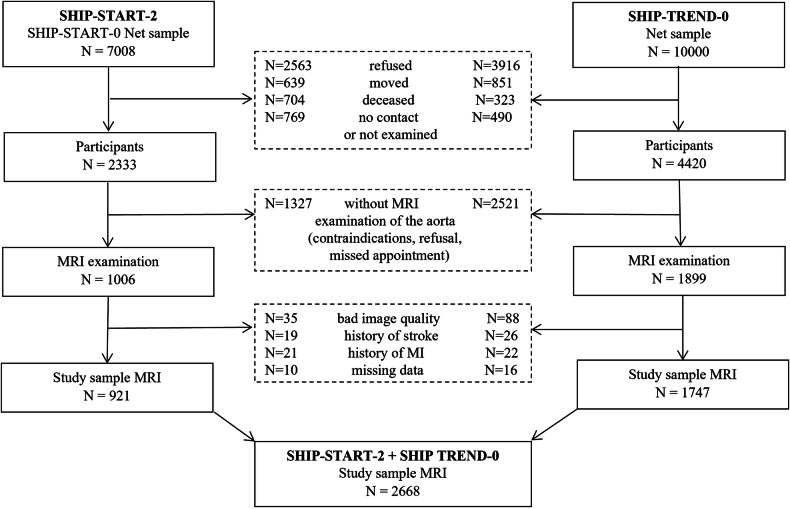
At these examination cycles, a total of 2905 participants received a cardiovascular MRI between 2008 and 2012, including imaging of the aorta. We excluded individuals with insufficient image quality (N = 123), persons with a history of stroke (N = 45), myocardial infarction (N = 43), and missing data for established cardiovascular risk factors (N = 26). The final analytical sample comprised 2668 Caucasian subjects (1336 women), aged 21–88 years (Fig. 1).
Fig. 1.
Flow-chart of the study sample drawn from the population-based cohorts SHIP-START-2 and SHIP-TREND-0
Clinical examination and assessment of covariates
Participants were examined at the SHIP study center by trained personnel. Information on age and sex was provided by the population registries. In standardized personal interviews, information was collected on smoking status (categorized as never, ex-smoker, and current smoker), history of stroke, myocardial infarction, and diabetes. For the definition of current diabetes, a self-reported physician’s diagnosis or a glycated hemoglobin (HbA1c) value ≥ 6.5% was used.
Waist circumference was measured horizontally with an inelastic tape in the middle between the lower rib margin and the iliac crest. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2). BSA was calculated according to the Du Bois formula: (BSA = 0.007184 × (height in cm)0.725 × (weight in kg)0.425 [11].
Systolic and diastolic blood pressure were measured three times at the right arm of seated participants using a digital blood pressure monitor (HEM-705CP, Omron Corporation) after a 5-min rest period, with each reading being followed by a further rest period of 3 min. The mean of the second and third measurements was calculated and used for present analyses.
Blood samples were drawn from the cubital vein in the supine position. The samples were taken between 07.00 a.m. and 04.00 p.m. and were analyzed immediately for standard laboratory parameters. Serum HbA1c levels were measured by high-performance liquid chromatography (Bio-Rad DiamatTM Analyzer, ClinRep® kit, RECIPE Chemicals + Instruments GmbH). Low-and high-density lipoprotein cholesterol (LDL-C and HDL-C), as well as triglyceride (TG) levels, were determined enzymatically using reagents from Dade Behring (Dimension VistaTM System, Flex® reagent cartridge, Dade Behring).
MRI-derived aortic diameters
MRI was performed on a 1.5-T MRI scanner (Magnetom Avanto; Siemens Healthcare) using integrated coil elements and phased-array surface coils. Aortic diameters were measured on plain axial 3D T1-weighted volumetric interpolated breath-hold examination images.
The outer diameters (wall to wall) of six predefined aortic segments (three thoracic and three abdominal aortic segments) were measured: the ascending and descending aorta (level of the pulmonary trunk), the aortic arch (proximal to the origin of the left subclavian artery), the subdiaphragmatic aorta (level of the aortic hiatus), and the supra- and infrarenal aorta (1 cm above/below the right renal artery origin. Diameters were measured on plain axial MRI- slices. Further technical details of the aortic diameter measurements are described in detail elsewhere [1, 12].
Mortality follow-up
Vital status information of study participants was collected regularly from population registries. Participants were censored at death or loss to follow-up. The number of months between baseline examination and censoring was used as the duration of follow-up. Death certificates were requested from the local health authorities and were coded by a certified nosologist according to the International Classification of Diseases, 10th revision (ICD-10). CVD was defined as ICD-10 codes I00 to I99 or R96. Two internists independently validated the underlying cause of death and performed a joint reading together with a third internist in cases of disagreement.
Statistical analyses
Baseline characteristics of the study sample were summarized by median and interquartile range (IQR) for continuous data and by number and percentage for categorical data. The different aortic parameters that were used as exposure variables in our hazard models, were modeled as continuous traits in our primary analyses and were dichotomized at the median in further analyses.
Overall and cardiovascular survival curves were calculated by Kaplan–Meier analysis and compared by log-rank test between individuals above vs equal or below the median for each aortic segment diameter. Cox-proportional hazard models were used to estimate hazard ratios (HR) with a 95% confidence interval (CI) for overall and cardiovascular mortality associated with the aortic diameters in 6 aortic segments (each segment considered separately). The aortic diameters were modeled as continuous traits (HR are provided per 1-unit increment of aortic diameter). We adjusted for age and sex (Model 1) and for age, sex, smoking status, BMI, HbA1c, HDL-C, LDL-C, and TG (Model 2). These parameters were selected as confounders because they were associated with aortic parameters in a previous study [7]. We didn’t adjust the models for blood pressure since it is regarded as a mediator for the association between aortic parameters and mortality. The Cox-proportional hazards assumption was checked by the parallelism of survival curves of log-log plots and by Schoenfeld’s residuals.
The improvement in predictive accuracy by adding aortic diameters to a model based on traditional risk factors including age, sex, smoking status, BMI, systolic blood pressure, diastolic blood pressure, HbA1c, HDL-C, LDL-C, and TG was evaluated by Harrell’s C-index and Likelihood-ratio test.
In sensitivity analyses, non-linear associations and effect modifications were tested by multivariable regression spline models and by the Stata module “MFPIGEN”, respectively. In secondary analyses, parameters of aortic diameters were indexed by BSA and BMI. Multivariable models were adjusted for “cohort” to exclude potential unobserved methodical differences in further sub-analyses. A value of p < 0.05 was considered statistically significant. In addition, we used a Bonferroni-corrected p < 0.0083 (0.05/6) due to the performed tests for six different aortic diameters. Statistical analyses were performed using Stata 16.1 (Stata Corporation).
Results
The main characteristics of the study sample (n = 2668 participants) are provided in Table 1. Participants of the SHIP-START-2 cohort (N = 921) were older and had higher average systolic BP compared to the SHIP-TREND-0 sample (N = 1747). In addition, all median diameters of the six different thoracic aortic sections were larger in SHIP-START-2 participants than in SHIP-TREND-0 participants.
Table 1.
Baseline characteristics of the study sample
| Total | SHIP-START-2 | SHIP-TREND-0 | |
|---|---|---|---|
| Parameter | N = 2668 | N = 921 | N = 1747 |
| Age (years) | 53 (43; 63) | 56 (45; 65) | 52 (41; 62) |
| Men | 1332 (49.9%) | 452 (49.1%) | 880 (50.4%) |
| Smoking status | |||
| Never-smoker | 1057 (39.6%) | 357 (38.8%) | 700 (40.1%) |
| Ex-smoker | 1034 (38.8%) | 396 (43%) | 638 (36.5%) |
| Current smoker | 577 (21.6%) | 168 (18.2%) | 409 (23.4%) |
| BMI (kg/m²) | 27.1 (24.5; 30.2) | 26.9 (24.6; 30.1) | 27.2 (24.4; 30.2) |
| Waist circumference (cm) | 89.4 (80; 98.6) | 90 (80.3; 98.9) | 89 (80; 98.5) |
| BSA (m2) | 1.91 (1.76; 2.1) | 1.90 (1.75; 2.0) | 1.92 (1.77; 2.1) |
| Systolic blood pressure (mmHg) | 128 (116; 139.5) | 131 (119.5; 142.5) | 126.5 (114.5; 138) |
| Diastolic blood pressure (mmHg) | 78 (71.5; 84.5) | 80 (74.0; 86.5) | 76.5 (70.5; 83.5) |
| Diabetes mellitus | 228 (8.6%) | 86 (9.3%) | 142 (8.1%) |
| HbA1c (%) | 5.3 (4.9; 5.6) | 5.3 (5;5.7) | 5.2 (4.9; 5.6) |
| HDL-C (mmol/L) | 1.41 (1.18; 1.69) | 1.40 (1.18; 1.69) | 1.41 (1.18; 1.69) |
| LDL-C (mmol/L) | 3.38 (2.77; 3.99) | 3.34 (2.73; 3.9) | 3.41 (2.81; 4.02) |
| Total cholesterol (mmol/L) | 5.5 (4.8; 6.2) | 5.5 (4.7; 6.1) | 5.5 (4.8; 6.2) |
| TGs (mmol/L) | 1.36 (0.95; 2.01) | 1.54 (1.01; 2.22) | 1.29 (0.92; 1.87) |
| Aortic parameters | |||
| Aortic ascending diameter (cm) | 3.36 (3.06; 3.66) | 3.40 (3.12; 3.69) | 3.33 (3.02; 3.64) |
| Aortic arch diameter (cm) | 2.84 (2.63; 3.08) | 2.88 (2.67; 3.12) | 2.83 (2.60; 3.06) |
| Aortic descending diameter (cm) | 2.50 (2.25; 2.74) | 2.53 (2.30; 2.79) | 2.47 (2.24; 2.71) |
| Aortic subphrenic diameter (cm) | 2.34 (2.12; 2.58) | 2.39 (2.17; 2.61) | 2.32 (2.09; 2.56) |
| Aortic suprarenal diameter (cm) | 2.22 (2.01; 2.41) | 2.26 (2.07; 2.44) | 2.19 (1.98; 2.39) |
| Aortic infrarenal diameter (cm) | 1.87 (1.72; 2.02) | 1.89 (1.74; 2.04) | 1.86 (1.70; 2.01) |
Data are given as number (percentage) or median (25th and 75th percentile)
HbA1c hemoglobin A1c, HDL-C high-density lipoprotein cholesterol, LDL-C low-density-lipoprotein cholesterol, BMI body mass index, BSA body surface area, TG triglyceride
The overall median of the ascending thoracic aorta was 3.36 cm (IQR: 3.06; 3.66) and of the descending thoracic aorta 2.50 cm (IQR: 2.25; 2.74) (Table 1).
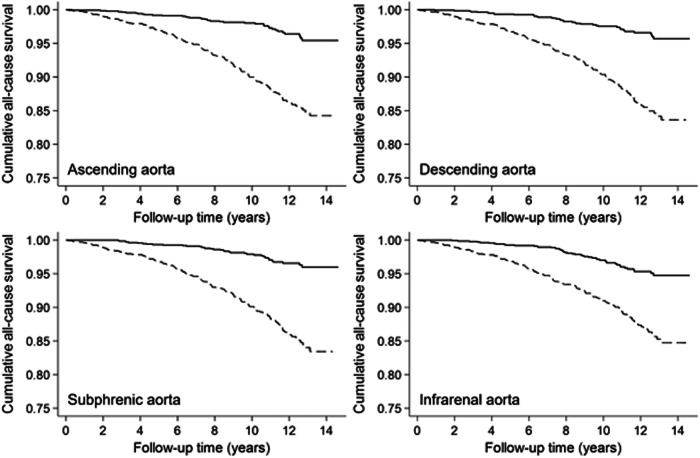
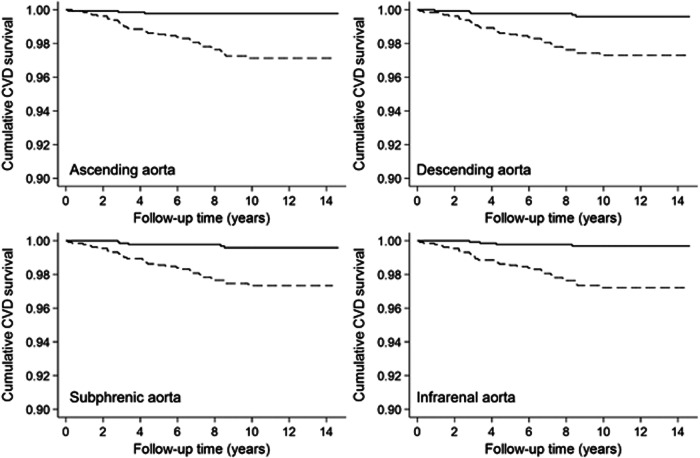
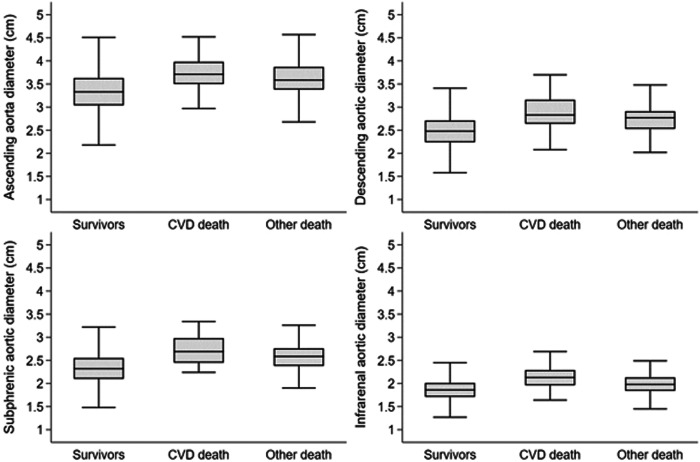
Mortality data were available for a median follow-up time of 10.6 years (IQR: 8.7; 12.4). During 27.702 person-years of follow-up, 188 participants (126 men and 62 women) died, 38 participants (32 men and 6 women) of cardiovascular causes with predominant acute myocardial infarction (n = 7, 18%), sudden cardiac death (n = 6, 16%), and unspecified heart failure (n = 5, 13%). Kaplan–Meier estimates revealed a higher cumulative all-cause mortality in participants with an aortic diameter above the median compared to lower diameters for all investigated aortic sections (all p < 0.001, Fig. 2). Similarly, cardiovascular mortality was higher in participants with a higher aortic diameter (all p < 0.001; Fig. 3). Aortic diameter distributions according to survivors, CVD death and other death after the observed follow-up time are displayed in Fig. 4.
Fig. 2.
Kaplan–Meier survival estimates for cumulative all-cause mortality for participants with levels above (dashed line) vs below (solid line) the median of selected aortic diameters (log-rank p < 0.001, respectively)
Fig. 3.
Kaplan–Meier survival estimates for cumulative CVD mortality for participants with levels above (dashed line) vs below (solid line) the median of selected aortic diameters (log-rank p < 0.001, respectively)
Fig. 4.
Distribution of aortic diameters displayed by boxplots according to survivors, CVD death, and other deaths after the observed follow-up time
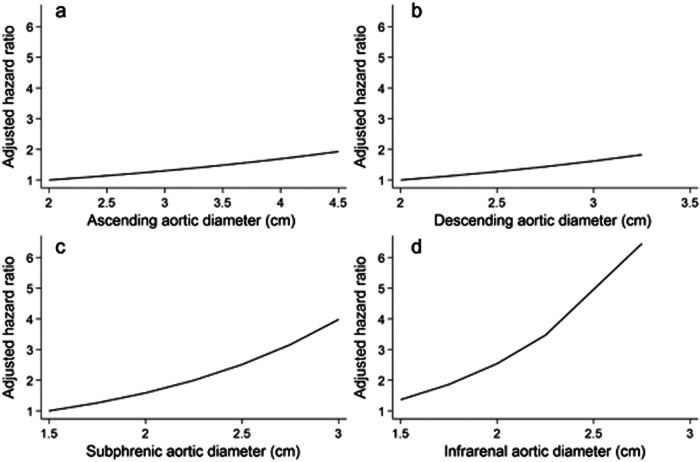
The ascending thoracic aorta diameter, modeled as a continuous trait, demonstrated an association with cardiovascular mortality (HR = 1.34 95% CI: 1.04; 1.72, p = 0.022 per 1-unit increment) but not with all-cause mortality (HR = 1.09 95% CI: 0.83; 1.43, p = 0.520) in fully adjusted Cox-proportional hazard regression models. A similar result concerning the association with cardiovascular mortality was found for the infrarenal aorta diameter in the age and sex-adjusted and fully-adjusted model (HR = 3.75 95% CI: 1.06; 13.3, p = 0.040; Table 2). The diameter of the subphrenic aorta was associated with higher cardiovascular mortality only in the age and sex-adjusted model (HR = 3.65 95% CI: 1.01; 13.3, p = 0.049). Further thoracic aortic section diameters were not associated with mortality of any cause (Table 2). Graphs of multivariable regression spline models displayed linearity of potential associations between thoracic aorta diameters and cardiovascular mortality (Fig. 5). An ascending thoracic aorta diameter above the median was significantly associated with higher cardiovascular mortality (HR = 3.52 95% CI: 1.01; 12.2, p = 0.049) and with all-cause mortality (HR = 1.49 95% CI: 1.01; 2.21, p = 0.047) in the age and sex-adjusted model but not after multivariable adjustment.
Table 2.
Association of MRI-derived aortic diameters with cardiovascular and all-cause mortality
| Aortic diameter | Cardiovascular mortality | All-cause mortality | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Model 1 | ||||
| Ascending | 1.23 (0.98; 1.54) | 0.077 | 1.06 (0.82; 1.38) | 0.657 |
| Arch | 1.44 (0.51; 4.03) | 0.487 | 0.94 (0.59; 1.51) | 0.807 |
| Descending | 1.77 (0.57; 5.50) | 0.325 | 1.12 (0.64; 1.94) | 0.688 |
| Subphrenic | 3.65 (1.01; 13.25) | 0.049 | 1.41 (0.76; 2.61) | 0.281 |
| Suprarenal | 2.11 (0.67; 6.65) | 0.201 | 0.81 (0.41; 1.64) | 0.565 |
| Infrarenal | 4.26 (1.41; 12.91) | 0.010 | 1.13 (0.56; 2.29) | 0.734 |
| Model 2 | ||||
| Ascending | 1.34 (1.04; 1.72) | 0.022 | 1.09 (0.83; 1.43) | 0.520 |
| Arch | 1.48 (0.47; 4.69) | 0.504 | 1.00 (0.60; 1.65) | 0.992 |
| Descending | 1.50 (0.40; 5.66) | 0.548 | 0.93 (0.51; 1.71) | 0.823 |
| Subphrenic | 2.46 (0.59; 10.3) | 0.217 | 1.08 (0.56; 2.08) | 0.814 |
| Suprarenal | 1.57 (0.38; 6.47) | 0.531 | 0.63 (0.31; 1.31) | 0.218 |
| Infrarenal | 3.75 (1.06; 13.3) | 0.040 | 0.93 (0.43; 2.01) | 0.858 |
HR per 1-unit increment in aortic diameter from Cox-proportional hazard regression models adjusted for age and sex (Model 1) and additionally for smoking status, BMI, HbA1c, HDL-C, LDL-C, and TG (Model 2)
Fig. 5.
HR of cardiovascular mortality by diameter levels of (a) ascending aorta, (b) descending aorta, (c) subphrenic aorta, and (d) infrarenal aorta using multivariable regression splines, adjusted for age and sex, smoking status, BMI, HbA1c, HDL-C, LDL-C, and TG
Effect modification analysis revealed a stronger impact of aorta diameters on cardiovascular mortality in participants with a higher BMI above the median (ascending thoracic aorta HR = 5.35 95% CI: 2.06; 13.9, p = 0.001) (Table 3).
Table 3.
Association of MRI-derived aortic diameters with cardiovascular and all-cause mortality according to BMI group
| Cardiovascular mortality | All-cause mortality | |||
|---|---|---|---|---|
| Aortic diameter | HR (95% CI) | p | HR (95% CI) | p |
| BMI > median | ||||
| Ascending | 5.35 (2.06; 13.9) | 0.001 | 1.34 (0.83; 2.15) | 0.227 |
| Arch | 8.26 (2.17; 31.4) | 0.002 | 1.29 (0.7; 2.38) | 0.417 |
| Descending | 9.89 (2.56; 38.2) | 0.001 | 1.39 (0.71; 2.72) | 0.337 |
| Subphrenic | 22.1 (3.97; 123.2) | < 0.001 | 1.09 (0.50; 2.35) | 0.831 |
| Suprarenal | 4.53 (1.89; 10.8) | 0.001 | 0.89 (0.41; 1.95) | 0.774 |
| Infrarenal | 13.7 (3.45; 54.6) | < 0.001 | 1.28 (0.56; 2.95) | 0.557 |
| BMI ≤ median | ||||
| Ascending | 1.08 (0.38; 3.09) | 0.889 | 1.13 (0.85; 1.51) | 0.410 |
| Arch | 0.33 (0.05; 2.04) | 0.234 | 1.30 (0.59; 2.84) | 0.519 |
| Descending | 0.37 (0.05; 2.74) | 0.333 | 1.28 (0.50; 3.23) | 0.607 |
| Subphrenic | 1.03 (0.14; 7.54) | 0.978 | 2.85 (1.11; 7.30) | 0.029 |
| Suprarenal | 0.74 (0.08; 7.38) | 0.801 | 1.60 (0.56; 4.55) | 0.382 |
| Infrarenal | 3.30 (0.44; 24.6) | 0.244 | 2.06 (0.71; 5.91) | 0.181 |
HR per 1-unit increment in aortic diameter from Cox-proportional hazard regression models adjusted for age, sex, smoking status, BMI, HbA1c, HDL-C, LDL-C, and TG
Model discrimination was improved slightly by adding the median-dichotomized ascending thoracic aorta diameter to a standard risk factor model for all-cause mortality (C-statistic, 0.8254 vs 0.8220, p = 0.019) and for cardiovascular mortality (C-statistic, 0.8854 vs 0.8732, p = 0.049).
In sensitivity analysis, a number of five deaths that occurred during the first year of follow-up were excluded without substantial change in the association between the ascending thoracic aortic diameter (HR = 1.34 95% CI: 1.05; 1.70, p = 0.019) and the infrarenal aortic diameter (HR = 4.45 95% CI: 1.28; 15.5, p = 0.019) with higher cardiovascular mortality.
Continuous aortic MRI parameters showed no significant associations with cardiovascular and all-cause mortality when indexed by BSA (Table 4 and BMI (Table 5).
Table 4.
Association of MRI-derived aortic diameters (BSA-indexed) with cardiovascular and all-cause mortality
| Cardiovascular mortality | All-cause mortality | |||
|---|---|---|---|---|
| Aortic diameter/BSA | HR (95% CI) | p | HR (95% CI) | p |
| Model 1 | ||||
| Ascending | 1.28 (0.71; 2.29) | 0.414 | 0.85 (0.42; 1.72) | 0.654 |
| Arch | 0.57 (0.07; 4.75) | 0.601 | 0.53 (0.21; 1.34) | 0.180 |
| Descending | 0.86 (0.07; 10.3) | 0.907 | 0.67 (0.22; 2.04) | 0.481 |
| Subphrenic | 2.48 (0.20; 31.1) | 0.481 | 0.92 (0.29; 2.93) | 0.888 |
| Suprarenal | 0.96 (0.06; 15.3) | 0.974 | 0.34 (0.10; 1.20) | 0.095 |
| Infrarenal | 7.46 (0.56; 99.9) | 0.129 | 0.59 (0.14; 2.42) | 0.463 |
| Model 2 | ||||
| Ascending | 1.48 (0.86; 2.55) | 0.156 | 1.03 (0.56; 1.91) | 0.921 |
| Arch | 0.83 (0.08; 8.12) | 0.871 | 0.75 (0.29; 1.97) | 0.563 |
| Descending | 1.17 (0.08; 16.8) | 0.907 | 0.68 (0.21; 2.19) | 0.523 |
| Subphrenic | 1.95 (0.14; 27.9) | 0.624 | 0.80 (0.24; 2.65) | 0.719 |
| Suprarenal | 0.80 (0.05; 13.3) | 0.878 | 0.32 (0.09; 1.14) | 0.079 |
| Infrarenal | 6.56 (0.46; 92.6) | 0.164 | 0.57 (0.13; 2.46) | 0.448 |
HR per 1-unit increment in aortic diameter from Cox-proportional hazard regression models adjusted for age and sex (Model 1) and additionally for smoking status, HbA1c, HDL-C, LDL-C, and TG (Model 2)
Table 5.
Association of MRI-derived aortic diameters (BMI-indexed) with cardiovascular and all-cause mortality
| Cardiovascular mortality | All-cause mortality | |||
|---|---|---|---|---|
| Aortic diameter/BSA | HR (95% CI) | p | HR (95% CI) | p |
| Model 1 | ||||
| Ascending | 1.02 (0.70; 1.49) | 0.904 | 0.95 (0.79; 1.15) | 0.602 |
| Arch | 0.86 (0.61; 1.22) | 0.393 | 0.92 (0.79; 1.07) | 0.268 |
| Descending | 0.86 (0.60; 1.24) | 0.421 | 0.94 (0.81; 1.1) | 0.466 |
| Subphrenic | 0.97 (0.68; 1.39) | 0.879 | 0.98 (0.84; 1.15) | 0.826 |
| Suprarenal | 0.90 (0.63; 1.29) | 0.567 | 0.91 (0.78; 1.06) | 0.226 |
| Infrarenal | 1.11 (0.80; 1.54) | 0.516 | 0.96 (0.83; 1.11) | 0.588 |
| Model 2 | ||||
| Ascending | 1.13 (0.85; 1.50) | 0.408 | 1.02 (0.86; 1.22) | 0.794 |
| Arch | 0.94 (0.64; 1.39) | 0.767 | 0.98 (0.84; 1.16) | 0.853 |
| Descending | 0.93 (0.62; 1.39) | 0.728 | 0.97 (0.82; 1.15) | 0.765 |
| Subphrenic | 1.00 (0.68; 1.49) | 0.991 | 1.00 (0.85; 1.19) | 0.981 |
| Suprarenal | 0.93 (0.63; 1.37) | 0.715 | 0.92 (0.78; 1.09) | 0.343 |
| Infrarenal | 1.16 (0.84; 1.62) | 0.363 | 0.98 (0.84; 1.16) | 0.848 |
HR per 1-SD increment in aortic diameter from Cox-proportional hazard regression models adjusted for age and sex (Model 1) and additionally for smoking status, HbA1c, HDL-C, LDL-C, and TG (Model 2)
Discussion
In this population-based analysis including 2668 participants (1336 women) free of known CVD, we assessed the associations of 6 different segments of the thoracic and abdominal aorta with all-cause and cardiovascular mortality. We observed that the non-indexed diameters of the ascending thoracic aorta and of the infrarenal aorta were statistically significantly associated with higher cardiovascular mortality in multivariable-adjusted models, but not with all-cause mortality.
The associations of aortic diameters with risk factors of CVD events and mortality were examined in several studies with partially conflicting results (3–6). In two cohorts of the Framingham Heart Study (mean follow-up 8.8 years, non-contrast computed tomography (CT) of the thoracic and abdominal aorta) increased diameter of the infrarenal and of the lower abdominal aorta conferred a 57%, respectively 53% greater risk for incident CVD events (including cardiovascular death) [6].
In the Rotterdam study (mean follow-up 9 years), an association between BMI-indexed ascending (33% higher risk) and descending thoracic aortic diameter (38% higher risk) and cardiovascular mortality for women, as well as all-cause mortality for women and men, was reported [5]. However, there was no association with mortality for non-indexed aortic diameters.
Our results partly support the observations of both studies, mentioned above. Consistent with the observations in the Framingham cohorts, the diameter of the infrarenal aorta was associated with increased CVD mortality in our sample. In Framingham, the association with a combined endpoint, consistent with CVD death, myocardial infarction, coronary insufficiency, index admission for heart failure, and stroke was investigated [6].
We also observed an association of the ascending aorta diameter with CVD mortality, as in the Rotterdam study. However, in contrast to the finding of the Rotterdam Study, we observed these associations only for the non-indexed aortic diameters, but not for BMI- or BSA-indexed diameters. However, the most appropriate method for normalizing aortic diameter measurements is still debated. While indexation for BSA is often used in the literature [13]. There is some evidence, that in underweight and overweight subjects, correction for BSA can distort the prevalence of left ventricular hypertrophy [14]. It is conceivable that this also applies to the aortic diameter measurements [15]. A reason for our findings could be a higher proportion of overweight participants in our cohort compared to the participants of the Rotterdam Study, with a subsequent underestimation of the indexed aortic diameter in obese patients.
The ascending aorta plays a central role in cushioning the highly pulsatile blood flow from the left ventricle into the steady, non-pulsatile flow, which is delivered to the target organs. Arterial aging throughout the lifespan is associated with a decrease of this function resulting in subclinical damage to high-flow, low-resistance organs such as the heart, brain, and kidney increasing the risk for cardiovascular mortality [8, 16].
The infrarenal part of the aorta and its association with cardiovascular mortality is interesting from a practical view, because of its easy approach using ultrasound and the fact that this aortic segment is prone to dilate, which makes it a common location for aneurysm formation. Ultrasound-based studies could confirm the association of increased infrarenal aortic diameter with future CVD events, as well as cardiovascular and all-cause mortality [17, 18].
However, our study does not support all the results of the above-mentioned studies. Differences in the samples (e.g., with respect to the distribution of age or of standard CVD risk factors), in the exact endpoints used (incident CVD as defined above, cardiovascular mortality, all-cause mortality), indexing of the exposure variables (the aortic diameters to BMI or BSA) and different imaging modalities (ultrasound vs CT vs MRI) might contribute to the observed differences.
In addition, we observed that in participants with a higher BMI (above the median), a stronger association between the ascending thoracic aorta on cardiovascular mortality was present, whereas in participants with a lower BMI (below the median), a stronger association of the ascending thoracic aorta with all-cause mortality was observed.
The reasons for the association of aortic diameter with all-cause mortality are not well understood. However, age-associated vascular remodeling including an increase in diameter activates a multifactorial, inflammatory response. This may explain the association of increased aortic diameters with nonvascular causes of death including malignancy, infection, and neurodegenerative disorders, that are also characterized by a chronic inflammatory state [8, 19, 20].
To our knowledge, this is the first study assessing the associations of cardiovascular and all-cause mortality risks with thoracic and abdominal aortic diameters in a population-based approach. A limitation of our study is the relatively small number of deaths, which could be observed during the follow-up period. However, several sensitivity analyses could confirm our main finding, even after considering the Bonferroni-adjusted level of significance. In addition, we had no information about connective tissue or aortic valve disease of the participants, both is associated especially with the diameter of the ascending aorta and we did not analyze the association of aortic diameter with CVD events (e.g., myocardial infarction or stroke) as other studies did previously. Finally, we used only plain diameter measurements, whereas the determination of the cross-sectional vascular area would produce more valid results.
In conclusion, the results of this study indicate that greater non-indexed diameters of the ascending thoracic aorta and of the infrarenal aorta are associated with higher cardiovascular mortality. Physicians analyzing cross-sectional images should be aware of this association. Future research is needed to answer the question if there is a prognostic role of aortic diameter measurement to predict cardiovascular mortality and to evaluate how they can be included in an improved prevention process.
Acknowledgements
The Community Medicine Research network of the University of Greifswald, Germany, covers several research projects that share data from the population-based SHIP (http://ship.community-medicine.de). The contributions to data collection made by field workers, technicians, interviewers, and computer assistants are gratefully acknowledged.
Abbreviations
- BMI
Body mass index
- BSA
Body surface area
- CI
Confidence interval
- CVD
Cardiovascular disease
- HbA1c
Glycated hemoglobin
- HDL-C
High-density lipoprotein cholesterol
- HR
Hazard ratios
- IQR
Interquartile range
- LDL-C
Low-densitiy lipoprotein cholesterol
- SHIP
Study of Health in Pomerania
- TG
Triglyceride
Funding
SHIP is part of the Community Medicine Research Net of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research (01ZZ9603, 01ZZ0103, 01ZZ0403, 01ZZ0701, and 03ZIK012), the Ministry of Cultural Affairs, as well as the Social Ministry of the Federal State of Mecklenburg-West Pomerania. Whole-body MR imaging was supported by a joint grant from Siemens Healthcare, Erlangen, Germany, and the Federal State of Mecklenburg-West Pomerania. The University of Greifswald is a member of the ‘Center of Knowledge Interchange’ program of Siemens AG. Contrast-enhanced MRI research is part of the entire whole-body MRI study and was supported by Bayer Healthcare. Furthermore, this work is part of the Greifswald Approach to Individualized Medicine (GANI_MED) research project. The GANI_MED consortium is funded by the German Federal Ministry of Education and Research, as well as by the Ministry of Cultural Affairs of the German Federal State of Mecklenburg-West Pomerania (03IS2061A). This study was further supported by the DZHK (German Center for Cardiovascular Research). Open Access funding enabled and organized by Projekt DEAL.
Compliance with ethical standards
Guarantor
The scientific guarantor of this publication is Birger Mensel.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Four of the authors have significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
No overlap with previous publications.
Methodology
Prospective
Observational
Performed at one institution
Footnotes
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mensel B, Hesselbarth L, Wenzel M et al (2016) Thoracic and abdominal aortic diameters in a general population: MRI-based reference values and association with age and cardiovascular risk factors. Eur Radiol 26:969–978 [DOI] [PubMed] [Google Scholar]
- 2.Rogers IS, Massaro JM, Truong QA et al (2013) Distribution, determinants, and normal reference values of thoracic and abdominal aortic diameters by computed tomography (from the Framingham Heart Study). Am J Cardiol 111:1510–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elefteriades JA, Farkas EA (2010) Thoracic aortic aneurysm. J Am Coll Cardiol 55:841–857 [DOI] [PubMed] [Google Scholar]
- 4.Solberg S, Forsdahl SH, Singh K, Jacobsen BK (2010) Diameter of the infrarenal aorta as a risk factor for abdominal aortic aneurysm: the Tromsø Study, 1994–2001. Eur J Vasc Endovasc Surg 39:280–284 [DOI] [PubMed] [Google Scholar]
- 5.Rueda-Ochoa OL, Bons LR, Zhu F et al (2022) Thoracic aortic diameter and cardiovascular events and mortality among women and men. Radiology 304:208–215 [DOI] [PubMed] [Google Scholar]
- 6.Qazi S, Massaro JM, Chuang ML et al (2017) Increased aortic diameters on multidetector computed tomographic scan are independent predictors of incident adverse cardiovascular events. Circ Cardiovasc Imaging 10:e006776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tadic M, Gherbesi E, Sala C et al (2022) Is Thoracic aortic diameter an independent predictor of cardiovascular disease and mortality? A narrative review. Front Cardiovasc Med 9:867026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierce GL, Coutinho TA, DuBose LE, Donato AJ (2022) Is it good to have a stiff aorta with aging? Causes and consequences. Physiology 37:154–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agmon Y, Khandheria BK, Meissner I et al (2003) Is aortic dilatation an atherosclerosis-related process? J Am Coll Cardiol 42:1076–1083 [DOI] [PubMed] [Google Scholar]
- 10.Völzke H, Schössow J, Schmidt CO et al (2022) Cohort profile update: the Study of Health in Pomerania (SHIP). Int J Epidemiology 51:e372–e383 [DOI] [PubMed] [Google Scholar]
- 11.Bois DD, Bois EFD (1989) A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 5:303–311 [PubMed] [Google Scholar]
- 12.Mensel B, Hegenscheid K, Heßelbarth L et al (2012) Thoracic and abdominal aortic diameter measurement by MRI using plain axial volumetric interpolated breath-hold examination in epidemiologic research a validation study. Acad Radiol 19:1011–1017 [DOI] [PubMed] [Google Scholar]
- 13.Girardi LN, Lau C, Gambardella I (2021) Aortic dimensions as predictors of adverse events. J Thorac Cardiovasc Surg 161:1193–1197 [DOI] [PubMed] [Google Scholar]
- 14.Lang RM, Bierig M, Devereux RB et al (2006) Recommendations for chamber quantification. Eur J Echocardiogr 7:79–108 [DOI] [PubMed] [Google Scholar]
- 15.Kamimura D, Suzuki T, Musani SK et al (2017) Increased proximal aortic diameter is associated with risk of cardiovascular events and all‐cause mortality in Blacks the Jackson Heart Study. J Am Heart Assoc 6:e005005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashimoto J, Ito S (2009) Some mechanical aspects of arterial aging: physiological overview based on pulse wave analysis. Ther Adv Cardiovasc Dis 3:367–378 [DOI] [PubMed] [Google Scholar]
- 17.Norman P, Le M, Pearce C, Jamrozik K (2004) Infrarenal aortic diameter predicts all-cause mortality. Arterioscler Thromb Vasc Biol 24:1278–1282 [DOI] [PubMed] [Google Scholar]
- 18.Freiberg MS, Arnold AM, Newman AB et al (2008) Abdominal aortic aneurysms, increasing infrarenal aortic diameter, and risk of total mortality and incident cardiovascular disease events. Circulation 117:1010–1017 [DOI] [PubMed] [Google Scholar]
- 19.Syed MBJ, Fletcher AJ, Dweck MR et al (2019) Imaging aortic wall inflammation. Trends Cardiovasc Med 29:440–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allison MA, Hsi S, Wassel CL et al (2012) Calcified atherosclerosis in different vascular beds and the risk of mortality. Arterioscler Thromb Vasc Biol 32:140–146 [DOI] [PubMed] [Google Scholar]