Abstract
Introduction
Biliary tract cancers (BTC) are rare and aggressive neoplasms. The current management of locally advanced or unresectable BTC is primarily based on chemotherapy (CHT) alone, linked to a median overall survival (OS) of approximately 12 months. However, international guidelines still consider concurrent chemoradiation (CRT) as an alternative treatment option. This study aims to review the current evidence on “modern” CRT for primary or recurrent unresectable BTC.
Materials and Methods
A comprehensive search was conducted on PubMed, Scopus, and Cochrane Library to identify relevant papers. Prospective or retrospective trials reporting outcomes after concurrent CRT of unresectable non‐metastatic, primary, or recurrent BTC were included. Only English‐written papers published between January 2010 and June 2022 were considered.
Results
Seventeen papers, comprising a total of 1961 patients, were included in the analysis. Among them, 11 papers focused solely on patients with primary unresectable BTC, while two papers included patients with isolated local recurrences and four papers encompassed both settings. In terms of tumor location, 12 papers included patients with intrahepatic, extrahepatic, and hilar BTC, as well as gallbladder cancer. The median CRT dose delivered was 50.4 Gy (range: 45.0–72.6 Gy) using conventional fractionation. Concurrent CHT primarily consisted of 5‐Fluorouracil or Gemcitabine. The pooled rates of 1‐year progression‐free survival (PFS) and OS were 40.9% and 56.2%, respectively. The median 1‐ and 2‐year OS rates were 63.1% and 29.4%, respectively. Grade ≥3 acute gastrointestinal toxicity ranged from 5.6% to 22.2% (median: 10.9%), while grade ≥3 hematological toxicity ranged from 1.6% to 50.0% (median: 21.7%).
Conclusion
Concurrent CRT is a viable alternative to standard CHT in patients with locally advanced BTC, offering comparable OS and PFS rates, along with an acceptable toxicity profile. However, prospective trials are needed to validate and further explore these findings.
Keywords: biliary tract cancers, brachytherapy, chemoradiation, literature review, systematic review
This is a systematic review of the current evidence on “modern” chemoradiation for primary or recurrent unresectable biliary tract cancers. We have critically compared chemoradiation outcomes with those of other treatment options, seeking to determine whether specific chemoradiation modalities provide distinct advantages in terms of treatment efficacy and patient safety. This analysis is pivotal for informing future treatment guidelines and optimizing patient care in this challenging clinical area.
1. INTRODUCTION
Biliary tract cancers (BTC) represent a significant clinical challenge due to their rarity and aggressive nature, contributing to approximately 3% of all gastrointestinal cancers. 1 These malignancies originate within the biliary tree, with classifications including intrahepatic cholangiocarcinoma (ICC), hilar cholangiocarcinoma (HCCA), extrahepatic cholangiocarcinoma (ECC), and gallbladder cancer (GBC). A key obstacle in the management of BTC is the frequent late‐stage diagnosis, which substantially limits treatment options and contributes to the dismally low 5‐year survival rates of 9%–16%. 2 This underscores the urgent need for improved therapeutic strategies.
At present, the primary treatment for advanced BTC involves chemotherapy (CHT), primarily using a combination of gemcitabine and cisplatin. 3 , 4 This regimen is linked to a median survival period of approximately 12 months. 3 , 4 Additionally, there is growing interest in exploring systemic therapies targeting specific molecular pathways involved in BTC. 5 , 6 For cases that are unresectable or locally recurrent, international guidelines have proposed chemoradiation (CRT) as a viable alternative. 7 In fact, the use of concurrent fluoropyrimidines‐ or gemcitabine‐based CRT has shown promising results in terms of both efficacy and tolerability. 8 , 9 , 10 , 11 However, there is a notable gap in the literature regarding optimal CRT target definition, 12 , 13 and comprehensive international guidelines for CRT in BTC are yet to be established. Furthermore, evidence on the use of CRT specifically for locally recurrent BTC remains limited and somewhat fragmented. 8 , 14 , 15
One of the most critical voids in the current understanding is the lack of randomized trials that directly compare CHT and CRT in the context of locally advanced BTC. This leaves a significant question unanswered: does one treatment modality offer distinct advantages over the other? Moreover, the comparative efficacy and safety of CRT against other treatment modalities, such as best supportive care, stereotactic radiotherapy, and transarterial‐radioembolization, have not been sufficiently explored. Additionally, there is a scarcity of robust evidence guiding the optimal planning and delivery of CRT, including considerations for dose, fractionation, technique, and the integration of concurrent or adjuvant systemic therapies.
Given these gaps in knowledge, this study aims to conduct a comprehensive review of the existing literature on CRT in the context of primary or recurrent unresectable BTC. We will critically compare CRT outcomes with those of other treatment options, seeking to determine whether specific CRT modalities—such as dose and fractionation, radiotherapy techniques, drug combinations, radiotherapy boost, and target definition—provide distinct advantages in terms of treatment efficacy and patient safety. This analysis is pivotal for informing future treatment guidelines and optimizing patient care in this challenging clinical area.
2. MATERIALS AND METHODS
The protocol for this analysis was registered in the PROSPERO international prospective register of systematic reviews on July 17 2020. 16 We followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) methodology. 17 The flowchart of paper selection is shown in Figure 1.
FIGURE 1.
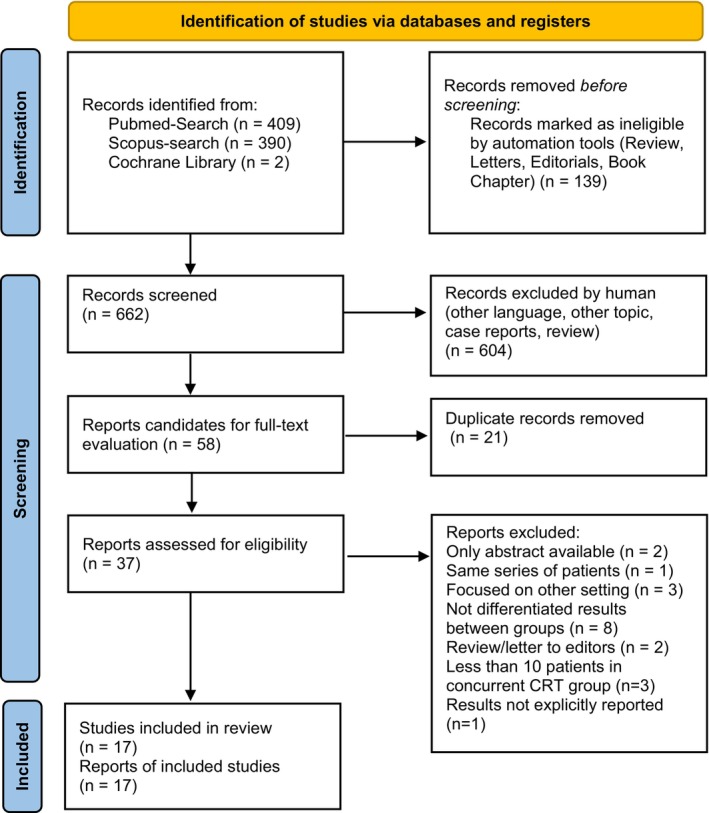
Flowchart of paper selection according to PRISMA 2020 diagram.
2.1. Bibliographic Search
We conducted a literature search in PubMed, Scopus, and Cochrane Library. We included retrospective and prospective papers published from January 2010 to June 2022, reporting outcomes after concurrent CRT for primary or recurrent BTC. Only English‐written papers with a minimum of 10 patients treated with concurrent CRT were considered. The search used keywords such as “biliary tract neoplasms,” “biliary cancer,” “cholangiocarcinoma,” “radio‐CHT,” “chemo‐radiotherapy,” and “chemoradiation.” The complete search strings are shown in Supplementary Material A.
2.2. Inclusion Criteria
Our research question was defined using the patient, intervention, comparison, outcome (PICO) model, 18 as shown in Figure 2. The primary outcome was overall survival (OS), while secondary outcomes were progression‐free survival (PFS) and toxicity. Trials including metastatic patients or reporting on CRT in the adjuvant or neo‐adjuvant setting were excluded. Studies including patients with other abdominal cancers (hepatocellular carcinoma, ampullary or pancreatic adenocarcinoma) were excluded if the results were not differentiated based on the primary tumors. Systematic or narrative reviews, meta‐analyses, guidelines, book chapters, studies on animal models, preclinical studies, study protocols, and case reports were also excluded.
FIGURE 2.
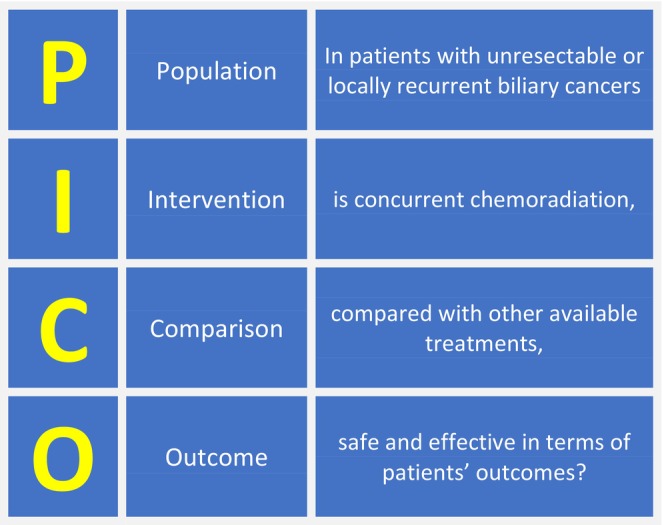
Research question framed in the PICO model.
2.3. Study selection
Papers were independently screened by FMa and EG based on title and abstract. After removing duplicates, full‐text evaluation was independently performed by SB and FMe. Any disagreements were resolved by a third author (AGM). Papers excluded from full‐text evaluation with reasons for exclusion are listed in Supplementary material B.
2.4. Data analysis
Data on the included population (disease site and stage) and the delivered treatment (radiation dose and fractionation, any boost, concurrent CHT) were collected. Outcomes included median and/or 1‐ to 5‐year survival rates, median and/or 1‐ to 2‐year PFS rates, and acute and late toxicity rates. The outcome analysis, based on actuarial OS and PFS, was performed only for the CRT population. Values including other subgroups were listed as not reported (NR) or marked separately. A meta‐regression analysis was conducted between OS and radiation total dose and biologically effective dose (BED).
2.5. Quality assessment
We assessed the risk of bias using the ROBINS‐I tool (risk of bias in non‐randomized studies of intervention). 19 Bias related to confounding factors, participant selection, intervention classification, deviations from intended intervention, missing data, outcome measurement, and selection of reported results were considered. Two authors (SB, FMa) independently ranked the included papers and resolved any disagreements through discussion. The results of this analysis were graphically reported using the robvis tool. 20
3. RESULTS
3.1. Search results
A total of 17 papers were included in the analysis, 8 , 10 , 11 , 14 , 15 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 comprising a total of 1961 patients. Among these studies, two were prospective trials, 21 , 23 while the rest were retrospective. The patients were treated between 1991 and 2018. Twelve studies focused on patients with primary unresectable non‐metastatic BTC, 10 , 21 , 22 , 23 , 25 , 26 , 27 , 29 , 30 , 31 , 32 two studies included patients with isolated local recurrence, 15 , 28 and four studies considered both settings. 8 , 11 , 14 , 24 One study exclusively included patients with GBC, 30 one study focused on ECC, 15 one study analyzed only cases of HCCA, 22 while two papers presented data on ICC. 31 , 32 The remaining papers included a mixed population of patients with various types of BTC. The stage of disease was reported in 15 studies, 8 , 10 , 11 , 14 , 15 , 21 , 22 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 32 with a median of 69.6% of patients presenting with T3–4 tumor stage. The percentage of lymph node involvement was reported in 11 papers, 8 , 10 , 11 , 14 , 15 , 22 , 24 , 26 , 27 , 28 , 30 , 32 with a median of 46%. Table 1 provides further details on the characteristics of the patients. Five studies compared CRT with radiotherapy (RT), 11 , 14 , 15 , 22 , 28 while four studies compared CRT with CHT. 23 , 30 , 31 , 32 One study compared definitive CRT to adjuvant and neoadjuvant CRT, 27 one study compared CRT to best supportive care, 26 and finally one study compared CRT to transarterial radioembolization or stereotactic RT. 29 Among the publications reporting results on patients with locally advanced tumors, only one specified the version of the TNM classification used, which was the AJCC 6th edition. 26 In the other publications, the stage classification was presented but the version of the TNM system used was not specified, 10 , 21 , 25 , 29 , 30 , 31 , 32 while in other no data on the stage classification were provided. 22 , 23 , 27
TABLE 1.
Studies and patients characteristics.
| Reference, year | Study design | N° patients included (total) | Treatment period | Diagnosis | Site (%) | T stage: 1–2 (%) | T stage: 3–4 (%) | cN1 (%) |
|---|---|---|---|---|---|---|---|---|
| Laughlin et al, 2022 27 | Retrosp. | 29 (65) | 1998–2019 | UR |
ECC: 90.0 HCCA: 10.0 |
64.0 | 36.0 | 38.0 |
| Koh et al, 2021 28 | Retrosp. | 61 (76) | 2001–2015 | LR |
ECC: 57.0 HCCA: 43.0 |
58.0 | 42.0 | 29.0 |
| Jethwa et al, 2020 10 | Retrosp. | 48 (48) | 1998–2018 | UR |
ECC: 85.0 GBC: 15.0 |
42.0 | 58.0 | 29.0 |
| Hung et al, 2020 11 | Retrosp. | 23 (30) | 2015–2017 | UR and LR |
ICC: 60.0 ECC: 30.0 GBC: 10.0 |
26.7 | 70.0 | 56.7 |
| Sebastian et al, 2019 29 | Retrosp. | 54 (141) | 2004–2014 | UR | NR | 51.9 | 48.1 | NR |
| Bisello et al, 2018 8 | Retrosp. | 61 (76) | 1991–2017 | UR and LR |
ICC: 3.9 HCCA: 51.3 ECC: 32.9 GBC: 3.9 LR: 7.8 |
21.5 | 78.5 | 36.8 |
| Verma et al, 2018 31 | Retrosp. | 666 (2842) | 2004–2013 | UR | ICC: 100.0 | NR | NR | NR |
| Verma et al, 2017 30 | Retrosp. | 327 (1199) | 2004–2013 | UR | GBC: 100.0 | 7.0 | 59.0 | 30.0 |
| Kim et al, 2017 15 | Retrosp. | 18 (23) | 2001–2013 | LR | ECC: 100.0 | 30.4 a | 69.6 a | 47.8 a |
| Jackson et al, 2016 32 | Retrosp. | 374 (1636) | 2001–2011 | UR | ICC: 100.0 | 28.6 b | 71.3 b | NR |
| Lee et al, 2016 21 | Prosp. | 18 (18) | 2007–2011 | UR |
ECC: 22.2 HCCA: 33.3 GBC: 44.5 |
22.2 b | 77.8 b | NR |
| Chen et al, 2015 22 | Retrosp. | 16 (34) | 2001–2010 | UR | HCCA: 100 | 31.0 | 69.0 | 50.0 |
| Phelip et al, 2014 23 |
Prosp. Phase II |
18 (34) | 2006–2010 | UR |
ICC: 56.0 ECC: 11.0 HCCA: 22.0 GBC: 11.0 |
NR | NR | NR |
| Moureau‐Zabotto et al, 2013 14 | Retrosp. | 18 (30) | 1995–2008 | UR and LR |
HCCA: 67.0 ECC: 33.3 b |
12.0 b | 75.0 b | 46.0 b |
| Yoshioka et al, 2014 25 | Retrosp. | 117 (498) | 2000–2011 | UR |
ICCA: 14.0 ECC: 35.0 HCC: 44.0 GBC: 8.0 a |
53.0 a | 32.0 a | NR |
| Yi et al, 2014 26 | Retrosp. | 106 (176) | 1995–2010 | UR |
ICC: 39.6 ECC: 29.2 GBC: 31.1 |
11.3 | 88.7 | 80.2 |
| Habermehl et al, 2012 24 | Retrosp. | 11 (25) | 2003–2010 | UR and LR |
ECC: 36.4 HCCA: 63.6 |
0.0 | 100.0 | 81.8 |
Abbreviations: BTC, biliary tract cancer; ECC, extrahepatic cholangiocarcinoma; GBC, gallbladder cancer; HCCA, hilar cholangiocarcinoma (Klatskin tumor); ICC, intrahepatic cholangiocarcinoma; LR, local recurrence; NR, not reported; UR, unresectable.
Related to the whole population included in the analysis;
Stage reported according to American Joint Committee on Cancer (AJCC) staging system.
3.2. Treatment
The CRT targets were described in 10 papers. 8 , 10 , 11 , 14 , 15 , 21 , 22 , 23 , 25 , 28 Five studies 10 , 11 , 15 , 22 , 25 defined the clinical target volume (CTV) as the sum of the gross tumor volume (GTV) and involved lymph nodes, while another five studies 8 , 14 , 21 , 23 , 28 included the GTV and prophylactic nodal irradiation in the CTV. The planning target volume (PTV) was defined with an isometric expansion of the CTV by 10–20 mm in seven cases, 8 , 14 , 15 , 22 , 23 , 25 , 28 and by 5 mm in two cases. 10 , 21 One study defined an internal target volume. 11 Photon‐based RT was used in all studies except for one that used proton beams. 11 The RT technique was reported in 11 papers, 8 , 10 , 14 , 15 , 22 , 23 , 24 , 26 , 27 , 28 with three‐dimensional conformal RT being used more frequently (10 studies), 8 , 14 , 15 , 22 , 23 , 24 , 26 , 28 while two studies used both three‐dimensional and intensity‐modulated RT techniques 10 , 27 and one study including also 2D technique (8). The median delivered dose was reported in 14 papers, 8 , 10 , 11 , 14 , 15 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 ranging from 45.0 to 72.6 Gy, with a median of 50.4 Gy. Conventional fractionation (1.8 or 2.0 Gy per fraction) was used in all studies 8 , 10 , 14 , 15 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 33 except for one series with patients receiving 72.6 Cobalt‐Gray Equivalent (CGE) in 3.3 CGE/fraction. 11 A brachytherapy boost was delivered in five studies 8 , 10 , 24 , 25 , 27 to a median of 17.0% of the study population, while two studies used an intraoperative RT boost to 2%–18% of the enrolled population. 24 , 25 The biologically effective dose (BED) ranged from 53.1 to 96.6 Gy (α/β = 10). Thirteen studies 8 , 10 , 11 , 14 , 15 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 reported the concurrent CHT schedule, primarily based on 5‐fluorouracil or gemcitabine, with some studies also using capecitabine 8 , 10 , 15 , 27 or 5‐fluorouracil and leucovorin. 28 Detailed treatment characteristics are provided in Table 2.
TABLE 2.
Treatment characteristics.
| Reference, year | CTV definition | PTV definition | RT‐technique | Median dose (range) Gy | Gy/Fraction | Boost %, Dose Gy | Median BEDα/ß10 (range) | Comparisons | CHT schedule (%) |
|---|---|---|---|---|---|---|---|---|---|
| Laughlin et al, 2022 27 | NR | NR |
3DRT IMRT |
50.4 (7.2–62.4) | 1.8–2.0 |
BRT 10.0% LDR 10.0(8.0–20.0) |
59.5 | nCRT vs. aCRT vs dCRT | 5‐FU, CAPE |
|
Koh et al, 2021 28 |
GTV+PNI | CTV+10–20 mm | 3DRT | 54.0 (40.0–64.0) | 1.8–3.0 | No | 64.8 (50.0–76.8) | CRT vs. RT | 5‐FU, CAPE, leucovorin |
| Jethwa et al, 2020 10 | GTV+CIN+5–10 mm | CTV + 5–10 mm |
3DRT IMRT |
50.4 (45.0–50.4) | 1.5–1.8 |
BRT 17.0% HDR 9.0 LDR 20.0–25.0 |
59.5 | CRT±BRT boost a |
5‐FU CAPE |
| Hung et al, 2020 11 | GTV+CIN+5 mm | ITV | PBT | 72.6 (39.6–73.4) CGE | 3.3 | No | 96.6 (52.7–109.6) CGE | CRT vs. RT |
GEM 63.3 5‐FU 13.3 b |
| Sebastian et al, 2019 29 | NR | NR | NR | 50.4 (45.0–54.0) | 1.8 | No | 59.5 | SBRT vs. CRT vs. TARE | NR |
| Bisello et al, 2018 8 | GTV+PNI+10 mm | CTV+10 mm |
2DRT 3DRT |
50.0 (16.0–75.0) | 1.8 |
BRT 51.3% 14.0 (14.0–50.0) |
59.0 | CRT±BRT boost |
5‐FU 29.5 GEM 67.2 CAPE 3.3 |
| Verma et al, 2018 31 | NR | NR | NR | NR | NR | No | NR | CRT vs. CHT | NR |
| Verma et al, 2017 30 | NR | NR | NR | NR | NR | No | NR | CRT vs. CHT | NR |
| Kim et al, 2017 15 | GTV+CNI+5–10 mm | CTV+10–20 mm | 3DRT | 54.0 (45.0–60.0) | 1.8–2.0 | No | 63.7 | CRT vs. RT | 5‐FU 55.5, GEM 22.2, CAPE 16.6, Tegafur/uracil 5.5 |
| Jackson et al, 2016 32 | NR | NR | NR | NR | NR | No | NR | CRT vs. CHT | NR |
| Lee et al, 2016 21 | GTV+PNI+bile duct | CTV+5 mm | 3DRT | 45.0 | 1.8 | No | 53.1 | CRT+CHT | GEM + cisplatin 100.0 |
| Chen et al, 2015 22 | GTV+CIN+5 mm | CTV+10 mm | 3DRT | 54.0 (40.0–66.6) b | 1.8–2.0 | Yes, not specified | 63.7 | CRT vs. RT | 5‐FU 100.0 |
| Phelip et al, 2014 23 | GTV+PNI | CTV+20 mm | 3DRT | 50.0 | 2.0 | No | 60.0 | CRT vs. CHT | 5‐FU+cisplatin |
| Moureau‐Zabotto et al, 2013 14 | GTV+PNI+15 mm | CTV+10 mm | 3DRT | 48.3 (30.0–78.0) | 1.8–2.0 | No | 57.4 | CRT vs. RT | 5‐FU±cisplatin |
| Yoshioka et al, 2014 25 | GTV+/− PNI | CTV+10–20 mm | NR | 50.0 (40.0–60.0) b | 1.8–2.0 |
BRT 14.0%–18.0% IORT 2.0% 20.0–25.0 b |
59.0 | RT±surgery±CHT |
GEM 5‐FU |
| Yi et al, 2014 26 | NR | NR | 3DRT | 46.0 (36.0–52.0) | 1.8–2.0 |
EBRT 100.0% 50.4 (45.0–60.0) |
59.5 | CRT vs. BSC |
GEM 27.4 5‐FU 72.6 |
| Habermehl et al, 2012 24 | NR | NR | 3DRT | 45.0 (39.0–50.4) c | 1.8–2.0 | BRT 18.2%‐24.0; IORT 18.2%‐24.0 | 53.1 | Surgery+CRT vs RT or CRT±BRT/IORT boost |
5FU 18.2 GEM 81.8 |
Abbreviations: 5‐FU 5‐fluorouracil, 3DRT 3D conformal radiotherapy; aCRT, adjuvant chemoradiation; BRT, brachytherapy; BSC, best supportive care; CAPE, capecitabine; CGE, cobalt Gray equivalent; CHT, chemotherapy; CHT‐RT, sequential chemo‐radiotherapy; CIN, clinically involved nodes; CRT, concurrent chemoradiation; CTV, clinical target volume; dCRT, definitive chemoradiation; GEM, gemcitabine; GTV, gross tumor volume; HDR, high dose rate brachytherapy; IMRT, intensity‐modulated radiotherapy; IORT, intra‐operative radiation therapy; ITV, internal target volume; LDR, low dose rate brachytherapy; nCRT, neoadjuvant chemoradiation; NR, not reported; PNI, prophylactic nodal irradiation; RT, radiotherapy; SBRT, stereotactic radiotherapy; TARE, trans‐arterial radioembolization.
CRT 94.0%, Prior CHT 4.0%, Adjuvant CHT 10.0%, BRT boost 17.0%;
Related to the whole population included in the analysis;
Two patients received 15 Gy with Intra‐operative radiotherapy, two patients received 24 Gy of brachytherapy boost.
3.3. Outcomes
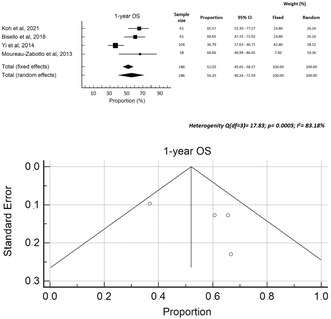
The median follow‐up was reported in 13 studies 10 , 11 , 14 , 15 , 22 , 23 , 24 , 27 , 28 , 29 , 30 , 31 , 32 and ranged between 9.0 and 27.9 months (median 13.0 months). Median OS rates were reported in 13 studies 8 , 10 , 11 , 21 , 22 , 23 , 24 , 25 , 26 , 29 , 30 , 31 , 32 and ranged between 9.6 and 20.0 months (median: 13.5 months). One‐year OS was reported in four 8 , 14 , 26 , 28 studies with rates ranging between 36.8% and 66.7% (median: 63.1%). Two‐year OS, reported in four studies, 8 , 10 , 15 , 32 ranged from 24.4% to 52.1% (median 29.4%). Two papers 10 , 27 reported 16.0% 27 and 20.0% 10 3‐year OS rates, respectively, while three papers 10 , 26 , 27 reported 5‐year OS rates, ranging from 0.0% to 7.9% (median: 1.9%). The median PFS was reported in six studies 10 , 21 , 22 , 23 , 24 , 26 with values ranging from 3.1 to 12.1 months (median: 8.2 months). Three papers 8 , 14 , 28 reported one‐year PFS (median: 44.1%), while other three papers 8 , 10 , 15 reported 2‐year PFS (median: 21.0%). A meta‐regression analysis was conducted on the impact of total dose and BED on OS, which showed no significant correlations, with a sample heterogeneity (I 2 test) of 55.8% and 54.0%, respectively. Finally, a Forrest plot and funnel plot of 1‐year OS ad 1‐year PFS were created ad are reported in Figure 3 and Figure 4, respectively. The heterogeneity test showed statistically significant values for OS but not for PFS. Moreover, an asymmetry is evident from the examination of the funnel plots, both for PFS and for OS, suggesting the possibility of publication bias. The pooled rates of 1‐year PFS and OS were 40.9% and 56.2%, respectively.
FIGURE 3.
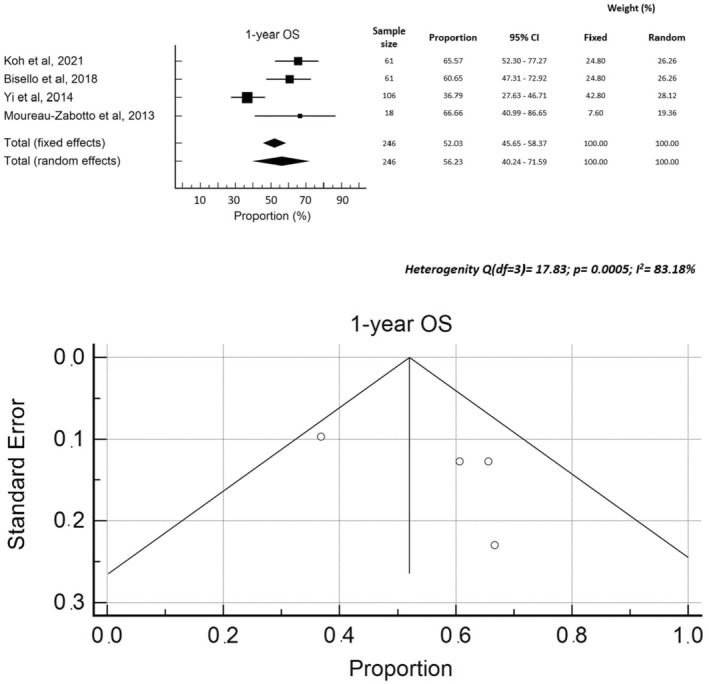
Forrest plot and funnel plot 1‐year OS.
FIGURE 4.
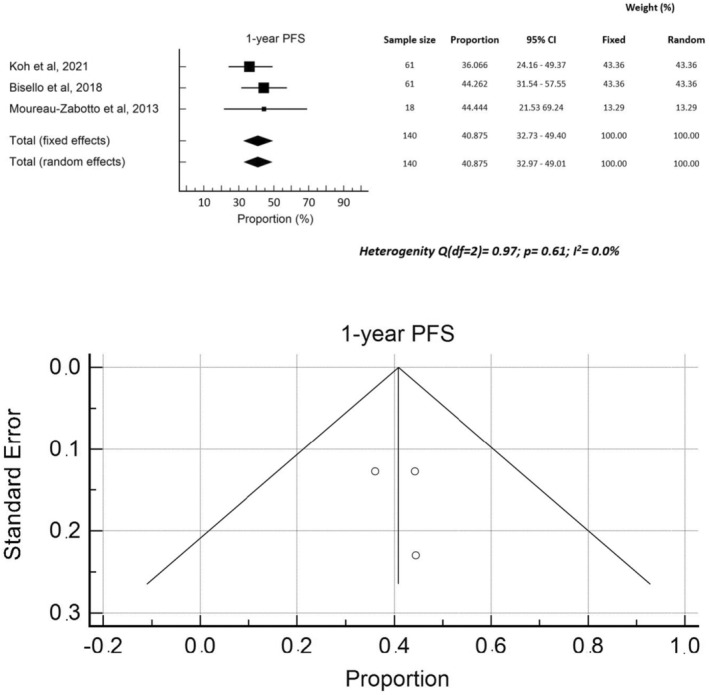
Forrest plot and funnel plot 1‐year PFS.
3.4. Response and treatment failures
Tumor response was assessed using the RECIST criteria 34 in two studies. 21 , 26 A partial response was observed in 19.8% 26 and 27.8% 21 of patients, while stable disease was seen in 69.8% 26 and 72.2% 21 of patients, respectively. Two papers 11 , 28 reported data on local control (LC) with a 1‐year LC rate of 88.0% 11 and freedom from local progression rate of 70.0%. 28 Three other papers 10 , 22 , 27 reported local failures as crude rates of 17.0% and 62.0%, 22 , 27 and a 2‐year rate of local progression of 27.0%. 10 Distant metastases were reported as a crude value of 18.0%, 22 and as a 2‐year rate of 33.0% in another study. 10 Finally, distant progression was reported as a crude value of 38.0% in one study. 27
3.5. Toxicity
Acute toxicity was scored according to the Common Terminology Criteria for Adverse Events (CTCAE), or the RTOG scale. 8 Acute gastrointestinal toxicity (Grade ≥3) was registered in eight studies 8 , 10 , 11 , 14 , 21 , 22 , 23 , 26 and ranged from 5.6% to 13.2% (median 10.9%). Seven papers 8 , 11 , 21 , 22 , 23 , 26 , 28 found Grade ≥3 acute hematologic toxicity, ranging from 1.6% to 50.0% (median 21.7%). Late toxicity was generally not reported. Treatment outcomes are summarized in Table 3.
TABLE 3.
Outcomes.
| Reference, year | Median FU, months (range) | Median OS, months, (range) | 1‐y OS (%) | 2‐y OS (%) | 3‐ y OS (%) | 5‐y OS (%) | Median PFS months, (range) | 1‐y PFS (%) | 2‐y PFS (%) | Tumor Response/Local Control (%) | Acute Toxicity G≥3, % (scale) | Findings |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Laughlin et al, 2022 27 | 9.0 a | NR | NR | NR | 16.0 |
0.0 |
NR | NR | NR |
LP 17.0 DP 38.0 |
NRS (CTCAE 5.0) |
Improved OS after nCRT + OLT compared to dCRT or aCRT |
|
Koh et al, 2021 28 |
13.0 (2.0–119.0) a | 16.0 (13.0–19.0) a | 66.0 | NR | NR | NR | 9.0 (7.0–11.0) a | 37.0 | NR |
1‐y FFLP 70.0 |
HAE 1.6 (CTCAE 4.0) |
Improved OS and PFS after CRT (compared to RT) or with BED >59.0 GY |
| Jethwa et al, 2020 10 | 13.0 (6.0–29.0) | 12.0 (2.3–73.2) | NR | 33.0 | 20.0 | 7.0 | 9.0 (1.7–73.2) | NR | 21.0 |
2‐ y LP 27.0 2‐y DM 33.0 |
GI 13.0 (CTCAE 4.0) |
Improved OS and PFS with BED >59.5 Gy |
| Hung et al, 2020 11 | 16.0 (3.0–36.0) | 20.6 | 83.0 a | 32.0 a | NR | NR | 12.1 | 47.0 a | NR |
1‐y LC 88.0 1‐y DMF 68.0 |
GI 10.0 HAE 21.7 (CTCAE 4.0) |
Improved OS, PFS, LC, and DMF after CRT compared to RT alone |
| Sebastian et al, 2019 29 | 17.0 a | 14.0 (11.0–20.0) | NR | NR | NR | NR | NR | NR | NR | NR | NR | Improved OS after SBRT compared to CRT or TARE |
| Bisello et al, 2018 8 | NR | 13.5 | 60.1 | 24.4 | NR | NR | 10.5 | 44.1 | 9.9 | NR |
GI 13.2 HAE 8.1 (RTOG) |
Improved PFS after 2D‐CRT compared to 3D‐CRT |
| Verma et al, 2018 31 | 10.0 (0–114.0) a | 13.6 (12.3–15.7) | NR | NR | NR | NR | NR | NR | NR | NR | NR | Improved OS after CRT compared to CHT |
| Verma et al, 2017 30 | 9.0 (0.0–123.0) a | 12.9 (11.0–14.7) | NR | NR | NR | NR | NR | NR | NR | NR | NR | Improved OS after CRT compared to CHT |
| Kim et al, 2017 15 | 14.2 (2.4–114.6) a | 18.4 (4.4–114.6) a | 62.6 a | 52.1 | NR | NR | 15.5 (1.6–114.6) a | 56.3 a | 53.3 | NR |
(CTCAE 4.03) NR |
Improved PFS after CRT compared to RT |
| Jackson et al, 2016 32 | 11.3 (2.0–121.8) a | 12.7 | NR | 25.8 | NR | NR | NR | NR | NR | NR | NR | Improved OS after CRT compared to CHT |
| Lee et al, 2016 21 | NR | 9.6 (5.4–30.4) | NR | NR | NR | NR | 6.8 (4.5–19.8) | NR | NR |
PR 27.8 SD 72.2 |
GI 5.6 HAE 50.0 (NCI CTC 4.0) |
/ |
| Chen et al, 2015 22 | 9.4 (2.4–47.4) a | 13.5 (9.4–17.7) | NR | NR | NR | NR | 8.8 (5.2–10.7) | NR | NR |
LP 62.0 DM 18.0 a |
GI 8.8 HAE 17.4 a (NCI CTC 3.0) |
Improved OS and PFS with CRT compared to RT alone |
| Phelip et al, 2014 23 | 27.9 (± 8.0) a | 13.5 (7.8–22.6) | NR | NR | NR | NR | 7.5 (2.8–12.5) | NR | NR | PD: 56.0 |
GI 11.8 HAE 23.0 (NCI‐CTC 2.0) |
Similar PFS and OS after CRT or CHT |
| Moureau‐Zabotto et al, 2013 14 | 12.0 (1.0–83.0) a | NRS | 66.7 ± 11.1 | NRS | NR | NR | NRS | 44.4 ± 11.7 | NRS | NRS |
GI 22.0 Systemic 15.0 (NCI CTC 3.0) b |
Similar OS after CRT compared to RT |
| Yoshioka et al, 2014 25 | NR | 15.0 (12.0–17.0) | NR | NR | NR | NR | NR | NR | NR | NR | NR | Improved OS after surgery plus RT/CHT compared to CRT/CHT |
| Yi et al, 2014 26 | NR | 10.5 (2.1–80.0) c | 36.8 | NR | NR | 1.9 | 7.5 (5.7–9.2) c | NR | NR |
PR 19.8 SD 69.8 PD 10.4 |
GI 9.4 HAE 21.7 (NCI CTC 3.0) |
Improved OS after CRT compared to BSC |
| Habermehl et al, 2012 24 | 13.0 | 13.6 (4.0–34.8) | NR | NR | NR | NR | 3.1 (2.3–24.8) | NR | NR | NR | NRS | Improved OS after surgery plus CRT compared to CRT and RT |
| Median | 13.0 | 13.5 | 63.1 | 29.4 | 18.0 | 1.9 | 8.2 | 44.1 | 21.0 |
GI 10.9 HAE 21.7 |
BED biological equivalent dose; BSC best supportive care, CHT chemotherapy, CI confidence interval, CR complete response, CRT chemoradiation, DFS disease free survival, DM distant metastasis, DMF distant metastasis free; FFLP freedom from Local Progression; FU follow‐up, GI gastrointestinal, HAE hematological, HR hazard ratio, LP local Progression, NCI CTC National Cancer Institute–Common Toxicity Criteria, NR not reported, NRS not reported separately, OLT orthotopic liver transplant, OS overall survival, PD progressive disease, PFS progression‐free survival, PR partial response, RECIST Response Evaluation Criteria in Solid Tumors, RT radiotherapy, RTOG Radiation Therapy Oncology Group, SD stable disease, TARE trans‐arterial radioembolization.
Related to the whole population included in the analysis, therefore not included in the final calculation of the median;
Pain, fever, asthenia.
Median OS and PFS are express in weeks in the original paper (median OS 42.6 (8.3–320.1), median PFS 29.9 (22.9–36.8)).
3.6. Comparisons
Considering the series enrolling patients with a single tumor site, the median OS is 12.9, 13.5, 18.4, and 12.7–13.6 months in patients with ICC, 31 , 32 GBC, 30 ECC, 15 and HCC, 22 respectively. In terms of tumor site, series enrolling only patients with local recurrence 15 , 28 showed higher median OS values (17.2 months; range 16.0–18.4 months) than those composed exclusively of patients with locally advanced disease (13.2 months; range: 9.6–15.0 months). 10 , 21 , 22 , 23 , 25 , 26 , 29 , 30 , 31 , 32 Four studies demonstrated a significant benefit in terms of PFS and OS with CRT compared to RT alone. 11 , 15 , 22 , 28 However, one retrospective study with a small sample size of only 18 patients treated with CRT found no differences. 14 Three studies reported significantly improved OS with CRT compared to CHT alone, 30 , 31 , 32 while a phase II trial with slow enrolment and only 18 patients treated with CRT showed no differences. 23 Additionally, CRT was found to be superior to best supportive care, 26 equivalent to transarterial radioembolization, 29 and inferior to stereotactic RT 29 in terms of OS. Notably, the superiority of stereotactic RT over CRT was observed in a series focusing only on ICC. 29 Finally, two studies analyzing the impact of RT dose found that a BED >59 Gy 28 or >59.5 Gy 10 correlated with better outcomes in terms of PFS and OS.
3.7. Quality assessment
Figures 5 and 6 display the traffic‐light plot and the summary plot based on the risk of bias in non‐randomized studies of intervention (ROBINS‐I) tool, respectively. The majority of the studies analyzed in this review had a moderate risk of bias, with only a few cases considered to have a serious risk. The domains that exhibited the highest risk of bias were “bias due to confounding” and “bias in classification of intervention.”
FIGURE 5.
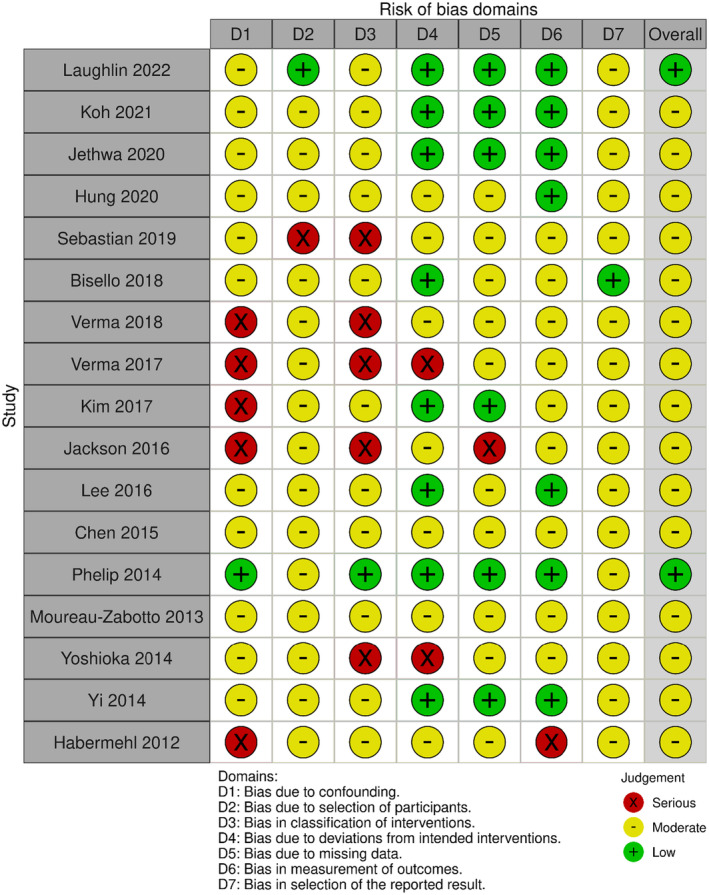
Risk of Bias in Non‐Randomized Studies—of Interventions (ROBINS‐I) traffic‐light plot.
FIGURE 6.
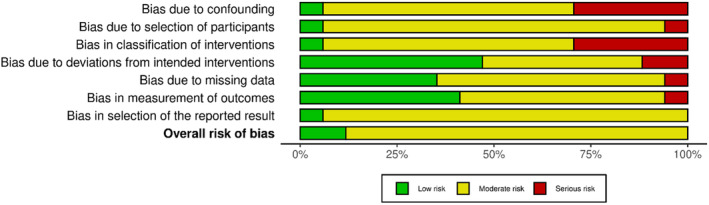
Risk of Bias in Non‐Randomized Studies—of Interventions (ROBINS‐I) summary plot.
4. DISCUSSION
Our systematic literature review aimed to evaluate the differences between CRT and other treatments for locally advanced BTC. The key findings indicate that CRT offers promising results, with pooled rates of 1‐year PFS and OS being 40.9% and 56.2%, respectively. Notably, the incidence of grade ≥3 gastrointestinal toxicity was less than 15% across all studies, underscoring CRT viability as a treatment option for these tumors. 8 , 10 , 11 , 14 , 15 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31
When comparing CRT with CHT, we observed different outcomes. One study reported similar results for both treatments, while three studies highlighted better OS following CRT. 23 , 29 , 30 , 31 Additionally, CRT seemed to offer superior PFS and OS compared to conventional fractionated RT in three studies. 11 , 22 , 28 However, one study with a small sample size showed no significant differences. 14 A separate study comparing CRT with stereotactic body radiation therapy (SBRT) reported better OS with SBRT, though it had certain limitations. 29 This suggests that the choice between SBRT and CRT might depend on specific patient characteristics. For instance, ICC, located in the liver, might respond better to SBRT, whereas tumors near hollow organs or with regional lymph node metastases might benefit more from CRT.
Interestingly, the efficacy of CRT did not appear to be significantly influenced by the tumor site, as median survival was similar across ICC, GBC, and ECC. 20 , 29 , 31 , 32 Higher doses of CRT were associated with better outcomes in two studies, 10 , 28 yet our meta‐regression analysis did not show a significant effect of CRT dose on OS, likely due to limited variability in administered doses across the studies.
This study, however, has its limitations. Most included studies were retrospective, and there was an absence of randomized controlled trials, which limits the strength of our conclusions. The funnel plot analysis suggested a risk of publication bias, and the included studies were heterogeneous in terms of stage, tumor site, and treatment techniques. In particular, with respect to tumor site, it is notable that patients with ECC presented a median OS of 18.4 months, 15 which appears superior to that of patients with ICC, GBC, and HCC (12.7–13.6 months 22 , 30 , 31 , 32 ). This variation underscores the importance of considering tumor site when evaluating outcomes and the potential benefits of treatment modalities. Furthermore, our analysis revealed heterogeneous survival outcomes between series that included only local recurrences 15 , 28 and those with only locally advanced tumors, 10 , 21 , 22 , 23 , 25 , 26 , 29 , 30 , 31 , 32 with a higher median survival observed in the former group (17.2 vs. 13.2 months). Interestingly, this heterogeneity was significant for OS but not for PFS, possibly due to the larger amount of data available for OS.
Furthermore, we must acknowledge that the previously reported comparisons between CRT and CHT are based on the evidence available during the period considered for analysis, when the standard CHT was represented by the combination of gemcitabine and cisplatin. However, two recent randomized trials have investigated the addition of immune checkpoint inhibitors (ICI) to standard CHT in advanced biliary cancer, demonstrating a modest but significant improvement in OS. 35
The TOPAZ‐1 trial, with 685 patients, evaluated durvalumab (a PD‐L1 inhibitor) combined with gemcitabine and cisplatin. The results showed a significant improvement in OS with durvalumab (12.8 vs. 11.5 months; hazard ratio 0.80; p = 0.021), along with better PFS and objective response rate (ORR), with similar toxicity between groups. 36
Similarly, the KEYNOTE‐966 trial studied pembrolizumab (a PD‐1 inhibitor) in 1069 newly diagnosed patients, also in combination with gemcitabine and cisplatin. Pembrolizumab significantly improved OS (12.7 vs. 10.9 months; hazard ratio 0.83; p = 0.0034) and PFS (6.5 vs. 5.6 months; p = 0.023) compared to placebo. Although the response rates were similar, the duration of response was longer with pembrolizumab. Survival benefits were consistent across all biliary cancer subtypes, and pembrolizumab did not significantly increase toxicity, maintaining health‐related quality of life. 37
Unfortunately, it is challenging to compare the results of these studies with those in our review, as both studies enrolled both patients with locally advanced disease and metastatic patients. Furthermore, it should be noted that in both studies, patients with locally advanced disease were the minority (11.8%–13.9%) and that in one of the studies, 36 no significant difference in terms of OS was recorded in the subgroup of non‐metastatic patients.
An important consideration in advanced BTC is that OS is often affected by complications like biliary obstructions and cholangitis, not just disease progression. This underscores the importance of considering variations in treatment approaches and the management of cancer‐related complications across different centers.
In conclusion, our analysis supports the potential role of CRT in inoperable BTC. However, there is a need for further research to identify patients who might benefit most from CRT, to confirm the impact of CRT dose on outcomes, and to determine the optimal treatment sequence. Considering the rarity of BTCs, conducting randomized studies in this field may be challenging. Alternative approaches like multi‐center data sharing and predictive modeling could be valuable in individualizing therapy based on patient characteristics.
AUTHOR CONTRIBUTIONS
Silvia Bisello: Conceptualization (equal); data curation (equal); formal analysis (equal); investigation (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Claudio Malizia: Conceptualization (equal); formal analysis (equal); methodology (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Filippo Mammini: Conceptualization (equal); data curation (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Erika Galietta: Conceptualization (equal); data curation (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Federica Medici: Conceptualization (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Gian Carlo Mattiucci: Conceptualization (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Francesco Cellini: Conceptualization (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Andrea Palloni: Conceptualization (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Luca Tagliaferri: Conceptualization (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Gabriella Macchia: Conceptualization (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Francesco Deodato: Conceptualization (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Savino Cilla: Conceptualization (equal); formal analysis (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Giovanni Brandi: Conceptualization (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Alessandra Arcelli: Conceptualization (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal). Alessio G. Morganti: Conceptualization (equal); data curation (equal); formal analysis (equal); methodology (equal); validation (equal); visualization (equal); writing – original draft (equal); writing – review and editing (equal).
FUNDING INFORMATION
This research received no external funding.
CONFLICT OF INTEREST STATEMENT
Not declared.
Supporting information
Data S1.
ACKNOWLEDGMENTS
We would like to express our gratitude to Carla Conti, Cinzia Giacometti, and Patrizia Volta who helped us during the writing of this manuscript.
Bisello S, Malizia C, Mammini F, et al. Chemoradiation of locally advanced biliary cancer: A PRISMA‐compliant systematic review. Cancer Med. 2024;13:e70196. doi: 10.1002/cam4.70196
DATA AVAILABILITY STATEMENT
All data supporting the reported results are included in this paper.
REFERENCES
- 1. Tariq NU, McNamara MG, Valle JW. Biliary tract cancers: current knowledge, clinical candidates and future challenges. Cancer Manag Res. 2019;11:11‐2642. doi: 10.2147/CMAR.S157092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. The American Cancer Society medical and editorial content team . Bile duct cancer early detection, diagnosis, and staging. CancerOrg. 2018;26‐27. [Google Scholar]
- 3. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273‐1281. doi: 10.1056/NEJMoa0908721 [DOI] [PubMed] [Google Scholar]
- 4. Morizane C, Okusaka T, Mizusawa J, et al. Combination gemcitabine plus S‐1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA‐BT (JCOG1113) randomized phase III clinical trial. Ann Oncol. 2019;30:30‐1958. doi: 10.1093/annonc/mdz402 [DOI] [PubMed] [Google Scholar]
- 5. Kam AE, Masood A, Shroff RT. Current and emerging therapies for advanced biliary tract cancers. Lancet. Gastroenterol Hepatol. 2021;6:6‐969. doi: 10.1016/S2468-1253(21)00171-0 [DOI] [PubMed] [Google Scholar]
- 6. Mirallas O, Verdaguer H, Tabernero J, Macarulla T. Advances in the systemic treatment of therapeutic approaches in biliary tract cancer. ESMO Open. 2022;7:100503. doi: 10.1016/j.esmoop.2022.100503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. NCCN guidelines hepatobiliary cancers . NCCN Clinical Practice Guidelines in Oncology—hepatobiliary cancers. Version 1.2022 2021.
- 8. Bisello S, Palloni A, Autorino R, et al. Radiotherapy or chemoradiation in unresectable biliary cancer: a retrospective study. Anticancer Res. 2019;39:3095‐3100. doi: 10.21873/anticanres.13445 [DOI] [PubMed] [Google Scholar]
- 9. Gkika E, Hawkins MA, Grosu AL, Brunner TB. The evolving role of radiation therapy in the treatment of biliary tract cancer. Front. Oncologia. 2020;10:10. doi: 10.3389/fonc.2020.604387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jethwa KR, Sannapaneni S, Mullikin TC, et al. Chemoradiotherapy for patients with locally advanced or unresectable extra‐hepatic biliary cancer. J Gastrointest Oncol. 2020;11:1408‐1420. doi: 10.21037/JGO-20-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hung SP, Huang BS, Hsieh CE, et al. Clinical outcomes of patients with unresectable cholangiocarcinoma treated with proton beam therapy. Am J Clin Oncol Cancer Clin Trials. 2020;43:180‐186. doi: 10.1097/COC.0000000000000646 [DOI] [PubMed] [Google Scholar]
- 12. Bisello S, Renzulli M, Buwenge M, et al. An atlas for clinical target volume definition, including elective nodal irradiation in definitive radiotherapy of biliary cancer. Oncol Lett. 2019;17:17‐1790. doi: 10.3892/ol.2018.9774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Socha J, Surdyka D, Kepka L. Nodal CTV selection according to primary tumour location and pT stage for biliary tract cancer. J Med Imaging Radiat Oncol. 2019;63:63‐828. doi: 10.1111/1754-9485.12937 [DOI] [PubMed] [Google Scholar]
- 14. Moureau‐Zabotto L, Turrini O, Resbeut M, et al. Impact of radiotherapy in the management of locally advanced extrahepatic cholangiocarcinoma. BMC Cancer. 2013;13:13. doi: 10.1186/1471-2407-13-568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim E, Kim YJ, Kim K, et al. Salvage radiotherapy for locoregionally recurrent extrahepatic bile duct cancer after radical surgery. Br J Radiol. 2017;90:90. doi: 10.1259/bjr.20170308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. PROSPERO . International prospective register of systematic reviews. Available at:https://www.crd.york.ac.uk/PROSPERO.
- 17. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;n71:372. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eriksen MB, Frandsen TF. The impact of patient, intervention, comparison, outcome (Pico) as a search strategy tool on literature search quality: a systematic review. J Med Libr Assoc. 2018;106:420‐431. doi: 10.5195/jmla.2018.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sterne JA, Hernán MA, Reeves BC, et al. ROBINS‐I: a tool for assessing risk of bias in non‐randomised studies of interventions. BMJ. 2016;355:355. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McGuinness LA, Higgins JPT. Risk‐of‐bias VISualization (robvis): an R package and shiny web app for visualizing risk‐of‐bias assessments. Res Synth Methods. 2021;12:55‐61. doi: 10.1002/jrsm.1411 [DOI] [PubMed] [Google Scholar]
- 21. Lee KJ, Yi SW, Cha J, et al. A pilot study of concurrent chemoradiotherapy with gemcitabine and cisplatin in patients with locally advanced biliary tract cancer. Cancer Chemother Pharmacol. 2016;78:841‐846. doi: 10.1007/s00280-016-3143-2 [DOI] [PubMed] [Google Scholar]
- 22. Chen SC, Chen MHH, Li CP, et al. External beam radiation therapy with or without concurrent chemotherapy for patients with unresectable locally advanced hilar cholangiocarcinoma. Hepato‐Gastroenterology. 2015;62:102‐107. doi: 10.5754/hge13076 [DOI] [PubMed] [Google Scholar]
- 23. Phelip JM, Vendrely V, Rostain F, et al. Gemcitabine plus cisplatin versus chemoradiotherapy in locally advanced biliary tract cancer: Fédération francophone de Cancérologie digestive 9902 phase II randomised study. Eur J Cancer. 2014;50:2975‐2982. doi: 10.1016/j.ejca.2014.08.013 [DOI] [PubMed] [Google Scholar]
- 24. Habermehl D, Lindel K, Rieken S, et al. Chemoradiation in patients with unresectable extrahepatic and hilar cholangiocarcinoma or at high risk for disease recurrence after resection: analysis of treatment efficacy and failure in patients receiving postoperative or primary chemoradiation. Strahlentherapie Und Onkol. 2012;188:795‐801. doi: 10.1007/s00066-012-0099-y [DOI] [PubMed] [Google Scholar]
- 25. Yoshioka Y, Ogawa K, Oikawa H, et al. Factors influencing survival outcome for radiotherapy for biliary tract cancer: a multicenter retrospective study. Radiother Oncol. 2014;110:546‐552. doi: 10.1016/j.radonc.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 26. Yi SW, Kang DR, Kim KS, et al. Efficacy of concurrent chemoradiotherapy with 5‐fluorouracil or gemcitabine in locally advanced biliary tract cancer. Cancer Chemother Pharmacol. 2014;73:191‐198. doi: 10.1007/s00280-013-2340-5 [DOI] [PubMed] [Google Scholar]
- 27. Laughlin BS, Petersen MM, Yu NY, et al. Clinical outcomes for hilar and extrahepatic cholangiocarcinoma with adjuvant, definitive, or liver transplant‐based neoadjuvant chemoradiotherapy strategies: a single‐center experience. J Gastrointest Oncol. 2022;13:288‐297. doi: 10.21037/jgo-21-615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Koh M, Park JH, Yoo C, et al. Radiation therapy for recurrent extrahepatic bile duct cancer. PLoS One. 2021;16:1‐12. doi: 10.1371/journal.pone.0253285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sebastian NT, Tan Y, Miller ED, Williams TM, Alexandra DD. Stereotactic body radiation therapy is associated with improved overall survival compared to chemoradiation or radioembolization in the treatment of unresectable intrahepatic cholangiocarcinoma. Clin Transl Radiat Oncol. 2019;19:66‐71. doi: 10.1016/j.ctro.2019.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Verma V, Surkar SM, Brooks ED, Simone CB, Lin C. Chemoradiotherapy versus chemotherapy alone for unresected nonmetastatic gallbladder cancer: national practice patterns and outcomes. JNCCN J Natl Compr Cancer Netw. 2018;16:59‐65. doi: 10.6004/jnccn.2017.7067 [DOI] [PubMed] [Google Scholar]
- 31. Verma V, Appiah AK, Lautenschlaeger T, Adeberg S, Simone CB, Lin C. Chemoradiotherapy versus chemotherapy alone for unresected intrahepatic cholangiocarcinoma: practice patterns and outcomes from the national cancer data base. J Gastrointest Oncol. 2018;9:527‐535. doi: 10.21037/jgo.2018.01.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jackson MW, Amini A, Jones BL, Rusthoven CG, Schefter TE, Goodman KA. Treatment selection and survival outcomes with and without radiation for unresectable, localized intrahepatic cholangiocarcinoma. Cancer J. 2016;22:237‐242. doi: 10.1097/PPO.0000000000000213 [DOI] [PubMed] [Google Scholar]
- 33. Chang WW, Hsiao PK, Qin L, Chang CL, Chow JM, Wu SY. Treatment outcomes for unresectable intrahepatic cholangiocarcinoma: Nationwide, population‐based, cohort study based on propensity score matching with the Mahalanobis metric. Radiother Oncol. 2018;129:284‐292. doi: 10.1016/j.radonc.2018.09.010 [DOI] [PubMed] [Google Scholar]
- 34. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:45‐247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 35. Almhanna K. Immune checkpoint inhibitors in combination with chemotherapy for patients with biliary tract cancer: what did we learn from TOPAZ‐1 and KEYNOTE‐966. Transl Cancer Res. 2024;13(1):22‐24. doi: 10.21037/tcr-23-1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oh DY, Ruth He A, Qin S, et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1(8):EVIDoa2200015. doi: 10.1056/EVIDoa2200015 [DOI] [PubMed] [Google Scholar]
- 37. Kelley RK, Ueno M, Yoo C, et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE‐966): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2023;401(10391):1853‐1865. doi: 10.1016/S0140-6736(23)00727-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
All data supporting the reported results are included in this paper.


