Abstract
BACKGROUND
The development of economically viable and environmentally neutral tools to control insects that consume or damage over 20% of global agriculture or vector human and animal disease represents one of the most important challenges of the 21st century. The suite of chemical‐based strategies currently employed to control insect populations rely primarily on insecticides, which are subject to rapid resistance and often have harmful off‐target environmental and health‐related impacts, and, to a lesser degree, repellents, which typically rely on masking attractive odors. The discovery and characterization of Vanderbilt University allosteric agonists (VUAAs), a family of small‐molecule agonists that target the highly conserved, insect‐specific odorant receptor coreceptor (Orco), raise the potential for the development of a novel repellent paradigm for vector/pest management. VUAAs have the potential to target nearly all insect olfactory sensory neurons, leading to highly aversive behavioral responses, but importantly have limited volatility, thereby reducing their utility as spatial repellents.
RESULTS
We have characterized VUAA thermolysis components and identified a suite of volatiles (VUAA‐based active ingredients, VUAIs) that act specifically in novel binary combinations as robust and long‐lasting spatial repellents against Anopheline mosquitoes. In mobility‐based behavioral experiments, VUAIs act synergistically as effective spatial repellents and outperform parent VUAA compounds against host‐seeking Anopheline mosquitoes.
CONCLUSIONS
VUAIs are volatile alternatives to Vanderbilt University allosteric agonists (VUAAs) that have the potential for use as spatial repellents in disease vector and agricultural pest control. The repellency observed is odorant receptor coreceptor (Orco)‐dependent, supporting the hypothesis that VUAIs and VUAAs similarly target an allosteric Orco recognition site. © 2024 The Author(s). Pest Management Science published by John Wiley & Sons Ltd on behalf of Society of Chemical Industry.
Keywords: Anopheles coluzzii, olfaction, Orco, VUAA1, spatial repellents, thermolysis components
Vanderbilt University allosteric agonists hyper‐stimulate odorant receptor co‐receptors and raise the potential for the development of novel spatial repellents for vector/pest management. Thermolysis‐derived volatile VUAA‐based active ingredients yield high repellency in mobility‐based behavioral assays.
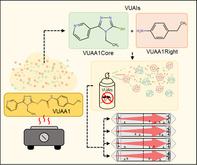
1. INTRODUCTION
Medically and economically important insects are responsible for catastrophic public health impacts through disease transmission and damage/destruction of agricultural crops. 1 , 2 These effects are dependent on food source/host preference and other behaviors that are largely driven by olfaction and other chemosensory modalities dedicated to detecting and discriminating a wide range of biological and environmental cues. 3
Female Anopheline mosquitoes transmit lymphatic filariasis as well as several alphaviruses and, importantly, are the sole vector for human malaria 1 transmission, which occurs during blood feeding, which is required for oocyte maturation. 4 , 5 In this context, olfactory signaling plays a large role in modulating the host‐preference and ‐seeking behaviors of blood‐feeding female mosquitoes. 6 , 7 As a result of their reproductive requirement for blood meals and their history of proximity to humans, it is not surprising that several species of mosquitoes, such as Anopheles coluzzii, have evolved a distinctive anthropophilic host preference to specifically target humans for blood feeding which, in turn, has given rise to their distinctive vectorial capacity. 8 , 9 , 10 , 11 While a variety of new prophylactic and therapeutic approaches are being developed to combat mosquito‐borne illnesses, preventing transmission by disrupting shared‐space interactions that most notably include mosquito biting remains a priority.
Many chemical‐based insecticides, the use of which remains the most broadly employed method to combat disease vectors and agricultural pests, have been developed to control insect populations. 12 However, significant concerns have been raised regarding collateral environmental and health effects as well as the rapid rise in insecticide resistance. 13 , 14 , 15 , 16 While technically defined as pesticides by the US Environmental Protection Agency (EPA), chemical repellents, which notably include N,N‐diethyl‐m‐toluamide (DEET), 17 IR 3535, and butan‐2‐yl 2‐(2‐hydroxyethyl)piperidine‐1‐carboxylate (icaridin, or picaridin), along with several synthetic pyrethroids, 18 represent an alternative to traditional insecticides for the control of insects of economic or medical importance. 19 , 20 However, in addition to similar environmental/health concerns associated with insecticides, the efficacy of repellent‐based, insect‐control strategies are also challenged by parallel and often redundant chemosensory organs/systems. These processes employ independent molecular pathways that collectively make it largely ineffective to selectively target a single modality with masking agents or chemical antagonists. More importantly, while DEET and pyrethroid‐based repellents are relatively effective (able to sufficiently prevent insect–human interactions in field or laboratory applications) against most, but crucially not all, biting insects, 21 , 22 , 23 mutation‐specific cross‐resistance modulates repellency in Anopheline mosquitoes. 13 Moreover, while the synthetic pyrethroid pesticides Transfluthrin and Metofluthrin have received EPA registration as indoor and outdoor spatial repellents (EPA Reg. Nos 71910‐11, 90098‐1‐5668847, 101563‐193, 91879‐1, and 10308‐30), DEET‐, IR 3535‐, and Icaridin‐based strategies have been formally classified as skin‐applied repellents by the EPA and are thus expected to be limited by their short‐range spatial efficacy. 19
A new class of insect repellents based on the novel activity of Vanderbilt University allosteric agonists (VUAAs) address several of the concerns associated with the limited repertoire of current insect‐control chemical agents. VUAAs specifically target the highly conserved, insect‐specific, and ubiquitously expressed odorant receptor coreceptor (Orco) subunit that comprises the majority of the insect odorant receptor (OR) ion channel tetrameric complex. 24 , 25 , 26 , 27 VUAAs target Orco in nearly all insects thereby providing an opportunity to develop next‐generation actives that can act across nearly all insect taxa without impacting humans and other unintended non‐insect animals, all of which lack Orco targets. 24 VUAA1 (2‐((4‐ethyl‐5‐(pyridin‐3‐yl)‐4H‐1,2,4‐triazol‐3‐yl)thio)‐N‐(4‐ethylphenyl) acetamide; Fig. 1(A)) was the first identified allosteric Orco agonist. 24 , 25 VUAA2, VUAA3, and VUAA4 agonists as well as several VUAA‐class Orco antagonists were subsequently synthesized as part of a broad examination of the structure–activity relationships (SAR) around VUAA1. 28 While the precise VUAA binding site on Orco has not been identified, recent insight into this association has been revealed. 29 Regardless of their Orco binding and/or recognition characteristics, VUAA agonists are hypothesized to act as insect repellents by eliciting aversive odorant receptor neuron (ORN) hyperstimulation, leading to altered behavior. However, the utility of the current suite of VUAAs as spatial repellents has been compromised as they are relatively large molecules that do not readily volatilize without undergoing heat treatment to >200 °C. 30 , 31 , 32
Figure 1.
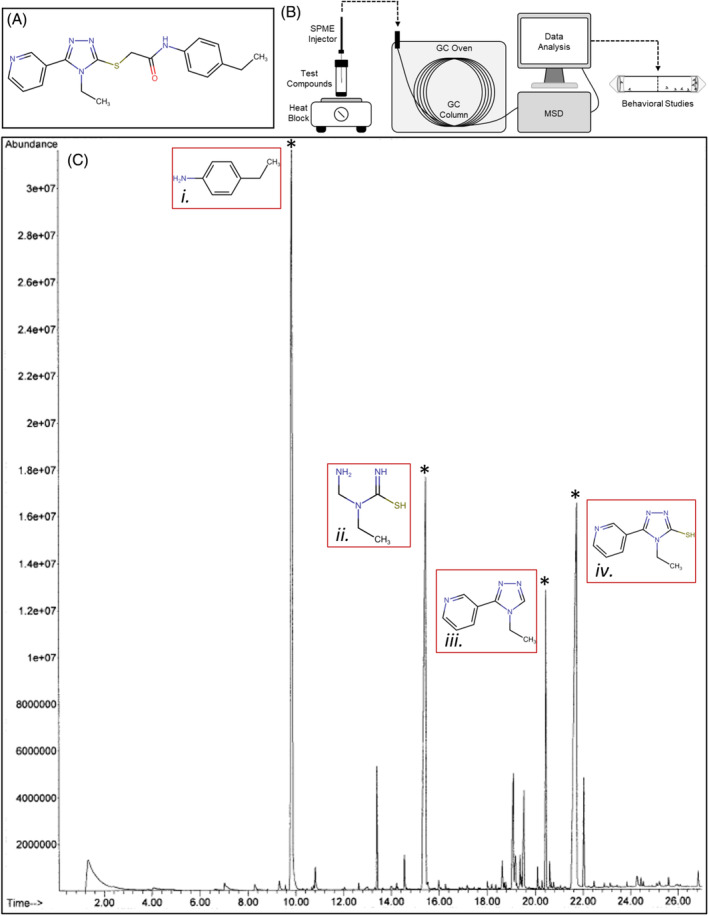
Thermolysis characterization of VUAA1. (A) Chemical structure of VUAA1. (B) Schematic diagram of headspace volatile identification and validation through GC‐MS and behavioral studies. (C) Gas chromatogram of VUAA1 thermolysis headspace volatiles. Peaks of interest (indicated by asterisks) were assigned proposed decomposition products: (i) an alkylated aniline product at 10 min, (ii) a thiourea product at 15 min (unconfirmed), (iii) a pyridinyl‐triazole product at 20 min (unconfirmed), and (iv) a pyridinyl‐triazole‐thiol product at 22 min.
In this study, we investigated the volatiles generated by heat treatment of VUAAs and their behavioral effect on An. coluzzii. To do thsi, we employed gas chromatography–mass spectrometry (GC–MS) of the headspace volatiles released from heating VUAAs to characterize the volatile thermolysis‐generated small molecule derivatives of VUAAs (Fig. 1(B)), which we term VUAA‐based active ingredients (VUAIs). We then used static‐air, glass‐tube repellency bioassays 33 to examine the impact of volatile VUAIs on adult female Anopheline mosquitoes. We demonstrate that binary VUAI formulations act as spatial repellents to directly modulate the behavior of adult female An. coluzzii mosquitoes.
2. MATERIALS AND METHODS
2.1. Mosquitoes
2.1.1. Rearing
All experiments were carried out using An. coluzzii mosquitoes, previously known as An. gambiae sensu stricto ‘M‐form’. 34 Larval and pupal rearing conditions were maintained in a dedicated insectary under a 12 h/12 h light/dark cycle at 27 °C, 75% relative humidity (RH) in walk‐in growth chambers (Percival Inc., Perry, Iowa, USA), with a diet of 2:1 commercial fish food (Kaytee Koi's Choice Premium, Kaytee Products Inc., Chilton, WI, USA) and active dry yeast. Adult mosquitoes were similarly maintained under a 12 h/12 h light/dark cycle at 27 °C, 75% RH and supplied with 10% sucrose solution. All physiological and behavioral assays were performed using 5‐ to 8‐day‐old mated and non‐blood‐fed adult female mosquitoes.
2.1.2. Strains
The behavioral experiments in this study were performed using wild‐type An. coluzzii (Nguosso, MRA‐1279 BEI/MR4) mosquitoes. Parallel control bioassays were conducted using an Orco −/− An. coluzzii (SUA, 2La/2La) Orco‐3xP3‐DsRed loss‐of‐function null mutant line generated via CRISPR/CAS9 gene editing. 35
2.2. Chemicals
All VUAA/VUAI volatiles used in these behavioral experiments were commercially sourced. VUAA1 (Fig. 1(A)) and VUAA4 (2‐[(4‐cyclopropyl‐5‐(4‐pyridinyl)‐4H‐1,2,4‐triazol‐3‐yl)thio]‐N‐[4‐(1‐methylethyl)phenyl] acetamide) (Fig. 2(A)) 28 , 36 were used for thermolysis decomposition experiments. VUAA4 (>99%) was also sourced from Aurorium (Indianapolis, IN, USA) for use in behavioral experiments. 4‐ethyl‐5‐(pyridine‐3‐yl)‐4H‐1,2,4‐triazole‐3‐thiol (VUAA1C) was synthesized on‐site for GC‐MS (confirmed using liquid chromatography MS and NMR) and synthesized by Enamine Ltd (Ukraine) (95%) for behavioral experiments. 4‐Ethylaniline (VUAA1R, 98%) and DEET (97%) were sourced from Sigma‐Aldrich (St. Louis, MO, USA). All test compound stock solutions for behavioral experiments were initially solubilized into dimethyl sulfoxide (DMSO) (molecular biology grade; Sigma‐Aldrich, St. Louis, MO, USA) and diluted into working solutions with final solvent ratios of 1:1 DMSO:acetone. All behavioral experiments were carried out with a dilution series across four concentrations: 1 mM, 25 mM, 50 mM, and 100 mM. Additional DEET experiments were carried out with concentrations of 200 mM, 500 mM, and 1500 mM. All VUAIs were assayed as unitary compounds and in binary combinations. Solvent controls were composed of 1:1 DMSO:acetone solution.
Figure 2.
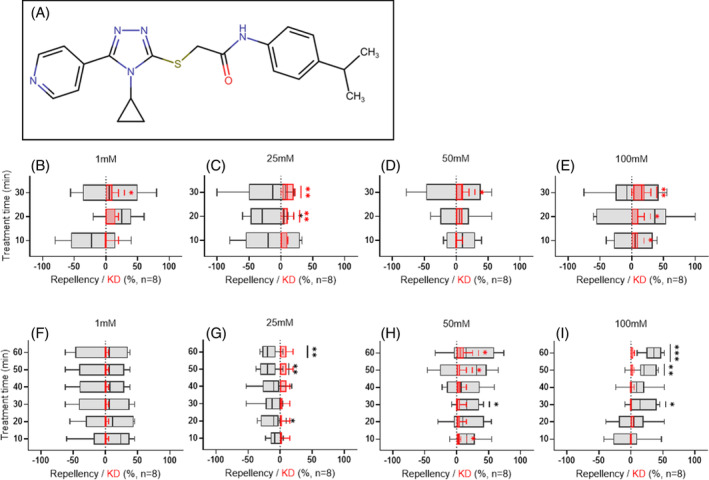
(A) Chemical structure of VUAA4. (B–E) VUAA4 elicits modest, statistically significant knockdown in small‐tube spatial preference assays. (F–I) In large‐tube spatial preference assays VUAA4 elicits modest and statistically significant attraction at 25 mM and robust repellency at 100 mM. Behavioral data (repellency) are displayed in black while knockdown (KD) data are displayed in red. Significance determined using one‐sample t‐tests comparing mean repellency percentage with a hypothetical mean (0), with P ≥ 0.05 indicating no significance; P < 0.05 (*), P < 0.01 (**), P < 0.001 (***), and P < 0.0001 (****) indicate significant differences.
2.3. Solid‐phase microextraction GC–MS
Before extraction, 2.5‐mg samples of solid VUAA compounds were placed in clear 15‐mL vials with a screw‐top, hole cap and PTFE/silicone septa (Supelco, Bellefonte, PA, USA), then placed on a heat block set to 200 °C for 5 min. VUAA headspace volatiles were sampled using 50/30 μm DVB/CAR/PDMS‐coated solid‐phase microextraction (SPME) fibers for manual injection (Supelco, Bellefonte, PA, USA) that had been conditioned in the GC injector port for 1 h before use. The conditioned SPME fiber was inserted through the septa and exposed to the headspace above the heated compounds for 5–10 s then immediately injected into an Agilent 5973A GC‐mass selective detector (MSD) with a DB‐5 capillary column (30 m long × 0.25 mm internal diameter × 0.25 μm film thickness; Agilent, Santa Clara CA, USA) for thermal desorption at 230 °C in splitless mode. The GC was operated as follows: temperature program (50 °C), 1 min; ramp of 10 °C min−1 to 280 °C; finally held at 280 °C for 2 min. Purified helium (7.65 psi), at a flow rate of 1 mL min−1, was used as the carrier gas. The single quadrupole MSD was operated in electron ionization mode at 70 eV, on full scan mode, with an acquisition range of m/z 50–250 and acquisition frequency of 2.38/s.
2.3.1. GC–MS data analysis
VUAA headspace volatiles were identified by assigning proposed decomposition products to the retention times and mass profiles of major GC peaks. The proposed fragments were examined using the same SPME‐GC‐MS paradigms as pure compounds. Proposed VUAA cores were solid at room temperature therefore were heated on a heat block at 200 °C for 5 min before headspace sampling for 5–10 s with the SPME fibers. Proposed VUAA right compounds were liquid at room temperature and thus were not heated before the headspaces were sampled for 1–2 s with the SPME fibers. The masses and retention times of the proposed fragments were compared with those of the VUAA headspace volatiles to confirm their identities.
2.4. Behavioral assays
2.4.1. Glass tube valence assay
Small (12.5 cm length, 2.5 cm diameter) and large (60 cm length, 9 cm diameter) glass‐tube spatial preference assays were performed 33 , 37 using adult female An. coluzzii mosquitoes (Fig. S1), approximately 10–15 individuals per small tube replicate and 20–25 individuals per large tube. Mosquitoes were transferred by aspirator into prepared tubes enclosed on both ends with nylon mesh netting fastened using vinyl ring‐caps, which were placed on a marked track designating the left, right, and middle (only for large tubes) zones of the tubes. Mosquitoes were maintained at 27 °C, 75% RH rearing conditions in a walk‐in growth chamber (Percival Inc.) during the setup and for the entire duration of the bioassays. Treatments and control samples were prepared independently in a double‐blind format to prevent bias during spatial preference measurements; active investigators were never aware of which compounds were being assayed or of the position of treatment and control solutions. Aliquots of 10.0 μL of test compound solutions or DMSO:acetone solvent control were spotted onto ~1‐cm 2 pieces of 3MM blotting paper, and solvents were allowed to evaporate for 10 min at room temperature before the odorant/control‐treated filter papers were placed inside clear caps and fixed to the ends of each tube and sealed with parafilm. Mosquitoes were given a 15‐min acclimation period within the tubes before the start of the experiments during sample preparation. Each treatment concentration was assayed in at least eight technical replicates. To eliminate adaptation/habituation effects, all mosquitoes were frozen and thereafter discarded after each assay.
2.4.2. Data collection
The spatial positions of the mosquitoes were recorded manually at 10‐min time intervals for a duration of 60 min. Knockdown (KD) corresponding to immobilized mosquitoes that fell to the bottom of the assay tube was also recorded. The repellency index (RI) in small‐tube assays was calculated as RI = [(C − T)/(C + T)] × 100, where C is the number of mosquitoes in the control area and T is the number of mosquitoes in the treatment area. A modified RI formula was used to calculate repellency in large‐tube assays: RI = [(C − T)/(C + M + T)] × 100, where M is the number of mosquitoes in the middle zone. The RI was classified as values >0, repellency; 0, neutral; and < 0, attraction (i.e., a value of 100 indicates full repellency and a value of −100 indicates full attraction). The KD index (KI) was calculated as KI = [KD/(C + T)] × 100 or KI = [KD/(C + M + T)] in small‐ and large‐tube assays, respectively.
2.4.3. Statistical analysis
All statistical analyses for repellency compared mean RI against a hypothetical mean of 0. Mean valence and KD values were plotted on box‐and‐whisker box plots using GraphPad Prism 10 to display the full data spread. Here the boxes extend from the 25th to the 75th percentiles, marking the median as a line inside the boxes, and the whiskers extend from the minimum to the maximum values encompassing the knockdown. Significant behavioral effects were determined using one sample t‐tests comparing the control and treatment datasets.
3. RESULTS
3.1. Characterization of volatile VUAA decomposition products
GC–MS chromatographs of VUAA1 thermolysis headspace volatiles displayed several prominent peaks of interest which eluted at 10, 15, 20, and 22 min (Fig. 1(C)). Proposed decomposition products were assigned to each peak based on their mass profiles (Fig. S2) as well as data and insights obtained during their chemical synthesis. 36 The peaks were initially hypothesized to be a C‐alkylated aniline product (10 min), a thiourea product (15 min), a pyridinyl‐triazole product (20 min), and a pyridinyl‐triazole‐thiol product (22 min). We focused on the aniline and pyridinyl‐triazole‐thiol products, which comprise the majority of the VUAA1 molecule, and examined them further by the comparing GC–MS profiles of pure compounds against the GC–MS profiles of the VUAA1 thermolysis headspace volatiles (Fig. S3). These analyses confirmed that the peaks at 10 and 22 min are indeed 4‐alkyl anilines and 4‐ethyl‐5‐(pyridine‐3‐yl)‐4H‐1,2,4‐triazole‐3‐thiol.
3.2. Repellency and knockdown in glass tube spatial preference assays
Initially, we employed a well‐established, high‐throughput, small (12.5 cm) glass‐tube‐based repellency bioassay 33 to rapidly assess the behavioral effects of VUAAs and VUAI volatiles on host‐seeking adult female An. coluzzii mosquitoes. In these studies, solvent‐alone negative control experiments with 1:1 DMSO:acetone on both sides of the tubes gave rise to mean RIs with substantially even distributions of mosquitoes in the glass tubes signifying neither attraction nor repellent effects (Fig. S4A and Table S1). Importantly, these negative control experiments had near zero mean knockdown percentages, suggesting that the solvent and/or handling conditions alone do not induce significant knockdown, independent of treatment compounds. To extend our analysis of VUAI‐induced spatial repellency we also carried out similar glass‐tube‐based bioassays in arenas that were over 60× larger by volume than the initial small‐tube studies. Once again, solvent‐alone negative control experiments failed to display any significant behavioral effect nor did they elicit mosquito knockdown (Figure S4C and Table S3).
3.2.1. VUAA4
Similar to solvent controls, unitary formulations of intact VUAA4, which was generated via a broad structure–activity relationship study of VUAA1 and has a 10‐fold enhancement in agonist potency compared to VUAA1, 28 did not elicit strong, statistically significant repellency at any concentration in either small‐tube (Fig. 2(B)–(E) and Table S1) or large‐tube (Fig. 2(F)–(I) and Table S3) bioassays. Interestingly, despite the lack of significant behavioral impact, VUAA4 demonstrated concentration‐dependent rates of knockdown in small tubes (Table S1).
3.2.2. VUAI volatiles
In small‐tube bioassays, both VUAA1C (Fig. 3(A)–(D)) and VUAA1R (Fig. 3(E)–(H)) inconsistently elicited modest but statistically significant repellency in small tubes between 1 and 100 mM. In contrast, treatments with binary (1:1) formulations of VUAA1R:VUAA1C elicited strong, sustained, concentration‐dependent and statistically significant repellency against female, host‐seeking An. coluzzii beginning at 25 mM (Fig. 3(I)–(N)). Using binary solutions of VUAA1R:VUAA1C at 50 and 100 mM concentrations, mean RIs were substantially greater than and distinct from those of negative control experiments (Fig. S5 and Table S1). Notably, a higher concentration of these binary formulations (100 mM) demonstrated diminished repellency, likely as a result of toxicity‐based knockdown associated with volatile saturation of the closed tubes (Table S1). Formulations of VUAA1C, VUAA1R, and 1:1 VUAA1R:VUAA1C all demonstrated concentration‐ and temporally dependent knockdown (Figs 3(N) and S6). Importantly, Orco−/− null mutant mosquitoes, which lack the hypothesized VUAI Orco target on their ORNs, are behaviorally indifferent to unitary solutions of VUAA1R and binary formulations of VUAA1R and VUAA1C, while displaying similar levels of knockdown as their wild‐type counterparts (Figs 3(O)–(R) and S7, Table S2).
Figure 3.
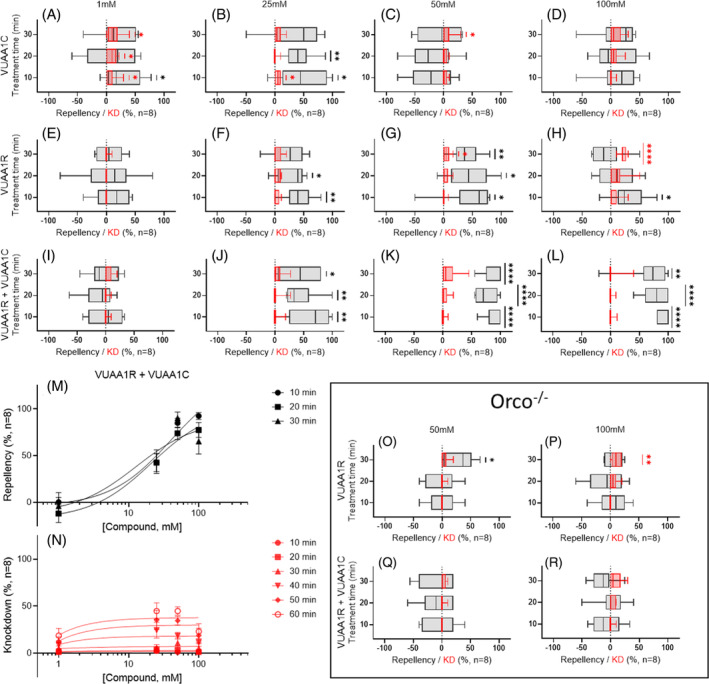
Small‐tube repellency and knockdown assays of VUAIs. Unitary solutions of VUAA1C (A–D) and VUAA1R (E–G) do not elicit strong repellency at any concentration. (I–L) Binary combinations of VUAA1R:VUAA1C elicit robust, significant, and concentration‐dependent repellency in An. coluzzii female mosquitoes. (M, N) Concentration–response curves for spatial repellency and knockdown of VUAA1R + VUAA1C on An. Coluzzii. Symbols are the mean ± standard error of the mean. (O–R) Behavioral responses to VUAA1R and VUAA1R:VUAA1C are absent in Orco−/− loss‐of‐function mutant mosquitoes. Behavioral data (repellency) are displayed in black while knockdown (KD) data are displayed in red. Significance determined by one‐sample t‐tests comparing mean repellency percentage with a hypothetical mean (0), with P ≥ 0.05 indicating no significance; P < 0.05 (*), P < 0.01 (**), P < 0.001 (***), and P < 0.0001 (****) indicate significant differences.
In large tube bioassays, VUAA1C (Figs 4(A)–(D) and S8, Table S3) elicits modest, but statistically significant attractive responses at 25 and 50 mM concentrations. In contrast, VUAA1R alone elicited robust repellency at concentrations of 50 and 100 mM (Figs 4(E)–(H) and S8, Table S3), while treatment with VUAA1R:VUAA1C in 1:1 binary formulations induced potent repellency at 25 mM and near‐complete repellency at 50 and 100 mM, which was not distinct from VUAA1R alone (Figs 4(I)–(N) and S8, Table S3). Unitary formulations of VUAA1C, VUAA1R, as well as a 1:1 binary mixture of VUAA1R:VUAA1C all demonstrated similarly low levels of knockdown in these large‐tube experiments (Fig. 4(A)–(L) and Table S3). In parallel control assays against these formulations, Orco−/− null mutants demonstrated similar but less pronounced behavioral effects than wild‐type mosquitoes, suggesting off‐target (i.e., non‐ Orco) induced repellency (Figs 4(O)–(R) and S9, Table S4).
Figure 4.
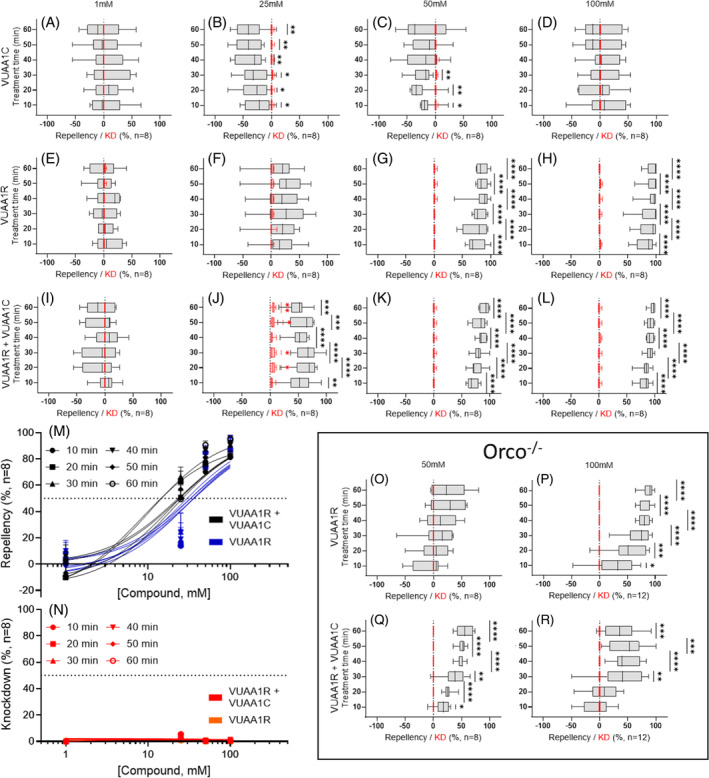
Large tube repellency and knockdown assays of VUAIs. (A–D) Unitary solutions of VUAA1C do not elicit repellency at any concentration, but (E–H) unitary solutions of VUAA1R do elicit strong repellency at concentrations of at least 50 mM in An. coluzzii female mosquitoes. Interestingly, very low concentrations of VUAA1R (1 mM) elicit moderate levels of attraction. (I–L) Binary combinations of VUAA1R:VUAA1C elicit strong, concentration‐dependent repellency. (M, N) Concentration–response curves for unitary VUAA1R and binary VUAA1R:VUAA1C on An. coluzzii, symbols are the mean ± standard error of the mean. (O–R) Behavioral responses to VUAA1R and VUAA1R:VUAA1C in Orco−/− loss‐of‐function mutant mosquitoes. This suggests that off‐target, non‐Orco‐centric (likely from aniline toxicity) contribute to repellency as well in large tube assays. Behavioral data (repellency) are displayed in black while knockdown (KD) data are displayed in red. Significance determined by one‐sample t‐tests comparing mean repellency percentage to a hypothetical mean (0) with P ≥ 0.05 indicating no significance; P < 0.05 (*), P < 0.01 (**), P < 0.001 (***), and P < 0.0001 (****) indicate significant differences.
3.2.3. DEET
In small tubes, solutions of DEET induced modest, concentration‐dependent repellency in wild‐type An. coluzzii mosquitoes at concentrations as low as 25 mM that increased with higher DEET concentrations and plateaued in efficacy from 100 to 1500 mM, with a mean RI of approximately 50% (Figs 5(A),(B) and S10A). This modest efficacy contrasts with the relative indifference displayed by An. coluzzii mosquitoes to 100% (~5.2 M) DEET concentrations in close‐proximity response assays; 38 and likely reflects salient differences in methodology. Furthermore, the considerable reduction in DEET's efficacy relative to previous small tube studies in Aedes aegypti 30 is consistent with the species‐specific sensitivity between anophelines and aedines that has been reported in field 39 and laboratory 40 studies. Looking beyond repellency, these DEET formulations elicited concentration‐ and temporally dependent knockdown exceeding KD effects of VUAA4 and VUAI (Figs 5(C)–(E) and S10A). Notably, Orco−/− null mutants demonstrated increased repellency and decreased knockdown in response to DEET (Fig. S10B). In large tubes, wild‐type mosquitoes displayed relatively modest DEET repellency at concentrations up to 1500 mM (Figa 6(A)–C and S10A). As was observed in the small‐tube paradigm, Orco−/− null mutants demonstrated greater repellency in response to DEET than their wild‐type counterparts (Fig. S11B). Neither wild‐type nor Orco−/− mosquitoes displayed significant DEET‐induced knockdown in large tubes (Figs 6(D)–(F) and S11A).
Figure 5.
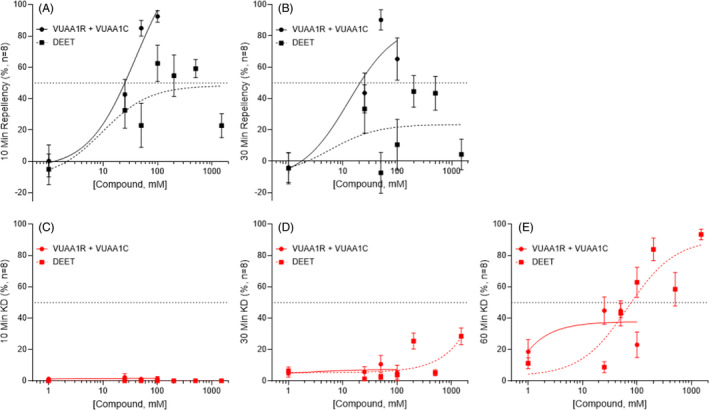
Comparison of DEET and binary VUAI mixtures in small tube repellency and knockdown bioassays. Concentration–response curves show modest concentration‐dependent repellency in response to DEET in wild‐type An. coluzzii female mosquitoes compared with DEET at 10 min (A) and 30 min (B). Wild‐type An. coluzzii females demonstrate similar measures of knockdown in response to DEET and VUAIs at 10 min (C), 30 min (D), and 60 min (E) at lower concentrations, but commercially relevant concentrations induced high KD percentages at 60 min. Symbols are the mean ± standard error of the mean (absent error bars are within the size of the symbol). The dashed horizontal line indicates the 50% repellency/KD level.
Figure 6.
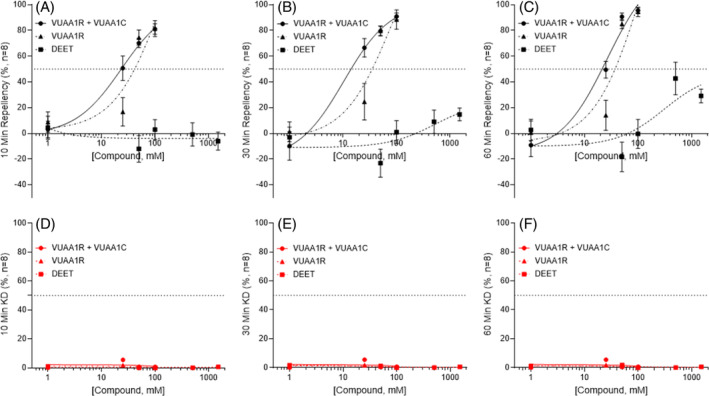
Comparison of repellency and knockdown of DEET and binary VUAI mixtures in large tubes. Concentration–response curves show no repellency in response to DEET in wild‐type An. coluzzii female mosquitoes at 10 min (A) and 30 min (B), and very mild concentration‐dependent repellency at 60 min (C) compared with VUAA1R:VUAA1C. Wild‐type An. coluzzii females do not demonstrate knockdown in response to DEET or VUAA1R:VUAA1C at 10 min (D), 30 min (E), and 60 min (F). Symbols are the mean ± standard error of the mean (absent error bars are within the size of the symbol). The dashed horizontal line indicates the 50% repellency/KD level.
4. DISCUSSION
The interactions of mosquitoes and indeed all insects with their environment and other biologicals are critically dependent on their ability to detect and respond to a wide range of chemical signals. Chemosensory responses are initiated in a variety of peripheral neurons by the activation of at least three large families of membrane‐bound receptors that notably include Orco‐dependent ORs that are broadly agonized by VUAA‐class actives. 24 , 25 , 28 Although intact VUAA molecules exhibit potent effects when directly exposed to Orco/Or complexes in cell‐based or larval assays, 24 , 25 , 28 which suggests they are amenable for the development of a wide range of formulations for contact repellency against insects of economic and medical importance, they lack adequate volatility to act as robust spatial repellents. 16
In this study, we used high‐throughput, laboratory‐based small‐ and large‐tube behavioral bioassays to illustrate the effectiveness of binary VUAI thermolysis mixtures to act as spatial repellents against host‐seeking Anopheline mosquitoes. In small‐tube assays, while unitary exposure to high concentrations of VUAA1R to An. coluzzii mosquitoes displayed some repellency, it also elicited significant knockdown toxicity. In contrast, mosquitoes exposed to 1:1 binary VUAA1R:VUAA1C mixtures responded with significant aversive behavior at 25 mM and near 100% repellency at 50 and 100 mM, with little to no knockdown toxicity (Fig. 3). Moreover, as expected, VUAI repellency was largely absent in small‐tube assays with Orco−/− loss‐of‐function mutant mosquitoes, in keeping with the hypothesis that VUAIs specifically target the Orco co‐receptor. The inconsistent VUAI behavioral effects in small‐tube assays observed after 30 min, especially at higher concentrations (Table S1), were interpreted to be the result of test compounds diffusing to saturation throughout the 12.5‐cm tubes, thereby preventing accurate measurements of any repellent effects. Under these conditions, KD measurements were collected for the full 60‐min duration of the experiments.
In large‐tube assays, both VUAA1R and the binary VUAA1R:VUAA1C mixture were able to induce significant behavioral repellency in wild‐type female mosquitoes (Fig. 5). This raised the possibility that VUAA1R alone might be sufficient to repel mosquitoes without the addition of VUAA1C. However, in large‐tube control assays with Orco−/− null mutant mosquitoes, repellency to VUAIs—and importantly to VUAA1R alone—is still present, albeit with lesser effectiveness than in wild‐type counterparts. These findings suggest that VUAA1R elicits off‐target, non‐Orco‐based repellency. We hypothesize this may, in part, be a response to behaviorally‐aversive silencing by VUAA1R (and not VUAA1C) of peripheral GR‐expressing, CO2‐sensitive maxillary peg A (cpA) neurons we have observed in An. coluzzii 41 (Fig. S12). This has been previously reported for ammonia and structurally similar aniline analogs, such as phenylethylamine. 42 Alternatively, this might be a simple aversive response to aniline toxicity.
The characterization of volatile and behaviorally disruptive VUAI thermolysis constituents that display increased efficacy as binary mixtures (Fig. 7) is a unique structure–activity relationship for an allosteric modulator pharmacophore. While future studies are required to fully characterize this relationship, it is reminiscent of in vitro fragment‐based drug discovery (FBDD) efforts to identify tandem sub‐site ligands, i.e., compounds that simultaneously occupy adjacent pockets within a receptor's recognition domain. 43 FBDD was designed to identify high‐complementarity small molecule ligands by linking low‐affinity lead fragments to form complex, target‐specific compounds and has proven effective for drug development. In contrast to non‐volatile parent VUAAs that have limited ability to act as spatial repellents, we have identified a pair of unlinked volatile compounds that act more effectively together to repel host‐seeking mosquitoes. Importantly, while the indifference of Orco−/− control mosquitoes is consistent with the hypothesis that VUAIs also target Orco, binding studies are required to determine whether VUAIs act by maintaining the binding orientation and specificity of VUAAs or target distinct allosteric sites. 44 , 45
Figure 7.
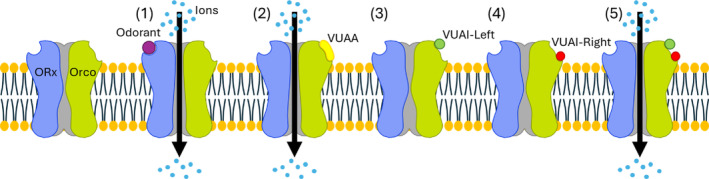
Hypothetical model for (1) odorant‐mediated orthosteric activation of OR/Orco complex and (2) VUAA‐mediated allosteric activation of OR/Orco complex. (3) VUAI‐Left is unable to activate of OR/Orco complex. (4) VUAI‐Right is unable to activate of OR/Orco complex. (5) Binary VUAI‐mediated allosteric activation of OR/Orco complex.
This study also provides evidence that VUAIs are more effective than DEET against Anopheline mosquitoes, which have previously demonstrated a decreased sensitivity compared with that of Aedes and other mosquito clades, 46 even at much higher and commercially relevant concentrations of 1500 mM (~28.6% v/v; Figs 5, 6, S10, and S11). Moreover, wild‐type mosquitoes were more susceptible to knockdown in response to DEET than to VUAA4 and VUAI volatiles. Surprisingly, and in contrast to previously published studies in Ae. aegypti indicating a loss of DEET sensitivity in Orco mutants, 47 An. coluzzii Orco−/− null mutant mosquitoes respond and indeed showed dramatically higher sensitivity to DEET repellency than wild‐type counterparts and substantially greater resistance to knockdown (Figs S10 and S11). While this may once again reflect the differences between mosquito taxa, the respective repellency assays as well as higher DEET concentrations, we hypothesize that these data also result from the multi‐modal mechanisms of action of DEET, which may be differentially impacting GRs and perhaps other targets when Orco/OR complexes are absent. 48 , 49
From a practical perspective, VUAIs offer a powerful new suite of actives that target the insect‐specific and highly conserved Orco co‐receptor, an obligate and universal component of insect OR functionality. 24 , 25 These characteristics raise the potential that VUAIs may have broad applicability against insects of both economic and medical importance. Furthermore, in contrast to the inherent lethality of insecticides that drives the rapid emergence of a variety of resistance mechanisms, the use of high‐efficacy, non‐lethal, VUAI‐based insect repellents may be expected to provide robust and long‐lived insect‐control strategies. Despite the limited volatility of parent VUAAs that constrain initial target product profiles to contact‐based applications, volatile VUAI binaries represent alternatives for the design and development of broadly active, next‐generation insect spatial repellents that have the potential to address a range of economic, ecological, and environmental concerns.
AUTHOR CONTRIBUTIONS
LAM and LJZ designed the study. LAM, AI, EdJ, and IR performed the experiments. LAM analyzed data and prepared the figures. LAM, AI, EdJ, IR, and LJZ interpreted the results and wrote the manuscript.
CONFLICT OF INTEREST
LJZ is an inventor on multiple patents granted and/or pending filed by Vanderbilt University on the chemical novelty and the use of VUAA and VUAI compounds for insect control applications.
Supporting information
Data S1. Supporting Information.
ACKNOWLEDGEMENTS
We thank Zhen Li for care, maintenance, and rearing of mosquitoes, Dr. Samuel Ochieng for preparing volatiles for mass spec analysis, Dr. H. Will Honegger for critical comments and suggestions, Dr. A.M. McAinsh for copy‐editing, and all members of the Zwiebel laboratory for their support and feedback. We are also grateful to the Vanderbilt University Mass Spectrometry Research Center for training and use of their facilities and equipment. This work was conducted with the support of Vanderbilt University via endowment funding to LJZ.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. World Health Organization , World Malaria Report (2022).
- 2. Food and Agriculture Organization of the United Nations , The State of Food Security and Nutrition in the World: Transforming Food Systems for Food Security, Improved Nutrition and Affordable Healthy Diets for All (2021).
- 3. Hansson BS and Stansky MC, Evolution of insect olfaction. Neuron 72:698–711 (2011). [DOI] [PubMed] [Google Scholar]
- 4. Allan SA, Day JF and Edman JD, Visual ecology of biting flies. Annu Rev Entomol 32:297–316 (1987). [DOI] [PubMed] [Google Scholar]
- 5. Garrett‐Jones C, Prognosis for interruption of malaria transmission through assessment of the mosquito's vectorial capacity. Nature 204:1173–1175 (1964). [DOI] [PubMed] [Google Scholar]
- 6. Raji JI and DeGennaro M, Genetic analysis of mosquito detection of humans. Curr Opin insect Sci 20:34–38 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takken W, Chemical signals affecting mosquito behaviour. Invertebr Reprod Dev 36:67–71 (1999). [Google Scholar]
- 8. Besansky NJ, Hill CA and Costantini C, No accounting for taste: host preference in malaria vectors. Trends Parasitol 20:249–251 (2004). [DOI] [PubMed] [Google Scholar]
- 9. McBride CS, Genes and odors underlying the recent evolution of mosquito preference for humans. Curr Biol 26:R41–R46 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bonizzoni M, Waterhouse RM, Ometto L, Marconcini M and Powell JR, An evolutionary perspective on vector‐borne diseases. Front Genet 10:1266 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Angeli Dutra D, Poulin R and Ferreira FC, Evolutionary consequences of vector‐borne transmission: how using vectors shapes host, vector and pathogen evolution. Parasitology 149:1667–1678 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moreno‐Gómez M, Miranda MA and Bueno‐Marí R, To kill or to repel mosquitoes? Exploring two strategies for protecting humans and reducing vector‐borne disease risks by using pyrethroids as spatial repellents. Pathogens 10:MDPI (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deletre E, Martin T, Duménil C and Chandre F, Insecticide resistance modifies mosquito response to DEET and natural repellents. Parasit Vectors 12:89 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Donley N, The USA lags behind other agricultural nations in banning harmful pesticides. Environ Health 18:44 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. World Health Organization , Global Malaria Programme., Global Plan for Insecticide Resistance Management in Malaria Vectors. World Health Organization, Geneva, Switzerland (2012). [Google Scholar]
- 16. Yang L, Norris EJ, Jiang S, Bernier UR, Linthicum KJ and Bloomquist JR, Reduced effectiveness of repellents in a pyrethroid‐resistant strain of Aedes aegypti (Diptera: culicidae) and its correlation with olfactory sensitivity. Pest Manag Sci 76:118–124 (2020). [DOI] [PubMed] [Google Scholar]
- 17. Syed Z and Leal WS, Mosquitoes smell and avoid the insect repellent DEET. Proc Natl Acad Sci USA 105:13598–13603 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang L, Richoux GM, Norris EJ, Cuba I, Jiang S, Coquerel Q et al., Pyrethroid‐derived acids and alcohols: bioactivity and synergistic effects on mosquito repellency and toxicity. J Agric Food Chem 68:3061–3070 (2020). [DOI] [PubMed] [Google Scholar]
- 19. Norris EJ and Coats JR, Current and future repellent technologies: the potential of spatial repellents and their place in mosquito‐borne disease control. Int J Environ Res Public Health 14:124 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Katz TM, Miller JH and Hebert AA, Insect repellents: historical perspectives and new developments. J Am Acad Dermatol 58:865–871 (2008). [DOI] [PubMed] [Google Scholar]
- 21. Canyon DV and Speare R, A comparison of botanical and synthetic substances commonly used to prevent head lice (Pediculus humanus var. capitis) infestation. Int J Dermatol 46:422–426 (2007). [DOI] [PubMed] [Google Scholar]
- 22. Boevé JL, Honraet K and Rossel B, Screening of repellents against vespid wasps. Insects 5:272–286 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Terriquez JA, Klotz SA, Meister EA, Klotz JH and Schmidt JO, Repellency of DEET, Picaridin, and three essential oils to Triatoma rubida (Hemiptera: Reduviidae: Triatominae). J Med Entomol 50:664–667 (2013). [DOI] [PubMed] [Google Scholar]
- 24. Jones PL, Pask GM, Rinker DC and Zwiebel LJ, Functional agonism of insect odorant receptor ion channels. Proc Natl Acad Sci U S A 108:8821–8825 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rinker DC, Jones PL, Pitts RJ, Rutzler M, Camp G, Sun L et al., Novel high‐throughput screens of Anopheles gambiae odorant receptors reveal candidate behaviour‐modifying chemicals for mosquitoes. Physiol Entomol 37:33–41 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhao J, Chen AQ, Ryu J and del Mármol J, Structural basis of odor sensing by insect heteromeric odorant receptors. Science 384:1460–1467 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Qiu L, Wang B, Guan Z, Dong Z, Zhang J et al., Structural basis for odorant recognition of the insect odorant receptor OR‐Orco heterocomplex. Science 384:1453–1460 (2024). [DOI] [PubMed] [Google Scholar]
- 28. Taylor RW, Romaine IM, Liu C, Murthi P, Jones PL, Waterson AG et al., Structure‐activity relationship of a broad‐spectrum insect odorant receptor agonist. ACS Chem Biol 7:1647–1652 (2012). [DOI] [PubMed] [Google Scholar]
- 29. Pacalon J, Audic G, Magnat J, Philip M, Golebiowski J, Moreau CJ et al., Elucidation of the structural basis for ligand binding and translocation in conserved insect odorant receptor co‐receptors. Nat Commun 14:8182 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang L, Liu Y, Richoux GM, Bernier UR, Linthicum KJ and Bloomquist JR, Induction coil heating improves the efficiency of insect olfactory studies. Front Ecol Evol 7:247 (2019). [Google Scholar]
- 31. Ferguson ST, Park KY, Ruff AA, Bakis I and Zwiebel LJ, Odor coding of nestmate recognition in the eusocial ant Camponotus floridanus . J Exp Biol 223:jeb215400 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sharma KR, Enzmann BL, Schmidt Y, Moore D, Jones GR, Parker J et al., Cuticular hydrocarbon pheromones for social behavior and their coding in the ant antenna. Cell Rep 12:1261–1271 (2015). [DOI] [PubMed] [Google Scholar]
- 33. Jiang S, Yang L and Bloomquist JR, High‐throughput screening method for evaluating spatial repellency and vapour toxicity to mosquitoes. Med Vet Entomol 33:388–396 (2019). [DOI] [PubMed] [Google Scholar]
- 34. Coetzee M, Hunt RH, Wilkerson R, Della Torre A, Coulibaly MB and Besansky NJ, Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa 3619:246–274 (2013). [PubMed] [Google Scholar]
- 35. Sun H, Liu F, Ye Z, Baker A and Zwiebel LJ, Mutagenesis of the Orco odorant receptor co‐receptor impairs olfactory function in the malaria vector Anopheles coluzzii HHS public access. Insect Biochem Mol Biol 127:103497 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Romaine IM, Taylor RW, Saidu SP, Kim K, Sulikowski GA, Zwiebel LJ et al., Narrow SAR in odorant sensing Orco receptor agonists. Bioorg Med Chem Lett 24:2613–2616 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Paluch G, Grodnitzky J, Bartholomay L and Coats J, Quantitative structure‐activity relationship of botanical sesquiterpenes: spatial and contact repellency to the yellow fever mosquito, Aedes aegypti . J Agric Food Chem 57:7618–7625 (2009). [DOI] [PubMed] [Google Scholar]
- 38. Afify A, Betz JF, Riabinina O, Lahondère C and Potter CJ, Commonly used insect repellents hide human odors from Anopheles mosquitoes. Current Biol 29:3669–3680.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Costantini C, Badolo A and Ilboudo‐Sanogo E, Field evaluation of the efficacy and persistence of insect repellents DEET, IR3535, and KBR 3023 against Anopheles gambiae complex and other Afrotropical vector mosquitoes. Trans R Soc Trop Med Hyg 98:644–652 (2004). [DOI] [PubMed] [Google Scholar]
- 40. Afify A and Potter CJ, Insect repellents mediate species‐specific olfactory behaviours in mosquitoes. Malar J 19:1–10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lu T, Qiu YT, Wang G, Kwon JY, Rutzler M, Kwon HW et al., Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae . Curr Biol 17:1533–1544 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Clark JT, Ganguly A, Ejercito J, Luy M, Dahanukar A and Ray A, Chemosensory detection of aversive concentrations of ammonia and basic volatile amines in insects. iScience 26:105777 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou AL, Jensen DR, Peterson FC, Thomas MA, Schlimgen RR, Dwinell MB et al., Fragment‐based drug discovery of small molecule ligands for the human chemokine CCL28. SLAS Discov 28:163–169 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kozakova D, Hall DR, Jehle S, Luo L, Ochiana SO, Jones EV et al., Ligand deconstruction: why some fragment binding positions are conserved and others are not. Proc Natl Acad Sci U S A 112:E2585–E2594 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hajduk PJ, Puzzling through fragment‐based drug design. Nat Chem Biol 2:658–659 (2006). [DOI] [PubMed] [Google Scholar]
- 46. Lupi E, Hatz C and Schlagenhauf P, The efficacy of repellents against Aedes, Anopheles, Culex and Ixodes spp. A literature review. Travel Med Infect Dis 11:374–411 (2013). [DOI] [PubMed] [Google Scholar]
- 47. Degennaro M, McBride CS, Seeholzer L, Nakagawa T, Dennis EJ, Goldman C et al., Orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 498:487 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guo H, Kunwar K and Smith D, Multiple channels of DEET repellency in Drosophila . Pest Manag Sci 76:880–887 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee Y, Kim SH and Montell C, Avoiding DEET through insect gustatory receptors. Neuron 67:555–561 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


