Abstract
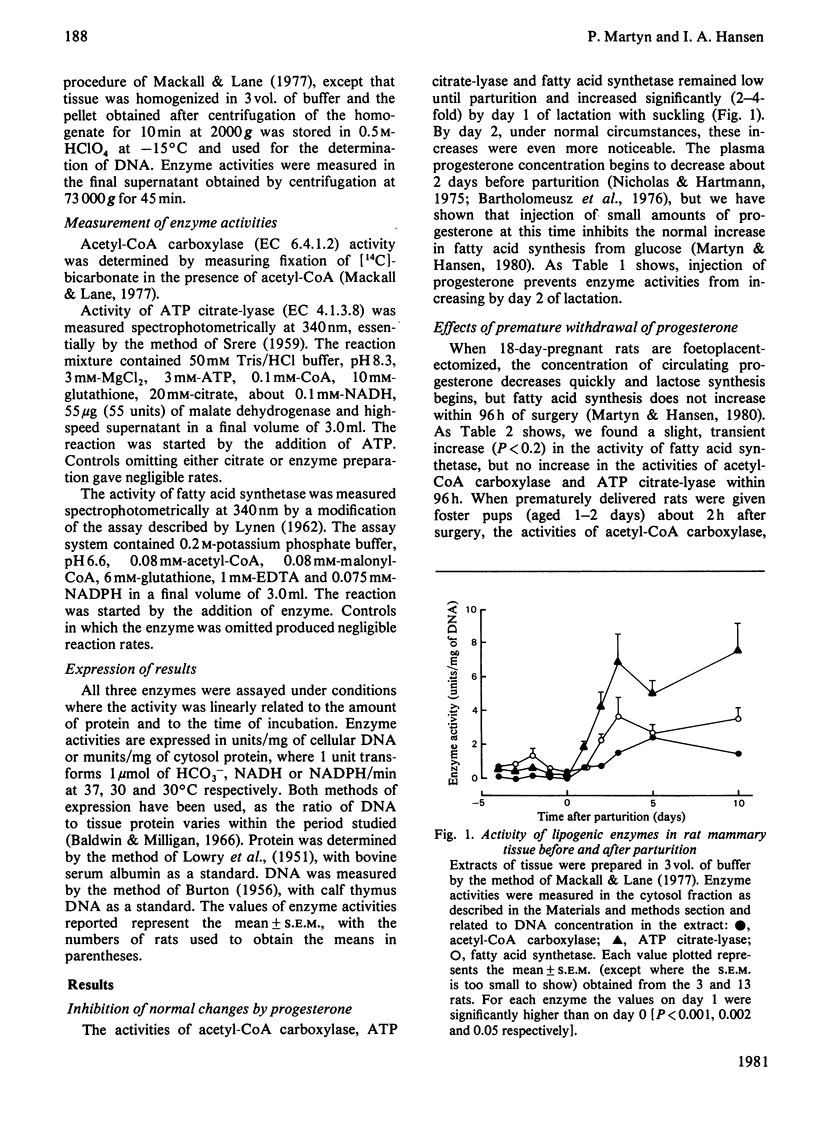
The activities of acetyl-CoA carboxylase, ATP citrate-lyase and fatty acid synthetase remained low until parturition at 22 days of gestation and increased significantly within 1 day post partum. Administration of progesterone on days 20 and 21 and at parturition abolished the increases for at least 48 h after parturition. Removal of the pups of normal rats prevented the increases in activities of acetyl-CoA carboxylase and ATP citrate-lyase, but not of fatty acid synthetase, and administration of prolactin corticosterone or insulin did not stimulate activity. Tissue from suckled glands in which the ducts had been ligated at parturition showed no increase in the activities of acetyl-CoA carboxylase and ATP citrate-lyase within 24 h, whereas fatty acid synthetase activity was similar to that in the sham-operated contralateral glands. Foetoplacentectomy on day 18 increased the activity of fatty acid synthetase but not of acetyl-CoA carboxylase and ATP citrate-lyase; suckling of these dams by foster pups increased both acetyl-CoA carboxylase and ATP citrate-lyase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM S., CADY P., CHAIKOFF I. L. Effect of insulin in vitro in pathways of glucose utilization, other than Embden-Meyerhof, in rat mammary gland. J Biol Chem. 1957 Feb;224(2):955–962. [PubMed] [Google Scholar]
- Agius L., Robinson A. M., Girard J. R., Williamson D. H. Alterations in the rate of lipogenesis in vivo in maternal liver and adipose tissue on premature weaning of lactating rats: a possible regulatory role of prolactin. Biochem J. 1979 Jun 15;180(3):689–692. doi: 10.1042/bj1800689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALMAIN J. H., FOLLEY S. J., GLASCOCK R. F. Relative utilization of glucose and acetate carbon for lipogenesis by mammary gland slices, studies with tritium, 13C and 14C. Biochem J. 1954 Feb;56(2):234–239. [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin R. L., Milligan L. P. Enzymatic changes associated with the initiation and maintenance of lactation in the rat. J Biol Chem. 1966 May 10;241(9):2058–2066. [PubMed] [Google Scholar]
- Bartholomeusz R. K., Bruce N. W., Martin C. E., Hartmann P. E. Serial measurement of arterial plasma progesterone levels throughout gestation and parturition in individual rats. Acta Endocrinol (Copenh) 1976 Jun;82(2):436–443. doi: 10.1530/acta.0.0820436. [DOI] [PubMed] [Google Scholar]
- GREENBAUM A. L., SLATER T. F. Studies on the particulate components of rat mammary gland. II. Changes in the levels of the nucleic acids of the mammary glands of rats during pregnancy, lactation and mammary involution. Biochem J. 1957 May;66(1):155–161. doi: 10.1042/bj0660155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumaa K. A., Greenbaum A. L., McLean P. Adaptive changes in satellite systems related to lipogenesis in rat and sheep mammary gland and in adipose tissue. Eur J Biochem. 1973 Apr 2;34(1):188–198. doi: 10.1111/j.1432-1033.1973.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Hamosh M., Clary T. R., Chernick S. S., Scow R. O. Lipoprotein lipase activity of adipose and mammary tissue and plasma triglyceride in pregnant and lactating rats. Biochim Biophys Acta. 1970 Sep 8;210(3):473–482. doi: 10.1016/0005-2760(70)90044-5. [DOI] [PubMed] [Google Scholar]
- Howanitz P. J., Levy H. R. Acetyl-CoA carboxylase and citrate cleavage enzyme in the rat mammary gland. Biochim Biophys Acta. 1965 Oct 4;106(2):430–433. doi: 10.1016/0005-2760(65)90056-1. [DOI] [PubMed] [Google Scholar]
- Jones E. A. Changes in the enzyme pattern of the mammary gland of the lactating rat after hypophysectomy and weaning. Biochem J. 1967 May;103(2):420–427. doi: 10.1042/bj1030420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsrud G. O., Baldwin R. L. Effects of endocrinectomy and hormone replacement therapies upon enzyme activities in lactating rat mammary glands. Biol Reprod. 1969 Apr;1(1):21–30. doi: 10.1095/biolreprod1.1.21. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J., Lowenstein J. M. Lactogenesis in the rat. Changes in metabolic parameters at parturition. Biochem J. 1967 Dec;105(3):995–1002. doi: 10.1042/bj1050995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J. Progesterone withdrawal as the lactogenic trigger in the rat. J Endocrinol. 1969 May;44(1):39–54. doi: 10.1677/joe.0.0440039. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mackall J. C., Lane M. D. Changes in mammary-gland acetyl-coenzyme A carboxylase associated with lactogenic differentiation. Biochem J. 1977 Mar 15;162(3):635–642. doi: 10.1042/bj1620635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martyn P., Hansen I. A. Initiation of fatty acid synthesis in rat mammary glands. Biochem J. 1980 Jul 15;190(1):171–175. doi: 10.1042/bj1900171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardacci N. J., Lee J. W., McGuire W. L. Differential regulation of alpha-lactalbumin and casein messenger RNA's in mammary tissue. Cancer Res. 1978 Sep;38(9):2694–2699. [PubMed] [Google Scholar]
- Nicholas K. R., Topper Y. J. Enhancement of alpha-lactabumin-like activity in mammary explants from pregnant rats in the absence of exogenous prolactin. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1424–1431. doi: 10.1016/0006-291x(80)90578-1. [DOI] [PubMed] [Google Scholar]
- Otway S., Robinson D. S. The significance of changes in tissue clearing-factor lipase activity in relation to the lipaemia of pregnancy. Biochem J. 1968 Feb;106(3):677–682. doi: 10.1042/bj1060677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plucinski T., Baldwin R. L. Effects of adrenalectomy and glucocorticoid therapy on enzyme activities in mammary and adipose tissues from lactating rats. J Dairy Sci. 1976 Jan;59(1):157–160. doi: 10.3168/jds.S0022-0302(76)84170-7. [DOI] [PubMed] [Google Scholar]
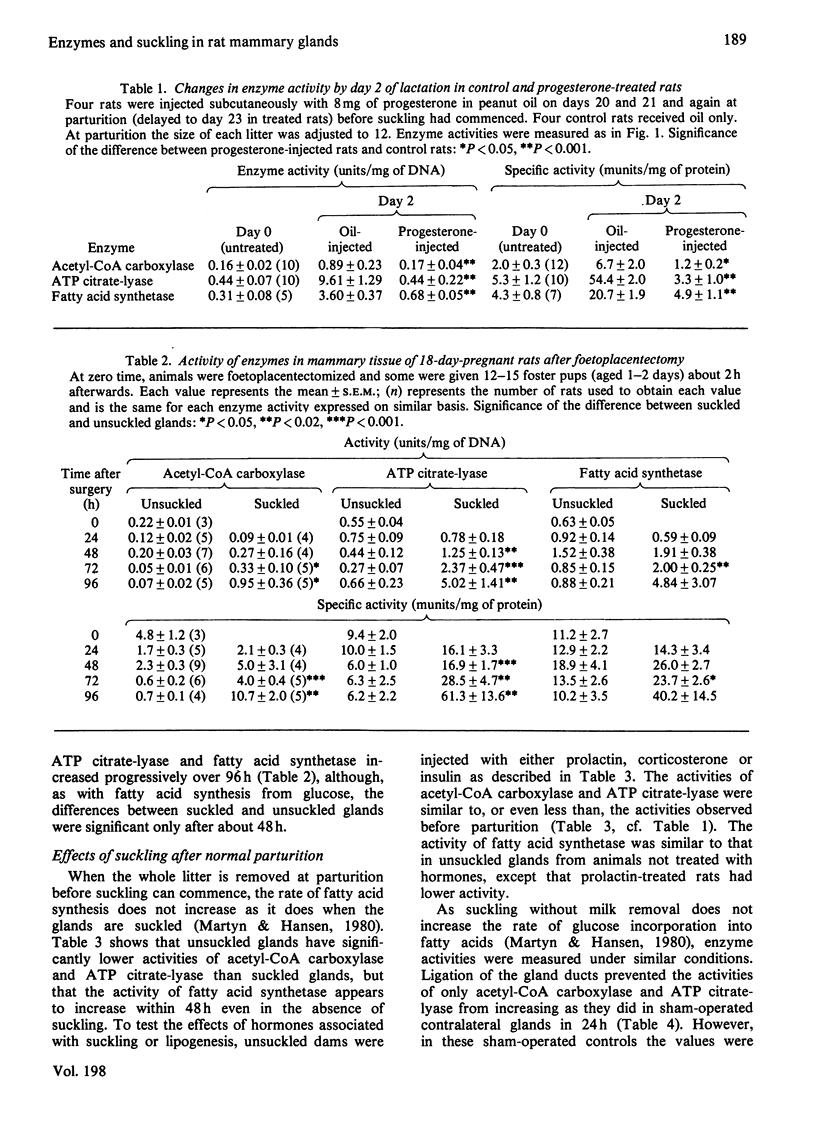
- Robinson A. M., Williamson D. H. Comparison of glucose metabolism in the lactating mammary gland of the rat in vivo and in vitro. Effects of starvation, prolactin or insulin deficiency. Biochem J. 1977 Apr 15;164(1):153–159. doi: 10.1042/bj1640153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRERE P. A. The citrate cleavage enzyme. I. Distribution and purification. J Biol Chem. 1959 Oct;234:2544–2547. [PubMed] [Google Scholar]
- Smith S., Ryan P. Asynchronous appearance of two enzymes concerned with medium chain fatty acid synthesis in developing rat mammary gland. J Biol Chem. 1979 Sep 25;254(18):8932–8936. [PubMed] [Google Scholar]
- Speake B. K., Dils R., Mayer R. J. Regulation of enzyme turnover during tissue differentiation. Interactions of insulin, prolactin and cortisol in controlling the turnover of fatty acid synthetase in rabbit mammary gland in organ culture. Biochem J. 1976 Feb 15;154(2):359–370. doi: 10.1042/bj1540359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian M. G., Reece R. P. Anterior pituitary and plasma prolactin in rats after 2 to 90 minutes of suckling. Proc Soc Exp Biol Med. 1975 Jul;149(3):754–756. doi: 10.3181/00379727-149-38892. [DOI] [PubMed] [Google Scholar]


