ABSTRACT
Background
The literature on fatigue in children and adolescents undergoing cancer treatment is highly variable, creating uncertainties about its prevalence and identifying those at higher risk.
Objectives
The primary purpose was to describe the prevalence of fatigue among patients (< 21 years) undergoing cancer treatment across cancer types. Secondary outcomes included the prevalence of severe fatigue and factors associated with fatigue.
Methods
Systematic searches of MEDLINE, Embase, Cochrane Central Register of Controlled Trials, CINAHL, and PsycINFO were conducted from inception to May 22, 2023. Two reviewers independently identified relevant citations and extracted data. Pooled prevalence estimates were derived using an inverse variance, random‐effects model. We used Joanna Briggs's critical appraisal checklist to assess study quality. (PROSPERO: CRD42020179307).
Results
We included 47 studies: 26 for prevalence and 29 for factors associated with fatigue. The pooled prevalence of fatigue was 73% (95% [Confidence Interval, CI: 66%–79%; I 2 96%; 26 studies; 2699 patients], and severe fatigue was 30% [95% CI 14%–46%, I 2 98%; 8 studies; 1027 patients]). Subgroup analyses based on cancer type, study design, fatigue scale, fatigue reporting personnel, sample frame, and response rate did not reveal significant differences in fatigue prevalence. Fatigue prevalence significantly differed by treatment setting (inpatient [83%] vs. outpatient [55%] vs. inpatient and outpatient [69%]; p: 0.02). Due to considerable heterogeneity among studies, data on fatigue‐associated factors are presented descriptively.
Conclusions
The prevalence of fatigue among children and adolescents undergoing cancer treatment is variable but notably high. Systematic evaluation of factors associated with fatigue is essential to understanding which children are at high risk of developing fatigue.
Trial Registration
PROSPERO: CRD42020179307
Keywords: adolescents, cancer, chemotherapy, children, fatigue, risk factors, treatment
1. Introduction
Fatigue is a debilitating symptom commonly experienced by children and adolescents with cancer throughout their cancer journey [1]. Fatigue can result from a range of factors, including cancer itself, treatment‐related toxicities such as pain, infection, and anorexia, as well as psychological distress, anxiety, depression, and sleep disturbances. Fatigue significantly impacts children's ability to engage in daily activities, such as attending school, participating in sports, and maintaining social relationships. Beyond physical tiredness, it also affects their cognitive and psychological functioning, leading to psychological‐emotional distress, particularly in adolescents with cancer [2, 3]. Fatigue has been reported to be one of the most crucial factors impacting the quality of life of these children and adolescents [4].
The reported prevalence of fatigue among children and adolescents with cancer during treatment is heterogeneous, varying from 14% to 67%, and has been shown to depend on various demographic and treatment‐related factors [5, 6, 7, 8]. While a few systematic reviews have analyzed interventions to mitigate fatigue in adults and pediatric patients with cancer, none have evaluated the prevalence and correlates of fatigue in the pediatric population during treatment [9, 10, 11]. Such a review can help assess the overall prevalence of fatigue in children with various cancer diagnoses during treatment by synthesizing data from multiple studies to provide a more precise estimate of fatigue prevalence. Investigating potential factors associated with fatigue is also essential to identify children and adolescents at risk of experiencing more fatigue during cancer treatment. Overall, this information can provide evidence‐based insights to inform the development of strategies for screening, preventing, and treating fatigue in children and adolescents during treatment.
Therefore, the primary objective of this systematic review and meta‐analysis was to determine the prevalence of fatigue, regardless of its severity and etiology, in children and adolescents receiving cancer treatment. The secondary objectives included assessing the prevalence of severe fatigue and identifying factors associated with the occurrence of fatigue of any severity in this patient population.
2. Methods
We conducted this systematic review following the methodological approaches outlined in the Cochrane Handbook for Systematic Reviews of Interventions and adhered to the Meta‐analysis of Observational Studies in Epidemiology (MOOSE) guidelines for reporting meta‐analyses of observational studies [12, 13]. This review was registered with PROSPERO (CRD42020179307) [14].
2.1. Search Strategy and Study Selection
A systematic search was conducted using Medline (Ovid), Embase (Ovid), Cochrane Central (Wiley), CINAHL (EBSCO), and PsycINFO (Ovid) from inception to May 22, 2023 (Table ). Reference lists of narrative and systematic reviews and the included studies were screened for additional citations. Additionally, we searched the gray literature to capture relevant studies.
2.2. Inclusion and Exclusion Criteria
Studies were included if they met the following criteria: (1) Participants were under 21 years of age with cancer or hematopoietic stem cell transplant (HCT) recipients; (2) the intervention included chemotherapy, radiotherapy, surgery, HCT, targeted therapy, or immunotherapy for cancer treatment; (3) reported prevalence, risk factors, or other variables associated with fatigue irrespective of the cause of fatigue; and (4) the study design was randomized controlled trials (RCTs), cohort studies, controlled before‐and‐after studies, or cross‐sectional studies. RCTs and controlled before‐and‐after studies had to report the prevalence of fatigue at baseline before the initiation of the intervention, provided they did not have the presence or severity of fatigue as an eligibility criterion for enrolling patients. Studies were excluded if they (1) included < 75% of children and adolescents with cancer; (2) exclusively included children and adolescents with relapsed/refractory cancer or those receiving palliative care or had > 25% of children and adolescents with relapsed/refractory cancer or those receiving palliative care; (3) solely included childhood cancer survivors or had > 25% of the population off cancer treatment; (4) did not report any outcomes of interest to this review; (5) reported fatigue only at cancer diagnosis; or (6) were non‐English language studies.
2.3. Outcomes
The primary outcome of interest was the prevalence of overall self‐ or proxy‐report fatigue measured by any fatigue assessment scale, except when fatigue was reported as toxicity using Common Terminology Criteria for Adverse Events (CTCAE) grading. Secondary outcomes included the prevalence of self‐ or proxy‐report study‐defined severe fatigue measured by any fatigue assessment scale, except for CTCAE, and study‐reported patient‐, disease‐, or treatment‐related factors associated with the presence of fatigue.
2.4. Data Extraction
Two reviewers (SO, RJ, BH, GO, or OL) independently evaluated the titles and abstracts identified by the search strategy. Any citation deemed potentially relevant by either reviewer was retrieved in full‐text format and assessed for eligibility. Discrepancies between the two reviewers were resolved through consensus, with adjudication by a third reviewer (GO or SO) if necessary. We extracted the following data: (a) Study‐level variables, including study design and setting (inpatient vs. outpatient), year of enrollment and publication, country of study, inclusion and exclusion criteria, and quality assessment items; (b) Population‐, disease‐, and treatment‐related variables: such as age at diagnosis, sex and ethnicity of participants, cancer type, cancer stage (nonmetastatic vs. metastatic), percentage of participants with relapsed/refractory disease, type of cancer treatment, and phase of cancer treatment; (c) Outcome‐related variables including the type of fatigue assessment scale used, outcome definition (i.e., specific diagnostic criteria or fatigue assessment scale cut‐off), time point of fatigue assessment, the person reporting fatigue, and the number of patients assessed for or presenting with fatigue and severe fatigue and factors associated with fatigue. In case of missing or unclear data, authors of the individual studies were contacted to provide the required information. Studies using the same patients or datasets were grouped as companion studies and treated as a single study in this systematic review. When studies reported fatigue prevalence from both patient and parent perspectives, the patient‐reported data were used in our meta‐analysis. We used the average prevalence for longitudinal studies that provided prevalence estimates at multiple time points during cancer treatment.
2.5. Study Quality Assessment
Two reviewers (SO and RJ, BH, OL, or GO) assessed the quality of observational studies using the Joanna Briggs Institute's (JBI) critical appraisal checklist for studies reporting prevalence data [15]. Nine domains of each study were considered for quality assessment, including sample frame, sampling method, sample size, study setting, sample coverage, validation of fatigue scale, fatigue measurement, statistical analysis, and response rate (Table S2).
2.6. Data Synthesis
We performed descriptive statistics using Microsoft Excel 2019 [Excel v15, Microsoft Corp., Redmond, WA, United States of America (USA)] and pooled aggregate data at the study level. If study‐level proportions and standard errors were not reported, they were calculated before meta‐analyses. We averaged the prevalence estimates for longitudinal studies reporting prevalence estimates at different time points during cancer treatment. The weighted summary proportions of fatigue and corresponding 95% confidence intervals (CI) were calculated by pooling the study‐specific estimates using generic inverse variance random‐effects models. Statistical heterogeneity between studies was assessed using the I 2 statistic, which describes the percentage of total variation across studies due to heterogeneity rather than chance; values around 30%–60% represent moderate heterogeneity, 50%–90% substantial heterogeneity, and 75%–100% considerable heterogeneity [16]. Potential publication bias was evaluated using visual inspection of funnel plot analysis and Egger's regression test for the primary outcome of fatigue prevalence [17, 18]. To explore sources of heterogeneity and to provide estimates of fatigue prevalence among relevant subgroups, stratified/subgroup analyses were planned based on a priori‐defined subgroups based on age, sex/gender, presence of mood or anxiety disorders and sleep disorders, cancer type and stage, treatment type, treatment setting, the person reporting fatigue, type of fatigue scale used, study type, and adequate sample frame, sampling and response rate [14]. These subgroup comparisons provided detailed insights into how fatigue varied within distinct patient populations and treatment contexts. We used the p value for subgroup difference to assess whether the prevalence of fatigue varied across subgroups, with a p value < 0.05 indicating a statistically significant effect. The meta‐analysis was performed using the metafor R package in R software (version 4.3.0) [19].
3. Results
We identified 12,589 citations and screened 9016 after removing duplicates. Following a full‐text review of 236 articles, 47 studies met the eligibility criteria and were included in the review (Figure 1).
FIGURE 1.
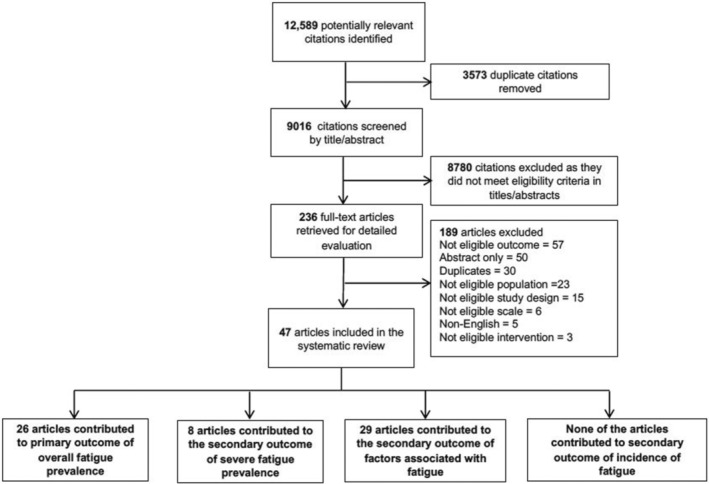
PRISMA flowchart of the selection of the included articles.
3.1. Study Characteristics
Table 1 lists the baseline characteristics of all included studies. Of the 47 studies [5, 6, 8, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64] 26 contributed to the primary outcome of overall fatigue prevalence [5, 6, 8, 20, 21, 22, 23, 25, 26, 27, 30, 33, 34, 35, 36, 37, 40, 42, 44, 45, 49, 50, 53, 54, 56, 62] 29 to factors associated with fatigue [5, 20, 21, 22, 24, 26, 28, 29, 32, 35, 36, 38, 39, 41, 42, 43, 44, 46, 47, 51, 52, 55, 56, 57, 58, 60, 61, 65, 66] and 8 to the prevalence of severe fatigue [8, 20, 40, 49, 53, 54, 62, 67]. Geographically, 47% of studies were conducted in the USA, 13% in the USA and Canada, 6% in China, 4% in Canada, and 4% in Turkey. Prospective cohort studies comprised 60%, while cross‐sectional and phase 3 RCTs comprised 30% and 9%, respectively. Among the 30 studies from the USA and Canada, 25 reported enrolments of non‐white participants with a median representation of 28% (range: 8%–75%). Sex/gender distribution was reported in all studies, with a median of 56% males (range: 41%–80%). Cancer diagnoses varied widely: 64% of studies included patients with different cancer types, 21% focused solely on acute lymphoblastic leukemia (ALL), and 4% included both acute myeloid leukemia (AML) and ALL. Two studies exclusively enrolled patients with Central Nervous System (CNS) tumors. Regarding the study setting, 47% included patients receiving inpatient and outpatient treatments, while 19% and 17% focused exclusively on inpatient or outpatient settings. Modalities of cancer treatment included chemotherapy (51%), chemotherapy/radiation/surgery (13%), chemotherapy/radiation (4%), radiation (2%), and HCT (2%).
TABLE 1.
Summary of studies included in the systematic review (N = 47).
| Study ID (author and year) | Country of study | Study type | Study setting | Inclusion of HSCT patients | Age of participants (years), mean (range) | Ethnicity (non‐White%) | Sex (% male) | Type of cancer | Total no. of participants in study | Person reporting fatigue | Fatigue prevalence reported |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gandy et al. 2022 [20] | USA | Prospective cohort | NR | No | 8.3 (3–16) | 54.5 | 57.6 | CNS tumor | 37 | Parent | Yes |
| Irestorm et al. 2022 [21] | Netherlands | Prospective cohort | NR | NR | 6.3 (NR) | NR | 56.5 | ALL | 127 | Parent | Yes |
| Jacobs et al. 2022 [22] | USA and Canada | Prospective cohort | Both | NR | 13.0 (7–18) | 22.2 | 51.4 | LL | 257 | Patient | Yes |
| Weaver et al. 2022 [23] | USA | Prospective cohort | Both | No | 13.0 (7–17) | 43.5 | 54.7 | Mixed a | 492 | Patient | Yes |
| Wu et al. 2022 [24] | China | Cross‐sectional | Both | NR | 8.9 (NR) | NR | 60.0 | Mixed a | 40 | Patient | No |
| Bradford et al. 2021 [25] | Australia | Cross‐sectional | Outpatient | No | 12 (8–18) | NR | 69.0 | Mixed a | 48 | Patient | Yes |
| Cheng et al. 2021 [26] | China | Cross‐sectional | Both | NR | NR | 100.0 | 66.8 | Mixed a | 187 | Patient | Yes |
| Cheng KK et al. 2021 [27] | Singapore | Prospective cohort | Outpatient | Yes | 13.7 (10–18) | NR | 62.0 | Mixed a | 50 | Patient | Yes |
| Brown et al. 2021 [28] | USA | Cross‐sectional | NR | NR | 8.5 (2.6–17.3) | 75.0 | 56.0 | ALL | 171 | Parent and patient | No |
| Daniel et al. 2020 [29] | USA | Cross‐sectional | Outpatient | NR | 10.1 (5–17) | 24.5 | 44.4 | Mixed a | 59 | Patient | No |
| Li et al. 2020 [30] | China | Cross‐sectional | Both | No | 8.9 (NR) | NR | 61.0 | ALL and AML | 159 | Parent and patient | Yes |
| Rostagno et al. 2020 [31] | Italy | Prospective cohort | Both | NR | 11.7 (5–17) | NR | 54.5 | Mixed a | 134 | Parent and patient | Yes |
| Steur et al. 2020 [32] | Netherlands | Prospective cohort | Outpatient | NR | (1–19) | NR | 57.0 | ALL | 151 | Parent | No |
| Cadamuro et al. 2020 [33] | Brazil | Cross‐sectional | Both | Yes | NR | 52.2 | 49.7 | Mixed a | 157 | Parent and patient | Yes |
| Kudubes et al. 2019 [34] | Turkey | Phase 3 RCT | Inpatient | NR | 9.4/9.1 (control and experimental group, NR) | NR | 57.5 | Mixed a | 80 | Parent and patient | Yes |
| Nagarajan et al. 2019 [35] | USA and Canada | Prospective cohort | Both | No | (2–18) | 17.0 | 52.6 | AML | 560 | Parent and patient | No |
| Rogers et al. 2019 [36] | USA | Phase 3 RCT | Inpatient | Yes | 9.5 (4–19) | 18.2 | 60.6 | Medulloblastoma | 43 | Parent and patient | Yes |
| Tomlinson et al. 2019 [8] | USA and Canada | Cross‐sectional | Both | Yes | NR | NR | 61.3 | Mixed a | 366 b | Patient | No |
| Hockenberry et al. 2018 [65] | USA | Prospective cohort | Both | NR | NR | 59.1 | 55.9 | ALL | 191 | Parent and patient | No |
| Macpherson et al. 2018 [37] | USA | Prospective cohort | Both | No | (8–18) | 56.3 | 54.1 | Mixed a | 96 | Patient | Yes |
| Dobrozsi et al. 2017 [38] | USA | Prospective cohort | Both | NR | 11.7 (5–21) | 28.0 | 58.0 | Mixed a | 41 | Patient | No |
| Rodgers et al. 2016 [39] | USA | Prospective cohort | NR | NR | (3–12) | 47.0 | 45.0 | ALL | 38 | Parent and patient | No |
| Bastani et al. 2015 [40] | Iran | Phase 3 RCT | Inpatient | NR | 10.0 (8–12) | NR | 68.3 | ALL | 120 | Patient | Yes |
| Crabtree et al. 2015 [41] | USA | Prospective cohort | NR | NR | 7.7 (2–18) | 19.0 | 51.0 | Mixed a | 170 | Parent and patient | No |
| MDR Nunes et al. 2015 [42] | USA | Descriptive with repeated measures | Outpatient | No | 12.8 (8–17) | 65.7 | 48.6 | Mixed a | 42 | Patient | Yes |
| Rogers et al. 2014 [43] | USA and Canada | Prospective cohort | Outpatient | NR | 8.8 (5–18) | 18.3 | 65.5 | ALL | 100 | Parent and patient | No |
| Ameringer et al. 2013 [44] | USA | Prospective cohort | Both | NR | 15.3 (13–18) | 66.7 | 55.6 | Mixed a | 9 | Patient | Yes |
| Hinds et al. 2013 [45] | USA | Cross‐sectional | Both | NR | 12.9 (8–17) | NR | 55.5 | Mixed a | 93 | Patient | Yes |
| Wesley et al. 2013 [46] | USA | Cross‐sectional | Both | NR | 15.6 (13–19) | 38.0 | 54.9 | NR | 123 | Patient | No |
| Hooke et al. 2011 [47] | USA | Prospective cohort | NR | NR | (6–17) | NR | 66.7 | Mixed a | 30 | Patient | No |
| Miller et al. 2011 [48] | USA | Prospective cohort | Inpatient | NR | 13.5 (10–17) | 59.0 | 43.6 | Mixed | 39 | Patient | Yes |
| Baggott et al. 2010 [5] | USA | Prospective cohort | Both | NR | 14.8 (10–18) | NR | 51.5 | Mixed a | 66 | Patient | Yes |
| Dupuis et al. 2010 [49] | Canada | Cross‐sectional | Both | NR | 9.4 (4–18) | NR | 55.0 | Mixed a | 200 | Parent | Yes |
| Erickson et al. 2010 [50] | USA | Prospective cohort | NR | NR | 16.1 (12–19) | 15.0 | 50.0 | Mixed | 20 | Patient | Yes |
| Hockenberry et al. 2010 [51] | USA | Prospective cohort | Both | NR | (7–18) | 52.0 | 57.0 | Mixed a | 67 | Parent and patient | No |
| Walker et al. 2010 [6] | USA | Prospective cohort | Both | NR | 14.2 (10–19) | 25.0 | 56.9 | Mixed a | 51 | Patient | Yes |
| Zupanec et al. 2010 [52] | Canada | Cross‐sectional | Outpatient | NR | (4–18) | 48.4 | 79.7 | ALL | 77 | Parent and patient | No |
| Sitaresmi et al. 2009 [53] | Indonesia | Prospective cohort | Both | NR | 7.1 (2–16) | NR | 63.0 | ALL | 51 | Parent | Yes |
| Yeh et al. 2009 [54] | Taiwan | Prospective cohort | Both | NR | 14.2 (10–18.9) | NR | 58.0 | Mixed a | 144 | Patient | Yes |
| Ekti Genc et al. 2008 [55] | Turkey | Phase 3 RCT | Inpatient | NR | 9 (7–12) | NR | 61.7 | ALL and AML | 60 | Parent and patient | No |
| Enskar et al. 2008 [56] | Sweden | Cross‐sectional | Both | No | 9.6 (NR) | NR | 59.0 | Mixed | 17 | Patient | Yes |
| Perdikaris et al. 2008 [57] | Greece | Prospective cohort | NR | NR | 8.9 (7–12) | NR | 65.0 | Mixed a | 40 | NR | No |
| Whitsett et al. 2008 [58] | USA and Canada | Prospective cohort | Inpatient | NR | 13.7 (9–17) | 8.3 | 66.7 | Mixed | 12 | Parent and patient | No |
| Yeh et al. 2008 [59] | Taiwan | Prospective cohort | Inpatient | NR | 11.5 (7–17) | NR | 54 | Mixed a | 48 | Parent and patient | No |
| Hinds et al. 2007 [60] | USA | Prospective cohort | Inpatient | NR | 12.5 (7–18) | 28.0 | 41.0 | Mixed a | 29 | Parent, patient and healthcare provider | No |
| Hinds et al. 2007 [61] (cancer journal paper) | USA and Canada | Prospective cohort | Outpatient | NR | (5–18) | 21.0 | 62.0 | ALL | 100 | Parent and patient | No |
| Williams et al. 2006 [62] | USA | Cross‐sectional | Inpatient | No | 10.4 (2–18) | 9.0 | 45.0 | Mixed a | 11 | Patient | Yes |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CNS, central nervous system; HCT, hematopoietic stem cell transplant; LL, leukemia and lymphoma; NR, not‐reported; USA, United States of America.
Mixed: mixed cancer types defined as a category where more than two types of patients with cancer were included.
Study enrolled 502 patients, of which, 366 were on treatment.
Regarding fatigue assessment, 49% of studies used patient‐reported measures, 11% utilized parent‐reported measures, and 38% employed both measures. Fatigue assessment across the 47 included studies [5, 6, 8, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64] utilized 13 scales (Table S3). The most commonly used scales were the Fatigue Scale‐Child (FS‐C), Fatigue Scale‐Adolescent (FS‐A), or Fatigue Scale‐Parent (FS‐P) in 35% of studies, followed by the Pediatric Quality of Life‐Multidimensional Fatigue Scale (PedsQL‐MFS) in 17% and the Patient‐Reported Outcomes Measurement Information System (PROMIS) scale in 14%.
3.2. Study Quality Assessment
Among all studies, 38% had an appropriate sampling frame, 17% had adequate sample size and 36% had suitable study settings (Figure S1). A valid fatigue measurement scale was utilized in 90% of studies, and standard and reliable outcome measurement was employed in 98%. However, blinding was inadequate due to non‐blinded outcome measures. Appropriate statistical methods were used for the overall prevalence analysis in all 26 studies reporting prevalence (Figure S2). Among the 29 studies exploring factors associated with fatigue as a secondary outcome, only 41% adjusted for potential confounders. The response or fatigue assessment rate was deemed adequate in 68% (N = 32) of studies, indicating minimal attrition bias.
3.3. Primary Outcome
In total, 26 studies, comprising 2699 patients (aged 2–18 years), reported the prevalence of overall fatigue, with 2522 patients assessed (Table S4). The reported fatigue prevalence varied from 45.8% to 100.0%. The pooled prevalence estimate across these studies was 73% (95% CI, 66%–79%; I 2 = 96%) (Figure 2).
FIGURE 2.
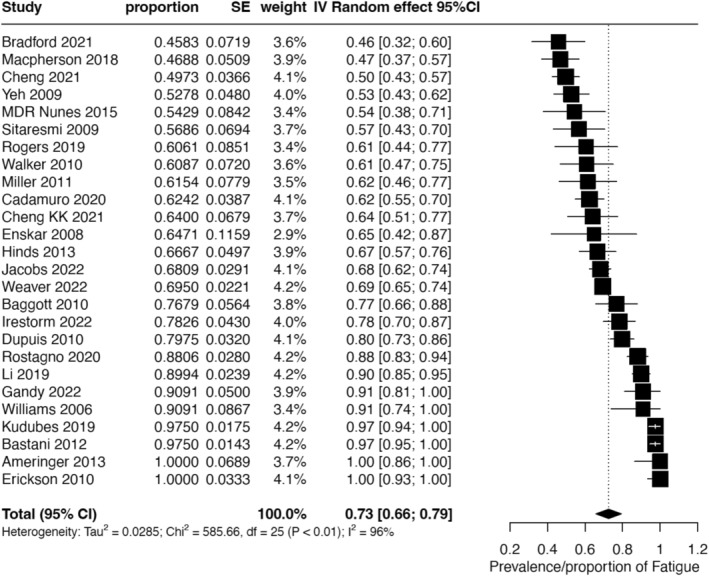
Forest plot of the meta‐analysis of overall fatigue prevalence in included studies (N = 26).
Subgroup analysis of overall fatigue prevalence based on predefined factors is detailed in Table 2. Significant differences in fatigue prevalence were observed only in subgroups categorized by treatment setting (p < 0.05). Subgroup analyses by age, sex, mood, or anxiety disorder, sleep disorder, and cancer stage were not feasible due to insufficient reporting in the included studies.
TABLE 2.
Subgroup analysis of the primary outcome of overall prevalence of fatigue.
| Subgroup | No. of studies | No. of participants enrolled | Prevalence (%) | 95% CI (%) | I 2 (%) | p Value for subgroup difference |
|---|---|---|---|---|---|---|
| Study type | 0.40 | |||||
| Cross‐sectional | 8 | 872 | 69 | 57–81 | 94 | |
| Prospective cohort | 15 | 1584 | 72 | 63–80 | 93 | |
| Phase 3 RCT | 3 | 243 | 87 | 64–100 | 89 | |
| Setting | 0.02 | |||||
| Inpatient | 5 | 293 | 83 | 66–99 | 90 | |
| Outpatient | 3 | 140 | 55 | 44–66 | 41 | |
| Both inpatient and outpatient | 15 | 2077 | 69 | 61–77 | 93 | |
| Type of cancer | 0.58 | |||||
| Mixed cancer types | 19 | 1905 | 70 | 62–79 | 95 | |
| Hematological malignancies | 5 | 714 | 79 | 65–93 | 97 | |
| CNS tumors | 2 | 80 | 77 | 47–100 | 89 | |
| Type of cancer treatment | 0.38 | |||||
| Chemotherapy | 11 | 1096 | 78 | 68–89 | 95 | |
| Chemotherapy/Surgery/Radiation | 4 | 475 | 68 | 47–89 | 98 | |
| Type of fatigue scale | 0.48 | |||||
| Childhood Fatigue Scale | 4 | 185 | 75 | 50–99 | 95 | |
| Memorial Symptom Assessment Scale | 5 | 365 | 72 | 60–84 | 88 | |
| Symptom Screening in Pediatrics Tool | 2 | 205 | 55 | 39–71 | 76 | |
| PROMIS Fatigue Subscale | 4 | 1029 | 64 | 55–73 | 87 | |
| PedsQL Multidimensional Fatigue Scale | 4 | 411 | 69 | 52–87 | 94 | |
| Person reporting fatigue | 0.33 | |||||
| Parent/Caregiver | 4 | 415 | 77 | 64–90 | 81 | |
| Patient | 17 | 1711 | 69 | 60–78 | 96 | |
| Patient and parent/Caregiver | 5 | 573 | 81 | 66–95 | 95 | |
| Adequate sample frame | 0.95 | |||||
| Yes | 11 | 1751 | 71 | 64–79 | 91 | |
| Unclear | 12 | 829 | 73 | 61–86 | 97 | |
| No | 3 | 119 | 73 | 45–100 | 94 | |
| Adequate sampling | 0.78 | |||||
| Yes | 11 | 1033 | 73 | 64–83 | 92 | |
| Unclear | 11 | 1206 | 74 | 63–85 | 97 | |
| No | 4 | 460 | 66 | 45–87 | 97 | |
| Adequate response rate | 0.90 | |||||
| Yes | 17 | 1388 | 74 | 65–83 | 96 | |
| Unclear | 3 | 425 | 71 | 42–100 | 98 | |
| No | 6 | 886 | 71 | 61–80 | 88 |
Abbreviations: CI, confidence interval; CNS, central nervous system; PedsQL, Pediatric Quality of Life; PROMIS, Patient‐Reported Outcomes Measurement Information System; RCT, randomized controlled trial.
3.4. Secondary Outcomes
3.4.1. Severe Fatigue
Severe fatigue prevalence was reported in 8 (17%) studies, encompassing 1027 patients (Figure 3 and Table S3) [20, 31, 40, 49, 53, 54, 62, 63]. These studies utilized six fatigue scales, with PedsQL‐MFS being the most frequently employed (25%, N = 2). Among the eight studies, 5 (62%) included patients with different cancer diagnoses. The pooled prevalence of severe fatigue across these studies was 30% (95% CI 14%–46%, I 2 = 98%).
FIGURE 3.
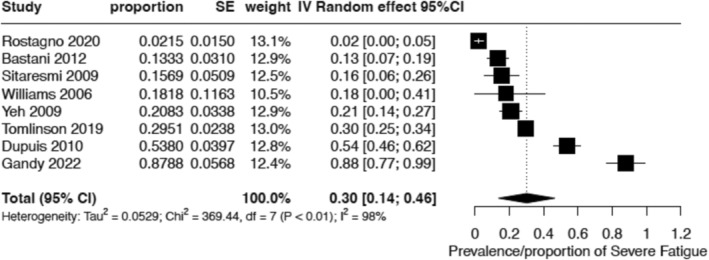
Forest plot of pooled prevalence of severe fatigue (N = 8).
3.4.2. Factors Associated With Fatigue
Twenty‐nine studies reported potential factors associated with fatigue among children and adolescents with cancer during active cancer treatment (Table 3). Meta‐analysis for this outcome was not conducted due to the considerable heterogeneity in the methods used to analyze and report (e.g., correlation, univariable, or multivariable analysis) and the study populations; therefore, data on the factors associated with fatigue are presented descriptively: (a) Demographic factors: Among six studies, three found older age associated with higher fatigue older age and higher fatigue [8, 22, 35, 36], two found no association [28, 60], and one found younger age associated with higher fatigue [38]. Seven studies examined the association of sex/gender with fatigue, with five finding no association and two identifying higher fatigue in females [22, 57]. Four studies explored race/ethnicity, with one finding Hispanic patients reporting higher fatigue, while the other three found no such association [22, 28, 35, 46]; (b) disease‐related factors: One study found that patients with CNS/solid tumors experienced less fatigue than those with leukemia/lymphoma [38], while another found no association with the type of leukemia/lymphoma [22]. Two studies in acute lymphoblastic leukemia (ALL) risk grouping showed mixed results, with one finding no association and the other reporting less fatigue in high‐risk and very high‐risk ALL patients compared to low and average‐risk patients [21, 28]; (c) treatment‐related factors: Among brain tumor patients undergoing radiotherapy, cranial spinal radiation dose was a significant predictor of fatigue in one study [20]. Dexamethasone pulse phases and steroid use during chemotherapy were associated with higher fatigue in ALL patients [32, 59]. In acute myeloid leukemia patients, the number of CTCAE toxicities was significantly associated with general fatigue [35]. Fatigue varied during treatment, decreasing over time in ALL patients and during the initial weeks of leukemia/lymphoma treatment [5, 41, 65]; (d) Sleep‐related factors: Seven studies found that poor sleep measures, such as inconsistent sleep habits, fragmented sleep, high caregiver‐reported sleep problems, frequent night awakenings and high nighttime activity, were associated with higher fatigue [32, 36, 44, 52, 60, 68, 69]; (e) Other clinical and psychosocial symptoms: Two studies found a positive association between fatigue and pain [26, 46] and two studies found a positive correlation between depressive symptoms and fatigue [44, 51, 58].
TABLE 3.
Factors associated with fatigue in the individual study included (N = 29).
| Study ID | Type of cancer | Factors analyzed | Type of analysis | Factors Associated/Correlated with greater fatigue | Factors Associated/Correlated with lower fatigue |
|---|---|---|---|---|---|
| Gandy et al. 2022 [20] | CNS tumor | Radiotherapy (proton vs. photon), hydrocephalus (None vs. Any), craniospinal radiation, age at radiation therapy | Multivariable regression analysis | Craniospinal radiation during first week of therapy | None |
| Irestorm et al. 2022 [21] | ALL | Couse of fatigue, sex, risk group at diagnosis, serious adverse event | Multivariable regression analysis | Course of fatigue during treatment predicted fatigue reported at follow‐up | None |
| Jacobs et al. 2022 [22] | Leukemia and lymphoma | Gender, age, ethnicity, time since diagnosis, diagnostic group (ALL/AML/NHL/HL), and caregiver's education level | Mixed effects model | Female, Hispanic patients | Male, older age, non‐Hispanic |
| Wu et al. 2022 [24] | Mixed | Quality‐of‐life distress | Multivariable regression analysis | Quality‐of‐ life distress | None |
| Cheng et al. 2021 [26] | Mixed | Pain interference, depression and lower mobility | Multivariable regression analysis | Greater pain interference, depressive symptoms and less mobility | None |
| Brown et al. 2021 [28] | ALL | Age at diagnosis, BMI, gender, race/ethnicity, type of leukemia, CNS involvement at diagnosis, high‐or very high‐risk ALL, and asparagine and gamma‐glutamyl glutamine in CSF | Multivariable regression analysis | Low‐risk and average‐risk ALL, asparagine and gamma‐glutamyl glutamine in CSF | High‐risk and very‐high risk ALL |
| Daniel et al. 2021 [29] | Mixed | Sleep timing, sleep consistency, technology use, presence of someone else, sleep disturbance, sleep‐related impairment, pain interference, nausea and sleep consistency | Correlation and Simple mediation models | Sleep disturbances, Sleep‐related impairment, pain interference and nausea | Consistent caregiver‐reported sleep routines |
| Steur et al. 2020 [32] | ALL | Dexamethasone pulses during maintenance chemotherapy, sleep–wake rhythm, stable sleep–wake rhythm, robust sleep–wake rhythm, more physical activity during the day and fragmented sleep–wake rhythm | Multivariable regression analysis | Dexamethasone pulses, fragmented sleep–wake rhythm | Robust sleep–wake rhythm, less fragmented sleep and higher physical activity during dexamethasone‐free periods |
| Nagarajan et al. 2019 [35] | AML | Age, sex, White race, Hispanic ethnicity, insurance status, high‐risk AML, bortezomib arm assignment, days of neutropenia, and number of submitted CTCAE toxicities | Multivariable regression analysis | Older age and number of submitted CTCAE toxicities | None |
| Rogers et al. 2019 [36] | Medulloblastoma | Age, percent sleep, longest sleep episode and nighttime activity score on actigraphy. Amplitude, 24 h auto‐correlation, intra‐daily variability, inter‐daily stability, dichotomy index on actigraphy | Linear mixed models and correlation | Higher age, lower percent nighttime sleep and higher nighttime activity scores (adolescent‐reported fatigue), longest nighttime sleep episode (child‐reported fatigue), dysregulated amplitude, 24 h auto‐correlation, and intra‐daily variability | Lower age |
| Hockenberry et al. 2018 [65] | ALL | 3NT (protein 3‐nitrotyrosine) in CSF and time since induction treatment | Latent class growth analysis and mixed models | Higher 3NT in CSF | Time from one treatment phase to another when measured from post‐induction to 12 months post‐induction chemotherapy |
| Dobrozsi et al. 2017 [38] | Mixed | Age, gender, type of cancer, time since diagnosis, and intensity of therapy | Linear mixed models | Leukemia/lymphoma | Older age and diagnosis of solid/CNS tumors |
| Rodgers et al. 2016 [39] | ALL | Reduced glutathione (GSH) and reduced/oxidized glutathione (GSH/GSSG) ratio in the CSF | Correlation | Low mean GSH/GSSG ratios in CSF | None |
| Crabtree et al. 2015 [41] | Mixed | Type of cancer, gender, age, and socioeconomic status, steroid use, radiation and chemotherapy, insomnia, sleep hygiene, bedtime, wake time, total sleep time, or restless sleep within 30 days of diagnosis and 8 weeks later | Univariable analysis and multivariable regression analysis | Longer sleep duration (6–12‐year‐old) | Younger children with leukemia/lymphoma had a significant decline in parent‐reported fatigue within 30 days of diagnosis and 8 weeks later than those with solid tumor/CNS tumors |
| MDR Nunes et al. 2015 [42] | Mixed | Age, gender, cancer type, sleep duration | Univariable analysis and correlation | Adolescents, females, sarcoma, less sleep duration | Younger children, males |
| Rogers et al. 2014 [43] | ALL | Circadian activity rhythm parameters—peak, midline estimating statistic of rhythm (MESOR), amplitude, acrophase and circadian quotient | Linear mixed models | None | Peak activity, MESOR and amplitude |
| Ameringer et al. 2013 [44] | Mixed | Anxiety and sleep disturbances | Correlation | Disturbed sleep | Higher trait anxiety |
| Wesley et al. 2013 [46] | NR | Age, gender, minority status, pain, nausea, positive and negative affect, stressful life events, family support, friend support and family functioning | Correlation | Pain, nausea, and positive affect | Negative affect |
| Hooke et al. 2011 [47] | Mixed | Gender, type of cancer and time since first three cycles of chemotherapy | Univariable analysis | None | For young children, fatigue significantly decreased during the first three cycles of chemotherapy, and the ALL group had a greater decrease in fatigue than the lymphoma or the solid tumor group from cycle 1 to cycle 3 of chemotherapy |
| Baggott et al. 2010 [5] | Mixed | Time since administration of a chemotherapy cycle | Multilevel logistic regression analysis | None | For each week following a cycle of chemotherapy, odds of reporting fatigue were lower than the previous week for 2 weeks after the initiation of chemotherapy cycle |
| Hockenberry et al. 2010 [51] | Mixed | Depression on day 7 of chemotherapy | Correlation | Depressive symptoms | None |
| Zupanec et al. 2010 [52] | ALL | Perceived problems in sleep, different sleep since diagnosis, different sleep place, sleep in 20 min, moving to different bed in the night and duration of weeknight sleep | Correlation | Sleep problems (4–12 years), Different sleep since diagnosis (4–7 years), moving to another bed (4–12 years) | None |
| Ekti Genc et al. 2008 [55] | ALL & AML | Sex, diagnosis, age, hemoglobin, mucositis, nausea and vomiting | Correlation | None | None |
| Enskar et al. 2008 [56] | Mixed | Life satisfaction | Univariable analysis | Less life satisfaction | None |
| Perdikaris et al. 2008 [57] | Mixed | Gender | Multivariable regression analysis | Females | None |
| Whitsett et al. 2008 [58] | Mixed | Depression | Correlation | Depression | None |
| Yeh et al. 2008 [66] | Mixed | Steroid use prior to start of chemotherapy cycle, steroids use per day of cycle, hemoglobin value, prior chemotherapy, cumulative doses of chemotherapy drugs in the cycle | Multivariable analysis | Steroids used before chemotherapy cycle, hemoglobin value, steroid use for each day of chemotherapy cycle and prior chemotherapy | None |
| Hinds et al. 2007 [60] | Mixed | Number of nocturnal awakenings during inpatient hospital stay, age, diagnosis, gender, baseline fatigue, or length of hospitalization, hematocrit and hemoglobin level | Mixed effect model | More night awakenings (20 or more) | None |
| Hinds et al. 2007 [61] | Mixed | Dexamethasone treatment, age, sex and ALL risk group | Correlation | Dexamethasone treatment | None |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CNS; central nervous system; CSF, cerebrospinal fluid; CTCAE, common terminology criteria for adverse events; HL, Hodgkin lymphoma; LSS‐C, Life Situation Scale for Children; NHL, non‐Hodgkin lymphoma.
3.5. Publication Bias
Visual inspection of the funnel plot exploring the publication bias of the studies contributing to the over fatigue prevalence showed that smaller studies reporting higher fatigue prevalence were underrepresented (Figure S3). Egger's regression test, assessing asymmetry in the funnel plot, demonstrated a statistically significant result (t = −3.77 and p—0.0009), indicating the presence of publication bias.
4. Discussion
In this review, we found high heterogeneity in summary effect measures, with the pooled prevalence of overall fatigue at 73% and severe fatigue at 30% in children and adolescents undergoing cancer treatment. These findings are comparable to a recent review demonstrating a prevalence of 62% among adults with cancer during treatment [70]. The high fatigue prevalence in our population also aligns with reports of increased fatigue among children and adolescents with chronic diseases such as cystic fibrosis and autoimmune diseases [71, 72]. Notably, this prevalence is seven times higher than the 10.1% prevalence of general fatigue among adolescents in the general population [73].
Stratified analyses by treatment setting revealed a lower prevalence among patients receiving outpatient treatment than those getting treatment either as an inpatient or both inpatient and outpatient. Typically, inpatient cancer treatments are more intensive than outpatient chemotherapy; plausibly, fatigue is more prevalent during inpatient chemotherapy cycles due to the increased intensity of therapy, increased distress associated with inpatient admissions impacting the psychological aspects of fatigue, fewer interactions with family and less time spent being physically active and more sleep disruptions due to night awakenings, and the noisy environment from the monitors [60, 74, 75, 76]. Additional studies are warranted to ascertain if outpatient delivery of a similar chemotherapy regimen leads to lesser fatigue than inpatient delivery.
Studies reporting on fatigue‐related factors examined various demographic, disease‐related, treatment‐related, and psychological factors but revealed inconsistent findings due to substantial heterogeneity. However, most studies found consistent associations between sleep patterns and fatigue, with stable sleep habits linked to lower fatigue levels. The literature indicates a reciprocal and strong relationship between cancer‐related fatigue and sleep disturbances through shared physiological pathways [77, 78]. Future research should target pediatric‐specific interventions to improve fatigue and sleep quality in children and adolescents with cancer.
Most studies demonstrated a positive association between pain, depression, and fatigue, in addition to sleep. This supports the concept of symptom clustering in oncology, an evolving concept that underscores the importance of clinicians recognizing the co‐occurrence of symptoms and the need to address multiple symptoms within a cluster to achieve better overall symptom control [51, 66].
Despite its heterogeneity, this systematic review's primary strength is in systematically presenting data on the prevalence of fatigue and associated factors among children and adolescents with cancer during treatment based on multiple studies conducted to date. A comprehensive search of several databases from their inception, utilizing broad search terms and robust methodology, was performed to avoid bias. Subgroup analyses were conducted to explore the causes of heterogeneity systematically. Finally, a multidisciplinary team provided expertise in conducting the review and interpreting the findings.
This review has several notable limitations. The included studies exhibited substantial heterogeneity in patient demographics, cancer diagnoses, treatment modalities, fatigue assessment tools, and follow‐up durations. Consequently, despite pooling the prevalence data, our confidence in the estimated pooled prevalence remains low. The secondary objective of identifying factors associated with fatigue also suffered from inconsistent adjustment for potential confounders across studies. Only 3 out of 27 studies scored well on all the domains of study quality assessment for external validity, with 60% failing to specify sampling methods, thus raising concerns about selection bias. The reliance on self‐reported fatigue questionnaires precluded participant blinding, resulting in high detection bias. Additionally, variations in fatigue prevalence estimates may result from inconsistent reporting sources, such as caregiver versus child self‐reports. Caregiver/proxy reports may overestimate child‐reported fatigue, leading to discrepancies in prevalence estimates among studies [79]. Fatigue, a multifaceted construct, was assessed in this review as a general or total measure, limiting our ability to comment on specific dimensions of fatigue. The restriction to English‐language studies may have introduced publication bias, and the exclusion of studies focusing on children and adolescents with relapsed/refractory cancer or those receiving palliative care limits the applicability of the findings to these populations.
5. Conclusions
In conclusion, our systematic review and meta‐analysis reveal that 73% of children and adolescents undergoing cancer treatment experience fatigue, with nearly 30% enduring severe fatigue. Despite the significant heterogeneity among studies, indicating a low certainty in these estimates, the high prevalence and variable nature of fatigue during treatment are evident. The high prevalence and significant impact of fatigue on the quality of life and other critical aspects of life for children and adolescents with cancer underscore the need for routine assessment using valid, reliable, and psychometrically robust fatigue scales in clinical settings [3, 80].
Since effective interventions such as physical activity, mindfulness, and relaxation interventions to mitigate fatigue even in younger children are available, it is paramount that healthcare professionals should not consider fatigue an inevitable toxicity of treatment and utilize effective approaches to address it with their patients [81, 82, 83]. Future research should focus on identifying patterns and predictors of fatigue persistence beyond treatment, employing consistent assessment methods and multivariable analyses while ensuring the inclusion of younger children to address current gaps.
Author Contributions
Sapna Oberoi: conceptualization (lead), data curation (lead), formal analysis (lead), investigation (lead), methodology (lead), project administration (lead), resources (lead), visualization (lead), writing – original draft (lead), writing – review and editing (lead). Beili Huang: conceptualization (supporting), data curation (supporting), formal analysis (supporting), project administration (supporting), writing – review and editing (supporting). Rasheda Rabbani: conceptualization (supporting), formal analysis (equal), investigation (supporting), methodology (equal), software (lead), visualization (equal), writing – review and editing (supporting). Nicole Askin: data curation (supporting), investigation (supporting), methodology (supporting), resources (supporting), writing – review and editing (supporting). George Okoli: data curation (supporting), formal analysis (supporting), investigation (supporting), methodology (supporting), project administration (supporting), resources (supporting), writing – review and editing (supporting). Richa Jain: data curation (supporting), formal analysis (supporting), investigation (supporting), project administration (supporting), writing – review and editing (supporting). Lillian Sung: conceptualization (supporting), investigation (supporting), methodology (supporting), supervision (supporting), writing – review and editing (supporting). Maya M. Jeyaraman: conceptualization (supporting), formal analysis (supporting), investigation (supporting), methodology (supporting), supervision (supporting), writing – review and editing (supporting). Alyson Mahar: conceptualization (supporting), formal analysis (supporting), investigation (supporting), investigation (supporting), methodology (supporting), methodology (supporting), project administration (supporting), project administration (supporting), supervision (equal), supervision (equal), writing – original draft (supporting), writing – original draft (supporting), writing – review and editing (supporting), writing – review and editing (supporting). Roberta Woodgate: conceptualization (supporting), formal analysis (supporting), investigation (supporting), methodology (supporting), project administration (supporting), supervision (equal), writing – original draft (supporting), writing – review and editing (supporting). Ryan Zarychanski: conceptualization (equal), formal analysis (supporting), investigation (supporting), methodology (equal), project administration (equal), supervision (lead), writing – original draft (supporting), writing – review and editing (supporting).
Ethics Statement
This study is exempt from ethics approval as it is a systematic review that utilized data from previously published data from trials.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Data S1.
Acknowledgements
The authors have nothing to report.
Data Availability Statement
The data supporting this study's findings are available from the corresponding author upon reasonable request.
References
- 1. Hockenberry‐Eaton M. and Hinds P. S., “Fatigue in Children and Adolescents With Cancer: Evolution of a Program of Study,” Seminars in Oncology Nursing 16, no. 4 (2000): 261–272. [DOI] [PubMed] [Google Scholar]
- 2. Hinds P. S., Hockenberry‐Eaton M., Gilger E., et al., “Comparing Patient, Parent, and Staff Descriptions of Fatigue in Pediatric Oncology Patients,” Cancer Nursing 22, no. 4 (1999): 277–288. [DOI] [PubMed] [Google Scholar]
- 3. Tomlinson D., Zupanec S., Jones H., O'Sullivan C., Hesser T., and Sung L., “The Lived Experience of Fatigue in Children and Adolescents With Cancer: A Systematic Review,” Supportive Care in Cancer 24, no. 8 (2016): 3623–3631, 10.1007/s00520-016-3253-8. [DOI] [PubMed] [Google Scholar]
- 4. Hicks J., Bartholomew J., Ward‐Smith P., and Hutto C. J., “Quality of Life Among Childhood Leukemia Patients,” Journal of Pediatric Oncology Nursing 20, no. 4 (2003): 192–200, 10.1177/1043454203253969. [DOI] [PubMed] [Google Scholar]
- 5. Baggott C., Dodd M., Kennedy C., et al., “Changes in Children's Reports of Symptom Occurrence and Severity During a Course of Myelosuppressive Chemotherapy,” Journal of Pediatric Oncology Nursing 27, no. 6 (2010): 307–315, 10.1177/1043454210377619. [DOI] [PubMed] [Google Scholar]
- 6. Walker A. J., Gedaly‐Duff V., Miaskowski C., and Nail L., “Differences in Symptom Occurrence, Frequency, Intensity, and Distress in Adolescents Prior to and One Week After the Administration of Chemotherapy,” Journal of Pediatric Oncology Nursing 27, no. 5 (2010): 259–265, 10.1177/1043454210365150. [DOI] [PubMed] [Google Scholar]
- 7. Collins J. J., Byrnes M. E., Dunkel I. J., et al., “The Measurement of Symptoms in Children With Cancer,” Journal of Pain and Symptom Management 19, no. 5 (2000): 363–377, 10.1016/s0885-3924(00)00127-5. [DOI] [PubMed] [Google Scholar]
- 8. Tomlinson D., Baggott C., Dix D., et al., “Severely Bothersome Fatigue in Children and Adolescents With Cancer and Hematopoietic Stem Cell Transplant Recipients,” Supportive Care in Cancer 27, no. 7 (2019): 2665–2671, 10.1007/s00520-018-4555-9. [DOI] [PubMed] [Google Scholar]
- 9. Oberoi S., Robinson P. D., Cataudella D., et al., “Physical Activity Reduces Fatigue in Patients With Cancer and Hematopoietic Stem Cell Transplant Recipients: A Systematic Review and Meta‐Analysis of Randomized Trials,” Critical Reviews in Oncology/Hematology 122 (2018): 52–59, 10.1016/j.critrevonc.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 10. Duong N., Davis H., Robinson P. D., et al., “Mind and Body Practices for Fatigue Reduction in Patients With Cancer and Hematopoietic Stem Cell Transplant Recipients: A Systematic Review and Meta‐Analysis,” Critical Reviews in Oncology/Hematology 120 (2017): 210–216, 10.1016/j.critrevonc.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 11. Patel P., Robinson P. D., van der Torre P., et al., “Guideline for the Management of Fatigue in Children and Adolescents With Cancer or Pediatric Hematopoietic Cell Transplant Recipients: 2023 Update,” EClinicalMedicine 63 (2023): 102147, 10.1016/j.eclinm.2023.102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins J. P. T., Thomas J., Chandler J., et al., eds., Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (London, UK: Cochrane, 2023). [Google Scholar]
- 13. Stroup D. F., Berlin J. A., Morton S. C., et al., “Meta‐Analysis of Observational Studies in Epidemiology: A Proposal for Reporting. Meta‐Analysis of Observational Studies in Epidemiology (MOOSE) Group,” JAMA 283, no. 15 (2000): 2008–2012, 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 14. Oberoi S., Zarychanski R., Woodagte R., et al., “Prevalence and Risk Factors of Fatigue in Children Receiving Cancer Treatment: A Systematic Review and Meta‐Analysis,” (2020), Prospero, https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020179307.
- 15. Munn Z., Moola S., Lisy K., Riitano D., and Tufanaru C., “Methodological Guidance for Systematic Reviews of Observational Epidemiological Studies Reporting Prevalence and Incidence Data,” International Journal of Evidence‐Based Healthcare 13 (2015): 147–153. [DOI] [PubMed] [Google Scholar]
- 16. Higgins J. P. and Thompson S. G., “Quantifying Heterogeneity in a Meta‐Analysis,” Statistics in Medicine 21, no. 11 (2002): 1539–1558, 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 17. Sterne J. A. and Egger M., “Funnel Plots for Detecting Bias in Meta‐Analysis: Guidelines on Choice of Axis,” Journal of Clinical Epidemiology 54, no. 10 (2001): 1046–1055, 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 18. Egger M., Davey Smith G., Schneider M., and Minder C., “Bias in Meta‐Analysis Detected by a Simple, Graphical Test,” BMJ 315, no. 7109 (1997): 629–634, 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Viechtbauer W., “Conducting Meta‐Analyses in R With the Metafor Package,” Journal of Statistical Software 36, no. 3 (2010): 1–48, 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 20. Gandy K., Chambers T., Raghubar K. P., et al., “A Prospective Evaluation of Fatigue in Pediatric Brain Tumor Patients Treated With Radiation Therapy,” Journal of Pediatric Hematology/Oncology Nursing 39, no. 6 (2022): 358–365, 10.1177/275275302110560011068754. [DOI] [PubMed] [Google Scholar]
- 21. Irestorm E., Steur L. M. H., Kaspers G. J. L., et al., “Fatigue Trajectories During Pediatric ALL Therapy Are Associated With Fatigue After Treatment: A National Longitudinal Cohort Study,” Supportive Care in Cancer 31, no. 1 (2022): 1, 10.1007/s00520-022-07456-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jacobs S. S., Withycombe J. S., Castellino S. M., et al., “Longitudinal Use of Patient Reported Outcomes in Pediatric Leukemia and Lymphoma Reveals Clinically Relevant Symptomatic Adverse Events,” Pediatric Blood & Cancer 69, no. 12 (2022): e29986, 10.1002/pbc.29986. [DOI] [PubMed] [Google Scholar]
- 23. Weaver M. S., Wang J., Greenzang K. A., McFatrich M., and Hinds P. S., “The Predictive Trifecta? Fatigue, Pain, and Anxiety Severity Forecast the Suffering Profile of Children With Cancer,” Supportive Care in Cancer 30, no. 3 (2022): 2081–2089, 10.1007/s00520-021-06622-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu W. W., Tang C. C., Jou S. T., and Yu T. H., “Associations Between Fatigue, Sleep Disturbance, Physical Activity, and Quality of Life for Children With Cancer: A Correlational Study,” Cancer Nursing 45, no. 6 (2022): 421–429, 10.1097/NCC.0000000000001001. [DOI] [PubMed] [Google Scholar]
- 25. Bradford N. K., Bowers A., Chan R. J., et al., “Documentation of Symptoms in Children Newly Diagnosed With Cancer Highlights the Need for Routine Assessment Using Self‐Report,” Cancer Nursing 44, no. 6 (2021): 443–452, 10.1097/NCC.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 26. Cheng L., Wang Y., Duan M., Wang J., Huang H., and Yuan C., “Self‐Reported Fatigue in Chinese Children and Adolescents During Cancer Treatment,” Journal of Pediatric Oncology Nursing 38, no. 4 (2021): 262–270, 10.1177/1043454221992304. [DOI] [PubMed] [Google Scholar]
- 27. Cheng K. K. and Tan L. M. L., “A Pilot Study of the Effect of a Home‐Based Multimodal Symptom‐Management Program in Children and Adolescents Undergoing Chemotherapy,” Cancer Reports 4, no. 3 (2021): e1336, 10.1002/cnr2.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brown A. L., Sok P., Taylor O., et al., “Cerebrospinal Fluid Metabolomic Profiles Associated With Fatigue During Treatment for Pediatric Acute Lymphoblastic Leukemia,” Journal of Pain and Symptom Management 61, no. 3 (2021): 464–473, 10.1016/j.jpainsymman.2020.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Daniel L. C., Meltzer L. J., Gross J. Y., Flannery J. L., Forrest C. B., and Barakat L. P., “Sleep Practices in Pediatric Cancer Patients: Indirect Effects on Sleep Disturbances and Symptom Burden,” Psycho‐Oncology 30, no. 6 (2021): 910–918, 10.1002/pon.5669. [DOI] [PubMed] [Google Scholar]
- 30. Li R., Ma J., Chan Y., Yang Q., and Zhang C., “Symptom Clusters and Influencing Factors in Children With Acute Leukemia During Chemotherapy,” Cancer Nursing 43, no. 5 (2020): 411–418, 10.1097/NCC.0000000000000716. [DOI] [PubMed] [Google Scholar]
- 31. Rostagno E., Marchetti A., Bergadano A., et al., “Concordance Between Paediatric Self‐Reports and Parent Proxy Reports on Fatigue: A Multicentre Prospective Longitudinal Study,” European Journal of Oncology Nursing 49 (2020): 101829, 10.1016/j.ejon.2020.101829. [DOI] [PubMed] [Google Scholar]
- 32. Steur L. M. H., Kaspers G. J. L., van Someren E. J. W., et al., “The Impact of Maintenance Therapy on Sleep‐Wake Rhythms and Cancer‐Related Fatigue in Pediatric Acute Lymphoblastic Leukemia,” Supportive Care in Cancer 28, no. 12 (2020): 5983–5993, 10.1007/s00520-020-05444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Andrade C. S., Onishi Franco J., Paiva C. E., Oliveira M. A., and Sakamoto Ribeiro Paiva B., “Association Between Multiple Symptoms and Quality of Life of Paediatric Patients With Cancer in Brazil: A Cross‐Sectional Study,” BMJ Open 10, no. 5 (2020): e035844, 10.1136/bmjopen-2019-035844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kudubes A. A., Bektas M., and Mutafoğlu K., “The Effect of Fatigue‐Related Education on Pediatric Oncology Patients' Fatigue and Quality of Life,” Journal of Cancer Education 34, no. 6 (2019): 1130–1141, 10.1007/s13187-018-1419-4. [DOI] [PubMed] [Google Scholar]
- 35. Nagarajan R., Gerbing R., Alonzo T., et al., “Quality of Life in Pediatric Acute Myeloid Leukemia: Report From the Children's Oncology Group,” Cancer Medicine 8, no. 9 (2019): 4454–4464, 10.1002/cam4.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rogers V. E., Zhu S., Ancoli‐Israel S., Liu L., Mandrell B. N., and Hinds P. S., “A Pilot Randomized Controlled Trial to Improve Sleep and Fatigue in Children With Central Nervous System Tumors Hospitalized for High‐Dose Chemotherapy,” Pediatric Blood & Cancer 66, no. 8 (2019): e27814, 10.1002/pbc.27814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Macpherson C. F., Wang J., DeWalt D. A., Stern E. D., Jacobs S., and Hinds P. S., “Comparison of Legacy Fatigue Measures With the PROMIS Pediatric Fatigue Short Form,” Oncology Nursing Forum 45, no. 1 (2018): 106–114, 10.1188/18.ONF.106-114. [DOI] [PubMed] [Google Scholar]
- 38. Dobrozsi S., Yan K., Hoffmann R., and Panepinto J., “Patient‐Reported Health Status During Pediatric Cancer Treatment,” Pediatric Blood & Cancer 64, no. 4 (2017): 295, 10.1002/pbc.26295. [DOI] [PubMed] [Google Scholar]
- 39. Rodgers C., Sanborn C., Taylor O., et al., “Fatigue and Oxidative Stress in Children Undergoing Leukemia Treatment,” Biological Research for Nursing 18, no. 5 (2016): 515–520, 10.1177/1099800416647794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bastani F., Khosravi M., Borimnejad L., and Arbabi N., “The Effect of Acupressure on Cancer‐Related Fatigue Among School‐Aged Children With Acute Lymphoblastic Leukemia,” Iranian Journal of Nursing and Midwifery Research 20, no. 5 (2015): 545–551, 10.4103/1735-9066.164508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Crabtree V. M., Rach A. M., Schellinger K. B., Russell K. M., Hammarback T., and Mandrell B. N., “Changes in Sleep and Fatigue in Newly Treated Pediatric Oncology Patients,” Supportive Care in Cancer 23, no. 2 (2015): 393–401, 10.1007/s00520-014-2356-3. [DOI] [PubMed] [Google Scholar]
- 42. Darezzo Rodrigues Nunes M., Jacob E., Adlard K., Secola R., and Nascimento L., “Fatigue and Sleep Experiences at Home in Children and Adolescents With Cancer,” Oncology Nursing Forum 42, no. 5 (2015): 498–506, 10.1188/15.ONF.498-506. [DOI] [PubMed] [Google Scholar]
- 43. Rogers V. E., Zhu S., Ancoli‐Israel S., and Hinds P. S., “Impairment in Circadian Activity Rhythms Occurs During Dexamethasone Therapy in Children With Leukemia,” Pediatric Blood & Cancer 61, no. 11 (2014): 1986–1991, 10.1002/pbc.25147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ameringer S., Elswick R. K., Shockey D. P., and Dillon R., “A Pilot Exploration of Symptom Trajectories in Adolescents With Cancer During Chemotherapy,” Cancer Nursing 36, no. 1 (2013): 60–71, 10.1097/NCC.0b013e318250da1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hinds P. S., Nuss S. L., Ruccione K. S., et al., “PROMIS Pediatric Measures in Pediatric Oncology: Valid and Clinically Feasible Indicators of Patient‐Reported Outcomes,” Pediatric Blood & Cancer 60, no. 3 (2013): 402–408, 10.1002/pbc.24233. [DOI] [PubMed] [Google Scholar]
- 46. Wesley K. M., Zelikovsky N., and Schwartz L. A., “Physical Symptoms, Perceived Social Support, and Affect in Adolescents With Cancer,” Journal of Psychosocial Oncology 31, no. 4 (2013): 451–467, 10.1080/07347332.2013.798761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hooke M. C., Garwick A. W., and Gross C. R., “Fatigue and Physical Performance in Children and Adolescents Receiving Chemotherapy,” Oncology Nursing Forum 38, no. 6 (2011): 649–657, 10.1188/11.ONF.649-657. [DOI] [PubMed] [Google Scholar]
- 48. Miller E., Jacob E., and Hockenberry M. J., “Nausea, Pain, Fatigue, and Multiple Symptoms in Hospitalized Children With Cancer,” Oncology Nursing Forum 38, no. 5 (2011): E382–E393, 10.1188/11.ONF.E382-E393. [DOI] [PubMed] [Google Scholar]
- 49. Dupuis L. L., Milne‐Wren C., Cassidy M., et al., “Symptom Assessment in Children Receiving Cancer Therapy: The Parents' Perspective,” Supportive Care in Cancer 18, no. 3 (2010): 281–299, 10.1007/s00520-009-0651-1. [DOI] [PubMed] [Google Scholar]
- 50. Erickson J. M., Beck S. L., Christian B., et al., “Patterns of Fatigue in Adolescents Receiving Chemotherapy,” Oncology Nursing Forum 37, no. 4 (2010): 444–455, 10.1188/10.ONF.444-455. [DOI] [PubMed] [Google Scholar]
- 51. Hockenberry M. J., Hooke M. C., Gregurich M., McCarthy K., Sambuco G., and Krull K., “Symptom Clusters in Children and Adolescents Receiving Cisplatin, Doxorubicin, or Ifosfamide,” Oncology Nursing Forum 37, no. 1 (2010): E16–E27, 10.1188/10.ONF.E16-E27. [DOI] [PubMed] [Google Scholar]
- 52. Zupanec S., Jones H., and Stremler R., “Sleep Habits and Fatigue of Children Receiving Maintenance Chemotherapy for ALL and Their Parents,” Journal of Pediatric Oncology Nursing 27, no. 4 (2010): 217–228, 10.1177/1043454209358890. [DOI] [PubMed] [Google Scholar]
- 53. Sitaresmi M. N., Mostert S., Purwanto I., Gundy C. M., and Sutaryo V. A. J., “Chemotherapy‐Related Side Effects in Childhood Acute Lymphoblastic Leukemia in Indonesia: Parental Perceptions,” Journal of Pediatric Oncology Nursing 26, no. 4 (2009): 198–207, 10.1177/1043454209340315. [DOI] [PubMed] [Google Scholar]
- 54. Yeh C. H., Wang C. H., Chiang Y. C., Lin L., and Chien L. C., “Assessment of Symptoms Reported by 10‐ to 18‐Year‐Old Cancer Patients in Taiwan,” Journal of Pain and Symptom Management 38, no. 5 (2009): 738–746, 10.1016/j.jpainsymman.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 55. Ekti Genc R. and Conk Z., “Impact of Effective Nursing Interventions to the Fatigue Syndrome in Children Who Receive Chemotherapy,” Cancer Nursing 31, no. 4 (2008): 312–317, 10.1097/01.NCC.0000305740.18711.c6. [DOI] [PubMed] [Google Scholar]
- 56. Enskär K. and von Essen L., “Physical Problems and Psychosocial Function in Children With Cancer,” Paediatric Nursing 20, no. 3 (2008): 37–41, 10.7748/paed2008.04.20.3.37.c6521. [DOI] [PubMed] [Google Scholar]
- 57. Perdikaris P., Merkouris A., Patiraki E., Papadatou D., Vasilatou‐Kosmidis H., and Matziou V., “Changes in Children's Fatigue During the Course of Treatment for Paediatric Cancer,” International Nursing Review 55, no. 4 (2008): 412–419, 10.1111/j.1466-7657.2008.00644.x. [DOI] [PubMed] [Google Scholar]
- 58. Whitsett S. F., Gudmundsdottir M., Davies B., McCarthy P., and Friedman D., “Chemotherapy‐Related Fatigue in Childhood Cancer: Correlates, Consequences, and Coping Strategies,” Journal of Pediatric Oncology Nursing 25, no. 2 (2008): 86–96, 10.1177/1043454208315546. [DOI] [PubMed] [Google Scholar]
- 59. Yeh C. H., Chiang Y. C., Lin L., et al., “Clinical Factors Associated With Fatigue Over Time in Paediatric Oncology Patients Receiving Chemotherapy,” British Journal of Cancer 99, no. 1 (2008): 23–29, 10.1038/sj.bjc.6604434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hinds P. S., Hockenberry M., Rai S. N., et al., “Nocturnal Awakenings, Sleep Environment Interruptions, and Fatigue in Hospitalized Children With Cancer,” Oncology Nursing Forum 34, no. 2 (2007): 393–402, 10.1188/07.ONF.393-402. [DOI] [PubMed] [Google Scholar]
- 61. Hinds P. S., Hockenberry M. J., Gattuso J. S., et al., “Dexamethasone Alters Sleep and Fatigue in Pediatric Patients With Acute Lymphoblastic Leukemia,” Cancer 110, no. 10 (2007): 2321–2330, 10.1002/cncr.23039. [DOI] [PubMed] [Google Scholar]
- 62. Williams P. D., Schmideskamp J., Ridder E. L., and Williams A. R., “Symptom Monitoring and Dependent Care During Cancer Treatment in Children: Pilot Study,” Cancer Nursing 29, no. 3 (2006): 188–197, 10.1097/00002820-200605000-00004. [DOI] [PubMed] [Google Scholar]
- 63. Tomlinson D., Hyslop S., Stein E., et al., “Development of Mini‐SSPedi for Children 4–7 Years of Age Receiving Cancer Treatments,” BMC Cancer 19, no. 1 (2019): 32, 10.1186/s12885-018-5210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hockenberry M. J., Pan W., Scheurer M. E., et al., “Influence of Inflammatory and Oxidative Stress Pathways on Longitudinal Symptom Experiences in Children With Leukemia,” Biological Research for Nursing 21, no. 5 (2019): 458–465, 10.1177/1099800419863160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hockenberry M. J., Moore I. M. K., Scheurer M. E., et al., “Influence of Nitrosative Stress on Fatigue During Childhood Leukemia Treatment,” Biological Research for Nursing 20, no. 4 (2018): 403–409, 10.1177/1099800418772907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Yeh C. H., Chiang Y. C., Chien L. C., Lin L., Yang C. P., and Chuang H. L., “Symptom Clustering in Older Taiwanese Children With Cancer,” Oncology Nursing Forum 35, no. 2 (2008): 273–281, 10.1188/08.ONF.273-281. [DOI] [PubMed] [Google Scholar]
- 67. Rostagno E., Bergadano A., Piredda M., and De Marinis M. G., “Italian Nurses Knowledge and Attitudes Towards Fatigue in Pediatric Onco‐Hematology: A Cross‐Sectional Nationwide Survey,” International Journal of Pediatrics and Adolescent Medicine 7, no. 4 (2020): 161–165, 10.1016/j.ijpam.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bisogno G., De Salvo G. L., Bergeron C., et al., “Vinorelbine and Continuous Low‐Dose Cyclophosphamide as Maintenance Chemotherapy in Patients With High‐Risk Rhabdomyosarcoma (RMS 2005): A Multicentre, Open‐Label, Randomised, Phase 3 Trial,” Lancet Oncology 20, no. 11 (2019): 1566–1575, 10.1016/S1470-2045(19)30617-5. [DOI] [PubMed] [Google Scholar]
- 69. Nunes M. D. R., Jacob E., Bomfim E. O., et al., “Fatigue and Health Related Quality of Life in Children and Adolescents With Cancer,” European Journal of Oncology Nursing 29 (2017): 39–46, 10.1016/j.ejon.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Al Maqbali M., Al Sinani M., Al Naamani Z., Al Badi K., and Tanash M. I., “Prevalence of Fatigue in Patients With Cancer: A Systematic Review and Meta‐Analysis,” Journal of Pain and Symptom Management 61, no. 1 (2021): 167–189, 10.1016/j.jpainsymman.2020.07.037. [DOI] [PubMed] [Google Scholar]
- 71. Nap‐van der Vlist M. M., Dalmeijer G. W., Grootenhuis M. A., et al., “Fatigue in Childhood Chronic Disease,” Archives of Disease in Childhood 104, no. 11 (2019): 1090–1095, 10.1136/archdischild-2019-316782. [DOI] [PubMed] [Google Scholar]
- 72. Houghton K. M., Tucker L. B., Potts J. E., and McKenzie D. C., “Fitness, Fatigue, Disease Activity, and Quality of Life in Pediatric Lupus,” Arthritis and Rheumatism 59, no. 4 (2008): 537–545, 10.1002/art.23534. [DOI] [PubMed] [Google Scholar]
- 73. Yoon J. H., Park N. H., Kang Y. E., Ahn Y. C., Lee E. J., and Son C. G., “The Demographic Features of Fatigue in the General Population Worldwide: A Systematic Review and Meta‐Analysis,” Frontiers in Public Health 11 (2023): 1192121, 10.3389/fpubh.2023.1192121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Getz K. D., Szymczak J. E., Li Y., et al., “Medical Outcomes, Quality of Life, and Family Perceptions for Outpatient vs Inpatient Neutropenia Management After Chemotherapy for Pediatric Acute Myeloid Leukemia,” JAMA Network Open 4, no. 10 (2021): e2128385, 10.1001/jamanetworkopen.2021.28385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Woodgate R. L., “A Different Way of Being: Adolescents' Experiences With Cancer,” Cancer Nursing 28, no. 1 (2005): 8–15, 10.1097/00002820-200501000-00002. [DOI] [PubMed] [Google Scholar]
- 76. Hockenberry‐Eaton M., Hinds P. S., Alcoser P., et al., “Fatigue in Children and Adolescents With Cancer,” Journal of Pediatric Oncology Nursing 15, no. 3 (1998): 172–182, 10.1177/104345429801500306. [DOI] [PubMed] [Google Scholar]
- 77. Wu H. S., Davis J. E., and Natavio T., “Fatigue and Disrupted Sleep‐Wake Patterns in Patients With Cancer: A Shared Mechanism,” Clinical Journal of Oncology Nursing 16, no. 2 (2012): E56–E68, 10.1188/12.CJON.E56-E68. [DOI] [PubMed] [Google Scholar]
- 78. Walter L. M., Nixon G. M., Davey M. J., Downie P. A., and Horne R. S., “Sleep and Fatigue in Pediatric Oncology: A Review of the Literature,” Sleep Medicine Reviews 24 (2015): 71–82, 10.1016/j.smrv.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 79. Mack J. W., McFatrich M., Withycombe J. S., et al., “Agreement Between Child Self‐Report and Caregiver‐Proxy Report for Symptoms and Functioning of Children Undergoing Cancer Treatment,” JAMA Pediatrics 174, no. 11 (2020): e202861, 10.1001/jamapediatrics.2020.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mooney‐Doyle K., “An Examination of Fatigue in Advanced Childhood Cancer,” Journal of Pediatric Oncology Nursing 23, no. 6 (2006): 305–310, 10.1177/1043454206293269. [DOI] [PubMed] [Google Scholar]
- 81. Smith C., Farhat R., Fern‐Buneo A., et al., “Effects of an Exercise Program During Pediatric Stem Cell Transplantation: A Randomized Controlled Trial,” Pediatric Blood & Cancer 69, no. 5 (2022): e29618, 10.1002/pbc.29618. [DOI] [PubMed] [Google Scholar]
- 82. Stössel S., Neu M. A., Wingerter A., et al., “Benefits of Exercise Training for Children and Adolescents Undergoing Cancer Treatment: Results From the Randomized Controlled MUCKI Trial,” Frontiers in Pediatrics 8 (2020): 243, 10.3389/fped.2020.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lam K. K. W., Li W. H. C., Chung O. K., et al., “An Integrated Experiential Training Programme With Coaching to Promote Physical Activity, and Reduce Fatigue Among Children With Cancer: A Randomised Controlled Trial,” Patient Education and Counseling 101, no. 11 (2018): 1947–1956, 10.1016/j.pec.2018.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
The data supporting this study's findings are available from the corresponding author upon reasonable request.


