Abstract
Objective
We developed a technique to determine deep surgical margins using radiofrequency identification markers. This study assessed the feasibility of this technique during extended segmentectomy of intersegmental lesions.
Methods
A single-center, prospective, single-arm study was performed from 2020 to 2023. Small pulmonary lesions suspicious for malignancy locating the virtual intersegmental plane based on 3-dimensional imagery were included. Markers were placed in the vicinity of the lesions using electromagnetic navigation bronchoscopy. In addition to indocyanine green injection, surgeons used wireless signal strength to determine the best resection line without lung palpation to obtain surgical margins of 10 mm or the same size as the tumor.
Results
We analyzed 75 lesions in 75 patients. Median lesion size and depth from the pleura were 12.0 mm and 23.6 mm, respectively. Three-dimensional imagery identified lesions at a median distance of 7.0 mm from the virtual intersegmental plane. The median marker-lesion and marker-virtual intersegmental plane distances were 5.8 mm and 4.9 mm, respectively. Complex segmentectomy was performed in 64 of 75 patients (85.3%). The indocyanine green and preoperative simulated intersegmental lines agreed in 92.0% (69/75). In 6 cases (8.0%), the resection line was determined using radiofrequency identification markers to obtain adequate margins because the indocyanine green undyed area was smaller than the preoperatively simulated one. In 1 patient, planned segmentectomy was converted to lobectomy because of a misplaced radiofrequency identification marker (1.3%). The successful tumor resection rate was 98.7%. The median surgical margin was 16.0 mm.
Conclusions
Use of radiofrequency identification markers enabled precise extended segmentectomy with adequate surgical margins.
Key Words: indocyanine green, intersegmental plane, radiofrequency identification, robot-assisted thoracoscopic surgery, segmentectomy
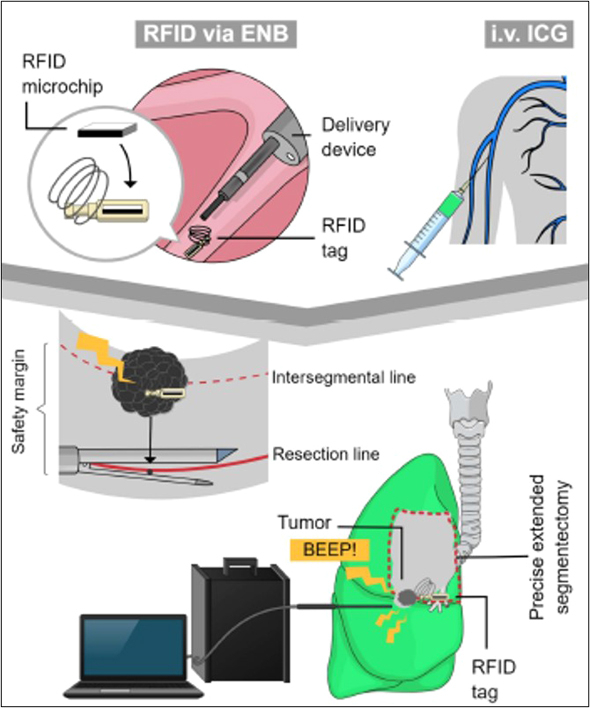
RFID markers secured deep surgical margins in segmentectomy for intersegmental lesions.
Central Message.
Adjacent subsegments can be precisely resected in a nonanatomic manner using wireless signals from RFID markers.
Perspective.
Use of bronchoscopically placed RFID markers enables precise nonanatomic extended thoracoscopic segmentectomy with adequate surgical margins.
With the introduction of multidetector computed tomography (CT), the number of incidentally detected small lung nodules has dramatically increased. Preoperative evaluation using high-resolution CT can help surgeons select the optimal patient groups for whom sublobar resection provides equivalent oncological outcomes to lobectomy.1, 2, 3, 4, 5, 6 Data from recent randomized controlled studies (JCOG 0802 and CALGB 140503) suggest that sublobar resection is reasonable for patients with small tumors 2 cm or less and negative sentinel nodes, provided that adequate surgical margins can be obtained.7,8
Although segmentectomy is still technically more challenging than standardized lobectomy under minimally invasive thoracoscopic settings, the combined use of preoperative 3-dimensional (3D) CT simulation and intravenous indocyanine green (ICG) with near-infrared light thoracoscopy has enabled precise preoperative assessment of surgical margins from the tumor to the virtual intersegmental plane of the adjacent segment.9, 10, 11, 12 However, when a nonpalpable tumor is located near the intersegmental plane deep within the pulmonary parenchyma, intraoperative assessment of the surgical margin requires more advanced surgical skills because the ICG demarcation line is limited on the visceral pleura.13 Particularly in complex segmentectomy, there is more concern about insufficient tumor-free distance at the surgical margins, which may lead to local relapse because adequate surgical margins must be achieved on several surfaces of the intersegmental planes. Therefore, to achieve adequate surgical margins, surgeons often consider extended segmentectomy and adjusting the optimal resection line beyond the ICG demarcation line that appears on the visceral pleura.14,15
To overcome the clinical issues regarding intraoperative assessment of surgical margins during segmentectomy, we developed a novel wireless localization technique using radiofrequency identification (RFID) markers,16, 17, 18, 19, 20 which can provide accurate localization of deeply located small pulmonary lesions. We prospectively investigated the feasibility of RFID marker-guided extended segmentectomy for nonpalpable intersegmental pulmonary lesions and evaluated the role of additional use of RFID markers in combination with systemic ICG injection.
Patients and Methods
Consecutive patients with potentially malignant pulmonary lesions who were scheduled to undergo segmentectomy via video-assisted thoracoscopic surgery (VATS) or robot-assisted thoracoscopic surgery (RATS) at our institution were prospectively enrolled from September 2020 to December 2023. All data were collected in a prospective manner. All patients provided written informed consent for study inclusion, and the institutional ethics committee approved this study (prospective observational study to assess the outcome of electromagnetic navigation bronchoscopy–guided RFID marker placement; approval Number R2599; approval date September 9, 2020).
Patients
Patients who met the following criteria were prospectively enrolled: (1) cTis, cT1mi, or cT1aN0 primary lung cancer; (2) deep lesions for which segmentectomy would be reasonable to achieve complete resection; and (3) intersegmental lesions located less than 10 mm from the virtual intersegmental plane on preoperative 3D-CT or spanning the virtual intersegmental plane; or (4) surgical procedure compromised by insufficient pulmonary function. To avoid unnecessary resection of potentially benign lesions, the indications for surgical resection were determined by the tumor board. Patients with less than 3 months of CT follow-up before surgery were excluded.
Electromagnetic Navigation Bronchoscopy–Guided Radiofrequency Identification Marker Placement
The details of the RFID marking system have been reported14, 15, 16, 17, 18 (Figure 1). Surgeons can determine the resection line without lung palpation based on the strength of wireless signals emitted from RFID markers which were bronchoscopically placed in the vicinity of the tumor under electromagnetic navigation bronchoscopy guidance (SuperDimension navigation system, version 7.0 (Medtronic Inc).21,22 To confirm the 3D relationship between the marker and the target lesion, CT was conducted in the maximum inspiratory state after bronchoscopic procedures.
Figure 1.
Components of the RFID lung marking system. A, Delivery device that is used through a bronchoscope with a 2-mm working channel. B, Passive RFID tag with nitinol coil (13.56 MHz; 3.2 × 1.6 × 0.9 mm). C, Detection probe (diameter, 10 mm) with 30-mm communication range. D, Signal-processing unit.
For segmentectomy when the lesion spans the intersegmental plane, the RFID marker was placed adjacent to the target lesion closer to the intersegmental plane from the targeted segmental bronchi so the intraoperative surgical margin could be precisely assessed (Figures 2 and E1).
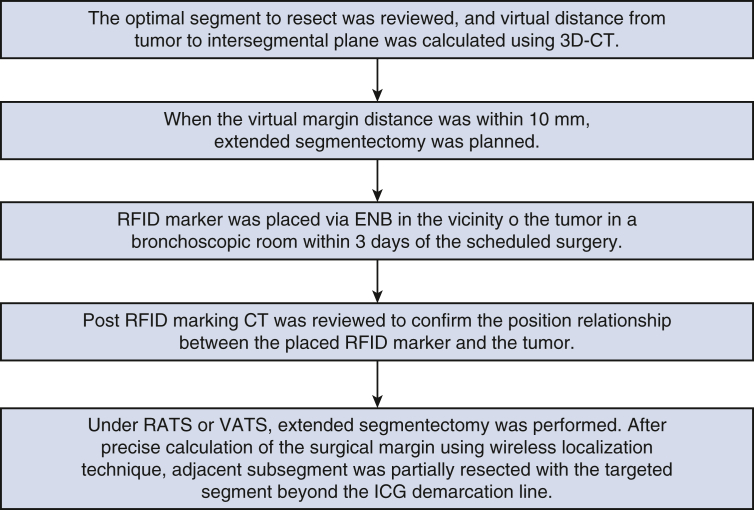
Figure 2.
Workflow of segmentectomy using RFID marking. 3D-CT, 3-Dimensional computed tomography; RFID, radiofrequency identification; CT, computed tomography; ENB, electromagnetic navigation bronchoscopy; RATS, robot-assisted thoracoscopic surgery; VATS, video-assisted thoracoscopic surgery; ICG, intravenous indocyanine green.
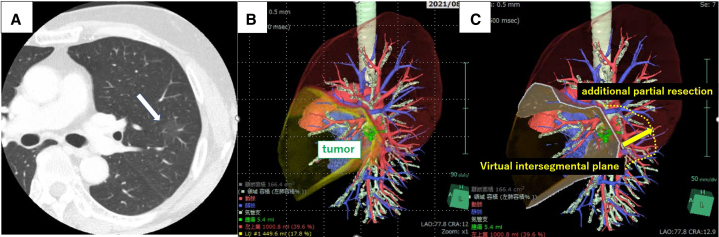
Figure E1.
Preoperative simulation using 3D-CT. A, CT showed pure ground-glass opacity (18.0 mm in size and 23.5 mm in distance from the pleura). The main part of the lesion was contained in S4; however, it partially spanned between S4 and S1+2c. The white arrow indicates the target lesion. B and C, 3D-CT simulation showed simple lingulectomy would be insufficient to ensure adequate surgical margins. Therefore, additional partial resection of S1+2c was planned using RFID marking. 3D-CT, 3-Dimensional computed tomography; CT, computed tomography.
Segmentectomy Using Wireless Localization on Top of the Intravenous Indocyanine Green Demarcation Line
VATS segmentectomy was performed via 3 ports: 1 at the 4th intercostal space at the anterior axillary line through a 40-mm incision, 1 at the 7th intercostal space at the mid-axillary line through a 15-mm incision, and 1 at the triangle of auscultation through a 15-mm incision. A Pinpoint thoracoscope (Stryker Corp) was used to obtain fluorescence image. RATS segmentectomy was performed using the da Vinci Surgical System (Xi) (Intuitive Surgical) through 4 ports: 3 port incisions were placed at the 8th intercostal space, and 1 port with a 40-mm incision was placed at the anterior axillary line at the 5th intercostal space. Firefly fluorescence imaging was used to identify the ICG demarcation line after dividing the targeted pulmonary artery and vein.
Intraoperative Assessment of Surgical Margins Using Radiofrequency Identification Markers
After insertion of the thoracoscope, RFID markers were first localized by a 10-mm diameter probe to prevent dislocation during peripheral dissection of the hilar structures. The precise marker position was indicated by the sound pitch, which became higher as the marker-probe distance became shorter. The pleural point where the highest sound pitch was detected was marked with a 4-0 polydioxanone suture. The tumor location was marked on the pleura using electrocautery by measuring the distance with a gauge inserted into the pleural cavity while referencing the 3D positional relationship between the tumor and the placed RFID marker.
After dividing the target artery and vein according to the preoperative simulation using the 3D reconstructed image, the targeted lung was inflated by the anesthesiologist to improve the intrapulmonary blood flow in the deflated lung. We injected 0.3 mg/kg of ICG into the systemic vein, and the intersegmental line on the pleural surface was marked with cauterization. After checking the positional relationship between the tumor and the ICG demarcated line, the surgeon cut into an adjacent segment in a nonanatomic manner using staplers beyond the line demarcated on the visceral pleura. This additional resection was performed while using wireless signals from the RFID marker to identify deep and lateral surgical margins in the inflated lung (Figure E2). Conversion to lobectomy depended on the sentinel lymph node status checked by intraoperative examination of frozen sections and whether there was incomplete resection of the targeted lesions. The distance from the tumor to the margin was measured using a cross-section of the lesion after cutting off the staples. The macroscopic surgical margin including the width of the staple line (3 mm) was recorded.
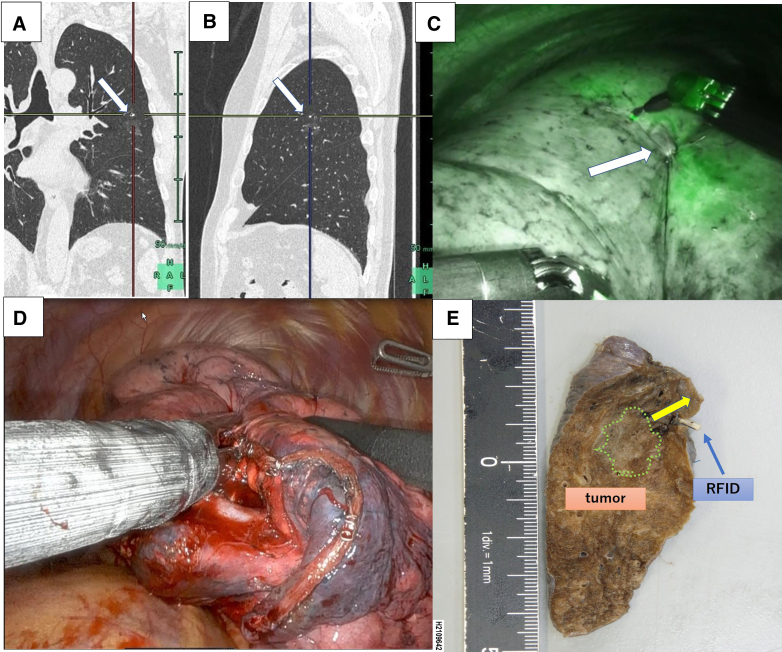
Figure E2.
Intraoperative findings during extended segmentectomy using RFID marking. A, RFID marker placement for segmentectomy. The marker was placed 7 mm cranial to the lesion to ensure adequate surgical margins. The white arrows indicate the placed markers. B, The 4-0 suture placed on the pleura was identified on the intersegmental line that was demarcated using ICG injections. C, The resection line was precisely adjusted using the marker position. D, Segmentectomy via RATS using a wireless localization technique resulted in a surgical margin of 12 mm in the deflated lung. E, The resected specimen.
Evaluated Outcomes
The primary outcome was the rate of successful resection of the tumor together with the placed RFID marker, with an adequate surgical margin (10 mm or the same size as the tumor). Secondary outcomes were (1) marking accuracy: the distance from the marker position to the tumor on postmarking CT; (2) marking safety: pneumothorax associated with marker placement and marker dislodgement; and (3) other parameters: bronchoscopy time and intraoperative localization time (time from the insertion of the detection probe to the time that the highest pitch was heard). Operation time, estimated blood loss, postoperative complications requiring additional medical treatment, interventions required, reoperation, and duration of chest drainage were also recorded.
Sample Size Calculation
The incidence of unsuccessful ICG staining was reported to be 10%.10 Among segmentectomy cases, half of the lesions were removed with intraoperative palpation in our historical data. The successful resection rate for intersegmental nonpalpable lesions using ICG was calculated as 50%. Therefore, to conclude that the RFID marking technique was effective in obtaining sufficient surgical margins in segmentectomy, we set a successful tumor resection rate of 60% as the threshold; the expected tumor resection rate was set at 80%. Based on this information, the required sample size was calculated as 64 patients, with a 2-sided type 1 error rate of 2.5% and a power of 90%.
Statistical Analysis
Numerical variables are presented as medians (interquartile range [IQR]). Categorical variables are summarized in a frequency distribution table. Descriptive statistics were used to describe the main features of numerical and categorical data, with simple summaries. Statistical analysis was conducted using JMP version pro 17.0 software (SAS Institute, Inc).
Results
Patient Characteristics
During the study period, 165 patients with 171 lesions underwent thoracoscopic segmentectomy and 75 of these patients (male 31, female 44) with 75 intersegmental lesions underwent segmentectomy using RFID markers (Table 1). The median age was 71 years (IQR, 55.0-74.5). Twenty-one patients (28.0%) were ex-smokers. Preoperative CT imaging showed emphysema in 13 patients (17.3%) and interstitial pneumonia in 5 patients (6.7%). Twenty-nine patients (38.7%) had a history of malignancy; among these, 18 patients (24.0%) had a history of thoracic intervention (lung cancer surgery in 14, radiation therapy in 3, and esophageal surgery in 1). Six patients (8.0%) had a history of surgery on the same side that was scheduled for surgery.
Table 1.
Patient and tumor characteristics
| Patient characteristics (75 patients) | Value | Range |
|---|---|---|
| Age, y | 71 (55-75) | 30-83 |
| Male:female | 31:44 | |
| Forced vital capacity, % | 105.5 (92.8-114.2) | 70.1-143.5 |
| Forced expiratory volume in 1 sec, % | 73.6 (64.45-80.8) | 45.7-89.2 |
| Diffusing capacity of lung carbon monoxide, % | 76.6 (71.1-86.4) | 52.8-119.4 |
| Smoking history (current or ex-smoker) | 21 (28.0%) | |
| Comorbidities of cancer | ||
| Lung cancer with prior lung resection | 18 (24.0%) | |
| Malignancies | 29 (38.7%) | |
| Patients with previous intrathoracic treatment on the same side as the scheduled thoracic surgery | 6 (8.0%) | |
| Antiplatelet or anticoagulation therapy | 12 (16.0%) | |
| Lesion characteristics (n = 75) | ||
| Size, mm | 12.0 (8.9-14.5) | 4.6-25.0 |
| Consolidation/tumor ratio | 0.08 (0-0.58) | 0-1.0 |
| Distance from the pleura, mm | 23.6 (19.3-30.9) | 15.3-52.2 |
| Distance from lesion to the virtual intersegmental plane | 7.0 (3.5-8.0) | 0-10.0 |
| Lesion distribution (n = 75) | ||
| Right upper lobe | 27 | |
| Right middle lobe | 0 | |
| Right lower lobe | 18 | |
| Left upper lobe | 22 | |
| Left lower lobe | 8 | |
Values are presented as medians (IQR), n, or n (%).
Preoperative Characteristics of the Pulmonary Lesions
Median lesion size including ground-glass opacity component and distance from the pleura were 12.0 mm (IQR, 8.9-14.5) and 23.6 mm (IQR, 19.3-30.9), respectively (Table 2). The median consolidation/tumor ratio was 0.08 (0-0.58). Lesion location was right upper lobe in 27 patients (36.0%), right lower lobe in 18 patients (24.0%), left upper lobe in 22 patients (29.3%), and left lower lobe in 8 patients (10.7%). Compromised segmentectomy was indicated in 6 patients (8.0%) with insufficient cardiopulmonary function.
Table 2.
Surgical approaches and segmentectomy details
| Approach | Simple segmentectomy (n = 11) | Complex segmentectomy (n = 64) |
|---|---|---|
| VATS (n = 28) | 3 | 25 |
| RATS (n = 47) | 8 | 39 |
| Right | n = 40 | Left | n = 35 |
|---|---|---|---|
| S1 | 9 | S1+2 | 16 |
| S2 | 5 | S3 | 5 |
| S1+2 | 4 | S1-3 | 3 |
| S2a+S3b | 1 | S4+5 | 2 |
| S3 | 8 | S6 | 2 |
| S6 | 2 | S8 | 4 |
| S8 | 4 | S10 | 3 |
| S9 | 3 | ||
| S7-10 | 2 | ||
| S9+10 | 2 |
Bold description is used for simple segmentectomy. Normal description is used for complex segmentectomy. VATS, Video-assisted thoracoscopic surgery; RATS, robot-assisted thoracoscopic surgery; S, segment.
Outcome of Radiofrequency Identification Marking Before Segmentectomy
The outcomes of RFID marking are summarized in Table E1. The RFID marking procedure was conducted within 3 days of the planned surgery (median interval: 23 hours [IQR, 4.5-24.5]). The median bronchoscopy time was 40 minutes (IQR, 33-50). Postmarking CT showed that the median distance between the lesion and the RFID marker, and the median distance between the marker and the pleura was 5.8 mm (IQR, 4.3-8.7) and 21.2 mm (IQR, 18.7-25.5), respectively. Regarding the positional relationships between the lesion, the marked site and the virtual intersegmental plane calculated using preoperative 3D-CT imagery, the median lesion-segmental plane distance was 7.0 mm (IQR, 3.5-8.0) and the median marked site-segmental plane distance was 4.9 mm (IQR, 3.0-7.4). In 1 patient with a left upper lesion, planned segmentectomy was converted to lobectomy because of a misplaced RFID marker. No hemothorax or pneumothorax caused by penetration of the visceral pleura occurred according to postmarking CT. All surgeries were conducted according to the planned schedule.
Surgical Outcomes of Radiofrequency Identification–Guided Segmentectomy
The successful tumor resection rate was 98.7%. The median surgical margin was 16.0 mm (IQR, 13.0-21.0). Surgeries were VATS (n = 28) or RATS (n = 47). Complex segmentectomy was performed in 64 patients (85.3%). Pathological examination identified the tumors as atypical adenomatous hyperplasia (n = 3), adenocarcinoma in situ (n = 19), minimally invasive adenocarcinoma (n = 17), adenocarcinoma (n = 22), squamous cell carcinoma (n = 4), metastatic tumor (n = 7), and other (n = 3). The pathological stages were 0 (n = 19), 1A1 (n = 34), 1A2 (n = 8), and 2B (n = 1). Although in 1 patient who underwent VATS left S1+2 segmentectomy for a 15.2-mm tumor and C/T ratio of 0.83, intraoperative frozen examination showed metastasis in the sentinel lymph node and lobectomy was not performed because of insufficient pulmonary function.
Although pleural invasion was not thoracoscopically identified in any patient, a slight change in color of the pleura induced by intrapulmonary hemorrhage caused by preoperative bronchoscopic marking was present in 6 patients (8.0%). The mean time to localize the placed RFID marker was 19 seconds (IQR, 12-26).
A total of 69 of 75 RFID markers (92.0%) inserted from the targeted bronchus were localized within or on the boundary of the area not dyed by ICG. However, there was a discrepancy between the preoperative simulated intersegmental line on the pleural surface and the actual ICG demarcation line in 6 patients (8.0%). In 1 patient with a 12-mm ground-glass opacity lesion in S6c who underwent right S6 segmentectomy via RATS, preoperative 3D-CT simulation showed an RFID marker was placed 2 mm caudal to the target, and the virtual surgical margin from the target to the simulated intersegmental plane was 0.3 mm. After division of the targeted segmental vessels, the ICG demarcation line on the visceral pleura showed that the marker was identified 10 mm caudal to the demarcated line on the visceral pleura. Therefore, in this patient, the resection line was adjusted according to the RFID markers placed in the vicinity of the tumor, which resulted in a successful tumor removal with a surgical margin of 15 mm (Figure E3). In the other 5 patients who underwent right S1, S2, S3, S8, or left S10 segmentectomy, because the RFID markers were localized outside the ICG undyed area, the resection line was also adjusted according to the marker position (Video 1).
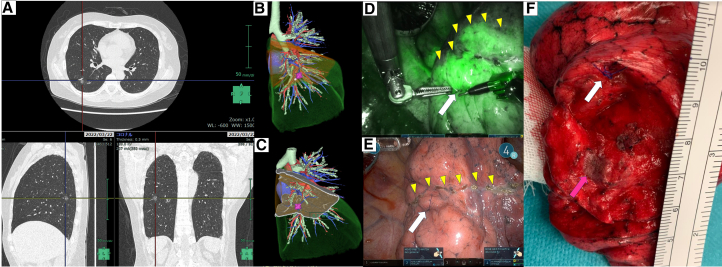
Figure E3.
Adjustment of the resection line in right S6 segmentectomy. A, CT suggested a pure ground-glass opacity lesion (12 mm) in S6c. B and C, Preoperative 3D-CT simulation showed the virtual surgical margin from the target to the simulated intersegmental plane was 0.3 mm and an RFID marker was placed 2 mm caudal to the target (white circle: RFID marker, pink mark: target). D and E, ICG demarcation line demonstrated the RFID marker was outside the undyed area, and the marker was identified 10 mm caudal to the ICG demarcated line on the visceral pleura (white arrow: RFID marker, yellow triangle: ICG line). F, The resection line was adjusted according to the marker, and the lesion was removed with a surgical margin of 15 mm (white arrow: RFID marker, pink arrow: tumor).
Median duration of postoperative chest tube drainage was 4 days (IQR, 3-5). Six patients (8.0%) developed postoperative pleuritis that required additional antibiotics. Two patients (2.7%) required postoperative pleurodesis because of prolonged air leak (>7 days), and 1 patient (1.3%) on anticoagulation therapy who experienced a delayed-onset air leak after hospital discharge required readmission for re-drainage followed by pleurodesis. Major complications (Clavien-Dindo grade IIIB and above) were not observed. No patient died within 30 days of surgery.
Discussion
This is the first feasibility study of RFID-guided extended segmentectomy for the resection of small nonpalpable intersegmental pulmonary lesions. In 8.0% of the segmentectomies for intersegmental lesions, the ICG undyed area was smaller than the virtual targeted segment to be resected, which showed that the combined use of preoperative 3D simulation and intravenous ICG injection after division of the pulmonary artery and vein was insufficient for obtaining adequate margins in some intersegmental lesions. These data suggest that, in addition to the ICG demarcation line on the visceral pleura, RFID markers could be a complementary tool to determine the intersegmental plane deep in the lung parenchyma to realize precise extended segmentectomies.
ICG-guided segmentectomy is safe and effective and has been associated with minimal complications. Although ICG allergy, which may be dose-dependent, has been reported, low-dose ICG (0.05-2 mg/kg) can achieve a clear demarcation line while avoiding anaphylactic shock and is generally considered safe.10 In a previous study, the rate of unsuccessful ICG demarcation was higher in complex segmentectomy than in simple segmentectomy (14.2% vs 9%).23 In complex segmentectomy where the arteries are divided more peripherally than those in simple segmentectomy, peripheral collateral blood flow in visceral pleura can contribute to the expansion of ICG dyed area into the resection area underestimate the intersegmental line.24 Unclear demarcation has been associated with the underlying pulmonary conditions with lower attenuation area and forced expiratory volume in 1 second in emphysematous lung. Although a larger amount or repeated use of ICG may be useful, additional ICG dye may enter the target lung tissue through the collateral or bronchial circulation, which can increase the risk of error during determination of the best resection line. In the current study, we judged the accuracy of the identification of the intersegmental line using intravenous injection of ICG from the positional relationship between placed RFID markers and ICG demarcation line. The rate of successful ICG demarcation was 92.0%. To minimize ICG demarcation error, we routinely inject ICG after inflation of the deflated lung to minimize atelectasis, which would improve intrapulmonary blood flow.
Anatomic segmentectomy is an essential surgical skill for resection of small pulmonary lesions that cannot be removed by wedge resection. However, concern remains as to whether it is possible to ensure surgical quality during segmentectomy and achieve adequate surgical margins. Unlike standardized lobectomy, greater flexibility is required in anatomic segmentectomy to manage the intersegmental planes and determine the extent of resection.14,15,25 To secure surgical margins, particularly when managing intersegmental pulmonary lesions, extended segmentectomy should be carefully simulated by preoperative 3D simulation. Although there is no consensus regarding the optimal additional sublobar resection procedures, for intersegmental lesions, the resection line can be extended beyond conventional anatomic segments, either by cutting into adjacent segments in a nonanatomic manner or by resecting a whole subsegment after additional division of hilar bronchovascular structures. In general, the latter method, additional subsegmentectomy, requires more advanced surgical skills and can hinder the matching of intraoperative anatomy with the simulated image. Therefore, we prefer segmentectomy with nonanatomic additional partial resection of the adjacent subsegment beyond the affected area.26
We believe that our procedure is a reasonable treatment option for patients with small lung lesions, but it is valid only when the lesion is peripherally located because intrapulmonary vessels or small bronchi may be stapled and, therefore, adequate margin resection can be ensured. Previous studies have demonstrated that malignant-positive margins were not found when the margin distance was greater than the tumor diameter.27 In our study, although stapler lavage cytology was not necessarily performed, the median tumor diameter in patients undergoing segmentectomy was 12 mm and the median surgical margin distance was 16.0 mm. Therefore, we consider our results to be reasonable and to show that RFID-guided segmentectomy can be successfully performed.
Limitations
This study has several limitations. The sample size was small and a control arm was lacking. A large prospective multicenter trial is required to confirm the reproducibility of the technique. In addition, we did not perform a cost-benefit analysis. Future studies should investigate the tumor characteristics or positions for which RFID marking can be most useful for achieving adequate surgical margins in segmentectomy for resection of intersegmental lesions.
Conclusions
Using RFID markers can be a complementary tool to obtain adequate surgical margins for resection of intersegmental lesions. However, future multicenter studies are needed to confirm.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
The authors thank the staff who assisted the present study, especially Drs Mamoru Takahashi, Hidenao Kayawake, Yoshito Yamada, and Masatsugu Hamaji. The authors thank Dr Kelly Zammit, BVSc, and Dr Leah Cannon, PhD, from Edanz (https://jp.edanz.com/ac), for editing a draft of this manuscript.
Footnotes
Institutional Approval Number: All patients provided written informed consent for study inclusion, and the institutional ethics committee approved this study. A prospective observational study to assess the outcome of electromagnetic navigation bronchoscopy-guided radiofrequency identification marker placement; approval Number R2599; approval date September 9, 2020.
Supplementary Data
RATS lingulectomy. After marker placement 7 mm cranial to the lesion, the tumor was accurately identified on the simulated intersegmental line that was demarcated using ICG injections. The resection line was precisely adjusted using the marker position, which resulted in a surgical margin of 12 mm in the deflated lung. Video available at: https://www.jtcvs.org/article/S2666-2507(24)00363-8/fulltext.
Appendix E1
Table E1.
Outcomes of 75 radiofrequency identification markings for 75 lesions in 75 patients
| Outcomes of RFID markings | Value | Range |
|---|---|---|
| Primary outcome | ||
| Tumor + RFID marker resection rate, % | 98.7 | |
| Macroscopic margin distance, mm | 16.0 (13.0-21.0) | 11.0-28.0 |
| Secondary outcome | ||
| RFID marking accuracy | ||
| Distance to the lesion, mm | 5.8 (4.3-8.7) | 0-15.3 |
| Distance to the pleura, mm | 21.2 (18.7-25.5) | 15.0-42.6 |
| Distance to the virtual intersegmental plane | 4.9 (3.0-7.4) | 0-13.8 |
| RFID marking safety | ||
| Complications associated with RFID marking procedure, n (%) | 1 (1.3%) | |
| Other parameters | ||
| Operating time, min | 209 (195-288) | 122-479 |
| Estimated blood loss, mL | 20 (0-50) | 0-200 |
| Complications | ||
| Reoperation | 0 | |
| Persistent air leak requiring pleurodesis | 2 (2.7%) | |
| Duration of chest tube drainage, d | 4 (3-5) | 1-13 |
Values are presented as medians (IQR) or n (%). RFID, Radiofrequency identification.
References
- 1.Lung Cancer Study Group. Ginsberg R.J., Rubinstein L.V. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann Thorac Surg. 1995;60(3):615–622. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 2.Kodama K., Doi O., Higashiyama M., Yokouchi H. Intentional limited resection for selected patients with T1 N0 M0 non-small-cell lung cancer: a single-institution study. J Thorac Cardiovasc Surg. 1997;114(3):347–353. doi: 10.1016/S0022-5223(97)70179-X. [DOI] [PubMed] [Google Scholar]
- 3.Okada M., Yoshikawa K., Hatta T., Tsubota N. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lung cancer of 2 cm or smaller? Ann Thorac Surg. 2001;71(3):956–960. doi: 10.1016/s0003-4975(00)02223-2. [DOI] [PubMed] [Google Scholar]
- 4.Sugarbaker D.J., Strauss G.M. Extent of surgery and survival in early lung carcinoma: implications for overdiagnosis in stage IA nonsmall cell lung carcinoma. Cancer. 2000;89(11 Suppl):2432–2437. doi: 10.1002/1097-0142(20001201)89:11+<2432::aid-cncr17>3.3.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki K., Watanabe S., Wakabayashi M., Saji H., Aokage K. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J Thorac Cardiovasc Surg. 2022;163(1):289–301. doi: 10.1016/j.jtcvs.2020.09.146. [DOI] [PubMed] [Google Scholar]
- 6.Yutaka Y., Sonobe M., Kawaguchi A., Hamaji M., Nakajima D. Prognostic impact of preoperative comorbidities in geriatric patients with early-stage lung cancer: significance of sublobar resection as a compromise procedure. Lung Cancer. 2018;125:192–197. doi: 10.1016/j.lungcan.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Saji H., Okada M., Tsuboi M., et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomized, controlled, non-inferiority trial. Lancet. 2022;399(10335):1607–1617. doi: 10.1016/S0140-6736(21)02333-3. [DOI] [PubMed] [Google Scholar]
- 8.Altorki N., Wang X., Kozono D., et al. Lobar or sublobar resection for peripheral stage IA non-small-cell lung cancer. N Engl J Med. 2023;388(6):489–498. doi: 10.1056/NEJMoa2212083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Misaki N., Chang S.S., Gotoh M., Yamamoto Y., Satoh K., Yokomise H. A novel method for determining adjacent lung segments with infrared thoracoscopy. J Thorac Cardiovasc Surg. 2009;138:613. doi: 10.1016/j.jtcvs.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Cui F., Liu J., Du M., et al. Expert consensus on indocyanine green fluorescence imaging for thoracoscopic lung resection (The Version 2022) Transl Lung Cancer Res. 2022;11(11):2318–2331. doi: 10.21037/tlcr-22-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardolesi A., Veronesi G., Solli P., Spaggiari L. Use of indocyanine green to facilitate intersegmental plane identification during robotic anatomic segmentectomy. J Thorac Cardiovasc Surg. 2014;148(2):737–738. doi: 10.1016/j.jtcvs.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Okusanya O.T., Hess N.R., Luketich J.D., Sarkaria I.S. Infrared intraoperative fluorescence imaging using indocyanine green in thoracic surgery. Eur J Cardiothorac Surg. 2018;53(3):512–518. doi: 10.1093/ejcts/ezx352. [DOI] [PubMed] [Google Scholar]
- 13.Kim Y., Rho J., Quan Y.H., Choi B.H., Han K.N. Simultaneous visualization of pulmonary nodules and intersegmental planes on fluorescent images in pulmonary segmentectomy. Eur J Cardiothorac Surg. 2020;58(Suppl_1):i77–i84. doi: 10.1093/ejcts/ezaa064. [DOI] [PubMed] [Google Scholar]
- 14.Tsubota N., Ayabe K., Doi O., et al. Ongoing prospective study of segmentectomy for small lung tumors. Study group of extended segmentectomy for small lung tumor. Ann Thorac Surg. 1998;66(5):1787–1790. doi: 10.1016/s0003-4975(98)00819-4. [DOI] [PubMed] [Google Scholar]
- 15.Yoshikawa K., Tsubota N., Kodama K., Ayabe H., Taki T., Mori T. Prospective study of extended segmentectomy for small lung tumors: the final report. Ann Thorac Surg. 2002;73(4):1055–1058. doi: 10.1016/s0003-4975(01)03466-x. [DOI] [PubMed] [Google Scholar]
- 16.Yutaka Y., Sato T., Zhang J., et al. Localizing small lung lesions in video-assisted thoracoscopic surgery via radiofrequency identification marking. Surg Endosc. 2017;31:3353–3362. doi: 10.1007/s00464-016-5302-2. [DOI] [PubMed] [Google Scholar]
- 17.Yutaka Y., Sato T., Matsushita K., et al. Three-dimensional navigation for thoracoscopic sublobar resection using a novel wireless marking system. Semin Thorac Cardiovasc Surg. 2018;30:230–237. doi: 10.1053/j.semtcvs.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Sato T., Yutaka Y., Nakamura T., Date H. First clinical application of radiofrequency identification (RFID) marking system—precise localization of a small lung nodule. JTCVS Tech. 2020;4:301–304. doi: 10.1016/j.xjtc.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yutaka Y., Sato T., Tanaka S., et al. Feasibility study of a novel wireless localization technique using radiofrequency identification markers for small and deeply located lung lesions. JTCVS Tech. 2022;12:185–195. doi: 10.1016/j.xjtc.2021.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yutaka Y., Sato T., Hidaka Y., Kato T., Kayawake H. Electromagnetic navigation bronchoscopy-guided radiofrequency identification marking in wedge resection for fluoroscopically invisible small lung lesions. Eur J Cardiothorac Surg. 2022;63(1) doi: 10.1093/ejcts/ezad006. [DOI] [PubMed] [Google Scholar]
- 21.Sato T., Yutaka Y., Ueda Y., et al. Diagnostic yield of electromagnetic navigational bronchoscopy: results of initial 35 cases in a Japanese institute. J Thorac Dis. 2018;10(Suppl 14):S1615–S1619. doi: 10.21037/jtd.2018.04.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yutaka Y., Sato T., Isowa M., et al. Electromagnetic navigation bronchoscopy versus virtual bronchoscopy navigation for improving the diagnosis of peripheral lung lesions: analysis of the predictors of successful diagnosis. Surg Today. 2022;52(6):923–930. doi: 10.1007/s00595-021-02398-z. [DOI] [PubMed] [Google Scholar]
- 23.Yotsukura M., Okubo Y., Yoshida Y., Nakagawa K., Watanabe S. Indocyanine green imaging for pulmonary segmentectomy. JTCVS Tech. 2021;6:151–158. doi: 10.1016/j.xjtc.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iizuka S., Kuroda H., Yoshimura K., et al. Predictors of indocyanine green visualization during fluorescence imaging for segmental plane formation in thoracoscopic anatomical segmentectomy. J Thorac Dis. 2016;8:985–991. doi: 10.21037/jtd.2016.03.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunelli A., Decaluwe H., Gonzalez M., et al. European Society of Thoracic Surgeons expert consensus recommendations on technical standards of segmentectomy for primary lung cancer. Eur J Cardiothorac Surg. 2023;63(6) doi: 10.1093/ejcts/ezad224. [DOI] [PubMed] [Google Scholar]
- 26.Yutaka Y., Ohsumi A., Nakajima D., Hamaji M., Menju T., Date H. Intraoperative margin assessment by wireless signals in thoracoscopic anterior (S3) segmentectomy using a radiofrequency identification marker. Gen Thorac Cardiovasc Surg. 2022;70(5):509–513. doi: 10.1007/s11748-021-01762-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawabata N., Ohta M., Matsumura A., et al. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg. 2004;77(2):415–420. doi: 10.1016/S0003-4975(03)01511-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RATS lingulectomy. After marker placement 7 mm cranial to the lesion, the tumor was accurately identified on the simulated intersegmental line that was demarcated using ICG injections. The resection line was precisely adjusted using the marker position, which resulted in a surgical margin of 12 mm in the deflated lung. Video available at: https://www.jtcvs.org/article/S2666-2507(24)00363-8/fulltext.