Abstract
Background
The paper presents an extensive dataset of the shallow reef fish communities and habitat characteristics in the Fernando de Noronha Archipelago (Southwest Atlantic). The data were collected from August to October 2006 in the Fernando de Noronha main island. To evaluate the shallow reef fish communities, 165 visual censuses were performed in eight different localities in the Fernando de Noronha Archipelago.
New information
The dataset reports a comprehensive compilation of the shallow reef fish abundance, of eight localities of the Fernando de Noronha Archipelago. The dataset reveals spatial heterogeneity amongst the selected localities in terms of fish abundance, composition and size.
Keywords: abundance, Brazil, endemic, oceanic island, Stegastesrocasensis , Thalassomanoronhanum
Introduction
Oceanic islands are ecologically relevant environments because of their high biodiversity, which is characterised by a high degree of endemism (Kier et al. 2009). Their geographic isolation contributes to providing relevant information on the distribution, dispersal and establishment of different species. Reef fishes are an economically and ecologically important group that inhabits oceanic islands, occupying a wide range of ecological niches and serving as models to study relevant ecological interactions in these environments (Fernández-Cisternas et al. 2021). Since the mid-1990s, knowledge on the marine fishes and biogeographic patterns of islands in the Southwest Atlantic has steadily increased. To date, there were several studies published that focused on the ichthyofauna of Fernando de Noronha Archipelago. Most of them focused on species spatial distribution, animal behaviour and/or ecological interactions (Souza et al. 2011 , Pereira et al. 2012, Souza and Ilarri 2014,Garla et al. 2017, Bettcher et al. 2022) and only a few deal with new occurrences (Garla and Garcia 2008, Pimentel et al. 2020, Bucair et al. 2022, Mincarone et al. 2022), the description of a new species (Sampaio et al. 2004, Smith-Vaniz et al. 2018, Carvalho-Filho et al. 2020, Villarins et al. 2023), biogeography (Mendes 2007) and compilation work (Soto 1997, Soto 2001, Schmid et al. 2020, Salvetat et al. 2022).
Although the Archipelago has been the subject of several studies of ichthyofauna, only a few of these have aimed to characterise the structure of fish assemblage (Krajewski and Floeter 2011, Krajewski et al. 2011, Medeiros et al. 2011, Ilarri et al. 2017). Therefore, it is important to provide detailed information on shallow reef fish species for the different localities of the Archipelago of Fernando de Noronha.
From August to October 2006, the ichthyofauna of the shallow reef of Fernando de Noronha Archipelago was assessed daily through visual censuses in eight selected localities. The aim of this study is to report and make available the data on the abundance of the shallow reef fish species collected through visual censuses in Fernando de Noronha Archipelago. In this study, we provide an extensive list of reef fish occurrences from an area of remarkable importance for Atlantic reef fishes that is currently under-represented in large-scale ecological studies.
Project description
Study area description
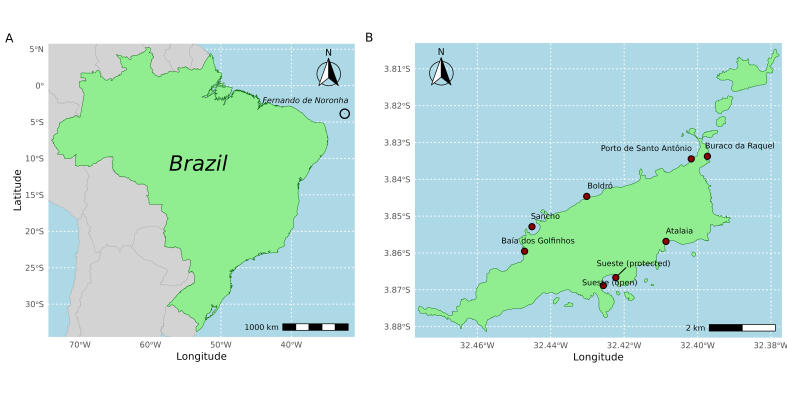
The study was conducted in Atlantic Southwest, more specifically in the Fernando de Noronha Archipelago, an isolated group of volcanic islands, with one main island (https://deims.org/030bec0b-f6ac-4840-b226-af813258b14b) and 19 smaller adjacent islands and an area of 26 km2, located 345 km off the northeast coast of Brazil (3°54’S, 32°25’W) (Fig. 1).
Figure 1.
Map showing A) the location of the Fernando de Noronha Archipelago (black circle) in relation to Brazilian mainland and B) the eight sampled localities in the Archipelago (red dots).
Data collection was conducted at eight different localities within the Fernando de Noronha main island: Atalaia, Baía dos Golfinhos, Boldró, Buraco da Raquel, Porto de Santo Antônio, Sancho, Sueste (open) and Sueste (protected). These sites were chosen to cover the diversity of habitats in the Archipelago. Atalaia is a small reef lagoon with a predominance of sandy and rocky habitats. Baía dos Golfinhos is a calm and sheltered rocky area characterised by large pebbles and volcanic sand. Boldró comprises a reef flat area with sand and a rocky plateau in the intertidal zone and is an area exposed to currents. Buraco da Raquel comprises a reef lagoon characterised by sand and rocks mainly, exposed to currents. Porto de Santo António is characterised by a reef area with sandy and rocky habitats mainly and is a calm and sheltered area. Sancho comprises a bay with sand and large pebbles and rocks and is a calm and sheltered area. Sueste (open) is a bay with rock and sand that has a central plateau and is a calm and sheltered area. Sueste (protected) is a bay with rocks, sand and pebbles and is a calm and sheltered area.
Funding
Financial support was provided by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) to the first author and by the Graduate Program in Biological Science (Zoology) of Universidade Federal da Paraíba. Martina I. Ilarri is currently supported by national funds through FCT – Foundation for Science and Technology, Portugal, within the scope of UIDB/04423/2020 and UIDP/04423/2020 and a research contract (DL57/2016/CP1344/CT0018). A. T. Souza is funded by eLTER PLUS (European Union's Horizon 2020 Research and Innovation Programme under grant agreement No 871128) and BioDT (https://doi.org/10.3030/101057437).
Sampling methods
Study extent
Study extent
Sampling description
To assess the shallow reef fish communities, a total of 165 visual censuses were performed from August to October 2006 (with a minimum of 20 censuses per locality). All observations were made by free diving in areas with depths up to six metres (m) during the day (from 0800 to 1800 h). To account for possible tidal and temporal influences, observations were distributed throughout the day (morning and afternoon) and different tidal regimes (ebb and flood). The fish assemblage was assessed using a belt-transect (30 m x 2 m), based on the belt-transect visual census method (Brock 1954). All censuses were made by the same diver, swimming at a constant speed, having previously stood by for five minutes to minimise disturbance caused by the diver's presence (Russ 1989). Fishes were identified according to Humann and Deloach (2002a), Carvalho-Filho (2023) and Floeter et al. (2023), counted and had their size visually estimated to the nearest centimetre (Fig. 2). Sampling was conducted randomly in selected areas of eight localities with predominantly (75%) consolidated substrate (Luckhurst and Luckhurst 1978, Ohlhorst et al. 1988, Littler et al. 1989, Rogers et al. 1994, Humann and Deloach 2002b).
Figure 2.
A plate with representative reef fish species observed during the visual censuses in the Fernando de Noronha Archipelago, Southwest Atlantic. A Stegastesrocasensis; B Bothuslunatus; C Sparisomafrondosum; D Caranxlatus; E Holocentrusadscensionis; F Muraenapavonina. Photos by Allan T. Souza.
Quality control
While visual censuses are often used to estimate fish populations on reefs, they have several limitations (Harvey et al. 2004, Bernard et al. 2013). Observer bias and varying skill levels can affect accuracy and lead to inconsistent data. Fish behaviour, such as avoiding divers or hiding, skews counts, especially for shy or nocturnal species, can vary significantly. Water clarity and light conditions also affect visibility and identification. In addition, visual censuses usually only cover small areas, so wider population trends may be missed. All of these factors limit the reliability and representativeness of visual censuses for the comprehensive assessments of fish populations on reefs and, therefore, these known issues must be taken into account when using this dataset. On the other hand, visual censuses have the advantage that they are non-lethal, providing instant access to the data on species and are also cheaper and easier to implement than other sampling methods.
Geographic coverage
Description
This study was carried out in eight different localities (Atalaia, Baía dos Golfinhos, Boldró, Buraco da Raquel, Porto de Santo Antônio, Sancho, Sueste (open), Sueste (protected) within the Archipelago of Fernando de Noronha, Southwest Atlantic, Brazil (Fig. 1).
Coordinates
-3.86886 and -3.83987 Latitude; -32.44712 and -32.40372 Longitude.
Taxonomic coverage
Description
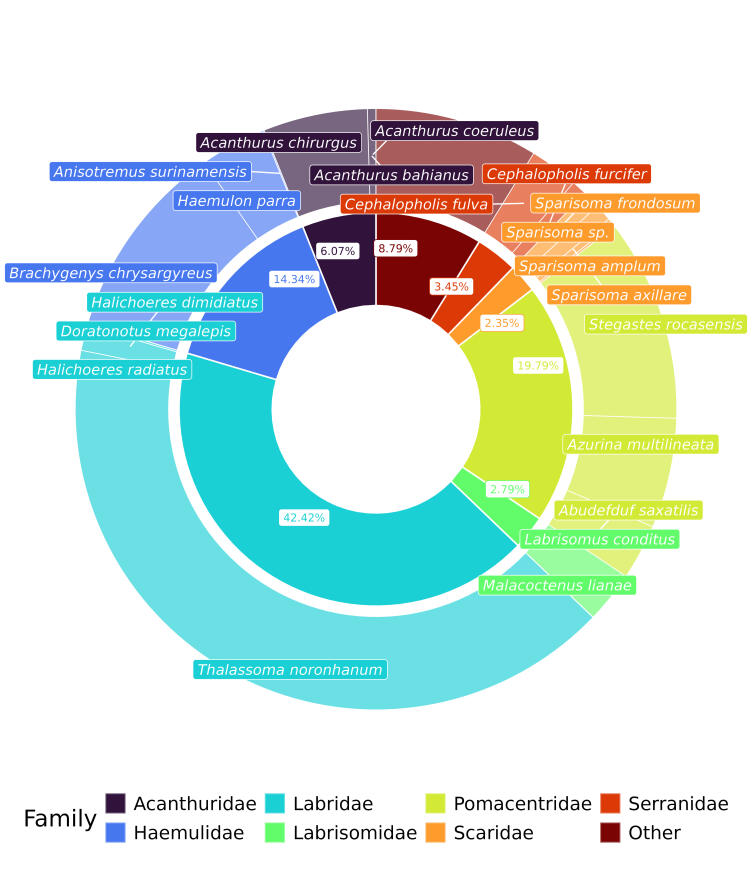
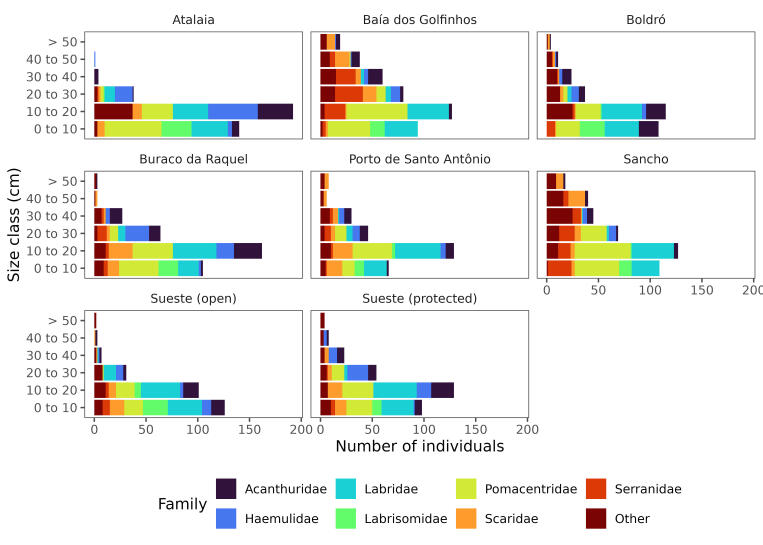
The dataset contains the records of 15,065 individuals belonging to 51 species and two unidentified species, from 29 families (Fig. 3) with Thalassomanoronhanum and Stegastesrocasensis representing the vast majority of the individuals (Fig. 3). On average, the largest individuals were recorded at Baía dos Golfinhos and Sancho, while Atalaia and Buraco da Raquel made the largest contribution of small fish individuals (Fig. 4). The taxa identification numbers (acceptedNameUsageID) were based on GBIF Backbone Taxonomy (GBIF Secretariat 2022). The scientific names of the taxa, their authorship and year and original descriptions followed Fricke et al. (2024), whereas the common name of the species was based on FishBase (Froese and Pauly 2024).
Figure 3.
Sunburst plot showing the proportion of the most representative species per family observed in the visual censuses made in the Fernando de Noronha Archipelago, Southwest Atlantic. The inner circle shows the percentage of individuals recorded by families, while the outer circle displays the proportional abundance of the most representative species recorded in this study. The seven most abundant fish families are displayed individually, while the remainder families are pooled together in the category named Other which contains fishes from 22 families (Aulostomidae, Balistidae, Belonidae, Blennidae, Bothidae, Carangidae, Carcharhinidae, Cheatodontidae, Clupeidae, Dasyatidae, Gobiidae, Hemiramphidae, Holocentridae, Kyphosidae, Lutjanidae, Monacanthidae, Mullidae, Muraenidae, Ophichthidae, Ostraciidae, Pomacanthidae and Sphyraenidae) and 32 species (Acanthostracionpolygonium, Aluterusscriptus, Aulostomusstrigosus, Bothuslunatus, Cantherhinespullus, Carangoidesbartholomaei, Caranxcrysos, C.latus, C.lugubris, Chaetodonocellatus, Coryphopterusglaucofraenum, Echidnacatenata, Gymnothoraxmiliaris, Harengulajaguana, Hemiramphusbrasiliensis, Holocentrusadscensionis, Hypanusberthalutzae, Kyphosus sp., Lactophrystrigonus, Lutjanusjocu, Melichthysniger, Mulloidichthysmartinicus, Muraenapavonina, Myrichthysocellatus, Myripristisjacobus, Negaprionbrevirostris, Ophioblenniustrinitatis, Platybeloneargalus, Pomacanthusparu, Pseudupeneusmaculatus, Sphyraenabarracuda and Sphyraenaguachancho).
Figure 4.
Size class (0-10; 10-20; 20-30; 30-40; 40-50; > 50 cm) per study area of the most representative fish families observed in the visual censuses made in the Fernando de Noronha Archipelago, SW Atlantic. Less representative families were pooled together and were displayed as Other in the graph. The Other category contains fishes from 22 families (Aulostomidae, Balistidae, Belonidae, Blennidae, Bothidae, Carangidae, Carcharhinidae, Cheatodontidae, Clupeidae, Dasyatidae, Gobiidae, Hemiramphidae, Holocentridae, Kyphosidae, Lutjanidae, Monacanthidae, Mullidae, Muraenidae, Ophichthidae, Ostraciidae, Pomacanthidae and Sphyraenidae) and 32 species (Acanthostracionpolygonium, Aluterusscriptus, Aulostomusstrigosus, Bothuslunatus, Cantherhinespullus, Carangoidesbartholomaei, Caranxcrysos, C.latus, C.lugubris, Chaetodonocellatus, Coryphopterusglaucofraenum, Echidnacatenata, Gymnothoraxmiliaris, Harengulajaguana, Hemiramphusbrasiliensis, Holocentrusadscensionis, Hypanusberthalutzae, Kyphosus sp., Lactophrystrigonus, Lutjanusjocu, Melichthysniger, Mulloidichthysmartinicus, Muraenapavonina, Myrichthysocellatus, Myripristisjacobus, Negaprionbrevirostris, Ophioblenniustrinitatis, Platybeloneargalus, Pomacanthusparu, Pseudupeneusmaculatus, Sphyraenabarracuda and Sphyraenaguachancho).
Taxa included
| Rank | Scientific Name | Common Name |
|---|---|---|
| species | Abudefdufsaxatilis (Linnaeus, 1758) | Sergeant-major |
| species | Acanthostracionpolygonium Poey, 1876 | Honeycomb cowfish |
| species | Acanthurusbahianus Castelnau, 1855 | Barber surgeonfish |
| species | Acanthuruschirurgus (Bloch, 1787) | Doctorfish |
| species | Acanthuruscoeruleus Bloch & Schneider, 1801 | Blue tang surgeonfish |
| species | Aluterusscriptus (Osbeck, 1765) | Scribbled leatherjacket filefish |
| species | Anisotremussurinamensis (Bloch, 1791) | Black margate |
| species | Aulostomusstrigosus Wheeler, 1955 | Trumpetfish |
| species | Azurinamultilineata (Guichenot, 1853) | Brown chromis |
| species | Bothuslunatus (Linnaeus, 1758) | Plate fish |
| species | Brachygenyschrysargyreus (Günther, 1859) | Smallmouth grunt |
| species | Cantherhinespullus (Ranzani, 1842) | Orangespotted filefish |
| species | Carangoidesbartholomaei (Cuvier, 1833) | Yellow jack |
| species | Caranxcrysos (Mitchill, 1815) | Blue runner |
| species | Caranxlatus Agassiz, 1831 | Horse-eye jack |
| species | Caranxlugubris Poey, 1860 | Black jack |
| species | Cephalopholisfulva (Linnaeus, 1758) | Coney |
| species | Cephalopholisfurcifer (Valenciennes, 1828) | Creole-fish |
| species | Chaetodonocellatus Bloch, 1787 | Spotfin butterflyfish |
| species | Coryphopterusglaucofraenum Gill, 1863 | Bridled goby |
| species | Doratonotusmegalepis Günther, 1862 | Dwarf wrasse |
| species | Echidnacatenata (Bloch, 1795) | Chain moray |
| species | Gymnothoraxmiliaris (Kaup, 1856) | Goldentail moray |
| species | Haemulonparra (Desmarest, 1823) | Sailor's grunt |
| species | Halichoeresdimidiatus (Agassiz, 1831) | |
| species | Halichoeresradiatus (Linnaeus, 1758) | Puddingwife wrasse |
| species | Harengulajaguana Poey, 1865 | Scaled herring |
| species | Hemiramphusbrasiliensis (Linnaeus, 1758) | Ballyhoo halfbeak |
| species | Holocentrusadscensionis (Osbeck, 1765) | Squirrelfish |
| species | Hypanusberthalutzae Petean, Naylor & Lima, 2020 | Lutz's stingray |
| genus | Kyphosus Lacepède, 1801 | |
| species | Labrisomusconditus Sazima, Carvalho-Filho, Gasparini & Sazima, 2009 | Masquerader hairy blenny |
| species | Lactophrystrigonus (Linnaeus, 1758) | Buffalo trunkfish |
| species | Lutjanusjocu (Bloch & Schneider, 1801) | Dog snapper |
| species | Malacoctenuslianae Carvalho-Filho, Almeida, Britto, Dias & Lima, 2020 | Saddled blenny |
| species | Melichthysniger (Bloch, 1786) | Black triggerfish |
| species | Mulloidichthysmartinicus (Cuvier, 1829) | Yellow goatfish |
| species | Muraenapavonina Richardson, 1845 | Whitespot moray |
| species | Myrichthysocellatus (Lesueur, 1825) | Goldspotted eel |
| species | Myripristisjacobus Cuvier, 1829 | Blackbar soldierfish |
| species | Negaprionbrevirostris (Poey, 1868) | Lemon shark |
| species | Ophioblenniustrinitatis Miranda Ribeiro, 1919 | |
| species | Platybeloneargalus (Lesueur, 1821) | Keeltail needlefish |
| species | Pomacanthusparu (Bloch, 1787) | French angelfish |
| species | Pseudupeneusmaculatus (Bloch, 1793) | Spotted goatfish |
| species | Sparisomaamplum (Ranzani, 1841) | Reef parrotfish |
| species | Sparisomaaxillare (Steindachner, 1878) | Gray parrotfish |
| species | Sparisomafrondosum (Agassiz, 1831) | Agassiz's parrotfish |
| genus | Sparisoma Swainson, 1839 | |
| species | Sphyraenabarracuda (Edwards, 1771) | Great barracuda |
| species | Sphyraenaguachancho Cuvier, 1829 | Guachanche barracuda |
| species | Stegastesrocasensis (Emery, 1972) | Rocas gregory |
| species | Thalassomanoronhanum (Boulenger, 1890) | Noronha wrasse |
Temporal coverage
Data range: 2006-8-28 – 2006-10-28.
Usage licence
Usage licence
Other
IP rights notes
CC BY 4.0
Data resources
Data package title
fdd-reef-fish
Resource link
Number of data sets
2
Data set 1.
Data set name
event
Data format
txt
Download URL
Description
The event dataset includes 17 terms that follow the Darwin Core standard (Darwin Core Maintenance Group 2021) whenever possible. The dataset contains 165 events, with Atalaia, Baía dos Golfinhos, Buraco da Raquel, Porto de Santo Antônio and Sueste (open) having 20 events in each, while Boldró and Sueste (protected) had 21 events in each, with Sancho having 23 events. Events ranged from 28 August to 28 October 2006, with the earliest events starting at 07:55 h and the latest event starting at 17:15 h.
Data set 1.
| Column label | Column description |
|---|---|
| eventID | An identifier for the set of information associated with a dwc:Event (something that occurs at a place and time). May be a global unique identifier or an identifier specific to the dataset. |
| eventDate | The date-time or interval during which a dwc:Event occurred. For occurrences, this is the date-time when the dwc:Event was recorded. Not suitable for a time in a geological context. |
| eventTime | The time or interval during which a dwc:Event occurred. |
| startDayOfYear | The earliest integer day of the year on which the dwc:Event occurred (1 for 1 January, 365 for 31 December, except in a leap year, in which case it is 366). |
| country | The name of the country or major administrative unit in which the dcterms:Location occurs. |
| countryCode | The standard code for the country in which the dcterms:Location occurs. |
| locality | The specific description of the place. |
| locationID | An identifier for the set of dcterms:Location information. May be a global unique identifier or an identifier specific to the dataset. |
| geodeticDatum | The ellipsoid, geodetic datum or spatial reference system (SRS) upon which the geographic coordinates given in dwc:decimalLatitude and dwc:decimalLongitude are based. |
| coordinateUncertaintyInMetres | The horizontal distance (in metres) from the given dwc:decimalLatitude and dwc:decimalLongitude describing the smallest circle containing the whole of the dcterms:Location. Leave the value empty if the uncertainty is unknown, cannot be estimated or is not applicable (because there are no coordinates). Zero is not a valid value for this term. |
| decimalLatitude | The geographic latitude (in decimal degrees, using the spatial reference system given in dwc:geodeticDatum) of the geographic centre of a dcterms:Location. Positive values are north of the Equator, negative values are south of it. Legal values lie between -90 and 90, inclusive. |
| decimalLongitude | The geographic longitude (in decimal degrees, using the spatial reference system given in dwc:geodeticDatum) of the geographic centre of a dcterms:Location. Positive values are east of the Greenwich Meridian, negative values are west of it. Legal values lie between -180 and 180, inclusive. |
| samplingProtocol | The names of, references to, or descriptions of the methods or protocols used during a dwc:Event. |
| samplingEffort | The amount of effort expended during a dwc:Event. |
| sampleSizeValue | A numeric value for a measurement of the size (time duration, length, area or volume) of a sample in a sampling dwc:Event. |
| sampleSizeUnit | The unit of measurement of the size (time duration, length, area or volume) of a sample in a sampling dwc:Event. |
| recordedBy | A list (concatenated and separated) of names of people, groups or organisations responsible for recording the original dwc:Occurrence. The primary collector or observer, especially one who applies a personal identifier (dwc:recordNumber), should be listed first. |
Data set 2.
Data set name
occurrence
Data format
txt
Download URL
Description
The occurrence dataset includes 21 terms that follow the Darwin Core standard (Darwin Core Maintenance Group 2021) whenever possible. The dataset contains 2735 observations, from 53 different taxonomic entities (51 species and 2 genera). A total of 15065 individuals were recorded, ranging from 3 to 130 cm of Total Length (TL).
Data set 2.
| Column label | Column description |
|---|---|
| eventID | An identifier for the set of information associated with a dwc:Event (something that occurs at a place and time). May be a global unique identifier or an identifier specific to the dataset. |
| ownerInstitutionCode | The name (or acronym) in use by the institution having ownership of the object(s) or information referred to in the record. |
| basisOfRecord | The specific nature of the data record. |
| occurrenceID | An identifier for the dwc:Occurrence (as opposed to a particular digital record of the dwc:Occurrence). In the absence of a persistent global unique identifier, construct one from a combination of identifiers in the record that will most closely make the dwc:occurrenceID globally unique. |
| occurrenceStatus | For dwc:Occurrences, the default vocabulary is recommended to consist of present and absent, but can be extended by implementers with good justification. This term has an equivalent in the dwciri: namespace that allows only an IRI as a value, whereas this term allows for any string literal value. |
| kingdom | The full scientific name of the kingdom in which the dwc:Taxon is classified. |
| phylum | The full scientific name of the phylum or division in which the dwc:Taxon is classified. |
| order | The full scientific name of the order in which the dwc:Taxon is classified. |
| family | The full scientific name of the family in which the dwc:Taxon is classified. |
| genus | The full scientific name of the genus in which the dwc:Taxon is classified. |
| specificEpithet | The name of the first or species epithet of the dwc:scientificName. |
| scientificName | The full scientific name, with authorship and date information if known. When forming part of a dwc:Identification, this should be the name in lowest level taxonomic rank that can be determined. This term should not contain identification qualifications, which should instead be supplied in the dwc:identificationQualifier term. |
| establishmentMeans | Statement about whether a dwc:Organism has been introduced to a given place and time through the direct or indirect activity of modern humans. |
| taxonRank | The taxonomic rank of the most specific name in the dwc:scientificName. |
| taxonID | A global unique identifier for the taxon (name in a classification). |
| identifiedBy | A list (concatenated and separated) of names of people, groups or organisations who assigned the dwc:Taxon to the subject. |
| dateIdentified | The date on which the subject was determined as representing the dwc:Taxon. |
| identificationReferences | A list (concatenated and separated) of references (publication, global unique identifier, URI) used in the dwc:Identification. |
| organismQuantity | A number or enumeration value for the quantity of dwc:Organisms. |
| organismQuantityType | The type of quantification system used for the quantity of dwc:Organisms. |
| dynamicProperties | A list of additional measurements, facts, characteristics or assertions about the record. Meant to provide a mechanism for structured content. |
Additional information
This work was carried out in accordance with Brazilian legal requirements, including those related to the conservation and protection of animals.
Acknowledgements
The authors would like to thank Águas Claras diving centre, for allowing free use of its facilities and to IBAMA for issuing research permits to the Fernando de Noronha National Marine Park. MII acknowledges support from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and by the Graduate Program in Biological Science (Zoology) of Universidade Federal da Paraiba. Additionally, MII is currently supported by national funds through FCT – Foundation for Science and Technology, Portugal, within the scope of the Strategic Funding to CIIMAR (UIDB/04423/2020 and UIDP/ 04423/2020) and research contract (DL57/2016/CP1344/CT0018). This study has received funding from the European Union's Horizon Europe Research and Innovation Programme under grant agreement No 101057437 (BioDT project, https://doi.org/10.3030/101057437). This project has also received funding from the European Union's Horizon 2020 Research and Innovation Programme under grant agreement No 871128 (eLTER PLUS). Open access funded by Helsinki University Library.
Contributor Information
Martina I. Ilarri, Email: martinailarri@gmail.com.
Allan T. Souza, Email: allan.souza@helsinki.fi.
Author contributions
The authors have contributed to this data paper according to the following CRediTstatements.
Conceptualisation: MII, ATS
Data curation: MII, ATS
Formal analysis: ATS
Funding acquisition: MII
Investigation: MII, LV, HMG, RSR, ATS
Methodology: MII, ATS
Project administration: MII
Resources: MII
Software: MII, ATS
Supervision: ATS
Validation: MII, ATS
Visualisation: MII, ATS
Writing - original draft: MII, ATS
Writing - review & editing: MII, LV, HMG, RSR, ATS
References
- Bernard A. T.F., Götz A., Kerwath S. E., Wilke C. G. Observer bias and detection probability in underwater visual census of fish assemblages measured with independent double-observers. Journal of Experimental Marine Biology and Ecology. 2013;443:75–84. doi: 10.1016/j.jembe.2013.02.039. [DOI] [Google Scholar]
- Bettcher Vanessa B., Bucair Nayara, Granville Marta, Góes Amanda, Mendes Liana de Figueiredo, Di Dario Fabio, de Moura Rodrigo Leão, Garla Ricardo, Rangel Bianca S. First record of mating behaviour and induced parturition of the Brazilian endemic Lutz's stingray Hypanusberthalutzae. Journal of Fish Biology. 2022;102(1):172–177. doi: 10.1111/jfb.15249. [DOI] [PubMed] [Google Scholar]
- Brock Vernon E. A preliminary report on a method of estimating reef fish populations. The Journal of Wildlife Management. 1954;18(3) doi: 10.2307/3797016. [DOI] [Google Scholar]
- Bucair Nayara, Mendonça Sibele, Araújo Camila, Rangel Bianca S., Gadig Otto B. F. Records of bentfin devil ray, Mobulathurstoni, in a marine protected area in Brazilian Equatorial Atlantic: implications for the species’ distribution and local conservation strategies. Environmental Biology of Fishes. 2022;105(5):653–661. doi: 10.1007/s10641-022-01266-0. [DOI] [Google Scholar]
- Carvalho-Filho Alfredo, Sazima Ivan, Lima Sergio Maia Queiroz, Almeida Daniel, Mendes Liana, Dias Ricardo Marques, Britto Marcelo R, Gasparini JoÃo Luiz. Review of the genus Malacoctenus (Actinopterygii: Labrisomidae) from the Southwestern Atlantic, with description of two new species. Zootaxa. 2020;4819(3):4. doi: 10.11646/zootaxa.4819.3.4. [DOI] [PubMed] [Google Scholar]
- Carvalho-Filho A. Fishes of the Brazilian coast. Literare Books International; São Paulo: 2023. 424. English. [Google Scholar]
- Group Darwin Core Maintenance. List of Darwin Core terms. Biodiversity Information Standards (TDWG) https://dwc.tdwg.org/list/ https://dwc.tdwg.org/list/
- Fernández-Cisternas Italo, Majlis Jorge, Ávila-Thieme M. Isidora, Lamb Robert W., Pérez-Matus Alejandro. Endemic species dominate reef fish interaction networks on two isolated oceanic islands. Coral Reefs. 2021;40(4):1081–1095. doi: 10.1007/s00338-021-02106-w. [DOI] [Google Scholar]
- Floeter SR, Krajewski JP, Fiuza TMJ, Rocha LA, Carvalho-Filho A. Peixesrecifaisbrasileiros: Brazilian reef fishes. Editora CRV; Curitiba: 2023. 320. Portuguese. [DOI] [Google Scholar]
- Fricke R., Eschmeyer W. N., Van der Laan R. Genera, Species, References. Fricke R., Eschmeyer W. N., Van der Laan R., editors. Eschmeyer's catalog of fishes 2024
- Froese R, Pauly D. FishBase. World Wide Web electronic publication. www.fishbase.org. [2024-04-29T00:27:33+00:00]. www.fishbase.org
- Garla RC, Garcia J. Occurrence of the ragged-tooth shark, Odontaspisferox, at Fernando de Noronha Archipelago, western equatorial Atlantic. Marine Biodiversity Records. 2008;1:e38. doi: 10.1017/S1755267206003952. [DOI] [Google Scholar]
- Garla Ricardo C., Gadig Otto B. F., Garcia Junior José, Veras Leonardo B., Garrone-Neto Domingos. Hunting tactics of the lemon shark, Negaprionbrevirostris, in shallow waters of an oceanic insular area in the western equatorial Atlantic. Neotropical Ichthyology. 2017;15(1) doi: 10.1590/1982-0224-20160119. [DOI] [Google Scholar]
- Secretariat GBIF. GBIF Backbone Taxonomy. Checklist dataset. [2024-04-29T00:27:33+00:00]. [DOI]
- Harvey Euan, Fletcher David, Shortis Mark R., Kendrick Gary A. A comparison of underwater visual distance estimates made by scuba divers and a stereo-video system: implications for underwater visual census of reef fish abundance. Marine and Freshwater Research. 2004;55(6) doi: 10.1071/mf03130. [DOI] [Google Scholar]
- Humann P, Deloach N. Reef Fish Identification: Florida, Caribbean and Bahamas. 3rd Edition. New World Publications; 2002. 481. [Google Scholar]
- Humann P, Deloach N. Reef creature identification: Florida, Caribbean and Bahamas. 2nd Edition. New World Publications; Jacksonville: 2002. 420 pp. [Google Scholar]
- Ilarri M. I., Souza A. T., Rosa R. S. Community structure of reef fishes in shallow waters of the Fernando de Noronha archipelago: effects of different levels of environmental protection. Marine and Freshwater Research. 2017;68(7) doi: 10.1071/mf16071. [DOI] [Google Scholar]
- Kier Gerold, Kreft Holger, Lee Tien Ming, Jetz Walter, Ibisch Pierre L., Nowicki Christoph, Mutke Jens, Barthlott Wilhelm. A global assessment of endemism and species richness across island and mainland regions. Proceedings of the National Academy of Sciences. 2009;106(23):9322–9327. doi: 10.1073/pnas.0810306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewski João Paulo, Floeter Sergio R. Reef fish community structure of the Fernando de Noronha Archipelago (Equatorial Western Atlantic): the influence of exposure and benthic composition. Environmental Biology of Fishes. 2011;92(1):25–40. doi: 10.1007/s10641-011-9813-3. [DOI] [Google Scholar]
- Krajewski João Paulo, Floeter Sergio R., Jones Geoffrey P., Leite Fosca P. P. Patterns of variation in behaviour within and among reef fish species on an isolated tropical island: influence of exposure and substratum. Journal of the Marine Biological Association of the United Kingdom. 2011;91(6):1359–1368. doi: 10.1017/s0025315410000111. [DOI] [Google Scholar]
- Littler DS, Littler MM, Bucher KE, Norris JN. Marine plants of the Caribbean: A field guide from Florida to Brazil. 1st Edition. Smithsonian Institution Press; Washington: 1989. 263 pp [Google Scholar]
- Luckhurst B. E., Luckhurst K. Analysis of the influence of substrate variables on coral reef fish communities. Marine Biology. 1978;49(4):317–323. doi: 10.1007/bf00455026. [DOI] [Google Scholar]
- Medeiros Paulo R., Rosa Ricardo S., Francini-Filho Ronaldo B. Dynamics of fish assemblages on a continuous rocky reef and adjacent unconsolidated habitats at Fernando de Noronha Archipelago, tropical western Atlantic. Neotropical Ichthyology. 2011;9(4):869–879. doi: 10.1590/s1679-62252011005000048. [DOI] [Google Scholar]
- Mendes LF. Ophioblenniustrinitatis (Pisces: Blenniidae) from the oceanic archipelagos of São Pedro e São Paulo, Fernando de Noronha and Atol das Rocas. Brazilian Journal of Oceanography. 2007;55(1):63–65. doi: 10.1590/S1679-87592007000100008. [DOI] [Google Scholar]
- Mincarone Michael Maia, Eduardo Leandro Nolé, Di Dario Fabio, Frédou Thierry, Bertrand Arnaud, Lucena‐Frédou Flávia. New records of rare deep‐sea fishes (Teleostei) collected from off north‐eastern Brazil, including seamounts and islands of the Fernando de Noronha Ridge. Journal of Fish Biology. 2022;101(4):945–959. doi: 10.1111/jfb.15155. [DOI] [PubMed] [Google Scholar]
- Ohlhorst SL, Liddell WD, Taylor RJ, Taylor JM. Evaluation of reef census techniques; Proceedings of the 6th International Coral Reef Symposium; Qld, Australia. 8 August 1988; Townsville: 1988. 319–324 [Google Scholar]
- Pereira Pedro Henrique C., Leal Isabela Carolina S., de Araújo Maria Elisabeth, Souza Allan T. Feeding association between reef fishes and the fire coral Millepora spp. (Cnidaria: Hydrozoa) Marine Biodiversity Records. 2012;5 doi: 10.1017/s1755267212000073. [DOI] [Google Scholar]
- Pimentel Caio R., Rocha Luiz A., Shepherd Bart, Phelps Tyler A. Y., Joyeux Jean-Christophe, Martins Agnaldo S., Stein Carlos Eduardo, Teixeira João B., Gasparini João Luiz, Reis-Filho José Amorim, Garla Ricardo C., Francini-Filho Ronaldo B., Delfino Stephanie D. T., Mello Thayná J., Giarrizzo Tommaso, Pinheiro Hudson T. Mesophotic ecosystems at Fernando de Noronha Archipelago, Brazil (South-western Atlantic), reveal unique ichthyofauna and need for conservation. Neotropical Ichthyology. 2020;18(4) doi: 10.1590/1982-0224-2020-0050. [DOI] [Google Scholar]
- Rogers C. S, Garrison G, Crober R, Hillis Z. M, Franke M. A. Coral reef monitoring manual for the Caribbean and Western Atlantic. 1994.
- Russ G R. Distribution and abundance of coral reefs fishes in the Sumilon Island Reserve, central Philipines, after nine years of protectionfrom fishing. Asian Marine Biology. 1989;6:59–71. [Google Scholar]
- Salvetat Julie, Bez Nicolas, Habasque Jeremie, Lebourges-Dhaussy Anne, Lopes Cristiano, Roudaut Gildas, Simier Monique, Travassos Paulo, Vargas Gary, Bertrand Arnaud. Comprehensive spatial distribution of tropical fish assemblages from multifrequency acoustics and video fulfils the island mass effect framework. Scientific reports. 2022;12(1):8787. doi: 10.1038/s41598-022-12409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio CLS, Nunes JAC, Mendes LF. Acyrtuspauciradiatus, a new species of clingfish (Teleostei: Gobiesocidae) from Fernando de Noronha Archipelago, Pernambuco state, Northeastern Brazil. Neotropical Ichthyology. 2004;2(4):205–208. [Google Scholar]
- Schmid Kurt, Silva Fábio Renan Miranda da, Santos Bárbara Janaína Vieira dos, Bezerra Natalia Priscila Alves, Garla Ricardo Clapis, Giarrizzo Tommaso. First fish fauna assessment in the Fernando de Noronha Archipelago with BRUVS: Species catalog with underwater imagery. Biota Neotropica. 2020;20(4) doi: 10.1590/1676-0611-bn-2020-1014. [DOI] [Google Scholar]
- Smith-Vaniz William F., Tornabene Luke, Macieira Raphael M. Review of Brazilian jawfishes of the genus Opistognathus with descriptions of two new species (Teleostei, Opistognathidae) ZooKeys. 2018;794:95–133. doi: 10.3897/zookeys.794.26789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto JMR. Tubarões e raias (Chondricthyes) encontrados no Arquipélago de Fernando de Noronha durante expedições Arfenor I e II. Alcance. 1997;2:71–80. [Google Scholar]
- Soto J M R. Peixes do arquipélago Fernando de Noronha. Mare Magnum. 2001;1(2):147–169. [Google Scholar]
- Souza Allan T., Ilarri Martina I., Rosa Ierecê L. Habitat use, feeding and territorial behavior of a Brazilian endemic damselfish Stegastesrocasensis (Actinopterygii: Pomacentridae) Environmental Biology of Fishes. 2011;91(2):133–144. doi: 10.1007/s10641-010-9765-z. [DOI] [Google Scholar]
- Souza A. T., Ilarri M. I. Behavioral changes of a Brazilian endemic damselfish Stegastesrocasensis when guarding egg clutches. Environmental Biology of Fishes. 2014;97(11):1295–1303. doi: 10.1007/s10641-013-0215-6. [DOI] [Google Scholar]
- Villarins Bárbara Teixeira, Fischer Luciano Gomes, Prokofiev Artem Mikhailovich, Mincarone Michael Maia. A New Species of the Dragonfish Genus Melanostomias (Stomiidae: Melanostomiinae) from the Western Tropical Atlantic. Ichthyology & Herpetology. 2023;111(2) doi: 10.1643/i2022082. [DOI] [Google Scholar]