Abstract
4‐hydroxy 2‐methyl‐N‐(5‐methyl‐2‐thiazolyl)‐2H‐1, 2‐benzothiazin‐3‐carboxamide 1,1‐dioxide (QP001), a novel long‐lasting meloxicam formulation, may provide adequate postoperative pain relief with a good safety profile. This study aimed to evaluate the efficacy and safety of QP001 for moderate‐to‐severe pain following abdominal surgery. This multicenter, randomized, double‐blind, placebo‐controlled phase III trial recruited patients undergoing abdominal surgery at 23 centers between October 30, 2022, and July 10, 2023. Patients were randomized to a QP001 or placebo group in a 2:1 ratio. Postoperative pain intensity was evaluated using the Numerical Rating Scale. The primary efficacy outcome was the area under curve (AUC) of pain intensity‐time 0–24 h after awakening from anesthesia (AUC0–24). Adverse events and drug reactions were recorded to evaluate safety. A total of 258 patients underwent randomization, and 255 patients received at least one trial drug, including 170 in the QP001 group and 85 in the placebo group. Among these patients, 250 completed the study. The AUC0–24 was significantly lower in the QP001 group than in the placebo group (50.5 vs. 85.19, difference of 34.69 [40.7%], p < 0.0001). This was accompanied by a decrease in morphine use and an increase in patient satisfaction. Moreover, the overall adverse events or adverse drug reaction rates were similar between the QP001 and placebo groups. Among patients undergoing abdominal surgery, postoperative pain was significantly lower in the QP001 group than in the placebo group. QP001 has a great analgesic effect of up to 24 h and satisfactory safety in patients with moderate‐to‐severe abdominal pain.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Postoperative pain is a common complication after surgery. Although numerous medicines and approaches have been used in clinical practice, postoperative pain management still faces challenges, such as insufficient analgesic efficacy, relatively short duration, and adverse drug reactions (ADRs) or side effects.
WHAT QUESTION DID THIS STUDY ADDRESS?
In this multicenter, large‐scale study conducted in 23 hospitals in China, we demonstrated that QP001 is a novel solution formulation of meloxicam. It has a great analgesic effect in Chinese patients with moderate‐to‐severe postoperative pain following abdominal surgery, with an analgesic effect of up to 24 h and a favorable safety profile.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
QP001 is a novel solution formulation of meloxicam, with an analgesic effect of up to 24 h. Its sustained‐release property reduces the frequency of administration, making postoperative pain management more convenient and effective. Therefore, QP001 provides a better option for the management of postoperative pain in hospitalized patients in clinical practice in China.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The newly designed long‐lasting analgesia QP001 is very suitable for postoperative pain management. Especially for the patients who have undergone hysterectomy or other abdominal surgeries, such as myomectomy, colorectal resection, and nephrectomy. The current situation of postoperative analgesia management for hospitalized patients is expected to improve after approval for use of QP001.
INTRODUCTION
Postoperative pain is a common complication after surgery, and it greatly reduces the quality of life of affected patients and delays the recovery of physical function, with an increase in the occurrence of negative outcomes, such as delayed wound healing, prolonged hospital stay, and increased medical costs. 1 , 2 , 3 , 4 Moreover, in some patients, it progresses to chronic pain. 5 , 6 , 7 Although numerous medicines, such as morphine and nonsteroidal anti‐inflammatory drugs (NSAIDs) represented by meloxicam, and approaches, such as analgesic pumps and multimodal analgesia, have been widely used in clinical practice, postoperative pain management still faces challenges, such as insufficient analgesic efficacy, relatively short duration of action, and adverse drug reactions (ADRs) or side effects. 8 , 9 Thus, it is very valuable and urgent to explore novel drugs to overcome these clinical challenges.
QP001 is a novel solution formulation of meloxicam, which has been specifically designed to address moderate‐to‐severe postoperative pain. It is expected to demonstrate improved water solubility, rapid onset of action, prolonged duration of action, and potent analgesic efficacy following intravenous administration. 10 , 11 A phase I clinical study of QP001 involving single dose and multiple administrations in Chinese healthy volunteers has been completed. 11 Moreover, a phase II clinical study has assessed the use of 30 and 60 mg of QP001 in patients with moderate‐to‐severe pain after abdominal surgery. 10 Both these trials provide encouraging evidence for the excellent analgesic characteristics and minimal side effects of QP001.
We sequentially undertook this phase III clinical trial to further assess the efficacy and safety of QP001 in patients undergoing abdominal surgery who are at risk for moderate‐to‐severe postoperative pain. Through this trial, we will attempt to answer the following questions: (1) Does QP001 injection result in a lower area under the curve (AUC) of pain intensity‐time from 0 to 24 h after awakening from anesthesia (AUC0–24) than placebo? (2) Does QP001 injection have a favorable safety profile with respect to the incidence of adverse events (AEs) and ADRs?
METHODS
Study design
This multicenter, randomized, double‐blind, placebo‐controlled phase III trial included patients with moderate‐to‐severe pain following abdominal surgery. The trial was registered at the Chinese Clinical Trial Register (ChiCTR2300075629). The study protocol was formulated through discussions among multiple experts and in compliance with laws and regulations. The trial was conducted by 23 hospitals in China.: Renji Hospital, Shanghai Jiao Tong University School of Medicine is the team leader unit. The participated hospitals were all central hospitals of the city and/or affiliated hospitals of medical colleges/universities. Among them, are 7 hospitals located in the eastern of China, 9 hospitals in the central region, and 7 hospitals in the western region. Detailed information about participated hospitals is listed in Table S1. This trial was approved by the Ethics Committee of Renji Hospital, Shanghai Jiao Tong University School of Medicine (2022‐031‐A) and was performed in accordance with the Helsinki Declaration and the International Conference on Harmonization Good Clinical Practice Guideline. All of the clinical steps were strictly conducted according to ethical standards. Moreover, the trial was approved by the National Medical Products Administration (2021LP00439).
Patients
This study enrolled adult inpatients undergoing abdominal surgery. All patients provided written informed consent. The inclusion criteria were as follows: (1) age 18–65 years (gender not limited), (2) ability to fully understand and voluntarily participate in the research and sign the informed consent form, (3) decision to undergo total hysterectomy under general anesthesia (surgical incision length not limited) or other abdominal surgery under general anesthesia (myomectomy, colectomy, etc., expected single incision length of ≥3 cm), with an expected operation time of 1–3 h, (4) American Society of Anesthesiologists (ASA) classification of I/II, (5) body mass index of ≥18 and ≤30 kg/m2, and (6) ability to understand the research process and pain scales and communicate effectively with researchers.
The exclusion criteria were as follows: (1) known allergies or contraindications to meloxicam, excipients, aspirin, other NSAIDs, and other drugs that may be used during the trial, (2) use of drugs within 5 half‐lives before randomization (7 days before randomization if the half‐life was unclear) and drugs that were judged to influence the analgesic effect (according to the actual drug instructions), including but not limited to NSAIDs, monoamine oxidase inhibitors, glucocorticoids (except aerosol inhalation), sedatives, antiepileptic drugs, antidepressants, anticonvulsants, antianxiety drugs, etc., (3) use of chemoradiotherapy, hyperthermic perfusion, or other biological therapy for cancer within 60 days before randomization or plan to receive these treatments during the study, (4) open or laparoscopic surgery and/or a history of myocardial infarction or coronary artery bypass grafting within 1 year before randomization, (5) high bleeding risk, including congenital bleeding disorders (such as hemophilia), abnormal platelet function (such as idiopathic thrombocytopenic purpura, disseminated intravascular coagulation, and congenital abnormal platelet function), significant active bleeding (except active bleeding due to the expected surgical lesion), or active bleeding disease complicated with a peptic ulcer, perforation, or other condition within 6 months before randomization, which might be aggravated by taking NSAIDs, (6) cerebral ischemic disease, seizures, and other central nervous system diseases, which were judged to influence the efficacy evaluation, (7) other pain and other physical pain conditions that may confound postoperative pain evaluations as judged by the investigators, (8) poor blood pressure control (sitting systolic blood pressure [SBP] ≥160 mmHg, sitting diastolic blood pressure ≥ 100 mmHg, and/or sitting SBP <90 mmHg) during screening, (9) abnormal laboratory test results during the screening period, including (a) random blood glucose ≥11.1 mmol/L, (b) aspartate aminotransferase or alanine aminotransferase ≥1.5 times the upper limit of normal (ULN) and/or total bilirubin ≥1.5 times the ULN, (c) serum creatinine (Cr) ≥1.5 times the ULN, (d) prothrombin time prolonged beyond the ULN for 3 s and/or activated partial thromboplastin time prolonged beyond the ULN for 10 s, (e) platelet count <80 × 109/L and/or hemoglobin <90 g/L, and (f) QTc >450 millisecond for male patients and >470 millisecond for female patients (QTc is calculated using the Fridericia formula, QTcF = QT/[RR0.33]), (10) syphilis antibody or human immunodeficiency virus antibody positivity during screening, (11) blood donation or blood loss of >400 mL within 3 months before randomization, blood transfusion, or use of blood products, (12) pregnancy or breastfeeding, (13) plan for reproduction during the trial and within 3 months after the trial, and unwillingness or inability to use effective contraception, (14) participation in other drug or device clinical studies and use of treatment within 30 days before randomization; (15) history of alcohol abuse, and a history of opioid use, and (16) other situations considered inappropriate for participation in this trial by the investigators.
Study procedure
The enrolled patients were randomly assigned in a 2:1 ratio to receive QP001 (30 mg, intravenous bolus) or placebo (same volume of normal saline).
The first injection was administered 10 min before the incision, and the second one was administered 24 h (±15 min) after the initial injection. During the surgery, propofol, sufentanil, remifentanil, and inhaled anesthetics were used to induce and maintain general anesthesia. Immediately after the surgery, remifentanil infusion was stopped and an additional injection of morphine (2.5 mg) was administered. Other opioid or nonopioid analgesics were prohibited throughout the anesthesia duration. Patients, health care providers, data collectors, and outcome adjudicators were unaware of the group assignment.
Pain intensity was assessed using the Numerical Rating Scale (NRS; 0–10 points [0, no pain; 10, worst pain]) immediately after the patients recovered from anesthesia. The NRS score was assessed at rest. Anesthesia resuscitation was recorded as 0 h, and pain intensity was evaluated at 0, 1, 2, 3, 6, 9, 12, 15, 18, 21, 24, 30, 36, 42, and 48 h sequentially. The AUC of pain intensity‐time was calculated as the pain index. If the patient complained of pain and the NRS score was over 4 at any point, a single dose of morphine (2 mg) was administered intravenously as remedial analgesia treatment. A 15‐min interval was recommended to ensure safety. The dose of morphine used for remedial analgesia was recorded and added to the total amount of morphine. The first‐time use and frequency of use of remedial analgesics, and the proportion of patients requiring remedial analgesia within 24 and 48 h after the first administration of QP001 were recorded. From 48 h after anesthesia recovery to 5 ± 1 days, patients were followed up and safety assessments, such as laboratory examination, electrocardiography, vital sign evaluation, and physical examination, were conducted.
Primary outcome
The primary outcome was the efficacy of QP001 injection for managing postoperative pain: AUC0–24.
Secondary outcomes
The secondary outcomes were as follows: (1) AUC of pain intensity‐time at different intervals (AUC24–48, AUC0–48, AUC18–24, and AUC42–48); (2) total use of morphine; (3) amount of remedial morphine; (4) first‐time use of remedial analgesics; (5) proportion of patients requiring remedial analgesia within 24 and 48 h after QP001 administration; (6) frequency of remedial analgesia within 24 and 48 h after QP001 administration; and (7) overall satisfaction score of patients.
Other outcomes
According to the Common Terminology Criteria for Adverse Events (CTCAE) 5.0, safety assessments were conducted for the following: (1) any spontaneously reported and all directly observed AEs, ADRs, and severe adverse events (SAEs) and (2) abnormalities or changes in vital signs, physical examination results, laboratory test results (blood routine, blood biochemistry, urinalysis, coagulation function, etc.), electrocardiogram findings, etc.
Randomization, blinding, and sample size estimation
Randomization was performed by means of a central randomization system (interactive web response system). The enrolled patients were randomly assigned in a 2:1 ratio to the QP001 or placebo group. Eligible patients received a unique random number on enrollment. The study adopted a double‐blind design, with the evaluation researchers and patients blinded to the group assignment (drug formulation and administration were carried out by a nonblinded researcher, and the syringe was shielded to keep the evaluators and patients blinded).
A t‐test was performed to compare the means between the QP001 and placebo groups. The sample size was estimated based on the results of the phase II trial (AUC0–24). 10 We assumed that the AUC0–24 in the QP001 group would not be equal to that in the placebo group. The AUC0–24 was 43 (43.22 ± 21.32, Mean ± SD) in the QP001 group and 51 (50.85 ± 19.42, Mean ± SD) in the placebo group, and the combined standard deviation (SD) of the groups was 20. According to a 2:1 ratio, an α value of 0.025 (single‐sided), a power of 80%, and a dropout rate of 15%, 258 events were required (172 in the QP001 group and 86 in the placebo group). The sample size calculation formulas for the experimental (QP001) group and placebo group are:
where n E, Sample size of experimental (QP001) group; , Sample size of placebo group; r, the ration of QP001 and placebo group; σ, combined standard deviation; μ E , the mean of experimental (QP001) group; μ C , the mean of placebo group.
Details of the trial design have been provided in the Chinese Clinical Trial Register (ChiCTR2300075629).
Statistical analysis
All statistical analyses were conducted using the Statistical Analysis System (version 9.4; Statistical Analysis System Institute, Cary, NC, USA). A statistically significant difference was considered as a p‐value of <0.05 (two‐sided) for all treatment comparisons.
Analysis of baseline data
Continuous variables have been expressed as mean ± SD, and categorical variables have been expressed as frequencies and percentages.
Effectiveness analysis
To assess quantitative indicators, one‐way ANOVA was used for comparisons between the two groups, while to assess qualitative indicators, the χ 2 test was used. To assess time‐event indicators, the Kaplan–Meier method was used for estimating the median time and 95% confidence interval, and the log‐rank test was used for comparisons between the two groups. The Wilcoxon rank sum test was used to assess the frequency indicators and the overall satisfaction scores for analgesia.
The efficacy analysis included a main analysis and a supplementary analysis. The main analysis was performed using the full analysis set (FAS), which included all randomized patients who received at least one dose of medication. The supplementary analysis was performed using the per‐protocol set (PPS), which included eligible, randomized patients with no major protocol deviations affecting treatment efficacy.
Safety analysis
The safety analysis was performed using the safe analysis set, which included all randomized patients who received at least one dose of the study drug and underwent at least one baseline safety assessment. AEs and ADRs have been expressed as frequencies and percentages. Fisher's exact probability method was used to compare adverse events between the two groups.
RESULTS
We recruited patients from October 30, 2022, to July 10, 2023, at 23 hospitals in China. A total of 302 patients were screened, and of these, 44 were excluded (25 did not meet the inclusion criteria or met the exclusion criteria and 19 withdrew informed consent), leaving 258 patients to be randomly assigned to the QP001 or placebo group. Three patients (2 in the QP001 group and 1 in the placebo group) failed to receive the investigational drug, and finally, 170 patients were in the QP001 group and 85 were in the placebo group. In the QP001 group, 8 patients withdrew from the trial, and in the placebo group, 6 patients withdrew. Finally, 241 patients (162 in the QP001 group and 79 in the placebo group) were included in the PPS. The detailed information and data set are shown in Figure 1.
FIGURE 1.
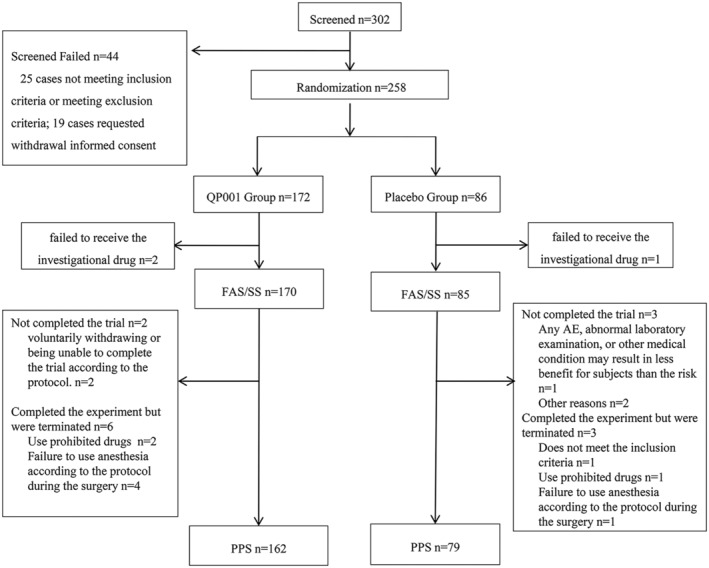
Patients screening and enrollment process diagram. FAS, full analysis set; PPS, per protocol set; SS, safety analysis set.
General characteristics
The baseline characteristics of the patients are presented in Table 1. The two groups were well‐matched at baseline. Overall, the average age and BMI of the patients were 47.9 years and 24.08 kg/m2, respectively. The types of surgeries, average size of the longest incision, surgical duration, and recovery time (hours) were similar between the groups.
TABLE 1.
Baseline characteristics of the patients.
| QP001 group (N = 170) | Placebo group (N = 85) | ||
|---|---|---|---|
|
Age (year) Mean ± SD |
48.1 ± 8.1 | 47.4 ± 7.6 | |
| Sex (n, %) | Male | 23 (13.5) | 10 (11.8) |
| Female | 147 (86.5) | 75 (88.2) | |
|
Height (cm) Mean ± SD |
160.2 ± 5.8 | 159.6 ± 6.6 | |
|
Weight (kg) Mean ± SD |
61.80 ± 8.22 | 61.66 ± 9.38 | |
|
BMI (kg/m2) Mean ± SD |
24.05 ± 2.71 | 24.13 ± 2.72 | |
| ASA grade (n, %) | I | 59 (34.7) | 28 (32.9) |
| II | 111 (65.3) | 57 (67.1) | |
| Type of surgery (n, %) | Hysterectomy | 104 (61.2) | 49 (57.6) |
| Excision of uterine lesions | 1 (0.6) | 0 (0.0) | |
| Myomectomy | 25 (14.7) | 20 (23.5) | |
| Colorectal or rectal resection | 15 (8.8) | 5 (5.9) | |
| Nephrectomy | 23 (13.5) | 9 (10.6) | |
| Gastrectomy | 2 (1.2) | 1 (1.2) | |
| Adnexectomy | 0 (0.0) | 1 (1.2) | |
| Type of anesthesia (n, %) | Intravenous anesthesia | 25 (14.7) | 15 (17.6) |
| General anesthesia | 145 (85.3) | 70 (82.4) | |
|
Surgical duration (h) Mean ± SD |
2.08 ± 1.07 | 2.06 ± 1.13 | |
|
Wake‐up time (h) Mean ± SD |
0.28 ± 0.19 | 0.27 ± 0.15 | |
|
The size of the longest incision (cm) Mean ± SD |
6.49 ± 4.04 | 7.04 ± 3.34 |
Abbreviations: BMI, body mass index; SD, standard deviation.
Primary outcome
AUC0 –24 results
The main analysis using the FAS showed that the AUC0–24 was significantly lower in the QP001 group than in the placebo group (50.50 ± 23.31 vs. 85.19 ± 28.85, difference of 34.69 [40.7%], p < 0.0001) (Table 2 and Figure 2a). The supplementary analysis using the PPS showed a similar finding (50.00 ± 22.87 vs. 83.23 ± 28.23, difference of 33.23 [39.9%], p < 0.0001). According to the pain intensity‐time curve within 48 h, the NRS score was significantly lower in the QP001 group than in the placebo group at each time point (Figure 2b).
TABLE 2.
Summary of efficacy endpoints.
| QP001 group, Mean ± SD | Placebo group, Mean ± SD | MD (95% CI) | p‐value | |
|---|---|---|---|---|
| Primary outcomes: The AUC0–24 of pain intensity‐time | ||||
| FAS main analysis |
N = 170 50.50 ± 23.31 |
N = 85 85.19 ± 28.85 |
−34.69 ± 3.36 (−41.30, 28.07) | <0.0001 |
| PPS supplementary analysis |
N = 162 50.00 ± 22.87 |
N = 79 83.23 ± 28.23 |
−33.23 ± 3.40 (−39.92, −26.54) | <0.0001 |
| Secondary outcomes (FAS) | ||||
| The AUC24–48 of pain intensity‐time | 36.3 ± 16.7 | 57.0 ± 22.8 | −20.7 ± 2.5 (−25.7, −15.8) | <0.0001 |
| The AUC0–48 of pain intensity‐time | 86.77 ± 35.90 | 142.19 ± 46.86 | −55.42 ± 5.30 (−65.85, −44.99) | <0.0001 |
| The AUC18–24 of pain intensity‐time | 8.67 ± 6.60 | 18.12 ± 9.20 | −9.45 ± 1.00 (−11.43, −7.47) | <0.0001 |
| The AUC42–48 of pain intensity‐time | 6.0 ± 5.5 | 10.4 ± 6.4 | −4.4 ± 0.8 (−5.9, −2.8) | <0.0001 |
| Total use of morphine within 24 h (mg) | 4.19 ± 3.25 | 7.35 ± 4.12 | −3.15 ± 0.47 (−4.08, −2.22) | <0.0001 |
| Total use of morphine within 48 h (mg) | 4.36 ± 3.77 | 8.08 ± 5.50 | −3.72 ± 0.59 (−4.87, −2.56) | <0.0001 |
| Total use of morphine within 24–48 h (mg) | 0.2 ± 1.0 | 0.7 ± 1.9 | −0.6 ± 0.2 (−0.9, −0.2) | 0.0027 |
| Amount of remedial morphine within 24 h (mg) | 1.7 ± 3.2 | 4.8 ± 4.1 | −3.2 ± 0.5 (−4.1, −2.2) | <0.0001 |
| Amount of remedial morphine within 48 h (mg) | 1.9 ± 3.8 | 5.6 ± 5.5 | −3.7 ± 0.6 (−4.9, −2.6) | <0.0001 |
| Amount of remedial morphine within 24–48 h (mg) | 0.2 ± 1.0 | 0.7 ± 1.9 | −0.6 ± 0.2 (−0.9, −0.2) | 0.0027 |
| First‐time use of remedial analgesics (median 95%CI) | ‐ | 3.3 (2.6, 4.0) | ‐ | <0.0001 |
| Frequency of remedial analgesia required within 24 h (n) | 0.8 ± 1.6 | 2.4 ± 2.1 | −1.6 ± 0.2 (−2.0, −1.1) | <0.0001 |
| Frequency of remedial analgesia required within 48 h (n) | 0.9 ± 1.9 | 2.8 ± 2.7 | −1.9 ± 0.3 (−2.4, −1.3) | <0.0001 |
| Proportion of patients requiring remedial analgesia within 24 h (%) | 36.5 | 82.4 | RD (95% CI) −45.9 (−56.7, −35.0) | <0.0001 |
| Proportion of patients requiring remedial analgesia within 48 h (%) | 37.6 | 82.4 | RD (95% CI) −44.7 (−55.6, −33.8) | <0.0001 |
| Overall satisfaction score, n (%) | ||||
| 0 bad | 0 (0.0) | 1 (1.2) | ||
| 1 average | 0 (0.0) | 3 (3.5) | ||
| 2 good | 8 (4.7) | 11 (12.9) | ||
| 3 very good | 33 (19.5) | 33 (38.8) | ||
| 4 excellent | 128 (75.7) | 37 (43.5) | ||
| Total | 169 (100.0) | 85 (100.0) | <0.0001 | |
Abbreviations: AUC, area under curve; FAS, full analysis set; MD, mean difference; PPS, per‐protocol set; RD, risk difference; SD, standard deviation.
FIGURE 2.
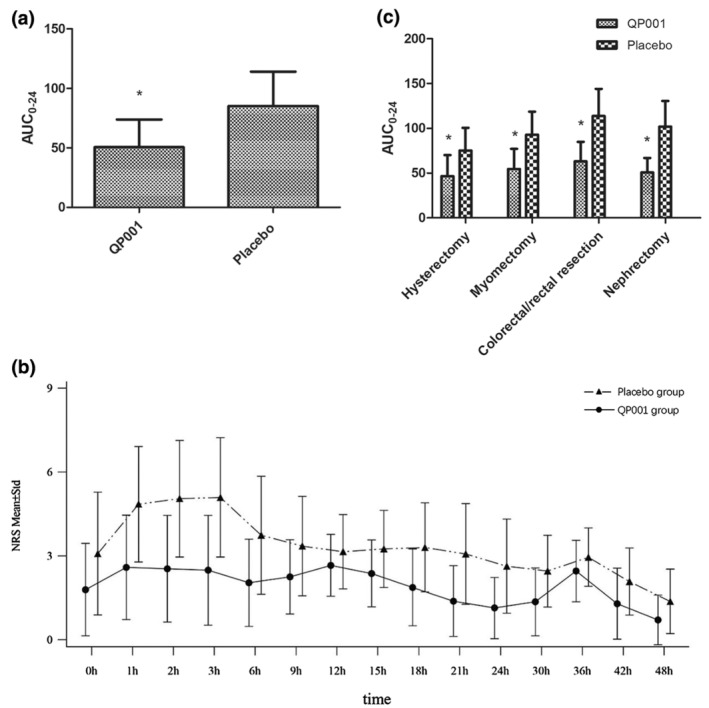
The AUC0–24 in the QP001 group was significantly decreased than that of the placebo group. (a) The AUC0–24 in the QP001 group was significantly decreased than the placebo group. (b) At each time point within 48 h the pain intensity‐time curve in the QP001 group was significantly lower than that of the placebo group. (c) Subgroup analysis showed that the AUC0–24 of each subgroup in the QP001 group was significantly reduced than the placebo group. AUC, area under curve; NRS, numerical rating scale. The data are expressed as the means ± SD. *p < 0.01 versus the Placebo group.
For the primary outcome, subgroup analysis was conducted according to the type of surgery. The AUC0–24 was significantly lower in the QP001 group than in the placebo group for hysterectomy (46.60 ± 23.53 vs. 75.21 ± 25.39, difference of 28.61 [38.0%], p < 0.0001), myomectomy (54.60 ± 22.54 vs. 92.90 ± 25.70, difference of 38.30 [41.2%], p < 0.0001), colorectal or rectal resection (63.03 ± 21.88 vs. 113.70 ± 30.32, difference of 50.67 [44.6%], p < 0.0001), and nephrectomy (50.70 ± 16.26 vs. 101.89 ± 28.65, difference of 51.19 [50.2%], p < 0.0001). Details are provided in Figure 2c and Table 3.
TABLE 3.
Subgroup analysis of Primary outcomes AUC0–24 (FAS).
| Subgroup | QP001 group (N = 170) | Placebo group (N = 85) | ||
|---|---|---|---|---|
| Surgical types | Hysterectomy |
Mean ± SD p‐value |
46.60 ± 23.53 | 75.21 ± 25.39 |
| <0.0001 | ||||
| Myomectomy | Mean ± SD | 54.60 ± 22.54 | 92.90 ± 25.70 | |
| p‐value | <0.0001 | |||
| Colorectal or rectal resection | Mean ± SD | 63.03 ± 21.88 | 113.70 ± 30.32 | |
| p‐value | 0.0007 | |||
| Nephrectomy | Mean ± SD | 50.70 ± 16.26 | 101.89 ± 28.65 | |
| p‐value | <0.0001 |
Secondary outcomes
AUC24 –48, AUC0 –48, AUC18 –24, and AUC42 –48 results
The AUC24–48 and AUC0–48 were significantly lower in the QP001 group than those in the placebo group (36.3 ± 16.7 vs. 57.0 ± 22.8, difference of 20.7 [36.3%], p < 0.0001 and 86.77 ± 35.90 vs. 142.19 ± 46.86, difference of 55.42 [39.0%], p < 0.0001, respectively) (Table 2 and Figure 2b).
The AUC18–24 and AUC42–48 were significantly lower in the QP001 group than those in the placebo group (8.67 ± 6.60 vs. 18.12 ± 9.20, difference of 9.45 [52.2%], p < 0.0001 and 6.0 ± 5.5 vs. 10.4 ± 6.4, difference of 4.4 [42.3%], p < 0.0001, respectively) (Table 2).
Total consumption of morphine and amount of remedial morphine
The total amounts of morphine used at 0–24, 0–48, and 24–48 h were significantly lower in the QP001 group than those in the placebo group (4.19 vs. 7.35 mg, difference of 3.15 [42.9%]; 4.36 vs. 8.08 mg, difference of 3.72 [46.0%]; and 0.2 vs. 0.7 mg, difference of 0.6 [85.7%], respectively, all p < 0.0001) (Table 2).
Moreover, the amounts of morphine used for remedial purposes at 0–24, 0–48, and 24–48 h were significantly lower in the QP001 group than those in the placebo group (1.7 vs. 4.8 mg, difference of 3.2 [66.7%], p < 0.0001; 1.9 vs. 5.6 mg, difference of 3.7 [66.1%], p < 0.0001; and 0.2 vs. 0.7 mg, difference of 0.6 [85.7%], p = 0.0027, respectively).
First‐time use of remedial analgesics
In the analysis of the first‐time use of remedial analgesics, as the proportion of patients using remedial analgesics in the QP001 group (37.6%) was less than 50%, the median time could not be calculated. However, the median time in the placebo group was 3.3 h. There was a significant difference between the two groups (p < 0.0001). The period of the first‐time use of remedial analgesics was longer in the QP001 group than in the placebo group, illustrating the effectiveness of QP001 (Table 2).
Proportion of patients requiring remedial analgesia within 24 and 48 h after QP001 administration
The proportions of patients requiring pain relief within 24 and 48 h were significantly lower in the QP001 group than those in the placebo group (36.5% vs. 82.4%, difference of 45.9% and 37.6% vs. 82.4%, difference of 44.7%, respectively, both p < 0.001) (Table 2).
Frequency of remedial analgesia within 24 and 48 h after QP001 administration
The frequencies of remedial analgesia required within 24 and 48 h were significantly lower in the QP001 group than those in the placebo group (0.8 vs. 2.4, difference of 1.6 [66.7%] and 0.9 vs. 2.8, difference of 1.9 [67.9%], respectively, both p < 0.001) (Table 2).
Overall satisfaction score of patients receiving analgesia
A 5‐level classification scale (0, poor; 1, average; 2, good; 3, very good; 4, excellent) was used to assess patients' satisfaction with pain relief at 48 h (±1 h). An excellent score was noted in 128 (75.7%) patients from the QP001 group and 37 (43.5%) from the placebo group (Table 2). No patient in the QP001 group had a “poor” or “average” score. Postoperative satisfaction with pain management was higher in the QP001 group than in the placebo group (p < 0.0001). The detailed information is shown in Table S7.
AE results
Overall incidences of AEs and ADRs
AEs mainly included nausea, vomiting, pelvic adhesions, abdominal adhesions, decreased blood pressure, anemia, bloating, and hypokalemia. Of the 255 patients, 215 (84.3%) experienced at least one AE, including 74 (87.1%) in the placebo group and 141 (82.9%) in the QP001 group.
The main ADRs included nausea, vomiting, decreased blood pressure, and anemia. Of the 255 patients, 145 (56.9%) had ADRs, including 52 (61.2%) in the placebo group and 93 (54.7%) in the QP001 group. No patient withdrew from the trial because of ADRs.
There were no significant differences in the incidences, types, and severities of AEs and ADRs between the two groups. Details of AEs and ADRs were provided in Tables S2–S5.
Almost all AEs and ADRs showed recovery or improvement. Only 1 patient in the placebo group had poor wound healing resulting in a SAE, which led to withdrawal from the trial (Table S6). There were no serious ADRs and no AEs leading to death during the study.
DISCUSSION
This trial provides promising evidence for the effectiveness and safety of QP001 injection. Among patients with moderate‐to‐severe pain after abdominal surgery, QP001 administration (30 mg) resulted in a significant decrease in the primary efficacy outcome (AUC0–24) compared with placebo administration. Additionally, in this large‐scale trial conducted across multiple centers, QP001 administration did not lead to a higher incidence of safety indicators (AEs and ADRs) compared with placebo administration, which strengthens the representativeness and persuasiveness of the analgesic efficacy of QP001.
As widely reported, NSAIDs are mainly used for postoperative pain management. 12 , 13 Among them, meloxicam is an excellent representative drug because of its strong analgesic effect and long‐lasting effect. 14 , 15 However, its limited water solubility renders it suboptimal for acute pain management. 14 , 15 , 16 QP001 injection has been designed as a novel solution formulation of meloxicam to address this drawback in patients with acute pain. A phase I study demonstrated that QP001 had rapid distribution, reaching peak concentrations immediately after intravenous administration, with the dose range of 15 to 60 mg being well tolerated. 11 A phase II study mainly focused on the duration of analgesic effects and showed that QP001 administration at either 30 or 60 mg provided effective and long‐lasting pain relief in patients with moderate‐to‐severe pain following abdominal surgery. 10 From the pharmacological perspective, when the effects are the same, it is advisable to choose the relatively lower doses to achieve effective treatment while avoiding side effects caused by the higher doses. Thus, this phase III study used a protocol of administering 30 mg once every 24 h after incision and twice consecutively. The results showed that the AUC values of pain intensity‐time were significantly decreased within 24 and 48 h, accompanied by a decrease in the amount of morphine used for remedial pain relief and an increase in patient satisfaction, with no serious AEs. Based on the results of the phase I, II, and III trials, QP001 injection has higher water solubility, faster onset, and long‐term analgesic effects compared with traditional NSAIDs, making it a preemptive candidate for managing acute postoperative pain after abdominal surgery.
However, when compared with long‐acting local anesthetics or nerve block analgesia, QP001 administration is more convenient, as pain relief can be achieved intravenously without the requirement of special operations such as local injections or nerve blocks. 17 , 18 Moreover, in cases where local anesthesia or nerve blocks are not suitable for certain types or areas of pain, effective and convenient injection is considered favorable and beneficial. Further, the finding that QP001 administration once every 24 h stably decreases the postoperative pain intensity in the later hours (18–24 and 42–48 h) of the 24‐h time windows without attenuation reaffirms its long‐term efficacy. QP001 has significant advantages in analgesic therapy, especially for postoperative pain management.
Abdominal surgery involves a wide range of surgical procedures, resulting in wide‐ranging pain and analgesia requirements. According to the literature, the degree of postoperative pain in laparoscopic hysterectomy may be moderate‐to‐severe. 19 In the study of gynecological laparoscopic postoperative pain conducted by Jong Bum Choi et al., almost all patients experienced various types of pain, with the majority of pain scores being above moderate. 20 The guidelines of “Postoperative pain management in nontraumatic emergency general surgery: WSES‐GAIS‐SIAARTI‐AAST (2022)” point out that postoperative pain in open or minimally invasive gastrointestinal surgery is mostly moderate‐to‐severe. 21 Therefore, the study selected participants who underwent hysterectomy or other abdominal surgeries, such as myomectomy and colorectal resection, with an expected single incision of over 3 cm, including subjects with moderate‐to‐severe postoperative abdominal pain.
The study designed normal saline as placebo based on the following factors: on the one hand, compared to placebo, the effectiveness of QP001 is more easily to be observed. The study designed the “remedial analgesia measures” to ensure that even subjects in the placebo group can also be well taken care of and experience good postoperative pain management. Actually, in our completed phase II abdominal surgery trial, normal saline was used as placebo control, combined with a morphine self‐control pump and morphine injection as remedial analgesia, no subjects experienced uncontrollable pain during the trial; on the other hand, the use of placebo was consistent with the “randomized, double‐blind, placebo‐controlled” standard which was recognized for the design of clinical trials. So, it is reasonable to choose normal saline as placebo for a parallel controlled trial.
In this study, the patients were from a relatively homogeneous surgical population, and there was no interference with other pain during the experiment. The use of other analgesic drugs during the perioperative period was strictly restricted. Therefore, the efficacy was evaluated without confounding variables, and the well‐controlled research design allowed monitoring of the efficacy and safety signals of the experimental drug. There are several other strengths of the trial. It is the first large‐scale trial of QP001 injection in East Asian populations. Moreover, it included 23 hospitals in China, and there was a high adherence to the experimental protocol and a high rate of follow‐up.
Limitations
There are some limitations of the study. Firstly, the trial was a multicenter study conducted in China rather than an international multicenter study; therefore, the results only represent the data of Chinese individuals. Secondly, the selected patients were not representative enough owing to the limitations of the types of surgeries, which may affect the inference of the results for the overall population. Third, the patients in the study were classified as ASA I/II, and the data of high‐risk individuals, such as obese and elderly individuals, are not yet clear.
CONCLUSIONS
QP001 injection has a great analgesic effect in patients with moderate‐to‐severe postoperative pain following abdominal surgery, with an analgesic effect of up to 24 h and a favorable safety profile. It is very suitable for pain relief in hospitalized patients who have undergone hysterectomy or other abdominal surgeries, such as myomectomy, colorectal resection, and nephrectomy. The current situation of postoperative analgesia is expected to improve after approval for the use of QP001.
AUTHOR CONTRIBUTIONS
X.L and D.S. wrote the manuscript. Y.Z., D.S., and W.Y. designed the research. Y.Z., D.S., and W.Y. analyzed the data. Y.Z., X.L., M.Y., J.R., W.O., S.W., Y.S., Y.G., L.Z., Z.Q., J.C., J.X., Ho.Z., Ha.Z.,J.Lv., Q.L., H.J., R.Z., K.Y., S.J., X.Z., C.W., Y.C., H.D., J.Li., S.Y., Y.J., Q.W., D.S., and W.Y. Yanhua Zhao, Xiaohua Liu and all of the other authors performed the research.
FUNDING INFORMATION
The study was supported by grants from the National Natural Science Foundation of China (81800553), Shanghai Municipal Health Commission Key Support Project (2023ZDFC0201).
CONFLICT OF INTEREST STATEMENT
The authors declared no competing interests for this work.
Supporting information
Table S1:
ACKNOWLEDGMENTS
The authors would like to acknowledge all investigators, nurses, and patients for their contributions to the development of this trial.
Liu X, Zhao Y, Yang M, et al. Efficacy and safety of QP001 , a fast‐acting meloxicam formulation, on moderate‐to‐severe pain following abdominal surgery: A phase III randomized controlled trial. Clin Transl Sci. 2024;17:e70081. doi: 10.1111/cts.70081
Yanhua Zhao and Xiaohua Liu contributed equally to this work.
Clinical trial registration: ChiCTR2300075629 (Chinese Clinical Trial Register).
Contributor Information
Diansan Su, Email: sudiansan@renji.com.
Weifeng Yu, Email: yuweifeng@renji.com.
REFERENCES
- 1. Kehlet H. Postoperative pain, analgesia, and recovery‐bedfellows that cannot be ignored. Pain. 2018;159(Suppl 1):S11‐s16. [DOI] [PubMed] [Google Scholar]
- 2. Rawal N. Organization, function, and implementation of acute pain service. Anesthesiol Clin North Am. 2005;23(1):211‐225. [DOI] [PubMed] [Google Scholar]
- 3. Argoff CE. Recent management advances in acute postoperative pain. Pain Pract. 2014;14(5):477‐487. [DOI] [PubMed] [Google Scholar]
- 4. Small C, Laycock H. Acute postoperative pain management. Br J Surg. 2020;107(2):e70‐e80. [DOI] [PubMed] [Google Scholar]
- 5. Macrae WA. Chronic post‐surgical pain: 10 years on. Br J Anaesth. 2008;101(1):77‐86. [DOI] [PubMed] [Google Scholar]
- 6. Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393(10180):1537‐1546. [DOI] [PubMed] [Google Scholar]
- 7. Chapman CR, Vierck CJ. The transition of acute postoperative pain to chronic pain: an integrative overview of research on mechanisms. J Pain. 2017;18(4):359.e351‐359.e338. [DOI] [PubMed] [Google Scholar]
- 8. Pirie K, Traer E, Finniss D, Myles PS, Riedel B. Current approaches to acute postoperative pain management after major abdominal surgery: a narrative review and future directions. Br J Anaesth. 2022;129(3):378‐393. [DOI] [PubMed] [Google Scholar]
- 9. Gan TJ. Poorly controlled postoperative pain: prevalence, consequences, and prevention. J Pain Res. 2017;10:2287‐2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou Y, Wang B, Duan K, et al. Preemptive QP001, a fast‐acting meloxicam formulation, provides analgesia and reduces opioid consumption following abdominal surgery: a randomized controlled trial. Inflammopharmacology. 2023;31(5):2401‐2410. [DOI] [PubMed] [Google Scholar]
- 11. Ma J, Huang J, Zou C, et al. A phase I study to evaluate the safety, tolerability, and pharmacokinetics of novel intravenous formulation of meloxicam (QP001) in healthy Chinese subjects. Drug Des Devel Ther. 2023;17:2303‐2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang RW, Tompkins DM, Cohn SM. Are NSAIDs safe? Assessing the risk‐benefit profile of nonsteroidal anti‐inflammatory drug use in postoperative pain management. Am Surg. 2021;87(6):872‐879. [DOI] [PubMed] [Google Scholar]
- 13. Gupta A, Bah M. NSAIDs in the treatment of postoperative pain. Curr Pain Headache Rep. 2016;20(11):62. [DOI] [PubMed] [Google Scholar]
- 14. Berkowitz RD, Mack RJ, McCallum SW. Meloxicam for intravenous use: review of its clinical efficacy and safety for management of postoperative pain. Pain Management. 2021;11(3):249‐258. [DOI] [PubMed] [Google Scholar]
- 15. Bekker A, Kloepping C, Collingwood S. Meloxicam in the management of post‐operative pain: narrative review. J Anaesthesiol Clin Pharmacol. 2018;34(4):450‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. IV meloxicam (Anjeso) for pain. Med Lett Drugs Ther. 2020;62(1601):100‐102. [PubMed] [Google Scholar]
- 17. Nestor CC, Ng C, Sepulveda P, Irwin MG. Pharmacological and clinical implications of local anaesthetic mixtures: a narrative review. Anaesthesia. 2022;77(3):339‐350. [DOI] [PubMed] [Google Scholar]
- 18. Lemoine A, Witdouck A, Beloeil H, Bonnet F. PROSPECT guidelines update for evidence‐based pain management after prostatectomy for cancer. Anaesthesia, Critical Care Pain Medicine. 2021;40(4):100922. [DOI] [PubMed] [Google Scholar]
- 19. Jiang B, Ye S. Pharmacotherapeutic pain management in patients undergoing laparoscopic cholecystectomy: a review. Adv Clin Exp Med. 2022;31(11):1275‐1288. [DOI] [PubMed] [Google Scholar]
- 20. Choi JB, Kang K, Song MK, Seok S, Kim YH, Kim JE. Pain characteristics after total laparoscopic hysterectomy. Int J Med Sci. 2016;13(8):562‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coccolini F, Corradi F, Sartelli M, et al. Postoperative pain management in non‐traumatic emergency general surgery: WSES‐GAIS‐SIAARTI‐AAST guidelines. World J Emerg Surg. 2022;17(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1:


