Abstract
Succinate dehydrogenase (SDH)-deficient renal cell carcinoma (RCC) is a rare subtype of RCC characterized by the presence of a germline mutation in one of the four subunits of the SDH enzyme complex (SDHA, SDHB, SDHC and SDHD). Together with a somatic second hit, these variants lead to the loss of function of the SDH complex. SDH-deficient RCC associated with SDHA mutation is a rare condition; to the best of our knowledge, there have been only four patients reported in the literature. The present study describes the case of a 22-year-old female patient with RCC associated with SDHA gene mutation. Next-generation sequencing and Sanger sequencing identified a novel heterozygous frameshift variant (NM_004168.4: c.992_999dup) in the SDHA gene. In the literature, this mutation has not previously been reported to be associated with RCC. The present description of a patient with a heterozygous SDHA frameshift variant expands the phenotypic spectrum of the SDHA gene, and provides further clinical, morphological and molecular data of SDHA-deficient RCC.
Keywords: succinate dehydrogenase, succinate dehydrogenase A, renal cell carcinoma
Introduction
Succinate dehydrogenase (SDH) is an enzyme complex located on the inner mitochondrial membrane that is composed of four subunits (SDHA, SDHB, SDHC and SDHD). This complex serves a dual role in converting succinate to fumarate during the Krebs cycle and participates in the electron transport chain (1). SDH deficiency leads to the accumulation of succinate, which inhibits proline hydroxylase activity and induces the accumulation of the hypoxia-inducible factor (HIF)-1α, thus activating vascular endothelial growth factor (VEGF) and insulin-like growth factor-1, and ultimately causing tumorigenesis (2). Germline mutations in any of the four subunits of SDH result in deficiency of the SDH complex associated with a group of hereditary tumors, including paraganglioma, phaeochromocytoma, gastrointestinal stromal tumor, pituitary adenoma and renal cell carcinoma (RCC) (3).
SDH-deficient RCC is a specific type of RCC that was first proposed in 2004 by Vanharanta et al (4), recognized by the International Society for Urological Pathology (ISUP) Vancouver in 2013 (5), and formally included as a subtype of RCC by the World Health Organization (WHO) in 2016 (6). SDH-deficient RCC is a rare malignancy with a high genetic correlation that accounts for 0.05–0.2% of all RCC cases. This subtype is usually caused by germline mutations with the addition of a somatic second hit, which leads to dysfunction of the SDH complex (7,8). The SDHB gene harbors most mutations, followed by SDHC and SDHD (9), while SDHA-deficient RCC is even rarer; to the best of our knowledge, only four patients have been reported in the literature to date (10–13).
The present study reports the clinical, morphological and molecular features of a new patient diagnosed with SDHA-deficient RCC harboring a novel SDHA mutation, and reviews the data of the four previously reported patients. Next-generation sequencing (NGS) and Sanger sequencing identified a novel heterozygous frameshift variant (NM_004168.4: c.992_999dup) in the SDHA gene, which has not been previously reported to be associated with RCC in the literature. This new case with a heterozygous SDHA frameshift variant expands the phenotypic spectrum of the SDHA gene, and provides further clinical, morphological and molecular data of SDHA-deficient RCC.
Case report
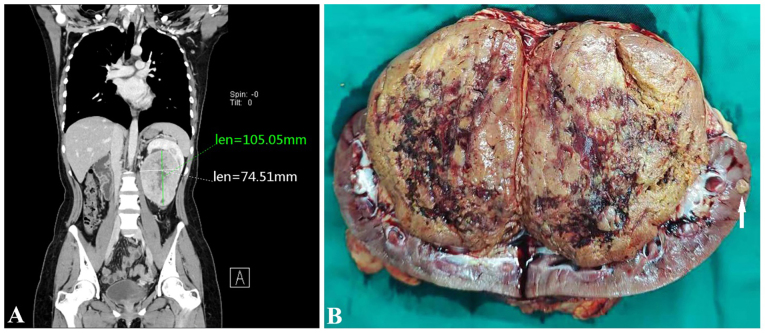
A 22-year-old female patient presented with a left kidney tumor and was admitted to Hubei Cancer Hospital (Tongji Medical College, Huazhong University of Science and Technology; Wuhan, China) in July 2021. An abdominal computerized tomography (CT) scan showed multiple nodular masses in the left kidney, with the larger one being ~10.5×7.5 cm in size (Fig. 1A). The left kidney was pushed upward, with no clear boundary between the mass and the renal pelvis. The patient had no family history of RCC or other tumors. After evaluation, radical nephrectomy was performed.
Figure 1.
CT examination and gross morphology of the present case. (A) Abdominal computerized tomography scan showed a 10.5×7.5 cm nodular mass with an uneven density in the left kidney. The boundary between the mass and the renal pelvis was not clear even after the left kidney was pushed upwards. (B) A brownish-yellow soft mass was present in the renal hilum and pelvis, with a clear boundary and shallow cut surface. Focal hemorrhage, cystic changes and small nodules (arrows) were seen in the renal parenchyma adjacent to the large mass. Len, length.
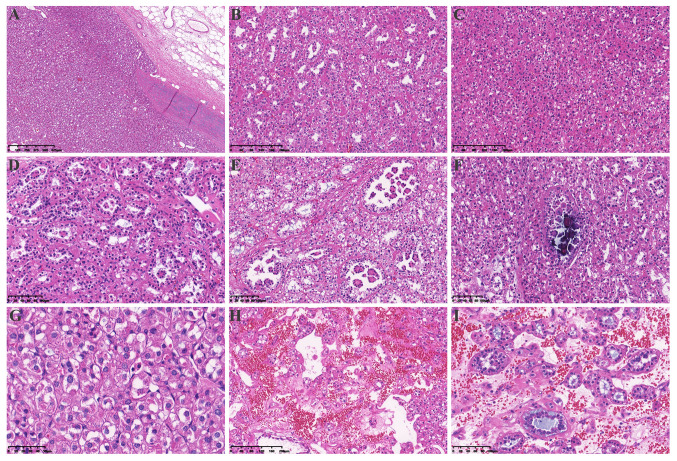
Gross examination revealed a 10×8×7 cm solid tan non-encapsulated tumor in the hilum of the kidney. The cut surface of the tumor was gray-brown with hemorrhagic and cystic foci. Several small nodules 1–2 cm in diameter were observed in the renal parenchyma adjacent to the largest mass (Fig. 1B). Examination of the histopathological staining with hematoxylin and eosin (H&E) as described in the supplementary information indicated that the tumor showed multiple nodules with a focally pushing border (Fig. 2A) and protruded into the renal pelvis. Single native renal tubules were entrapped at the periphery of the tumor. The tumor was mainly composed of dense tubular and vesicular structures (Fig. 2B), some of which were solid (Fig. 2C). In some areas, the tubules gradually expanded and tumor cells protruded into the lumen, forming characteristic annular tubular structures around eosinophilic hyaline bodies. Calcification of the eosinophilic hyaline bodies could also be detected (Fig. 2D-F). The tumor cells contained low-grade (ISUP grade 2) nuclei, which were round or oval, and relatively uniform in size, with abundant eosinophilic, somewhat flocculent cytoplasm, as well as small nucleoli. The tumor stroma was rich in thin-walled vascular networks, and portions of the stroma showed signs of loose edema and bleeding. Single tumor cells with intracellular mucus and small to medium-sized round, or dilated and twisted glandular ducts, were present in the surrounding area. The glandular lumen was filled with gray-blue mucus (Fig. 2G-I). The morphology of the small nodules around the largest mass in the renal parenchyma was consistent with that of the largest mass itself.
Figure 2.
Hematoxylin and eosin staining of the tumor tissue. (A) A pushing border surrounded the tumor (magnification, 4×). (B) The tumor was mainly composed of dense tubular and vesicular structures (magnification, 10×). (C) The tumor was arranged in solid vesicles (magnification, 10×). (D) Some tubules were dilated (magnification, 20×), with (E) tumor cells protruding into the lumen and forming characteristic annular tubular structures around eosinophilic hyaline bodies (magnification, 20×) or (F) calcifying in the lumen (magnification, 20×). (G) The morphology of the tumor cells was characterized by eosinophilic and flocculent cytoplasm (magnification, 40×). (H) The tumor stroma was rich in a thin-walled vascular network and some stroma showed signs of loose edema and bleeding (magnification, 10×). (I) Tumor cells in and around the stroma were diverse, including single cells rich in intracellular mucus, small to medium-sized round or dilated and twisted glandular ducts, and glandular lumen filled with gray-blue mucus magnification, (20×).
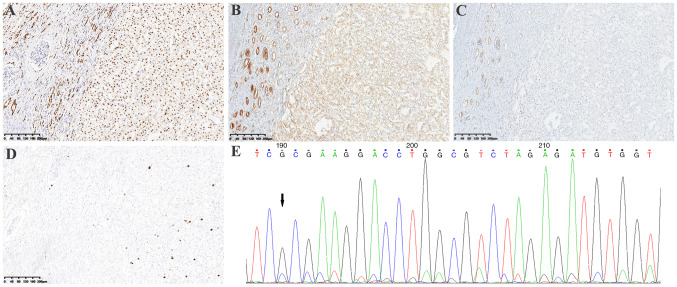
Immunohistochemistry (IHC) was performed on 4-µm thick 4% neutral formaldehyde solution fixed and paraffin-embedded (FFPE) tumor tissue blocks using the automated immunostained Autostainer Link48 (Dako; Agilent Technologies, Inc.) according to the manufacturer's protocol. The IHC protocol was described in the supplementary information and the information of primary antibodies was stated in detail in Table SI. Results showed that PAX8 was positive (Fig. 3A), SDHA was weakly positive (Fig. 3B) and SDHB was negative (Fig. 3C). In addition, CAIX, CK7, CD10, CD117, P504S, Vimentin, E-Cadherin, HMB45, Cathepsin K, Melan-A, S-100, TFE3 and TFEB were negative; fumarate hydratase (FH) was positive (data not shown); and the Ki67 index was ~5% (Fig. 3D). Fluorescence in situ hybridization (FISH) was performed on 4 µm-thick formalin-fixed paraffin-embedded tissues to detect TFE3 (Xp11.2) and TFEB (6p21) gene rearrangement using a dual-color TFE3 (Xp11.2) and TFEB (6p21) break-apart rearrangement probe (IBP Group) according to the manufacturer's protocols. Results showed that TFE3 (Xp11.2) and TFEB (6p21) rearrangement were both negative (data not shown).
Figure 3.
Immunohistochemical staining of the tumor. Immunostaining showed tumor cells were (A) positive for PAX8 (magnification, 10×), (B) weakly positive for SDHA (the non-neoplastic renal tubules showed medium to strong positive staining; magnification, 10×), but (C) negative for SDHB (magnification, 10×). (D) Ki67 index was ~5% (magnification, 10×). (E) Sanger sequencing of SDHA: c.992_999dup (CCCCTGTC) (arrow). SDH, succinate dehydrogenase.
NGS analysis was performed as described in the previous study (14) using the 4% neutral formaldehyde formalin-fixed and paraffin-embedded tumor tissue to detect 425 cancer-relevant genes (Geneseeq Technology Inc.), including SDHA, SDHB, SDHC, SDHD, FH, TSC1/2, ARID1A, POLE, CHEK2 and GATA2, and a novel SDHA (RefSeq accession number: SDHA NM_004168.4) frameshift variant: c.992_999dup (p.A334Pfs*17) was identified. This variant of SDHA has been submitted to the ClinVar database (http://www.ncbi.nlm.nih.gov/clinvar), under the accession number SCV004035231. The NGS analysis also examined copy number alterations of the genes, and no copy number alterations were detected. Bioinformatics analysis using the bcl2fastq (v2.19) software (Illumina, Inc.) revealed it was most likely a somatic event in the SDHA gene. Sanger sequencing also confirmed the novel SDHA frameshift variant (NM_004168.4): c.992_999dup (CCCCTGTC) (Fig. 3E).
The patient did not receive adjuvant chemotherapy and was followed-up by CT examinations every 6 months in the first 2 years and then once a year after resection. There was no apparent recurrence or metastasis in July 2024 (Fig. S1).
Discussion
SDH-deficient RCC is a specific type of RCC generally caused by a pathogenic germline variant with the addition of a somatic second hit in every one of the four SDH subunits, which leads to dysfunction of the SDH complex. A germline mutation of the SDHB gene is usually detected, and somatic mutation of SDHB is rarely reported in SDH-deficient RCC (3). In addition, a few cases of SDHC or SDHD gene mutations have been reported (9), whereas SDHA-deficient RCC is rare. SDH-deficient RCC often occurs in young adults (age range, 14–76 years; median age, 35 years), with a slight male predominance (7–8). The majority of SDH-deficient RCC cases are well-circumscribed or lobulated with a pushing border, and are sometimes characterized by the presence of a pseudocapsule (15). The tumors consist of sheets or compact nests of bland cells with eosinophilic cytoplasm, which may have a pale, bubbly appearance, and lack prominent cell borders (16). The most distinctive feature is the presence of cytoplasmic inclusions containing eosinophilic or pale flocculent material, despite being absent in some cases (15,16). Most cases of SDH-deficient RCC are consistent with a low-grade morphology, but an increasing number of high-grade tumors have been reported (15,16). Cases associated with SDHA mutations more commonly show a higher nuclear grade, and demonstrate papillary, solid, cribriform or desmoplastic architecture (10–12).
The clinicopathological features of the four cases of SDHA-deficient RCC previously reported in the literature (10–13) are summarized in Table I. All four patients were male, aged 23–62 years, with a median age of 49.5 years. The tumors were usually large, ranging between 8 and 11 cm in diameter. In three out of the four patients, the tumor was arranged in papillary, tubular, vesicular or solid structures with eosinophilic cytoplasm. The tumor cells contained high-grade nuclei (ISUP grade 3/4), with large pleomorphic nuclei, coarse chromatin and prominent nucleoli, even with sarcomatoid dedifferentiation. The fourth case exhibited a low-grade morphology, similar to chromophobe RCC. The patient described in the present study was a young woman, with low-grade nuclear morphology, which were different from the four previously reported patients with SDHA-deficient RCC. The distinctive morphological features of SDH-deficient RCC, such as the tumor cells with abundant eosinophilic, somewhat flocculent cytoplasm, were seen in the present patient. The tumor was mainly arranged in tubular, vesicular and solid structures, without an obvious papillary structure. Elongated papillae tumor cells protruded into the dilated tubular lumen and formed a characteristic annular tubule structure surrounding the eosinophilic hyaline bodies, with focal calcification. The morphology of the small nodules in the renal parenchyma outside the tumor body was consistent with that of the main tumor. A wide panel of IHC studies was performed in order to rule out other renal neoplasms, and as loss of SDHB expression is a prerequisite for the diagnosis of SDH-deficient RCC. Furthermore, the tumors usually show positive staining for PAX8, and negative staining for CD117 and CK7, and FH expression is consistently preserved. To the best of our knowledge, the present study is the first to detect annular tubule structures surrounding the eosinophilic hyaline bodies in SDH-deficient RCC.
Table I.
Clinical and molecular features of SDHA-deficient RCC cases.
| IHC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| First author, year | Sex/Age, years | Tumor location | Tumor size, cm |
|
||||||
| SDHA | SDHB | Molecular genetics | Germline/somatic | Treatment | Outcome | (Refs.) | ||||
| McEvoy, 2018 | Male/45 | NA | 11 | - | - | SDHA biallelic mutations | Germline and somatic | Laparoscopic nephrectomy + PD-1 inhibitor and TKIs | Recurrence at 10 months | (10) |
| Yakirevich, 2015 | Male/54 | Right | 10 | - | - | SDHA homozygous deletion | Somatic | Radical nephrectomy | NA | (11) |
| Ozluk, 2015 | Male/62 | Right | 8 | - | - | Single-nucleotide splice site deletion in SDHA gene | Somatic | Radical nephrectomy and lymphadenectomy | Local recurrence at 10 months | (12) |
| Jiang, 2015 | Male/23 | Left | 4 | +, weak | - | SDHA heterozygous mutation | Germline | Partial nephrectomy + sunitinib | No recurrence within 2 years | (13) |
| Present study | Female/22 | Left | 11 | +, weak | - | SDHA frameshift mutation | Somatic | Radical nephrectomy | No recurrence 36 months after resection | - |
IHC, immunohistochemistry; NA, not available; PD-1, programmed cell death protein 1; SDH, succinate dehydrogenase; TKIs, tyrosine kinase inhibitors.
A previous study has shown that pathogenic mutations of SDH complex subunits lead to loss of expression of the SDHB protein (9). SDHA-deficient RCC is caused by a mutation in the SDHA gene resulting in the dysfunction of the SDH complex and loss of expression of both SDHA and SDHB proteins. The four cases of SDHA-deficient RCCs reported in the literature contained SDHA gene pathogenic mutations, including one case with a germline truncating variant in conjunction with a somatic missense variant; another case with biallelic homozygous deletion; and one case with a single nucleotide splice site deletion. These three cases expressed neither SDHB nor SDHA protein. The last low-grade chromophobe cell carcinoid RCC consisted of a germline heterozygous mutation in SDHA, with IHC showing a weak positive expression of the SDHA protein and the loss of SDHB. Similarly, in the present case report, the tumor presented with a frameshift variant in the SDHA gene that showed a weak positive expression of SDHA and led to the loss of SDHB expression. These two cases were both low-grade, with a heterozygous mutation in the SDHA gene, which might explain the decreased (but still present) expression of the SDHA protein and the defective SDH complex with negative SDHB expression. This finding enriches the known molecular mechanisms associated with SDHA-deficient RCC. Among the four cases of SDHA-deficient RCCs reported in the literature, three were high-grade (two of which resurged 10 months after surgery) and one low-grade. The present case was low-grade, with no recurrence and/or metastasis 36 months after surgery.
Notably, not all mutations in the SDHA gene lead to SDH complex deficiency. In a recent cohort study (17), NGS and Sanger sequencing were used to detect SDHA gene status in 107 cases of clear cell RCC (ccRCC), 17 cases of papillary RCC type 2 (pRCC2), 3 cases of chromophobe cell carcinoma and 2 cases of collecting duct carcinoma. Single nucleotide variants (SNVs) of the SDHA gene causing amino acid sequence variants (missense mutations) were detected in six pRCC2 and five ccRCC cases, but no mutations of the SDHB, SDHC or SDHD genes were detected. In addition, IHC indicated a decrease in SDHA and SDHB protein expression in the six pRCC2 and five ccRCC cases, but none showed a complete loss of SDHA or SDHB. In this previous study, the authors suggested that SDHA SNVs could lead to decreased expression of the SDHA protein, but not cause SDH complex dysfunction as manifested by the complete loss of SDHB protein expression. Consequently, the 11 RCC cases were not classified as SDHA-deficient RCCs. In addition, the diagnosis of pRCC2 is no longer recommended according to the 5th edition of the WHO Classification of Tumors of Urinary System and Male Genital Organs published in 2022 (16). Accordingly, the 17 cases of type 2 pRCC2s in this previous study require further molecular characterization for unbiased classification.
The present case should be distinguished from other similar tumors. Specifically, TFE3-rearranged/TFEB-altered RCC represents a group of RCC with a variety of morphologies that characteristically include single native renal tubules at their periphery (16). The most distinctive pattern of TFEB-rearranged RCC is a biphasic structure composed of nests of larger epithelioid cells and smaller cells clustered around the hyaline basement membrane in the center (18). TFE3-rearranged/TFEB-altered RCC cases consistently express melanocytic markers, such as HMB45, Melan A and Cathepsin K (19). In addition, strong nuclear labeling for TFE3 and TFEB, TFE3/TFEB arrangement identified by break-apart FISH, or TFE3/TFEB gene fusion identified by RNA sequencing, can be used to easily distinguish them from other neoplasms (20). Although in the present case, the structure of the tumor mimics the biphasic appearance, with some cells present at the periphery of the nests and other cells clustering around the hyaline basement membrane, TFE3, TFEB, HMB45, Melan A and Cathepsin K were negative, and TFE3/TFEB was not identified by break-apart FISH in the present study. Accordingly, a diagnosis of TFE3-rearranged/TFEB-altered RCC could not be made for the present case.
FH-deficient RCC typically demonstrates multiple admixed morphological patterns and characteristic prominent eosinophilic nucleoli. A previous study has reported a few cases of low-grade FH-deficient RCC with oncocytic morphology, which resemble SDH-deficient RCC, but retain SDHB expression (21). Negative immunohistochemical staining for FH in tumor cells is highly specific, and positive staining for 2SC is highly sensitive for FH-deficient RCC. In the case presented in the current study, FH was positive and SDHB was negative, supporting a diagnosis of SDH-deficient RCC. Oncocytoma commonly grows in solid nests with a central stellate scar, and is composed of round to polygonal cells with densely granular eosinophilic cytoplasm and round uniform nuclei with a central small nucleolus. In addition, these tumors are typically positive for CD117, whereas CD117 was negative in the present case. RCC with TSC/mTOR gene mutations belongs to a group of tumors with eosinophilic cytoplasm and often contains vacuolar structures and prominent nucleoli; these tumors also typically contain TSC2/mTOR gene mutations (22). The aforementioned tumors overlap morphologically with the present case, but the specific immunophenotype and molecular genetics can be well differentiated.
The majority of SDH-deficient RCC cases demonstrate low-grade morphology and have a favorable prognosis with a low metastatic rate; however, for a few cases with high-grade features (i.e. coagulative necrosis and sarcomatoid transformation), the rate of metastasis is as high as 70% (7), and adjuvant treatment is necessary for advanced patients. As previously mentioned (2), tumorigenesis caused by SDH deficiency is achieved via a pseudohypoxic pathway involving HIFs and VEGF; therefore, targeted therapy could be the first-line therapy for advanced RCC. Tyrosine kinase inhibitors (TKIs) are involved in the inhibition of VEGF-induced angiogenesis, and remain the mainstay first-line treatment for advanced RCC (23,24). Immunotherapy, such as immune checkpoint inhibitors against programmed cell death protein 1 or its ligand (25), has become the standard first-line treatment for a number of patients with RCC. For a substantial proportion of patients who are not suitable for immunotherapy, TKI treatment remains an appropriate first-line therapy, and is widely used as second-line and subsequent-line therapy. In a recent large, multicenter, phase 2/3 trial (STAR), which aimed to assess the potential benefits of a treatment break strategy compared with a conventional treatment continuation strategy in patients with RCC receiving TKI therapy, the results demonstrated that a drug-free interval strategy was non-inferior to a conventional continuation strategy for first-line treatment with TKIs, and treatment breaks may be a feasible and cost-effective option with lifestyle benefits for patients during TKI therapy (26). Furthermore, in a recently published phase 3, multicenter, open-label, active-controlled study, belzutifan, a HIF-2α inhibitor, exhibited a significant benefit over everolimus, an inhibitor of mammalian target of rapamycin, with respect to progression-free survival and objective response in participants with advanced RCC who had previously received antiangiogenic and immune checkpoint therapies (27).
In conclusion, SDH-deficient RCC is a specific type of RCC with genetic associations. SDHA-deficient RCC is rare, and the present case enriches the histological morphology, and immunohistochemical and molecular characteristics of SDHA-deficient RCC. When the protein expression of SDHA and/or SDHB is abnormal, further molecular analysis is required to confirm a pathogenic mutation of the SDHA gene and to avoid misdiagnosis. Future studies regarding the mechanism of this type of cancer are needed to strengthen the overall understanding of SDHA-deficient RCC.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
This study was partially supported by the Special Fund for Clinical Research of the Wu Jieping Medical Foundation (grant no. 320.6750.2021-21-15).
Availability of data and materials
The data generated in the present study may be found in the ClinVar database (http://www.ncbi.nlm.nih.gov/clinvar) under accession number SCV004035231 at the following URL: http://www.ncbi.nlm.nih.gov/clinvar/variation/2580151/?oq=SCV004035231&m=NM_004168.4(SDHA):c.992_999dup%20(p.Ala334fs). All other data generated in the present study may be requested from the corresponding author.
Authors' contributions
JQY and LFF conceived and designed the study. MH acquired and analyzed the data. XTW, XXX and QR performed the research and experiments, and interpreted the data. MH and JQY performed the writing, review and revision of the paper. MH and JQY confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the patient.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Aldera AP, Govender D. Gene of the month: SDH. J Clin Pathol. 2018;71:95–97. doi: 10.1136/jclinpath-2017-204677. [DOI] [PubMed] [Google Scholar]
- 2.Bardella C, Pollard PJ, Tomlinson I. SDH mutations in cancer. Biochim Biophys Acta. 2011;1807:1432–1443. doi: 10.1016/j.bbabio.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Gill AJ. Succinate dehydrogenase (SDH)-deficient neoplasia. Histopathology. 2018;72:106–116. doi: 10.1111/his.13277. [DOI] [PubMed] [Google Scholar]
- 4.Vanharanta S, Buchta M, McWhinney SR, Virta SK, Peçzkowska M, Morrison CD, Lehtonen R, Januszewicz A, Järvinen H, Juhola M, et al. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am J Hum Genet. 2004;74:153–159. doi: 10.1086/381054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srigley JR, Delahunt B, Eble JN, Egevad L, Epstein JI, Grignon D, Hes O, Moch H, Montironi R, Tickoo SK, et al. The international society of urological pathology (ISUP) vancouver classification of renal neoplasia. Am J Surg Pathol. 2013;37:1469–1489. doi: 10.1097/PAS.0b013e318299f2d1. [DOI] [PubMed] [Google Scholar]
- 6.Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: Renal, penile, and testicular tumours. Eur Urol. 2016;70:93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Gill AJ, Hes O, Papathomas T, Šedivcová M, Tan PH, Agaimy A, Andresen PA, Kedziora A, Clarkson A, Toon CW, et al. Succinate dehydrogenase (SDH)-deficient renal carcinoma: A morphologically distinct entity: a clinicopathologic series of 36 tumors from 27 patients. Am J Surg Pathol. 2014;38:1588–1602. doi: 10.1097/PAS.0000000000000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williamson SR, Eble JN, Amin MB, Gupta NS, Smith SC, Sholl LM, Montironi R, Hirsch MS, Hornick JL. Succinate dehydrogenase-deficient renal cell carcinoma: Detailed characterization of 11 tumors defining a unique subtype of renal cell carcinoma. Mod Pathol. 2015;28:80–94. doi: 10.1038/modpathol.2014.86. [DOI] [PubMed] [Google Scholar]
- 9.Tsai TH, Lee WY. Succinate dehydrogenase-deficient renal cell carcinoma. Arch Pathol Lab Med. 2019;143:643–647. doi: 10.5858/arpa.2018-0024-RS. [DOI] [PubMed] [Google Scholar]
- 10.McEvoy CR, Koe L, Choong DY, Leong HS, Xu H, Karikios D, Plew JD, Prall OW, Fellowes AP, Fox SB. SDH-deficient renal cell carcinoma associated with biallelic mutation in succinate dehydrogenase A: Comprehensive genetic profiling and its relation to therapy response. NPJ Precis Oncol. 2018;2:9. doi: 10.1038/s41698-018-0053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yakirevich E, Ali SM, Mega A, McMahon C, Brodsky AS, Ross JS, Allen J, Elvin JA, Safran H, Resnick MB. A novel SDHA-deficient renal cell carcinoma revealed by comprehensive genomic profiling. Am J Surg Pathol. 2015;39:858–863. doi: 10.1097/PAS.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 12.Ozluk Y, Taheri D, Matoso A, Sanli O, Berker NK, Yakirevich E, Balasubramanian S, Ross JS, Ali SM, Netto GJ. Renal carcinoma associated with a novel succinate dehydrogenase A mutation: A case report and review of literature of a rare subtype of renal carcinoma. Hum Pathol. 2015;46:1951–1955. doi: 10.1016/j.humpath.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Q, Zhang Y, Zhou YH, Hou YY, Wang JY, Li JL, Li M, Tong HX, Lu WQ. A novel germline mutation in SDHA identified in a rare case of gastrointestinal stromal tumor complicated with renal cell carcinoma. Int J Clin Exp Pathol. 2015;8:12188–12197. [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, He P, Zhou X, Wang C, Fu J, Zhang D, Liao D, Zhou Z, Wu C, Gong W. Gene mutation profiling and clinical significances in patients with renal cell carcinoma. Clinics (Sao Paulo) 2023;78:100259. doi: 10.1016/j.clinsp.2023.100259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuchs TL, Maclean F, Turchini J, Vargas AC, Bhattarai S, Agaimy A, Hartmann A, Kao CS, Ellis C, Bonert M, et al. Expanding the clinicopathological spectrum of succinate dehydrogenase-deficient renal cell carcinoma with a focus on variant morphologies: A study of 62 new tumors in 59 patients. Mod Pathol. 2022;35:836–849. doi: 10.1038/s41379-021-00998-1. [DOI] [PubMed] [Google Scholar]
- 16.WHO Classification of Tumours Editorial Board, corp-author. 5th edition. Vol 8. International Agency for Research on Cancer; Lyon, France: 2022. Urinary and male genital tumours: WHO classification of tumours. [Google Scholar]
- 17.Kamai T, Higashi S, Murakami S, Arai K, Namatame T, Kijima T, Abe H, Jamiyan T, Ishida K, Shirataki H, Yoshida KI. Single nucleotide variants of succinate dehydrogenase A gene in renal cell carcinoma. Cancer Sci. 2021;112:3375–3387. doi: 10.1111/cas.14977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Argani P, Hawkins A, Griffin CA, Goldstein JD, Haas M, Beckwith JB, Mankinen CB, Perlman EJ. A distinctive pediatric renal neoplasm characterized by epithelioid morphology, basement membrane production, focal HMB45 immunoreactivity, and t(6;11)(p21.1;q12) chromosome translocation. Am J Pathol. 2001;158:2089–2096. doi: 10.1016/S0002-9440(10)64680-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akgul M, Williamson SR, Ertoy D, Argani P, Gupta S, Caliò A, Reuter V, Tickoo S, Al-Ahmadie HA, Netto GJ, et al. Diagnostic approach in TFE3-rearranged renal cell carcinoma: A multi-institutional international survey. J Clin Pathol. 2021;74:291–299. doi: 10.1136/jclinpath-2020-207372. [DOI] [PubMed] [Google Scholar]
- 20.Wang XM, Shao L, Xiao H, Myers JL, Pantanowitz L, Skala SL, Udager AM, Vaishampayan U, Mannan R, Dhanasekaran SM, et al. Lessons from 801 clinical TFE3/TFEB fluorescence in situ hybridization assays performed on renal cell carcinoma suspicious for MiTF family aberrations. Am J Clin Pathol. 2023;160:549–554. doi: 10.1093/ajcp/aqad089. [DOI] [PubMed] [Google Scholar]
- 21.Smith SC, Sirohi D, Ohe C, McHugh JB, Hornick JL, Kalariya J, Karia S, Snape K, Hodgson SV, Cani AK, et al. A distinctive, low-grade oncocytic fumarate hydratase-deficient renal cell carcinoma, morphologically reminiscent of succinate dehydrogenase-deficient renal cell carcinoma. Histopathology. 2017;71:42–52. doi: 10.1111/his.13183. [DOI] [PubMed] [Google Scholar]
- 22.Tjota M, Chen H, Parilla M, Wanjari P, Segal J, Antic T. Eosinophilic renal cell tumors with a TSC and MTOR gene mutations are morphologically and immunohistochemically heterogenous: Clinicopathologic and Molecular Study. Am J Surg Pathol. 2020;44:943–954. doi: 10.1097/PAS.0000000000001457. [DOI] [PubMed] [Google Scholar]
- 23.Lalani AA, Heng DYC, Basappa NS, Wood L, Iqbal N, McLeod D, Soulières D, Kollmannsberger C. Evolving landscape of first-line combination therapy in advanced renal cancer: A systematic review. Ther Adv Med Oncol. 2022;14:17588359221108685. doi: 10.1177/17588359221108685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moran M, Nickens D, Adcock K, Bennetts M, Desscan A, Charnley N, Fife K. Sunitinib for metastatic renal cell carcinoma: A systematic review and meta-analysis of real-world and clinical trials data. Target Oncol. 2019;14:405–416. doi: 10.1007/s11523-019-00653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aggen DH, Drake CG, Rini BI. Targeting PD-1 or PD-L1 in metastatic kidney cancer: Combination therapy in the first-line setting. Clin Cancer Res. 2020;26:2087–2095. doi: 10.1158/1078-0432.CCR-19-3323. [DOI] [PubMed] [Google Scholar]
- 26.Brown JE, Royle KL, Gregory W, Ralph C, Maraveyas A, Din O, Eisen T, Nathan P, Powles T, Griffiths R, et al. Temporary treatment cessation versus continuation of first-line tyrosine kinase inhibitor in patients with advanced clear cell renal cell carcinoma (STAR): An open-label, non-inferiority, randomised, controlled, phase 2/3 trial. Lancet Oncol. 2023;24:213–227. doi: 10.1016/S1470-2045(22)00793-8. [DOI] [PubMed] [Google Scholar]
- 27.Choueiri TK, Powles T, Peltola K, de Velasco G, Burotto M, Suarez C, Ghatalia P, Iacovelli R, Lam ET, Verzoni E, et al. Belzutifan versus everolimus for advanced renal-cell carcinoma. N Engl J Med. 2024;391:710–721. doi: 10.1056/NEJMoa2313906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in the present study may be found in the ClinVar database (http://www.ncbi.nlm.nih.gov/clinvar) under accession number SCV004035231 at the following URL: http://www.ncbi.nlm.nih.gov/clinvar/variation/2580151/?oq=SCV004035231&m=NM_004168.4(SDHA):c.992_999dup%20(p.Ala334fs). All other data generated in the present study may be requested from the corresponding author.