Abstract
Background
The current standard for the surgical management of lung cancer involves anatomic lung resection combined with systemic lymph node dissection/sampling. The purpose of this study was to investigate the patterns of pathological lymph nodes in invasive non-small cell lung cancer (NSCLC), explore the occurrence in lymph node metastasis (LNM), and provide recommendations for optimal lymph node resection/sampling in lung cancer operation.
Methods
There were 1,678 patients with NSCLC who underwent lobectomy between 2018 and 2021 at the Taizhou Hospital of Zhejiang Province were reviewed retrospectively. The location and incidence of LNM and postoperative pathological findings were studied. We analysed the metastasis rates of lymph node dissection stations using Pearson’s χ2 and Fisher’s exact tests.
Results
There were 1,308 patients assessed as eligible and included in the study. The median number of lymph nodes cleared in the cohort was 11.2±5.1. In patients with lung adenocarcinoma, the rate of LNM was significantly higher in central than in peripheral lung cancer, especially in 2R/2L, L7, L9, L10, L11, and L12. Lung cancer patients with tumors ≤1 cm had no N2 lymph node metastases but few (2/191, 1.1%) N1 lymph node metastases. The likelihood of N2 metastasis increased (T1a, 0%, 0/191; T1b 3.5%, 22/625; T1c, 5.6%, 14/249; T2 and above, 18.9%, 46/243) with increasing tumor diameter. Thirty-four patients with stage N2 lung adenocarcinoma and 1–3 cm tumors displayed lobe-specific lymph node metastases in the mediastinum. In patients diagnosed with squamous cell carcinoma, no significant differences were observed in mediastinal LNM across various parameters (central versus peripheral location, tumor site, and tumor size).
Conclusions
Our study proposes recommendations for lymph node resection according to the pathological type of lung cancer, tumor location, lung lobes affected and tumor size, which may provide a certain reference value for the clinical work.
Keywords: Non-small cell lung cancer (NSCLC), lymph node dissection, lymph node metastasis (LNM), surgical technique
Highlight box.
Key findings
• For patients with different characteristics of non-small cell lung cancer, lymph node metastasis may show a characteristic distribution.
What is known and what is new?
• Mediastinal lymph node dissection is not necessary for patients with tumor ≤1 cm.
• The likelihood of N2 metastasis increased with increasing tumor diameter.
• Lobe-specific lymph node dissection may reduce the N-stage of patients with central lung cancer.
What is the implication, and what should change now?
• Our study proposes recommendations for lymph node resection according to the pathological type of lung cancer, tumor location, lung lobes affected and tumor size.
Introduction
Lung cancer is one of the most prevalent malignancies worldwide and the leading cause of cancer-related deaths. In China, lung cancer has the highest incidence, morbidity, and mortality rates out of all cancers (1). The National Comprehensive Cancer Network (NCCN) guidelines advocate the use of anatomic lung resection combined with systemic lymph node dissection (SLND) in the treatment of patients with lung cancer. SLND can help with staging lymph nodes accurately to assess prognosis and guide postoperative treatment and reduce postoperative recurrence rates (2).
Analysing lymph node metastasis (LNM), especially in mediastinal lymph nodes, is crucial in accurate staging for non-small cell lung cancer (NSCLC) patients and provides a basis for their subsequent treatment (2). The presence of mediastinal LNM can influence the prognosis of NSCLC patients; therefore, complete intraoperative clearance of pathological mediastinal lymph nodes plays a crucial role in improving overall survival and disease-free survival (2). It also detects occult metastases and improves the overall survival of lung cancer patients. Previous studies have suggested that LNM patterns may be lobe-specific, and lobe-specific lymph node dissection (L-SLND) may be an intraoperative alternative to SLND for early-stage NSCLC (3,4). L-SLND may be effective and suitable for selected patients, reducing the risk of complications associated with surgery. Patients may benefit from shorter operative times, reduced blood loss, and shorter hospital stays.
There is no conclusive evidence on the role of L-SLND in treating NSCLC. It is important to note that the recommended level of lymph node clearance for different stations based on LNM rates currently lacks high-level evidence in lung cancer surgery and definitive cutoff values. Therefore, we conducted this study to identify the metastasis rate of different lymph node stations in NSCLC to explore the priority of lymph node stations to be cleared during radical lung cancer surgery. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-450/rc).
Methods
Ethical statement
The study was approved by the Ethics Review Committee of Taizhou Hospital, Zhejiang Province (No. K20221222). The written patient informed consent was waived for this retrospective study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Date source and patient selection
We retrospectively collected 1,678 patients’ data with NSCLC who underwent lobectomy in the Department of Cardiothoracic Surgery, Taizhou Hospital, Zhejiang Province, between January 2018 and April 2021 through the electronic medical record system. Inclusion criteria included (I) histologically confirmed primary invasive NSCLC [Lung Cancer International TNM Staging (8th edition) T1-4N0-2M0]; (II) patients who had undergone lobectomy and lymph node dissection/sampling; and (III) patients with complete clinical and pathological data. Of these patients, 370 were excluded because they (I) were not diagnosed with primary NSCLC; (II) lacked complete pathological information on LNM; or (III) received salvage, palliative surgery, or neoadjuvant therapy (Figure 1).
Figure 1.
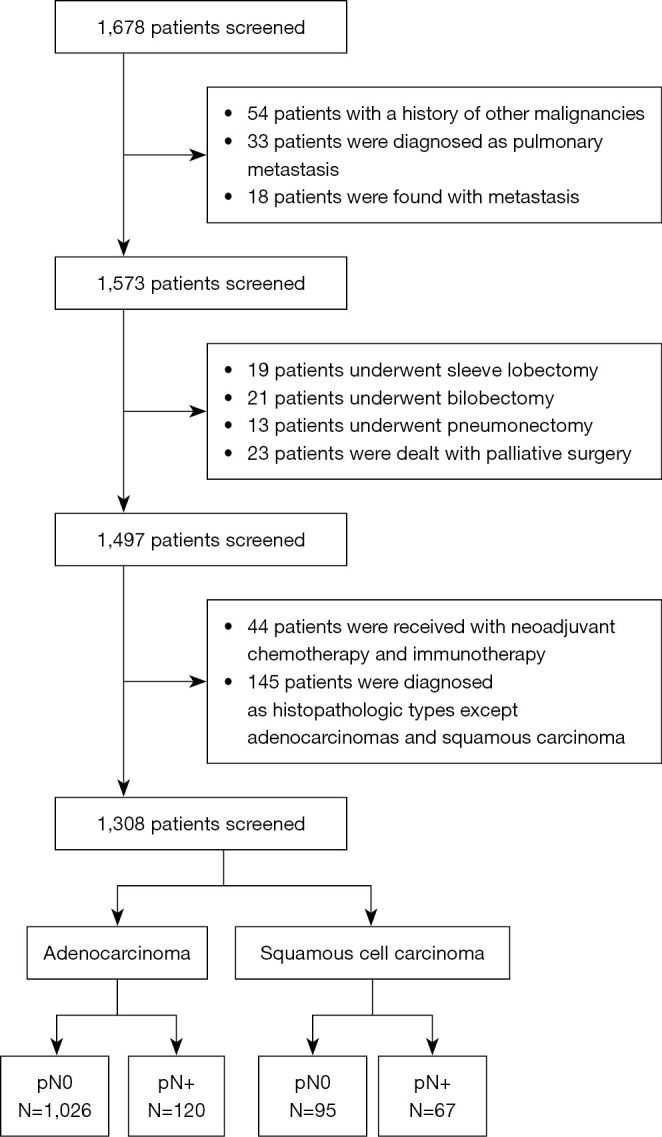
Flow diagram of the enrolled patient.
These patients were independently reviewed by two investigators and disagreements were resolved through discussions with a third investigator. Data on postoperative pathology and LNM, including lymph node location and lymph node station metastasis, were retrieved for each patient. We calculated the metastasis rate for each station of lymph nodes that were routinely resected separately based on the TNM staging system.
Variables
According to our clinical data, we analyzed the presence of LNM in the subgroups according to the following variables: tumor location, the affected lobe and tumor size. Central lung cancer is referred to as lung cancer that occurs in the segment and above the bronchus, while peripheral lung cancer is referred to as lung cancer that occurs below the segment bronchus. Both are distinguished according to the location of tumourigenesis.
Surgery
In keeping with the relevant clinical guidelines for lung cancer surgery (5), all patients with lung cancer included in this study underwent lobectomy and mediastinal lymph node dissection/sampling was routinely performed during lobectomy (4L, L5, L6, L7, L8, L9 stations for left-sided and 2R, 4R, L7, L8, L9 stations for right-sided lung cancer).
Results measurement
We analyzed the patients’ demographic and relevant clinical data using their electronic medical records. The patients’ lymph node metastases were evaluated using the histopathology of resected specimens. Tumor metastasis was recorded according to the 8th edition TNM staging system, and LNM rate was calculated for each lymph node station. There is no definite and well-accepted cutoff value for LNM rate reported in the literature, so we based on the lymph node clearance data reported in the relevant literature and analyzed them (4,6). To facilitate the interpretation of the data, we color-coded the lymph node metastases according to the metastasis rate: <2%, yellow; 2–4%, green; >4%, blue, and mapped the distribution of lymph node metastases.
Statistical analysis
For the analysis of descriptive variables, quantitative data following a normal distribution were expressed using the mean (standard deviation, SD), and qualitative variables were described using frequency (composition ratio) and compared using Pearson’s 2 test and Fisher’s exact test. Based on our clinical data, we compared LNM rates between groups according to the following grouping variables: tumor location, lung lobes affected and cT stage. We used the Bonferroni method for correction when multiple group comparisons were made. Statistical significance was determined by two-sided P<0.05. Data analysis was performed using the SPSS version 26.0 (the Statistical Package for the Social Sciences) software.
Results
Patient and baseline characteristics
Among the 1,308 patients who underwent lobectomy, 191 (14.6%) were stage pT1a, 625 (47.8%) stage pT1b, 249 (19.0%) stage pT1c, and a total of 243 (18.6%) were stage pT2 and above. Seventy-six patients (5.8%) were pN1, and 82 patients (6.3%) were pN2. The cohort’s median number of lymph nodes dissected was 11.2 (±5.1). Of these, the median number of lymph nodes dissected was 10.9 (±4.7) for adenocarcinoma and 13.2 (±6.9) for squamous cell carcinoma. The baseline characteristics of the enrolled are shown in Table 1.
Table 1. Patient characteristics of enrolled patients (N=1,308).
| Variables | All (N=1,308) | Adenocarcinomas (N=1,146, 87.6%) |
Squamous cell carcinomas (N=162, 12.4%) | P value |
|---|---|---|---|---|
| Mean age (SD), years | 60.3 (9.3) | 59.7 (9.4) | 64.5 (7.6) | <0.001 |
| Sex, n (%) | <0.001 | |||
| Male | 634 (48.5) | 475 (41.4) | 159 (98.1) | |
| Female | 674 (51.5) | 671 (58.6) | 3 (1.9) | |
| Smoking history, n (%) | <0.001 | |||
| Smokers/ever | 395 (30.2) | 270 (23.7) | 123 (75.9) | |
| Never | 913 (69.8) | 874 (76.3) | 39 (24.1) | |
| 8th clinical T status, n (%) | <0.001 | |||
| cT1a | 204 (15.6) | 196 (17.1) | 8 (4.9) | |
| cT1b | 620 (47.4) | 572 (49.9) | 48 (29.6) | |
| cT1c | 293 (22.4) | 263 (22.9) | 30 (18.5) | |
| cT2 and above | 191 (14.6) | 115 (10.1) | 76 (47.0) | |
| Tumour location, n (%) | <0.001 | |||
| Central | 245 (18.7) | 130 (11.3) | 115 (71.0) | |
| Peripheral | 1,063 (81.3) | 1016 (88.7) | 47 (29.0) | |
| Lobes of lung, n (%) | <0.001 | |||
| Left upper lobe | 305 (23.3) | 267 (23.3) | 38 (23.5) | |
| Left lower lobe | 211 (16.1) | 169 (14.7) | 42 (25.9) | |
| Right upper lobe | 417 (31.9) | 385 (33.6) | 32 (19.8) | |
| Right middle lobe | 100 (7.6) | 96 (8.4) | 4 (2.5) | |
| Right lower lobe | 275 (21.0) | 229 (20.0) | 46 (28.4) | |
| Surgical type, n (%) | <0.001 | |||
| VATS | 1230 (94.0) | 1,104 (96.3) | 126 (77.8) | |
| Open surgery | 78 (6.0) | 42 (3.7) | 36 (22.2) | |
| 8th pathological T status, n (%) | <0.001 | |||
| pT1a | 191 (14.6) | 181 (15.8) | 10 (6.2) | |
| pT1b | 625 (47.8) | 591 (51.6) | 34 (21.0) | |
| pT1c | 249 (19.0) | 214 (18.7) | 35 (21.6) | |
| pT2 and above | 243 (18.6) | 160 (13.9) | 83 (51.2) | |
| 8th pathological N status, n (%) | <0.001 | |||
| pN0 | 1,150 (87.9) | 1,027 (89.6) | 123 (75.9) | |
| pN1 | 76 (5.8) | 49 (4.3) | 27 (16.7) | |
| pN2 | 82 (6.3) | 70 (6.1) | 12 (7.4) | |
| 8th pathological stage, n (%) | <0.001 | |||
| IA | 993 (75.9) | 928 (81.0) | 65 (40.1) | |
| IB | 56 (4.3) | 30 (2.6) | 26 (16.1) | |
| IIA | 77 (5.9) | 64 (5.6) | 13 (8.0) | |
| IIB | 75 (5.7) | 46 (4.0) | 30 (18.5) | |
| IIIA | 95 (7.3) | 70 (6.1) | 24 (14.8) | |
| IIIB | 12 (0.9) | 8 (0.7) | 4 (2.5) |
VATS, video-assisted thoracoscopic surgery.
LNM rate and subgroup analysis
We estimated the incidence of LNM based on the location of lymph nodes in 1,308 patients with NSCLC who underwent surgical resection. The results for adenocarcinomas and squamous cell carcinomas can be found in Tables S1,S2, respectively. We found no significant differences in lesion location, lung lobes affected, and tumor T-stage in squamous cell carcinomas. Therefore we evaluated the locations and incidence of LNM in adenocarcinomas.
In patients with adenocarcinomas, lymph nodes with 2–4% metastasis rate were marked in green, including 2R/2L, 3A/3P, L5, L6, L7, L8, L10, L11, and L12 (Figure 2A). And in patients with squamous cell carcinomas (Figure 2B), the highest frequency of LNM was observed in blue-labeled L10, L11, L12, and L13.
Figure 2.
Location and incidence of lymph node metastasis rates in patients with (A) adenocarcinoma and (B) squamous cell carcinoma. The numbers above the horizontal lines in each colored bubble represent the lymph node stations, while the numbers below the lines represent the rate of lymph node metastasis.
LNM rate in central and peripheral adenocarcinomas
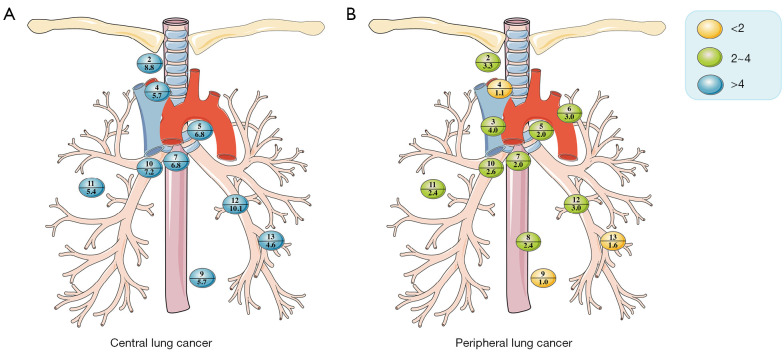
Of the 130 patients included with central adenocarcinomas, 93 were pN0, and 37 were pN+. Among these pN+ patients, we found that all lymph nodes had a LNM rate greater than 4%, as 2R/2L (8.8%) and L12 (10.1%) had the highest LNM rates (Figure 3A). Among the subgroups, there were only a few stations with less than 4% lymph nodes, as shown in Table S3. In contrast, among the 1,016 patients with peripheral adenocarcinomas, 934 were pN0, and 82 were pN+. Compared with central adenocarcinomas, the LNM rate was lower, and none of the lymph nodes were marked as blue nodes (Figure 3B).
Figure 3.
Location and incidence of lymph node metastasis rates in patients with (A) central lung cancer and (B) peripheral adenocarcinoma. The numbers above the horizontal lines in each colored bubble represent the lymph node stations, while the numbers below the lines represent the rate of lymph node metastasis.
In patients with adenocarcinomas, the rate of LNM was significantly higher in central lung cancer than in peripheral lung cancer, especially in 2R/2L (8.8 vs. 3.3, P=0.002), L7 (6.8 vs. 2.0, P<0.001), L9 (5.7 vs. 1.0, P=0.041), L10 (7.2 vs. 2.6, P<0.001), L11 (5.4 vs. 2.4, P=0.005), and L12 (10.1 vs. 3.0, P<0.001).
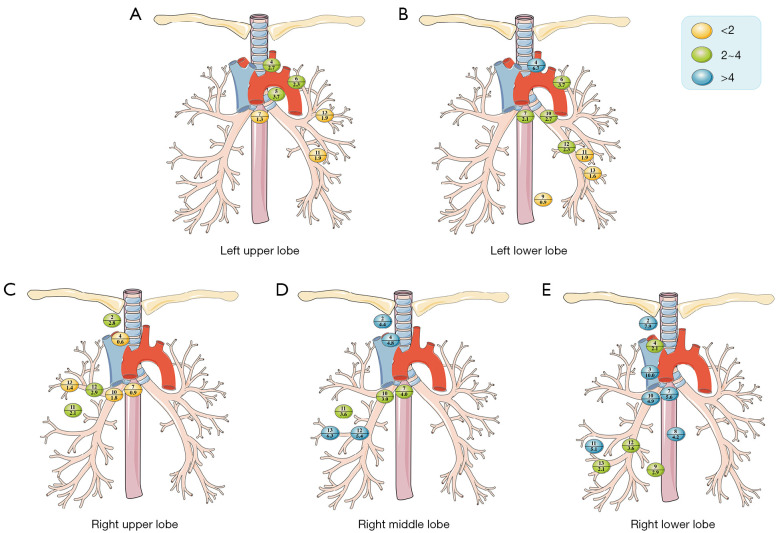
LNM rate in lung lobes affected
The highest rate of LNM was found in L12 (5.7%) for left upper lobe (Figure 4A) and 4L (6.7%) for left lower lobe (Figure 4B). In patients with adenocarcinoma of the right upper lobe, 2R (2.8%), L11 (2.1%), and L12 (2.9%) were labeled in green, while there were no nodes labeled in blue (Figure 4C). 2R (4.4%), 4R (4.8%), L12 (5.4%), and L13 (6.3%) for right middle lobe (Figure 4D) and 2R (7.9%), 3A/3P (10.0%), L7 (5.6%), L8 (4.2%), L10 (4.9%) and L11 (5.1%) for right lower lobe (Figure 4E) were labeled as blue nodes.
Figure 4.
Location and incidence of lymph node metastasis rates for lung lobes affected in adenocarcinomas. The numbers above the horizontal lines in each colored bubble represent the lymph node stations, while the numbers below the lines represent the rate of lymph node metastasis.
The probability of LNM was higher in patients with right lower lobe cancer relative to patients with cancer in other lung lobes, especially in 2R (7.9%, P=0.007), L7 (5.6%, P<0.001), L10 (4.9%, P=0.009), and L11 (5.1%, P=0.009). The LMN rate in L7 was significantly lower in left upper lobe (1.3%) and right upper lobe cancer (0.9%) than in other lung lobes (P<0.001).
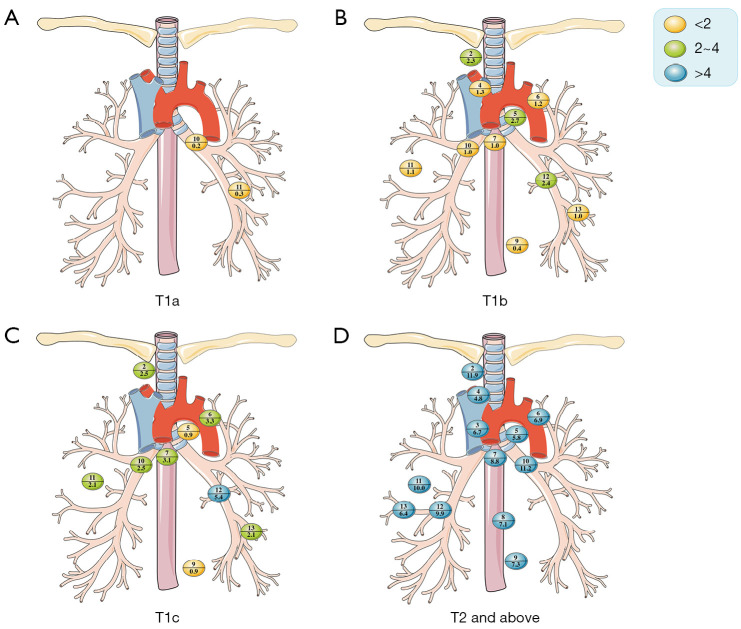
LNM rate in T-stage
In patients with lung cancer, the potential of LNM grew with increasing tumor diameter (T1a, 1.1%, 2/191; T1b, 6.1%, 38/625; T1c, 12.9%, 32/249; T2 and above, 35.4%, 86/243; Table S4; Figure 5), and the potential of N2 metastasis increased (T1a 0%, 0/191; T1b, 3.5%, 22/625; T1c, 5.6%, 14/249; T2 and above, 18.9%, 46/243).
Figure 5.
Location and incidence of lymph node metastasis rates for T-stage in adenocarcinomas. The numbers above the horizontal lines in each colored bubble represent the lymph node stations, while the numbers below the lines represent the rate of lymph node metastasis.
Lung cancer patients with tumors ≤1 cm had no pN2+ and few had pN1+.
Mediastinal LNM rate of patients with pT1b-cN2 adenocarcinoma in lung lobes affected
The rates of LNM in 34 patients with stage pT1b-cN2 adenocarcinomas are shown in Table 2. Among the 8 patients with pT1bN2 left upper lobe cancer, L5 metastasis occurred in 6 patients. Among the pT1b-cN2 patients, there was only 1 patient had left lower lobe cancer. All patients with right upper lung cancer had 2R metastasis (8/8). In 4 patients with right middle lobe cancer pT1bN2, L7 metastasis was present in all of them. Nine patients with right lower lobe cancer pT1b-cN2, had L7 metastasis (9/10).
Table 2. Mediastinal LNM in patients with pT1bN2 and pT1cN2 adenocarcinomas (N=34).
| Nodal station | pT1b (N=21) | pT1c (N=13) | pT1bN2/pT1cN2 (N=34) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LUL (N=8) | LLL (N=0) | RUL (N=4) | RML (N=4) | RLL (N=5) | LUL (N=2) | LLL (N=1) | RUL (N=4) | RML (N=1) | RLL (N=5) | LUL (N=10) | LLL (N=1) | RUL (N=8) | RML (N=5) | RLL (N=10) | |||
| 2R/2L | – | – | 4/4 | 1/3 | 3/3 | – | – | 4/4 | 1/1 | 0/2 | – | – | 8/8 | 2/4 | 3/5 | ||
| 3A/3P | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ||
| 4R/4L | 1/2 | – | 0/1 | 1/1 | – | 0/1 | – | 0/1 | – | 0/2 | 1/3 | – | 0/2 | 1/1 | 0/2 | ||
| L5 | 6/8 | – | – | – | – | 1/2 | – | – | – | – | 7/10 | – | – | – | – | ||
| L6 | 1/4 | – | – | – | – | 1/1 | – | – | – | – | 2/5 | – | – | – | – | ||
| L7 | 1/8 | – | 0/3 | 4/4 | 4/5 | 1/2 | 1/1 | 1/4 | 0/1 | 5/5 | 2/10 | 1/1 | 1/7 | 4/5 | 9/10 | ||
| L8 | – | – | – | – | 0/2 | – | – | 0/1 | – | – | – | – | 0/1 | – | 0/2 | ||
| L9 | 0/2 | – | – | 0/3 | 1/4 | – | 0/1 | 0/1 | 0/1 | 1/2 | 0/2 | 0/1 | 0/1 | 0/4 | 2/6 | ||
LNM, lymph node metastasis; LUL, left upper lobe; LLL, left lower lobe; RUL, right upper lobe; RML, right middle lobe; RLL, right low lobe.
The pathological subtype was predominantly adenocarcinoma with a predominantly acinar pattern (21/34), followed by a predominantly solid/micropapillary pattern (10/34). Interestingly, we found that 7 out of 10 patients with solid/micropapillary predominance had undergone 2L/2R dissection/sampling, and 6 of them had 2L/2R metastasis [lymph node metastases/total lymph nodes (9/18)]; all 10 patients had undergone L7 dissection/sampling, and 6 of them had L7 metastasis [lymph node metastases/total lymph nodes (7/16)]. Among the 10 patients with upper left lobe adenocarcinoma, 7 had L5 metastasis [lymph node metastases/total lymph nodes (8/19)].
Discussion
The current surgical approach for NSCLC is anatomic lung resection combined with SLND. In recent years, with the development of the concept of reducing parenchymal resection to improve postoperative lung function, the use of L-SLND in patients with early-stage NSCLC has become an area of research hot-spot. Several retrospective studies have shown that L-SLND is an alternative to SLND for selected clinical stage I-II patients and can potentially become the standard treatment for NSCLC surgery (3,4,7-9). Meanwhile, several studies have recommended that SLND be routinely performed intraoperatively in patients with resectable NSCLC (10,11). On the one hand, the prognostic impact of SLND on overall survival and disease-free survival remains controversial (8,9). On the other hand, it is not yet clear whether SLND reduces the actual N stage (12). In addition, L-SLND has been reported to increase the likelihood of recurrence (13). However, with the publication of more and more literature, lobe-specific lymphatic lead patterns are becoming increasingly plausible and practical (3,14,15). Therefore, we conducted this study on primary NSCLC patients treated with lobectomy combined with SLND to validate the role of L-SLND in NSCLC patients based on LNM rates.
We found that patients with central lung cancer were more likely to have mediastinal LNM than patients with peripheral lung cancer, especially in 2R/2L, L7 and L9. Therefore, L-SLND may reduce the N-stage of patients with central lung cancer, which can impact patients’ follow-up treatment plans.
Also, we found that the cohort’s median number of lymph nodes dissected between adenocarcinomas and squamous cell carcinomas varied. The histological origin of squamous cell carcinomas is most often found in the bronchial squamous epithelium or in sites where squamous epithelial hyperplasia occurs due to a history of regular smoking, with the central type of lung cancer being more common. This is also similar to our results, in which the central type was more common in squamous cell carcinomas and had a higher chance of LNM; therefore, surgeons are paying more attention to the extent of intraoperative lymph node dissection in squamous cell carcinomas.
In 2006, the European Society of Thoracic Surgeons (ESTS) proposed an L-SLND strategy for patients with early-stage NSCLC. For patients with peripheral clinical T1N0M0 lung cancer, they recommend L-SLND; for right upper and right middle lobe tumor clearance: 2R, 4R, L7; for right lower lobe: 4R, L7, L8, L9; for left upper lobe: L5, L6, L7; and for left lower lobe: L7, L8, L9 stations (15). The International Association for the Study of Lung Cancer (IASLC) suggests that for right-sided lung cancer, the ipsilateral stations 2R, 4R, L7, L10, and L11 should be cleared; for left-sided, stations L5, L6, L7, L10, and L11; and for if the lower lobe is involved, L9 should be added (16). Several studies consistently recommended 4L, L5, and L6 for left upper lobe, 2R and 4R for right upper, and L7, L8, and L9 for bilateral lower lobes (8,9,17).
In our study, 4L, L5, and L6 had high metastasis rates among the mediastinal lymph nodes in patients with left upper lobe cancer. The rate of 2R metastasis was higher in patients with right upper lobe cancer. The LNM rate in L7 was significantly lower in the left upper (1.3%) and right upper (0.9%) than in other lung lobes (P<0.001), so intraoperative clearance of subcarinal lymph nodes was not necessary, in keeping with the findings of previous studies (18). Mediastinal LNM in the right middle lobe cancer was similar to that in the right upper, and all had 2R, 4R, and L7 metastasis. Left lower lobe cancer tended to metastasis in 4L, L6, and L7, and although the rate of metastasis in L7 lymph nodes was not very high, it was most prone to jump metastasis among the mediastinal lymph nodes (40%, 4/10). This may be because the segmental and subpleural lymphatics originating from the lower lobe can drain directly to the subcarinal lymph nodes without involving the intrapulmonary or hilar lymph nodes (19). Patients with right lower lobe cancer had a high probability of LNM relative to patients with cancer in other lung lobes, especially in 2R (7.9%, P=0.007) and L7 (5.6%, P<0.001). We found a high rate of LNM in 3A/3P, which may be due to some bias in the small number of patients included, with intraoperative clearance of lymph nodes in 3A/3P stations.
Patients with lung cancer stage pT1a did not have any N2 lymph node metastases but had a few N1 lymph node metastases (2/191, 1.1%), consistent with the previous findings of Meng et al. (18) and suggests that mediastinal lymph node dissection may not be required for tumor size ≤1 cm. In patients with stage T1b-c adenocarcinomas, mediastinal LNM showed lobe-specific features. In patients with left upper lobe cancer, 4L, L5, and L6 were the most commonly metastasized lymph node stations and occasionally metastasized to L7 (2/12). In patients with lower left lobe cancer, L7 was the only lymph node with metastasis, which may be due to the small number of patients with left lower lobe cancer included in the study and needs to be verified further by future studies. All patients with right upper lobe cancer had LNM in the 2R group. Considering that 2R and 4R stations will fuse intraoperatively, the 4R group should be cleared simultaneously with the 2R. The L7 metastasis rate is significantly lower in both upper lung lobes than in others, so intraoperative clearance of the subcarinal lymph nodes is unnecessary (18). Patients with right middle lobe cancer had the highest rate of metastasis in L7, followed by the 2R and 4R groups. In patients with right lower lobe cancer, the highest metastasis rate was observed in L7 (9/10), followed by 2R and L9 groups. The probability of LNM in patients with tumor >3 cm was significantly higher (86/243, 35.4%) and the metastasis rate in each lymph node station was significantly higher compared with the T1 stage. Several studies have shown that after propensity score matching, the positive LNM rate increases with increasing T stage (8,9,20,21).
Based on our findings, patients with central lung cancer are more likely to develop LNM than patients with peripheral lung cancer, and SLND is recommended. There is no necessity for mediastinal lymph node dissection for patients with stage pT1a. L-SLND may be inappropriate for lung cancer patients with tumors >3 cm. If preoperative positron emission computed tomography-scan/endobronchial ultrasonography [positron emission tomography (PET-scan)/endobronchial ultrasound (EBUS)] considers the absence of N2 metastases, then L-SLND can be performed in patients with T1b-c lung adenocarcinoma. L-SLND is recommended for patients based on the location of the tumor: left upper lobe: 4L, L5, L6; right upper lobe: 2R, 4R; right middle lobe: 2R, 4R, L7 and 2R, 4R, L7, L9 for right lower lobe.
There are some limitations in this retrospective study. Firstly, our findings are based on a single center of an Asian population, which may have some bias in the distribution of lymph node metastases compared to other populations and may have potential selection bias. Secondly, for the analysis of patients with pT1bN2 and pT1cN2 stage adenocarcinoma, the number of patients included was minimal, and further studies are needed for validation. In addition, our study only described the rate of LNM and did not assess the prognosis, which needs further follow-up to identify local mediastinal recurrence. However, the pattern of lymph node invasion directly affects the staging and adjuvant treatment of patients and is, therefore, still of some importance.
Conclusions
Our study proposes recommendations for lymph node dissection according to the pathological type of lung cancer, tumor location, lung lobes affected and tumor size. Recommendations are also made for mediastinal lymph node dissection in patients with pT1bN2 and pT1cN2 stages in different lung lobes. A proper L-SLND can significantly improve the perioperative outcome of patients without compromising their long-term survival prognosis. For patients with different characteristics of NSCLC, LNM may show a characteristic distribution, which may provide a certain reference value for clinical work.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: The present study was supported by the National Natural Science Foundation of China (No. 82002420), Natural Science Foundation of Zhejiang Province (No. LTGY23H160028), Medical and Health Research Project of Zhejiang Province (No. 2023KY1311), Medical and Health Research Project of Zhejiang Province (No. 2020KY353), The Zhejiang Province’s Vanguard Geese Leading Plan Project (No. 2022C03152) and Key Research Project of Traditional Chinese Medicine in Zhejiang Province (No. 2022ZZ006).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The written patient informed consent was waived by the Ethics Review Committee of Taizhou Hospital, Zhejiang Province (No. K20221222).
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-450/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-450/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-450/dss
References
- 1.Wu F, Wang L, Zhou C. Lung cancer in China: current and prospect. Curr Opin Oncol 2021;33:40-6. 10.1097/CCO.0000000000000703 [DOI] [PubMed] [Google Scholar]
- 2.Adachi H, Ito H, Nagashima T, et al. Mediastinal lymph node dissection in segmentectomy for peripheral c-stage IA (≤2 cm) non-small-cell lung cancer. J Thorac Cardiovasc Surg 2024;S0022-5223(24)00786-4. [DOI] [PubMed]
- 3.Deng HY, Qin CL, Li G, et al. Can lobe-specific lymph node dissection be an alternative to systematic lymph node dissection in treating early-stage non-small cell lung cancer: a comprehensive systematic review and meta-analysis? J Thorac Dis 2018;10:2857-65. 10.21037/jtd.2018.04.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng HY, Zhou J, Wang RL, et al. Lobe-Specific Lymph Node Dissection for Clinical Early-Stage (cIA) Peripheral Non-small Cell Lung Cancer Patients: What and How? Ann Surg Oncol 2020;27:472-80. 10.1245/s10434-019-07926-3 [DOI] [PubMed] [Google Scholar]
- 5.Oncology Society of Chinese Medical Association. Chinese Medical Association Publishing House . [Chinese Medical Association guideline for clinical diagnosis and treatment of lung cancer (2022 edition)]. Zhonghua Zhong Liu Za Zhi 2022;44:457-90. 10.3760/cma.j.cn112152-20220413-00255 [DOI] [PubMed] [Google Scholar]
- 6.Yang MZ, Hou X, Liang RB, et al. The incidence and distribution of mediastinal lymph node metastasis and its impact on survival in patients with non-small-cell lung cancers 3 cm or less: data from 2292 cases. Eur J Cardiothorac Surg 2019;56:159-66. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro M, Kadakia S, Lim J, et al. Lobe-specific mediastinal nodal dissection is sufficient during lobectomy by video-assisted thoracic surgery or thoracotomy for early-stage lung cancer. Chest 2013;144:1615-21. 10.1378/chest.12-3069 [DOI] [PubMed] [Google Scholar]
- 8.Hishida T, Miyaoka E, Yokoi K, et al. Lobe-Specific Nodal Dissection for Clinical Stage I and II NSCLC: Japanese Multi-Institutional Retrospective Study Using a Propensity Score Analysis. J Thorac Oncol 2016;11:1529-37. 10.1016/j.jtho.2016.05.014 [DOI] [PubMed] [Google Scholar]
- 9.Adachi H, Sakamaki K, Nishii T, et al. Lobe-Specific Lymph Node Dissection as a Standard Procedure in Surgery for Non-Small Cell Lung Cancer: A Propensity Score Matching Study. J Thorac Oncol 2017;12:85-93. 10.1016/j.jtho.2016.08.127 [DOI] [PubMed] [Google Scholar]
- 10.Graham AN, Chan KJ, Pastorino U, et al. Systematic nodal dissection in the intrathoracic staging of patients with non-small cell lung cancer. J Thorac Cardiovasc Surg 1999;117:246-51. 10.1016/S0022-5223(99)70419-8 [DOI] [PubMed] [Google Scholar]
- 11.Manfredini B, Zirafa CC, Filosso PL, et al. The Role of Lymphadenectomy in Early-Stage NSCLC. Cancers (Basel) 2023;15:3735. 10.3390/cancers15143735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao W, Chen T, Feng J, et al. Comparison of lymph node dissection and lymph node sampling for non-small cell lung cancers by video-assisted thoracoscopic surgery. J Thorac Dis 2019;11:505-13. 10.21037/jtd.2019.01.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamigaichi A, Aokage K, Ikeno T, et al. Long-term survival outcomes after lobe-specific nodal dissection in patients with early non-small-cell lung cancer. Eur J Cardiothorac Surg 2023;63:ezad016. 10.1093/ejcts/ezad016 [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Qi Z, Cheng D, et al. Lobe-Specific Node Dissection Can Be a Suitable Alternative to Systematic Lymph Node Dissection in Highly Selective Early-Stage Non-Small-Cell Lung Cancer Patients: A Meta-Analysis. Ann Thorac Cardiovasc Surg 2021;27:143-50. 10.5761/atcs.oa.20-00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Leyn P, Lardinois D, Van Schil P, et al. European trends in preoperative and intraoperative nodal staging: ESTS guidelines. J Thorac Oncol 2007;2:357-61. 10.1097/01.JTO.0000263722.22686.1c [DOI] [PubMed] [Google Scholar]
- 16.Rusch VW, Asamura H, Watanabe H, et al. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568-77. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y, Mao Y, He J, et al. Lobe-specific Lymph Node Dissection in Clinical Stage IA Solid-dominant Non-small-cell Lung Cancer: A Propensity Score Matching Study. Clin Lung Cancer 2021;22:e201-10. 10.1016/j.cllc.2020.09.012 [DOI] [PubMed] [Google Scholar]
- 18.Meng S, Liu G, Wang S, et al. Nodal Involvement Pattern in Clinical Stage IA Non-Small Cell Lung Cancer According to Tumor Location. Cancer Manag Res 2020;12:7875-80. 10.2147/CMAR.S262623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Cheng J, Huang W, et al. Skip metastasis in mediastinal lymph node is a favorable prognostic factor in N2 lung cancer patients: a meta-analysis. Ann Transl Med 2021;9:218. 10.21037/atm-20-3513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang C, Xiang Y, Han W. Preoperative risk factors of lymph node metastasis in clinical N0 lung adenocarcinoma of 3 cm or less in diameter. BMC Surg 2022;22:153. 10.1186/s12893-022-01605-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuroda H, Ichinose J, Masago K, et al. Permissible Outcomes of Lobe-Specific Lymph Node Dissection for Elevated Carcinoembryonic Antigen in Non-Small Cell Lung Cancer. Medicina (Kaunas) 2021;57:1365. 10.3390/medicina57121365 [DOI] [PMC free article] [PubMed] [Google Scholar]