Abstract
Background
Rearrangement in anaplastic lymphoma kinase (ALK) occurs in 4–7% of non-small cell lung cancer (NSCLC) cases. Despite improved survival with tyrosine kinase inhibitors (TKIs), treatment resistance remains challenging. This retrospective study analyzed advanced ALK-positive NSCLC patients, focusing on clinical aspects, treatments, resistance, and outcomes.
Methods
Patients diagnosed between January 2009 and December 2021 who received at least one ALK-TKI line at the Karolinska University Hospital, were included. We evaluated crizotinib or 2nd generation ALK-TKI effectiveness in first-line treatment and lorlatinib in subsequent lines. Overall survival (OS) was defined as from the date of advanced lung cancer diagnosis until the date of last follow-up (April 22, 2022) or the date of death from any cause. Progression-free survival (PFS), from the date of starting ALK-TKI until the date of progression, death, or last follow-up. Resistance mechanisms were assessed through re-biopsies utilizing next-generation sequencing (NGS).
Results
Out of 160 eligible patients, 10 were excluded. Median follow-up was 54.0 months from diagnosis and 45.0 months from initial ALK-TKI treatment. Crizotinib showed a median PFS of 8.0 months and a median OS of 35.0 months. Second generation ALK-TKIs demonstrated a median PFS of 52.0 months [OS was not reached (NR)]. Overall, the median OS was 65.0 months. Poor prognostic factors included male sex, thromboembolism, crizotinib treatment, and chronic obstructive pulmonary disease (COPD)/asthma. Rebiopsies in 18 cases revealed secondary ALK mutations in 8 patients, correlating with a shorter median PFS in subsequent ALK-TKI treatment (1.0 vs. 7.0 months).
Conclusions
This comprehensive study, spanning over a decade, provides crucial insights into the clinical characteristics, treatment patterns, and resistance mechanisms of advanced ALK-positive NSCLC, where median OS exceeds 5 years. Re-biopsies during treatment are essential for advancing our understanding of resistance mechanisms and the tumor dynamics evolving during ALK-TKI therapy.
Keywords: Advanced non-small cell lung cancer (advanced NSCLC), anaplastic lymphoma kinase (ALK), tyrosine kinase inhibitor (TKI), real-world data, long-term survival outcomes
Highlight box.
Key findings
• This retrospective study provides valuable insights into the real-world clinical experience and outcomes of patients treated with anaplastic lymphoma kinase (ALK)-tyrosine kinase inhibitors (TKIs), offering guidance for the optimization of treatment strategies.
• The median overall survival was 65.0 months for patients treated with ALK-TKI as first-line treatment. Poor prognostic factors identified included: male gender, thromboembolism, crizotinib as initial treatment, and chronic lung disease.
What is known and what is new?
• While clinical trials play a pivotal role in advancing cancer therapies, they often fall short in addressing the myriad complexities surrounding patient outcomes and treatment resistance.
• Our comprehensive analysis of clinicopathologic and molecular characteristics, treatment patterns, and discontinuation reasons after progression on initial ALK-TKI provides practical insights into the challenges faced during treatment. The identification of specific resistance mechanisms, including secondary ALK kinase domain mutations and off-target genetic aberrations, highlights the need for personalized therapeutic approaches to overcome resistance.
What is the implication, and what should change now?
• The findings emphasize the need for continued research into personalized and sequential treatment strategies to enhance long-term survival outcomes in this patient population.
• Access to ALK-TKIs with good central nervous system penetration and tolerability, is important to achieve prolonged survival. Re-biopsies during treatment are crucial for enhancing our understanding of resistance mechanisms.
Introduction
Rearrangement in the anaplastic lymphoma kinase (ALK) gene is a driving oncogenic event causing non-small cell lung cancer (NSCLC) and is detected in approximately 4–7% of all cases (1). There are more than 90 different ALK fusion partners described where echinoderm microtubule-associated protein-like 4 (EML4) is the most common one and accounts for 80% of the ALK fusions (2). At least 15 different EML4-ALK variants have been identified where variants 1, 2, and 3a/b have been reported to represent 33%, 10%, and 29% of the cases, respectively (3). These patients are often younger with light or never smoking history and adenocarcinoma histology (4). They are at higher risk of developing brain metastasis which affects up to 60% of the patients during the disease (5).
Targeted therapy with tyrosine kinase inhibitors (TKIs) has significantly improved survival for advanced ALK-positive lung cancer patients, replacing the previous standard of care with conventional systemic chemotherapy due to improved response rates and clinical outcomes (6). The first ALK-TKI approved was crizotinib which showed a significantly better response rate and progression-free survival (PFS) compared to chemotherapy (7). However, many patients develop brain metastases due to the drug’s limited penetration into the central nervous system (CNS) (8). Today, the 2nd generation ALK-TKIs alectinib, ceritinib, and brigatinib are all approved in the first-line setting with better penetration in the brain (9-11). In a substantial fraction of patients, patients develop drug resistance due to secondary ALK kinase domain mutations leading to clinical relapse, these “on-target” mutations include C1156Y, L1196M, G1269A, and G1202R (12,13). Other resistance mechanisms described are the occurrence of “bypass signaling” or “off-target” genetic aberrations, such as in epidermal growth factor receptor (EGFR) and Kirsten rat sarcoma virus (KRAS) (14). Lorlatinib is a third-generation ALK-TKI that is also indicated in first-line but is also designed to overcome secondary resistance mutations within the ALK tyrosine kinase domain (15). The introduction of these drugs has prolonged the median overall survival (OS) for patients with metastatic ALK-positive NSCLC to over 7 years (16,17).
Even though several drugs provide impressive results, the most effective sequential treatment strategy is yet to be known. In this retrospective study, we aimed to analyze the real-life cohort of Swedish patients with advanced ALK-positive NSCLC treated at Karolinska University Hospital in Stockholm to evaluate the roles of different ALK-TKIs when used in the first line and to identify potential unmet needs of these treatments. Evaluation of resistance mechanisms was also performed on re-biopsies using next-generation sequencing (NGS). Our study provides a comprehensive picture of clinical characteristics, treatment pathways, and outcomes of real-life ALK-rearranged NSCLC patients with advanced disease in clinical practice. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-396/rc).
Methods
Study design and study population
A retrospective cohort study of NSCLC patients was identified in electronic health records (EHRs) at the Karolinska University Hospital in Stockholm. All patients diagnosed with ALK-rearranged NSCLC between January 2009 and December 2021 were reviewed. To minimize selection bias, we used uniform inclusion and exclusion criteria. Only patients with unresectable stage III, stage IV, or recurrence from earlier stages were included in the study. Patients treated with at least one line of ALK-TKI were included and subdivided into either receiving crizotinib or 2nd generation ALK-TKI in the first line in the subsequent analyses.
The ALK-positivity of the tumors was assessed by either fluorescent in situ hybridization (FISH) or immunohistochemistry (IHC). If enough tumor specimens were at hand, EML4-ALK fusion variants were also determined by reverse transcription polymerase chain reaction (RT-PCR). Patients diagnosed after 2015 were analyzed by NGS. Resistance mechanisms were evaluated in patients who underwent a re-biopsy of resistant tumor tissue by using NGS.
ALK treatment scenario in Sweden
During the study period, five different ALK-TKIs were approved by the European Medical Agency (EMA) and reimbursed in Sweden: crizotinib (after progression on chemotherapy and as first-line treatment), ceritinib (after progression on crizotinib or as first-line treatment), alectinib (after crizotinib treatment and first-line treatment), brigatinib (as first-line treatment or after crizotinib), and lorlatinib (as second-line treatment after progression on 2nd generation ALK-TKIs, after the study period also approved in first-line).
Data collection
The study was approved by the external Ethical Review Authority (Dnr 2022-00323-01) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013) (18). Individual consent for this retrospective analysis was waived. The data were collected using patients’ EHRs and pseudo-anonymized with identification codes. The following variables were retrospectively collected: demographic data, e.g., age and gender; tumor stage according to the eighth edition of the tumor-node-metastasis (TNM) classification, date of diagnosis of advanced disease; metastatic sites; oncological treatment including start and stop dates; histopathology; smoking status at diagnosis; thromboembolic events; physicians’ evaluations of Eastern Cooperative Oncology Group (ECOG) performance status (PS); and date of death or last follow-up.
Detection of ALK status, fusion variants, and ALK secondary mutations
A molecular testing algorithm was established and tested for the most reported EML4-ALK fusion variants. All the tests were performed on formalin-fixed paraffin-embedded (FFPE) material (4 µm thick). Tumor cell content was confirmed to be >20 % and around 500 cells per section at minimum was evaluated. All samples were deparaffinized before RNA extraction and further analysis. The test algorithm included in this report consisted of four techniques, FISH, IHC (19), NGS (20), and semi-quantitative RT-PCR (21), according to the standard protocol for clinical routine testing.
Statistical analysis
Categorical data were presented as percentages and continuous data was described by medians. The Chi-squared test was used to analyze categorical/ordinal variables and the Mann-Whitney test for continuous variables to determine the significance of clinicopathological differences between patients receiving either crizotinib or a 2nd generation ALK-TKI as initial treatment.
OS was defined as from the date of advanced lung cancer diagnosis until the date of last follow-up (April 22, 2022) or the date of death from any cause. In a subsequent step, OS was also evaluated from the start of the initial ALK-TKI until the date of the last follow-up or death. PFS, from the date of starting ALK-TKI until the date of progression, death, or last follow-up. Both OS and PFS were estimated using the time-to-event approach, described in Kaplan-Meier curves, and differences were analyzed by log-rank tests. In the next step, univariable Cox regression models were performed and statistically significant prognostic factors were further included in a multivariable Cox regression model to assess potential variables that were associated with poor prognosis and to control for confounders. Results from the models were presented as hazard ratios (HRs) with 95% confidence intervals (CIs).
Swimmer’s plots were used to summarize sequential therapy on those patients who underwent a re-biopsy after progression on ALK-TKI with the duration of treatment and occurrence of ALK secondary mutations.
All statistical tests were two-sided and a P value of <0.05 was considered statistically significant. All analyses were performed using the Statistical Package for Social Sciences (SPSS) version 27, except for the Swimmer plot that was created using the swim plot package in R version 4.2.0.
Results
Patient and tumor characteristics
We identified a total cohort of 160 patients with metastatic ALK-positive NSCLC treated at Karolinska University Hospital between January 2009 and December 2021. Only patients treated with at least one line of ALK-TKI were included in the final analysis, 10 patients were excluded (five patients without any systemic treatment and five patients treated with only chemotherapy). The patient characteristics and demographics are described in Table 1.
Table 1. Clinical and demographic characteristics of patients with advanced ALK-positive non-small cell lung cancer treated at Karolinska University Hospital between January 2009 and December 2021.
| Variables | Total cohort (n=150) | Initial ALK-TKI | P value† | |
|---|---|---|---|---|
| Crizotinib (n=74) | 2nd generation ALK-TKI (n=76) | |||
| Age (years) | 61 [23–89] | 61.5 [30–85] | 60.5 [23–89] | 0.63 |
| Age category (years) | 0.07 | |||
| ≤70 | 118 (78.7) | 63 (85.1) | 55 (72.4) | |
| >70 | 32 (21.3) | 11 (14.9) | 21 (27.6) | |
| Sex | 0.72 | |||
| Male | 61 (40.7) | 29 (39.2) | 32 (42.1) | |
| Female | 89 (59.3) | 45 (60.8) | 44 (57.9) | |
| Histology | 0.54 | |||
| Adenocarcinoma | 143 (95.3) | 71 (95.9) | 72 (94.7) | |
| Squamous carcinoma | 5 (3.3) | 2 (2.7) | 3 (3.9) | |
| Undifferentiated carcinoma | 1 (0.7) | 0 (0.0) | 1 (1.3) | |
| Adenosquamous carcinoma | 1 (0.7) | 1 (1.4) | 0 (0.0) | |
| ECOG PS | 0.32 | |||
| 0–1 | 141 (94.0) | 71 (95.9) | 70 (92.1) | |
| ≥2 | 9 (6.0) | 3 (4.1) | 6 (7.9) | |
| Smoking history | 0.09 | |||
| Never smoker | 86 (57.3) | 43 (58.1) | 43 (56.6) | |
| Ex-smoker | 49 (32.7) | 20 (27.0) | 29 (38.2) | |
| Smoker | 15 (10.0) | 11 (14.9) | 4 (5.3) | |
| Chronic lung disease | 0.44 | |||
| COPD/asthma | 10 (6.7) | 6 (8.1) | 4 (5.3) | |
| No | 140 (93.3) | 68 (91.9) | 72 (94.7) | |
| M stage | 0.10 | |||
| M0 (advanced) | 7 (4.7) | 4 (5.4) | 3 (3.9) | |
| M1a | 35 (23.3) | 21 (28.4) | 14 (18.4) | |
| M1b | 42 (28.0) | 24 (32.4) | 18 (23.7) | |
| M1c | 66 (44.0) | 25 (33.8) | 41 (53.9) | |
| Brain metastasis | <0.001* | |||
| Primary | 30 (20.0) | 6 (8.1) | 24 (31.6) | |
| Secondary | 41 (27.3) | 37 (50.0) | 4 (5.3) | |
| No | 79 (52.7) | 31 (41.9) | 48 (63.1) | |
| Skeletal metastasis | 0.10 | |||
| Primary | 59 (39.3) | 29 (39.2) | 30 (39.5) | |
| Secondary | 13 (8.7) | 10 (13.5) | 3 (3.9) | |
| No | 78 (52.0) | 35 (47.3) | 43 (56.5) | |
| Liver metastasis | 0.27 | |||
| Primary | 35 (23.3) | 18 (24.3) | 17 (22.4) | |
| Secondary | 20 (13.3) | 13 (17.6) | 7 (9.2) | |
| No | 95 (63.3) | 43 (58.1) | 52 (68.4) | |
| Adrenal metastasis | 0.34 | |||
| Primary | 18 (12.0) | 7 (9.5) | 11 (14.5) | |
| Secondary | 8 (5.3) | 5 (6.8) | 3 (3.9) | |
| No | 124 (82.7) | 62 (83.4) | 62 (81.6) | |
| First-line ALK-TKI | ||||
| Crizotinib | 28 (18.7) | 28 (37.8) | 0 (0.0) | |
| Ceritinib | 7 (4.7) | 0 (0.0) | 7 (9.2) | |
| Alectinib | 60 (40.0) | 0 (0.0) | 60 (78.9) | |
| Brigatinib | 1 (0.7) | 0 (0.0) | 1 (1.3) | |
| Chemotherapy cycles before ALK-TKI | <0.001* | |||
| 0 | 96 (64.0) | 28 (37.8) | 68 (89.5) | |
| 1 | 39 (26.0) | 35 (47.3) | 4 (5.3) | |
| 2–6 | 15 (10.0) | 11 (14.9) | 4 (5.3) | |
| Total number of ALK-TKIs | 0.003* | |||
| One or two ALK inhibitors | 125 (83.3) | 54 (73.0) | 71 (93.4) | |
| ≥ Three ALK inhibitors | 25 (16.7) | 20 (27.0) | 5 (6.6) | |
| Brain radiotherapy | 0.003* | |||
| SRS | 26 (17.3) | 14 (18.9) | 12 (15.8) | |
| WBRT | 10 (6.7) | 10 (13.5) | 0 (0.0) | |
| None | 114 (76.0) | 50 (69.4) | 64 (84.2) | |
| EML4-ALK fusion type | 0.03* | |||
| Variant 1 | 37 (24.7) | 19 (25.7) | 18 (23.7) | |
| Variant 2 | 7 (4.7) | 5 (6.8) | 2 (2.6) | |
| Variant 3a/b | 29 (19.3) | 19 (25.7) | 10 (13.2) | |
| Other variants | 18 (12.0) | 11 (14.9) | 7 (9.2) | |
| Non-EML4 | 1 (0.7) | 0 (0.0) | 1 (1.3) | |
| Missing | 58 (38.7) | 20 (27.0) | 38 (50.0) | |
| PD-L1 status | <0.001* | |||
| Positive (≥1%) | 42 (28.0) | 11 (14.0) | 31 (40.8) | |
| Negative (<1%) | 34 (22.7) | 3 (4.1) | 31 (40.8) | |
| Not determined | 74 (49.3) | 60 (81.1) | 14 (18.4) | |
| Thromboembolism | 0.002* | |||
| Yes | 57 (38.0) | 43 (58.1) | 14 (18.4) | |
| No | 93 (62.0) | 31 (41.9) | 62 (81.6) | |
| Deceased | <0.001* | |||
| Yes | 76 (50.7) | 60 (81.1) | 16 (21.1) | |
| No | 74 (49.3) | 14 (18.9) | 60 (78.9) | |
| Follow-up from diagnosis (months) | 54.0 (40.9–67.1) | 117.0 (86.5–147.5) | 28.0 (20.9–35.1) | |
| Follow-up from ALK-TKI (months) | 45.0 (41.4–48.6) | 87.0 (69.0–105.0) | 26.0 (20.3–31.7) | |
Results are presented as median [range], n (%), or median (95% CI). †, P value for the statistical difference between patients receiving crizotinib and 2nd generation ALK-TKI; *, clinical variables that are statistically significant between the groups. ALK, anaplastic lymphoma kinase; TKI, tyrosine kinase inhibitor; ECOG, Eastern Cooperative Oncology Group; PS, performance status; COPD, chronic obstructive pulmonary disease; SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy; EML4, echinoderm microtubule-associated protein-like 4; PD-L1, programmed cell death-ligand 1; CI, confidence interval.
The median age in our cohort was 61 (range, 23–89) years and 89 (59.3%) were females. The majority of the patients had adenocarcinoma histology (95.3%), were never smokers (57.3%), and had a good ECOG PS of 0–1 (94.0%). Ten patients had chronic lung disease, either chronic obstructive pulmonary disease (COPD) or asthma, as comorbidity.
Most of the patients (44.0%) had multiple metastases at baseline (M1c). Thirty patients presented with brain metastases at diagnosis (20.0%) and 41 patients (27.3%) developed brain metastases while on treatment. Radiotherapy to the brain, stereotactic radiosurgery (SRS) (17.3%), or whole-brain radiotherapy (WBRT) (6.7%), was given in 36 patients (24.0%).
Most patients received ALK-TKI as first-line treatment (64.0%), mostly alectinib (40.0%) and crizotinib (18.7%). Fifty-four patients (36.0%) received a TKI as a subsequent line after progression on chemotherapy. All patients were treated with a median of one line of ALK-TKI (range, 1–6).
The most common fusion variants detected in the cohort were variant 1 (24.7%) and variant 3a/b (19.3%). Programmed cell death-ligand 1 (PD-L1) status was checked in 76 patients and 42 (28.0%) had positive results with a staining threshold of 1%. Re-biopsies were performed in 18 cases during progression on ALK-TKI and secondary ALK mutations were found in eight patients. Fifty-seven patients developed thromboembolism throughout their disease and 76 patients were still alive on the day of analysis.
Clinical outcome analyses of ALK-TKI treatments
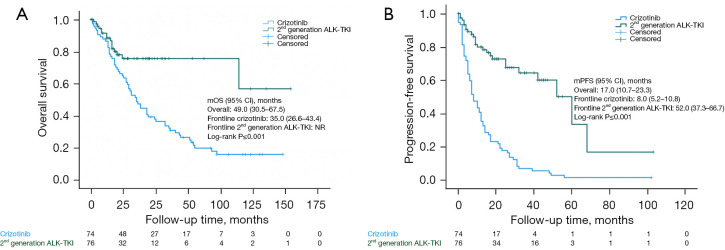
The median follow-up time from initiation of an initial ALK-TKI is presented in Table 1. The median PFS and median OS were significantly longer for 2nd generation ALK-TKI compared to crizotinib (Figure 1). The median PFS for 2nd generation ALK-TKI was 52.0 months (95% CI: 37.3–66.7) but not reached (NR) for the median OS.
Figure 1.
Kaplan-Meier curves of all patients. OS (A) and PFS (B) were significantly different between ALK-positive NSCLC patients receiving crizotinib and a 2nd generation ALK-TKI. ALK, anaplastic lymphoma kinase; TKI, tyrosine kinase inhibitor; mOS, median overall survival; CI, confidence interval; NR, not reached; mPFS, median progression-free survival; OS, overall survival; PFS, progression-free survival; NSCLC, non-small cell lung cancer.
The median PFS and median OS for crizotinib were 8.0 months (95% CI: 5.2–10.8) and 35.0 months (95% CI: 26.6–43.4), respectively. Median OS in the crizotinib group who were treated with 2nd generation ALK-TKI upon progression was 50.0 months compared to those who were not (14.0 months, P<0.001). For the full cohort, 60% were treated with a 2nd generation ALK-TKI upon progression on either another 2nd generation ALK-TKI or crizotinib with a median OS of 57.0 vs. 16.0 months (P<0.001) (Table S1).
Of the 150 patients, 134 were available for response evaluation. Overall response rate (ORR) was significantly higher in patients receiving 2nd generation ALK-TKI compared to crizotinib, 91% (95% CI: 82–97%) vs. 69% (95% CI: 56–79%).
Median OS for the whole cohort was 49.0 months (95% CI: 30.5–67.5), but was higher in the group who received ALK-TKI as first-line treatment at 65.0 months (95% CI: 22.7–107.3) compared to those who were treated with chemotherapy before ALK-TKI with 44.0 months (95% CI: 22.8–65.2); this was not statistically significant between the groups (log-rank P=0.76) (Table S1). Patients treated with at least three ALK inhibitors had significantly longer median OS compared to those treated with one or two (79.0 vs. 43.0 months, log-rank P=0.008; HR =0.46, 95% CI: 0.25–0.83) (Figure S1 and Table S2).
Univariate and multivariable analyses of OS and PFS for initial ALK-TKI
We studied the impact of clinical and molecular factors on PFS and OS on initial ALK-TKI. Both univariate and multivariable analyses are shown in Table 2.
Table 2. Univariate and multivariable Cox proportional hazards regression analysis of covariables associated with OS and PFS.
| Variables | Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| OS | |||||
| Age (≥70 years) | 1.79 (1.03–3.12) | 0.04* | 1.45 (0.75–2.80) | 0.27 | |
| Sex (male) | 1.74 (1.09–2.77) | 0.02* | 1.64 (1.10–2.70) | 0.050* | |
| PS† | 1.47 (1.06–2.02) | 0.02* | 1.43 (0.98–2.08) | 0.07 | |
| Tumor stage M1c (yes) | 1.33 (0.84–2.11) | 0.22 | – | – | |
| Primary brain metastasis (yes) | 0.74 (0.40–1.37) | 0.33 | – | – | |
| Smokers (yes) | 2.41 (1.30–4.49) | 0.005* | 1.24 (0.63–2.42) | 0.53 | |
| TE (yes) | 2.80 (1.77–4.44) | <0.001* | 2.09 (1.27–3.46) | 0.004* | |
| Prior chemotherapy (yes) | 1.10 (0.68–1.71) | 0.76 | – | – | |
| Initial ALK-TKI (crizotinib) | 2.64 (1.51–4.61) | <0.001* | 2.17 (1.21–3.89) | 0.009* | |
| Chronic lung disease (yes) | 5.13 (2.27–11.59) | <0.001* | 3.71 (1.59–8.70) | 0.003* | |
| Variant 3a/b (yes) | 1.03 (0.60–1.75) | 0.92 | – | – | |
| PD-L1 negative (yes) | 0.68 (0.32–1.43) | 0.31 | – | – | |
| PFS | |||||
| Age (≥70 years) | 1.24 (0.76–2.04) | 0.40 | – | – | |
| Sex (male) | 1.26 (0.84–1.88) | 0.27 | – | – | |
| PS† | 1.17 (0.91–1.50) | 0.23 | – | – | |
| Tumor stage M1c (yes) | 0.95 (0.63–1.42) | 0.78 | – | – | |
| Primary brain metastasis (yes) | 0.57 (0.33–0.99) | 0.046* | 1.14 (0.61–2.14) | 0.68 | |
| Smokers (yes) | 1.90 (1.05–3.41) | 0.03* | 1.01 (0.53–1.93) | 0.98 | |
| No. previous lines of CT† | 1.17 (0.97–1.40) | 0.10 | – | – | |
| Prior chemotherapy (yes) | 1.76 (1.18–2.62) | 0.006* | 0.66 (0.41–1.08) | 0.10 | |
| Initial ALK-TKI (crizotinib) | 4.60 (2.91–7.27) | <0.001* | 5.41 (2.95–9.94) | <0.001* | |
| Chronic lung disease (yes) | 3.07 (1.40–6.73) | 0.005* | 1.92 (0.81–4.53) | 0.14 | |
| Variant 3a/b (yes) | 1.37 (0.86–2.19) | 0.19 | – | – | |
| PD-L1 negative (yes) | 0.35 (0.18–0.67) | 0.002* | 0.72 (0.35–1.51) | 0.39 | |
†, PS as ordinal variable and No. previous lines of CT as a continuous variable; *, clinical variables that are statistically significant between the groups. OS, overall survival; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; PS, performance status; TE, thromboembolism; ALK, anaplastic lymphoma kinase; TKI, tyrosine kinase inhibitor; PD-L1, programmed cell death-ligand 1; No., number; CT, chemotherapy.
Univariate analysis of OS showed that age ≥70 years, males, PS as ordinal variable, smokers, thromboembolism, crizotinib treatment, and chronic lung disease were significant factors. Furthermore, multivariable analysis of OS showed that poor prognostic factors were males (HR =1.64; 95% CI: 1.10–2.70; P=0.050), thromboembolism (HR =2.09; 95% CI: 1.27–3.46; P=0.004), patients who started crizotinib in the first line (HR =2.17; 95% CI: 1.21–3.89; P=0.009), and patients with COPD or asthma (HR =3.71; 95% CI: 1.59–8.70; P=0.003).
Univariate analysis of PFS showed that patients with baseline brain metastases or PD-L1 negative tumors had better PFS than those without brain metastases and PD-L1 positive tumors, respectively. Patients smoking, crizotinib treatment, or with prior chemotherapy had worse PFS. Patients receiving crizotinib as initial ALK-TKI was the only significant prognostic factor in the multivariable analysis (HR =5.41; 95% CI: 2.95–9.94; P<0.001).
Treatment patterns, metastatic sites, and discontinuation reasons after progression on initial ALK-TKI
We analyzed the different sequences of ALK-TKIs in the crizotinib and 2nd generation ALK-TKI groups (Table S3). The majority of the patients in the cohort were treated with one ALK inhibitor (57%, n=85) and the most common ALK inhibitor was alectinib (37%, n=56). Forty patients (27%) received two ALK-TKIs and 25 patients (17%) were treated with 3–6 lines of ALK-inhibitors. Patients treated with more than one ALK inhibitor had predominantly crizotinib-initiated sequences (32%, n=48) followed by alectinib-initiated sequences (9%, n=13) and ceritinib-initiated sequences (3%, n=4). There was only one patient who started with brigatinib. Forty-nine patients (67%) received a 2nd generation ALK-TKI after progression on crizotinib (Figure S2).
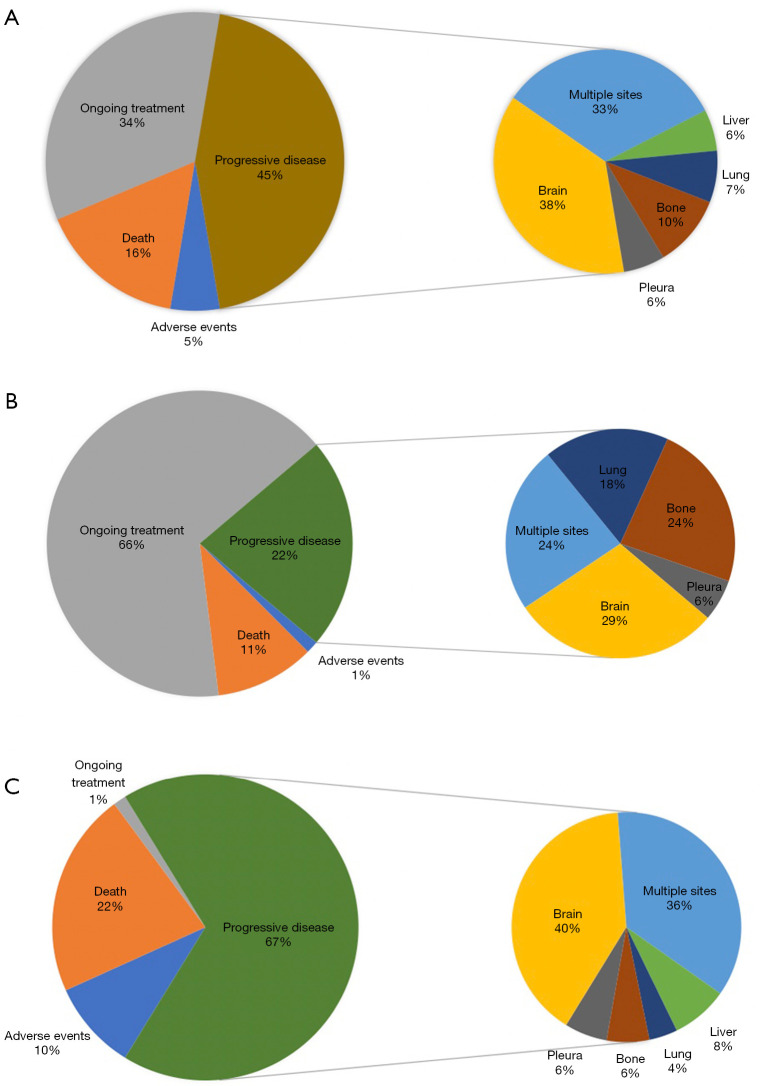
We next analyzed treatment status after the start of initial ALK-TKI, discontinuation reasons, and sites of progressive disease for the total cohort, 2nd generation ALK-TKI, and crizotinib (Figure 2). Most of the patients in the 2nd generation group had still ongoing therapy (66%, n=50), while only one patient was still on crizotinib at the time of analysis. The most common discontinuation reason was progressive disease in both groups. Brain metastasis after progression on initial ALK-TKI was the most common metastatic spread and was more common in the crizotinib group (40%, n=20 vs. 29%, n=5). The second most common metastatic spread was to multiple organs, 36% for crizotinib and 24% for 2nd generation ALK-TKI. The proportion of progression with skeletal metastasis was higher in patients receiving a 2nd generation ALK-TKI (24%, n=4 vs. 6%, n=3). Adverse events were more common in the crizotinib group (10%, n=7 vs. 1%, n=1) and death was the discontinuation reason in 16 patients in the crizotinib group (22%) and eight patients in the 2nd generation group (11%).
Figure 2.
ALK-TKI status, discontinuation reasons, and sites of progressive disease: (A) total cohort, (B) 2nd generation ALK-TKI, and (C) crizotinib. ALK, anaplastic lymphoma kinase; TKI, tyrosine kinase inhibitor.
Impact of secondary ALK mutations and EML4-fusion variants
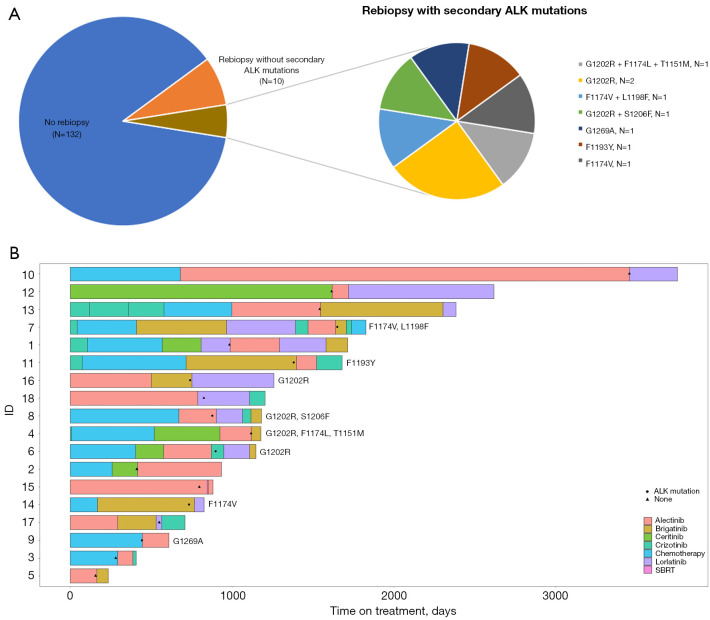
Eighteen patients underwent a re-biopsy after progression on an ALK inhibitor and eight patients were found to have secondary ALK mutations while the rest were without resistance mutations (Figure 3). All patients were treated with a subsequent line of ALK-TKI except for one patient (ID 15) who had oligoprogression and continued with the same TKI with the addition of stereotactic body radiotherapy (SBRT).
Figure 3.
The results of rebiopsy after ALK-TKI progression. (A) The occurrence of secondary ALK mutations after progression on ALK-TKI treatment. (B) Individual swimmer plots for patients who underwent re-biopsy after progression on ALK-TKI, a black circle indicates secondary ALK mutation and a black triangle if no mutation was found. Patient ID 15 had oligoprogression during treatment with alectinib and received stereotactic body radiation therapy without change of TKI treatment. ALK, anaplastic lymphoma kinase; SBRT, stereotactic body radiation therapy; TKI, tyrosine kinase inhibitor.
Patients with secondary ALK mutations experienced worse median PFS during the next treatment line of ALK inhibitors compared to those without existing resistance mutations (7.0 vs. 1.0 months, log-rank P=0.01). We also observed a longer median post-biopsy survival in those without ALK mutations, but the result was not statistically significant (11.0 vs. 5.0 months, log-rank P=0.17) (Figure S3).
We analyzed 91 patients with known EML4-ALK fusion variants (V1 found in 37 patients, V2 in seven patients, V3a/b in 29 patients, and 18 patients had the other EML4-fusion variants 4–7). We did not find any statistically significant difference between the EML4-fusion variants regarding OS and PFS on initial ALK-TKI (Figure S4). Patients with variant 2 had longer mPFS compared to EML4-non V2 (27.0 vs. 12.0 months, P=0.63; HR =0.80; 95% CI: 0.32–2.00).
Lorlatinib therapy and clinical outcomes
In a separate analysis, we included 23 patients who received lorlatinib (Table 3). Fifteen patients received ALK-TKI as first-line treatment without any previous chemotherapy. The majority of the patients had brain metastases (n=19, 83%). The median previous lines of ALK-TKI before lorlatinib were two. Eleven patients received lorlatinib after progression on a 2nd generation ALK-TKI and 12 patients after progression on both crizotinib and a 2nd generation ALK-TKI (Figure S2). The median follow-up time from diagnosis was 93.0 months (95% CI: 72.9–113.1). ORR was 57% and disease control rate (DCR) was 65%. Median PFS was 9.0 months (95% CI: 0.77–17.2), median post-lorlatinib survival was 13.0 months (95% CI: 0.0–29.7) and median OS was 79.0 months (95% CI: 26.6–131.4). Median PFS was higher in patients who received a 2nd generation ALK-TKI before lorlatinib compared to those who received crizotinib and a 2nd generation ALK-inhibitor (9.0 vs. 5.0 months, log-rank P=0.69) (Figure S5).
Table 3. Descriptive patient characteristics of advanced ALK-positive NSCLC patients receiving lorlatinib therapy and clinical outcome measures.
| Variables | Results (n=23) |
|---|---|
| Age (years) | 67.0 [30–84] |
| Sex | |
| Male | 7 (30.4) |
| Female | 16 (69.6) |
| Histology | |
| Adenocarcinoma | 22 (95.7) |
| Adenosquamous carcinoma | 1 (4.3) |
| ECOG PS | |
| 0–1 | 15 (65.2) |
| ≥2 | 8 (34.8) |
| Smoking history | |
| Never smoker | 11 (47.8) |
| Ex-smoker | 11 (47.8) |
| Smoker | 1 (4.3) |
| Brain metastasis | |
| Yes | 19 (82.6) |
| No | 4 (17.4) |
| First-line treatment | |
| TKI | 15 (65.2) |
| Chemotherapy | 8 (34.8) |
| Previous lines of ALK-TKI | 2.0 [1–3] |
| Prior ALK-inhibitors | |
| Crizotinib + 2nd generation ALK-TKI | 12 (52.2) |
| 2nd generation ALK-TKI | 11 (47.8) |
| Objective response rate | 13 (56.5) |
| DCR | 15 (65.2) |
| Complete response | 2 (8.7) |
| Partial response | 11 (47.8) |
| Stable disease | 2 (8.7) |
| Progressive disease | 5 (21.7) |
| Not evaluable | 3 (13.0) |
| Censored | 12 (52.2) |
| Follow-up (months) | 93.0 (72.9–113.1) |
| PFS (months) | 9.0 (0.77–17.2) |
| Post-lorlatinib survival (months) | 13.0 (0.0–29.7) |
| OS (months)† | 79.0 (26.6–131.4) |
Results are presented as median [range], n (%), or median (95% CI). †, median OS from advanced or metastatic NSCLC diagnosis. ALK, anaplastic lymphoma kinase; NSCLC, non-small cell lung cancer; ECOG, Eastern Cooperative Oncology Group; PS, performance status; TKI, tyrosine kinase inhibitor; DCR, disease control rate; PFS, progression-free survival; OS, overall survival; CI, confidence interval.
Discussion
This study investigates the real-world clinical experience and outcomes of ALK-TKI-treated metastatic ALK-positive NSCLC in a Swedish cohort from 2009 to 2021, including the impact of sequenced therapy, fusion variants, and resistance mutations.
We present detailed real-world data from one of the largest described single-center cohorts with advanced ALK-positive NSCLC. The strength of this study includes a long median follow-up time of 54.0 months from advanced lung cancer diagnosis and 45.0 months from the start of initial ALK-TKI treatment. The 10-month gap is attributed to the inclusion of patients diagnosed before the approval of ALK-TKI as first-line treatment in Sweden. These patients initially received chemotherapy before transitioning to ALK-TKI therapy upon its subsequent approval. The long timespan, in this study, also allows for a more balanced cohort with a mixture of both advantageous and disadvantageous cases with the sequential use of multiple generations of ALK-TKIs. The majority of the patients in the total cohort received ALK-TKI as first-line treatment and the most common ALK-TKI used was alectinib. This results in a modern representative cohort with up-to-date real-world treatment patterns in the present era with targeted therapy reflecting high generalizability and external validity.
Despite similar therapeutic indications and treatment guidelines, global differences are described in real-world cohorts (RWCs) with advanced ALK-positive NSCLC who receive ALK-TKI treatment. A recently published study showed an overview of ALK-TKI treatment patterns in Sweden but was conducted from the prescribed drug register (22). Since there is a lack of real-world clinical data based on detailed information from EHRs from Sweden, we chose to conduct this study for patients with advanced ALK-positive NSCLC at the time of their first exposure to either crizotinib or a 2nd generation ALK-TKI treatment.
The patients in the study aligned with previously published cohorts and consisted of predominantly younger female never smokers with adenocarcinoma histology, good PS, and high risk of brain metastases (23-27). However, in Sweden, routine computed tomography (CT)/magnetic resonance imaging (MRI) of the brain at the time of diagnosis for ALK-positive NSCLC patients (diagnosed before 2015) was not performed if the patients were asymptomatic. This practice is reflected in our data, where only 20% of patients had brain metastases at diagnosis. This lower percentage is likely due to the absence of routine brain imaging in asymptomatic patients rather than a true lower incidence of brain metastases. The higher incidence of 31.6% observed in the 2nd generation ALK-TKI cohort aligns more closely with other reported ALK-positive cohorts, as routine brain imaging at diagnosis was implemented for all ALK-positive NSCLC patients.
The prolonged median OS and PFS observed in this study underscore the significant efforts made in the discovery and availability of ALK-TKIs for these patients with metastatic NSCLC. Our survival results, with a median OS of 65.0 months, are higher than those reported in other RWCs where median OS ranges from 24.8 to 30.9 months across the USA, China, France, and India (28-31). Median OS, in line with our study, was seen in two other RWCs in Italy and Canada (23,32), and an impressive median OS of 81.0 months was reported in one study with 110 advanced ALK-positive NSCLC patients by Pacheco et al. (17).
In our study, median OS was higher in those patients who received ALK-TKI as first-line treatment compared to those treated with previous chemotherapy but was not statistically significant. Consistent with other studies, we found a superior median OS in the 2nd generation ALK-TKI group compared to crizotinib but median survival data was NR in the first group. The median OS was influenced by access to a 2nd generation ALK-TKI post-failure. We also observed a prolonged survival in those who received at least three lines of ALK-TKIs compared to one or two lines. This was also reported by Pacheco et al. where patients receiving a next-generation ALK-TKI after progression on crizotinib had a median OS of 86.0 months instead of 52.0 months for those who did not. One possible explanation for the higher median OS found in their study is that they had a higher proportion of patients who were further treated with a 2nd generation ALK-TKI drug (78%). Still, their results were not statistically significant (P=0.09) (17). Another study by Waterhouse et al. also showed an increase in OS with the increasing number of ALK-TKIs used, suggesting that multiple ALK-TKIs were achievable in those who lived long enough to be able to receive several lines of treatment and supports the use of sequential ALK therapies (33). Further analysis to investigate the best optimal sequencing was difficult to perform in our study because of the small subgroups and additional studies will be required for a better understanding of the best strategy for therapy sequencing. A reason for this heterogeneity in treatment options is that the choice of subsequent next-line ALK-TKI is often not guided by molecular factors upon treatment failure.
We found superior PFS of initial treatment with 2nd generation ALK-TKI compared to crizotinib, 52.0 vs. 8.0 months, where alectinib was the most frequently used drug. This extended PFS compared to crizotinib was higher in the present study than that observed in the ALEX clinical trial (34.8 vs. 10.9 months) (11) and the observed median PFS of crizotinib was lower than in the PROFILE 1014 study (10.9 months) (6).
Our study demonstrated a higher aggregated ORR of 79% compared to the phase 3 trial of Peters et al. (ORR, 60%) comparing first-line alectinib and crizotinib (11). The ORR (91%) of the 2nd generation ALK-TKI group aligns with the ORR (92%) of alectinib in the J-ALEX study (11). Discrepancies between real-world response evaluations and Response Evaluation Criteria in Solid Tumors (RECIST)-based assessments in clinical trials may explain these variations.
We analyzed prognostic factors in the whole cohort and found that male gender, thromboembolism, chronic lung disease at diagnosis, and crizotinib as initial ALK-TKI treatment were associated with negative effects on survival outcomes. The latter group included patients who received it as a first-line therapy and those who received it after progressing on chemotherapy. We did not separate these two groups in our analysis. However, our univariate analysis indicated that receiving prior chemotherapy before transitioning to ALK-TKI did not significantly affect OS. This suggests that the poor prognostic impact of crizotinib is consistent regardless of its sequence relative to chemotherapy. One possible explanation could be the pattern of progression. The most common reason for discontinuing the initial ALK-TKI treatment was progressive disease, mostly due to progression in the brain. CNS-progression was higher post-crizotinib than post-2nd generation ALK-TKI. This emphasizes the necessity of brain-penetrating ALK-TKIs as the first-line therapy.
During treatment with either a 2nd generation ALK-TKI or crizotinib, the only prognostic factor for PFS was the ALK-TKI used as the initial treatment. Brain metastasis at baseline, current smokers, previous chemotherapy, PD-L1 negative tumors, and chronic lung disease were all significant prognostic factors in the univariate analysis but did not hold in the multivariable Cox regression model. Our results need validation in a larger cohort.
A recent study by Li et al. found that ALK fusion variant 3a/b, concomitant mutations, and high PD-L1 expression were associated with poor clinical response to 2nd generation ALK-TKI (34). Positive PD-L1 expression was also correlated with poor response in crizotinib-treated patients reported by Yang et al. (35) and that there is an association between high PD-L1 expression and poor prognosis in ALK-rearranged NSCLC patients (36). These studies suggest that high PD-L1 expression is associated with fusion variant 3a/b and concomitant mutations leading to resistance to ALK-TKIs, but underlying mechanisms need to be further investigated. Regarding CNS involvement in our study, the observed better PFS in the univariate analysis is likely attributable to confounding factors, for example most patients with brain metastases at diagnosis received treatment with a 2nd generation ALK-TKI, and thus the result did not hold in the multivariable analysis.
We did not find any significant association in terms of OS or PFS between different fusion variants. ALK-fusion variant was not reported in 38.7% of the patients, either because it was not tested for using PCR/NGS or that fusion variant was detected but not specified in the pathological report. This lack of data is primarily because advanced diagnostic techniques, such as RNA-NGS, were not widely available or implemented during the earlier years of the study period. Conflicting data have been reported regarding the predictive roles of ALK fusion variants, where some studies report that variant non-3a/b is associated with longer PFS on crizotinib compared to other variants. In contrast, others show that there is no difference (37,38). These conflicting results could be explained by smaller cohorts, variations across different geographic areas, and heterogeneity in terms of ALK-TKI-treated patients.
We identified secondary ALK mutations, predominantly emerging after progression on 2nd generation ALK-TKIs. This finding aligns with resistance mutations previously reported in the literature (39). We found superior median PFS on subsequent ALK-TKI in those who lacked secondary ALK mutations compared to those with resistance mutations, but no statistically significant effect in post-biopsy survival. This is in contrast to what was found by Zou et al. with longer PFS on subsequent ALK-TKI in those patients who had secondary ALK mutations after treatment with alectinib compared to those who had not (40). One explanation could be the heterogeneity in our group with re-biopsies after progression on different ALK-TKIs and the use of ineffective “off-target” drugs in subsequent lines since the sensitivity of ALK-TKIs is different for each secondary mutation in the ALK kinase domain. The majority of our patients with secondary ALK mutations were also treated with several ALK-TKIs before the re-biopsy was performed and poorer PFS has been described the more prior ALK-TKIs that have been administered (41). The rebiopsies were crucial in guiding subsequent treatment choices. Lorlatinib was specifically utilized when the G1202R mutation was detected, as this mutation is known to confer resistance that lorlatinib can effectively overcome. However, in the case of patient ID 4, lorlatinib was not accessible at the time of progression on alectinib.
We also analyzed the patients who received lorlatinib in our cohort and where real-world data is scarce. Most of the patients had brain metastases before initiation of lorlatinib, ORR and median PFS were consistent with previously described data from RWCs with baseline brain metastases of 70–84%, ORR 33–67%, and median PFS 6.2–9.7 months (42-46). These findings suggest that the real-world efficacy of lorlatinib is comparable with results found in clinical trials (15). The observed decreasing percentage of patients receiving lorlatinib after progression on initial ALK-TKI can be primarily attributed to the fact that many patients in the crizotinib group did not live long enough to receive lorlatinib after progressing on both crizotinib and second-generation ALK-TKIs. Additionally, lorlatinib was not available at the time of progression for some patients. However, prior treatment to lorlatinib did not affect PFS even if there was a trend towards superior PFS in those patients who only received 2nd generation ALK-TKIs.
A limitation of this study is its single-center retrospective nature with missing data in some parameters, such as EML4-ALK fusion type and PD-L1 expression. Another limitation of the study is the immature survival data in the 2nd generation ALK-TKI group. Radiological progression in the real world is also not as frequently measured as in prospective clinical trials which might affect PFS. Even if the data were obtained retrospectively, we attempted to ensure the validity of the patients’ characteristics and clinical outcome assessments.
Conclusions
This study of patients with advanced ALK-positive NSCLC showed prolonged survival with a median OS exceeding 5 years. Access to ALK-TKIs with good penetration to the CNS, effective against multiple resistance mutations, and with good long-term tolerability are necessary to achieve prolonged survival. Re-biopsies during treatment are important to enhance our understanding of resistance mechanisms and the tumor dynamics that develop during ALK-TKI therapy and to increase the individualized management of this disease within the era of precision medicine.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This project was supported by the grant from the Swedish Cancer Society [Nos. CAN2023/2919 (S.E.) and CAN2021/1469 (R.L.)], the Stockholm Cancer Society [Nos. 221212 (R.L.) and 221383 (K.V.)], the King Gustaf V Jubilee Fund [No. 204053 (S.E.)], the Stockholm County Council [contract Nos. FoUI-966345 (R.L. and K.V.), 909121 and 750032 (R.L. and K.V.), and 987911 (S.E.)], the Sjöberg Foundation [No. 2022-2024 (S.E.)], the Erling Persson Family Foundation (K.V. and R.L.), and the Swedish Foundation for Strategic Research [No. SIP21-0106 (R.L.)].
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the external Ethical Review Authority (Dnr 2022-00323-01) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-396/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-396/coif). G.T. reports honoraria from MSD, Roche AB for lectures and reports paid participation at ELCC 2023 from Roche AB. K.V. is supported for salary and other project related costs by the indicated private funding bodies or region grants Stockholm Cancer Society (contract No. 221383), Stockholm County Council (contract Nos. 909121, 750032, and FoUI-966345), and Erling Persson Family Foundation. R.L. reports grant from the Swedish Cancer Society (No. CAN2021/1469), the Stockholm Cancer Society (No. 221212), the Stockholm County Council (contract Nos. FoUI-966345, 909121, and 750032), the Erling Persson Family Foundation, and the Swedish Foundation for Strategic Research (No. SIP21-0106). S.E. reports grants from the Swedish Cancer Society (grant No. CAN2023/2919), the King Gustaf V Jubilee Fund (grant No. 204053), the Sjöberg Foundation (No. 2022-2024), and the Stockholm County Council (No. 987911). The other authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-396/dss
References
- 1.Du X, Shao Y, Qin HF, et al. ALK-rearrangement in non-small-cell lung cancer (NSCLC). Thorac Cancer 2018;9:423-30. 10.1111/1759-7714.12613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ou SI, Zhu VW, Nagasaka M. Catalog of 5' Fusion Partners in ALK-positive NSCLC Circa 2020. JTO Clin Res Rep 2020;1:100015. 10.1016/j.jtocrr.2020.100015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabir SR, Yeoh S, Jackson G, et al. EML4-ALK Variants: Biological and Molecular Properties, and the Implications for Patients. Cancers (Basel) 2017;9:118. 10.3390/cancers9090118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol 2009;27:4247-53. 10.1200/JCO.2009.22.6993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang I, Zaorsky NG, Palmer JD, et al. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer. Lancet Oncol 2015;16:e510-21. 10.1016/S1470-2045(15)00013-3 [DOI] [PubMed] [Google Scholar]
- 6.Solomon BJ, Kim DW, Wu YL, et al. Final Overall Survival Analysis From a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:2251-8. 10.1200/JCO.2017.77.4794 [DOI] [PubMed] [Google Scholar]
- 7.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. 10.1056/NEJMoa1214886 [DOI] [PubMed] [Google Scholar]
- 8.Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011;29:e443-5. 10.1200/JCO.2010.34.1313 [DOI] [PubMed] [Google Scholar]
- 9.Shaw AT, Engelman JA. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:2537-9. 10.1056/NEJMc1404894 [DOI] [PubMed] [Google Scholar]
- 10.Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2027-39. 10.1056/NEJMoa1810171 [DOI] [PubMed] [Google Scholar]
- 11.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. 10.1056/NEJMoa1704795 [DOI] [PubMed] [Google Scholar]
- 12.Lin YT, Yu CJ, Yang JC, et al. Anaplastic Lymphoma Kinase (ALK) Kinase Domain Mutation Following ALK Inhibitor(s) Failure in Advanced ALK Positive Non-Small-Cell Lung Cancer: Analysis and Literature Review. Clin Lung Cancer 2016;17:e77-94. 10.1016/j.cllc.2016.03.005 [DOI] [PubMed] [Google Scholar]
- 13.Pan Y, Deng C, Qiu Z, et al. The Resistance Mechanisms and Treatment Strategies for ALK-Rearranged Non-Small Cell Lung Cancer. Front Oncol 2021;11:713530. 10.3389/fonc.2021.713530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gainor JF, Varghese AM, Ou SH, et al. ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer. Clin Cancer Res 2013;19:4273-81. 10.1158/1078-0432.CCR-13-0318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shaw AT, Solomon BJ, Besse B, et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2019;37:1370-9. 10.1200/JCO.18.02236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pacheco JM, Camidge DR. Is long-term survival possible for patients with stage IV ALK+ non-small cell lung cancer? Expert Rev Respir Med 2019;13:399-401. 10.1080/17476348.2019.1596028 [DOI] [PubMed] [Google Scholar]
- 17.Pacheco JM, Gao D, Smith D, et al. Natural History and Factors Associated with Overall Survival in Stage IV ALK-Rearranged Non-Small Cell Lung Cancer. J Thorac Oncol 2019;14:691-700. 10.1016/j.jtho.2018.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Medical Association Declaration of Helsinki : ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 19.van der Wekken AJ, Pelgrim R, 't Hart N, et al. Dichotomous ALK-IHC Is a Better Predictor for ALK Inhibition Outcome than Traditional ALK-FISH in Advanced Non-Small Cell Lung Cancer. Clin Cancer Res 2017;23:4251-8. 10.1158/1078-0432.CCR-16-1631 [DOI] [PubMed] [Google Scholar]
- 20.Canterbury CR, Fernandes H, Crapanzano JP, et al. ALK Gene Rearrangements in Lung Adenocarcinomas: Concordance of Immunohistochemistry, Fluorescence In Situ Hybridization, RNA In Situ Hybridization, and RNA Next-Generation Sequencing Testing. JTO Clin Res Rep 2021;2:100223. 10.1016/j.jtocrr.2021.100223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamichi S, Seike M, Miyanaga A, et al. RT-PCR for Detecting ALK Translocations in Cytology Samples from Lung Cancer Patients. Anticancer Res 2017;37:3295-9. 10.21873/anticanres.11696 [DOI] [PubMed] [Google Scholar]
- 22.Lauppe R, Nilsson FOL, Fues Wahl H, et al. Use of ALK-tyrosine kinase inhibitors (ALK TKI) in clinical practice, overall survival, and treatment duration - a Swedish nationwide retrospective study. Acta Oncol 2022;61:1354-61. 10.1080/0284186X.2022.2133972 [DOI] [PubMed] [Google Scholar]
- 23.Gibson AJW, Box A, Dean ML, et al. Retrospective Real-World Outcomes for Patients With ALK-Rearranged Lung Cancer Receiving ALK Receptor Tyrosine Kinase Inhibitors. JTO Clin Res Rep 2021;2:100157. 10.1016/j.jtocrr.2021.100157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moskovitz M, Dudnik E, Shamai S, et al. ALK Inhibitors or Chemotherapy for Third Line in ALK-positive NSCLC? Real-world Data. Oncologist 2022;27:e76-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito K, Yamanaka T, Hayashi H, et al. Sequential therapy of crizotinib followed by alectinib for non-small cell lung cancer harbouring anaplastic lymphoma kinase rearrangement (WJOG9516L): A multicenter retrospective cohort study. Eur J Cancer 2021;145:183-93. 10.1016/j.ejca.2020.12.026 [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson S, Gupta A, Scheuer N, et al. Assessment of Alectinib vs Ceritinib in ALK-Positive Non-Small Cell Lung Cancer in Phase 2 Trials and in Real-world Data. JAMA Netw Open 2021;4:e2126306. 10.1001/jamanetworkopen.2021.26306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popat S, Brustugun OT, Cadranel J, et al. Real-world treatment outcomes with brigatinib in patients with pretreated ALK+ metastatic non-small cell lung cancer. Lung Cancer 2021;157:9-16. 10.1016/j.lungcan.2021.05.017 [DOI] [PubMed] [Google Scholar]
- 28.Duruisseaux M, Besse B, Cadranel J, et al. Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective study. Oncotarget 2017;8:21903-17. 10.18632/oncotarget.15746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin Y, Chen Y, Yu X, et al. A real-world study of treatment patterns and survival outcome in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. Oncol Lett 2018;15:8703-10. 10.3892/ol.2018.8444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel A, Batra U, Prasad KT, et al. Real world experience of treatment and outcome in ALK-rearranged metastatic nonsmall cell lung cancer: A multicenter study from India. Curr Probl Cancer 2020;44:100571. 10.1016/j.currproblcancer.2020.100571 [DOI] [PubMed] [Google Scholar]
- 31.Davis KL, Kaye JA, Masters ET, et al. Real-world outcomes in patients with ALK-positive non-small cell lung cancer treated with crizotinib. Curr Oncol 2018;25:e40-9. 10.3747/co.25.3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Britschgi C, Addeo A, Rechsteiner M, et al. Real-World Treatment Patterns and Survival Outcome in Advanced Anaplastic Lymphoma Kinase (ALK) Rearranged Non-Small-Cell Lung Cancer Patients. Front Oncol 2020;10:1299. 10.3389/fonc.2020.01299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waterhouse DM, Espirito JL, Chioda MD, et al. Retrospective Observational Study of ALK-Inhibitor Therapy Sequencing and Outcomes in Patients with ALK-Positive Non-small Cell Lung Cancer. Drugs Real World Outcomes 2020;7:261-9. 10.1007/s40801-020-00207-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li M, Hou X, Chen J, et al. ALK fusion variant 3a/b, concomitant mutations, and high PD-L1 expression were associated with unfavorable clinical response to second-generation ALK TKIs in patients with advanced ALK-rearranged non-small cell lung cancer (GASTO 1061). Lung Cancer 2022;165:54-62. 10.1016/j.lungcan.2022.01.006 [DOI] [PubMed] [Google Scholar]
- 35.Yang CY, Liao WY, Ho CC, et al. Association of Programmed Death-Ligand 1 Expression with Fusion Variants and Clinical Outcomes in Patients with Anaplastic Lymphoma Kinase-Positive Lung Adenocarcinoma Receiving Crizotinib. Oncologist 2020;25:702-11. 10.1634/theoncologist.2020-0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian X, Li Y, Huang Q, et al. High PD-L1 Expression Correlates with an Immunosuppressive Tumour Immune Microenvironment and Worse Prognosis in ALK-Rearranged Non-Small Cell Lung Cancer. Biomolecules 2023;13:991. 10.3390/biom13060991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Batra U, Sharma M, Nathany S, et al. Are all ALK variants created equal? Clinicopathologic features and outcomes: a propensity-matched study. Int J Clin Oncol 2021;26:1221-8. 10.1007/s10147-021-01916-w [DOI] [PubMed] [Google Scholar]
- 38.Lin YT, Liu YN, Shih JY. The Impact of Clinical Factors, ALK Fusion Variants, and BIM Polymorphism on Crizotinib-Treated Advanced EML4-ALK Rearranged Non-small Cell Lung Cancer. Front Oncol 2019;9:880. 10.3389/fonc.2019.00880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sharma GG, Mota I, Mologni L, et al. Tumor Resistance against ALK Targeted Therapy-Where It Comes From and Where It Goes. Cancers (Basel) 2018;10:62. 10.3390/cancers10030062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou Z, Hao X, Zhang C, et al. Clinical outcome, long-term survival and tolerability of sequential therapy of first-line crizotinib followed by alectinib in advanced ALK+NSCLC: A multicenter retrospective analysis in China. Thorac Cancer 2022;13:107-16. 10.1111/1759-7714.14232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yanagitani N, Uchibori K, Koike S, et al. Drug resistance mechanisms in Japanese anaplastic lymphoma kinase-positive non-small cell lung cancer and the clinical responses based on the resistant mechanisms. Cancer Sci 2020;111:932-9. 10.1111/cas.14314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baldacci S, Besse B, Avrillon V, et al. Lorlatinib for advanced anaplastic lymphoma kinase-positive non-small cell lung cancer: Results of the IFCT-1803 LORLATU cohort. Eur J Cancer 2022;166:51-9. 10.1016/j.ejca.2022.01.018 [DOI] [PubMed] [Google Scholar]
- 43.Lee PH, Chen KC, Hsu KH, et al. Real-world efficacy and safety of lorlatinib in treating advanced ALK-positive non-small cell lung cancer patients. Anticancer Drugs 2021;32:1099-104. 10.1097/CAD.0000000000001107 [DOI] [PubMed] [Google Scholar]
- 44.Lee J, Sun JM, Lee SH, et al. Efficacy and Safety of Lorlatinib in Korean Non-Small-Cell Lung Cancer Patients With ALK or ROS1 Rearrangement Whose Disease Failed to Respond to a Previous Tyrosine Kinase Inhibitor. Clin Lung Cancer 2019;20:215-21. 10.1016/j.cllc.2018.12.020 [DOI] [PubMed] [Google Scholar]
- 45.Frost N, Christopoulos P, Kauffmann-Guerrero D, et al. Lorlatinib in pretreated ALK- or ROS1-positive lung cancer and impact of TP53 co-mutations: results from the German early access program. Ther Adv Med Oncol 2021;13:1758835920980558. 10.1177/1758835920980558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu VW, Lin YT, Kim DW, et al. An International Real-World Analysis of the Efficacy and Safety of Lorlatinib Through Early or Expanded Access Programs in Patients With Tyrosine Kinase Inhibitor-Refractory ALK-Positive or ROS1-Positive NSCLC. J Thorac Oncol 2020;15:1484-96. 10.1016/j.jtho.2020.04.019 [DOI] [PubMed] [Google Scholar]