Abstract
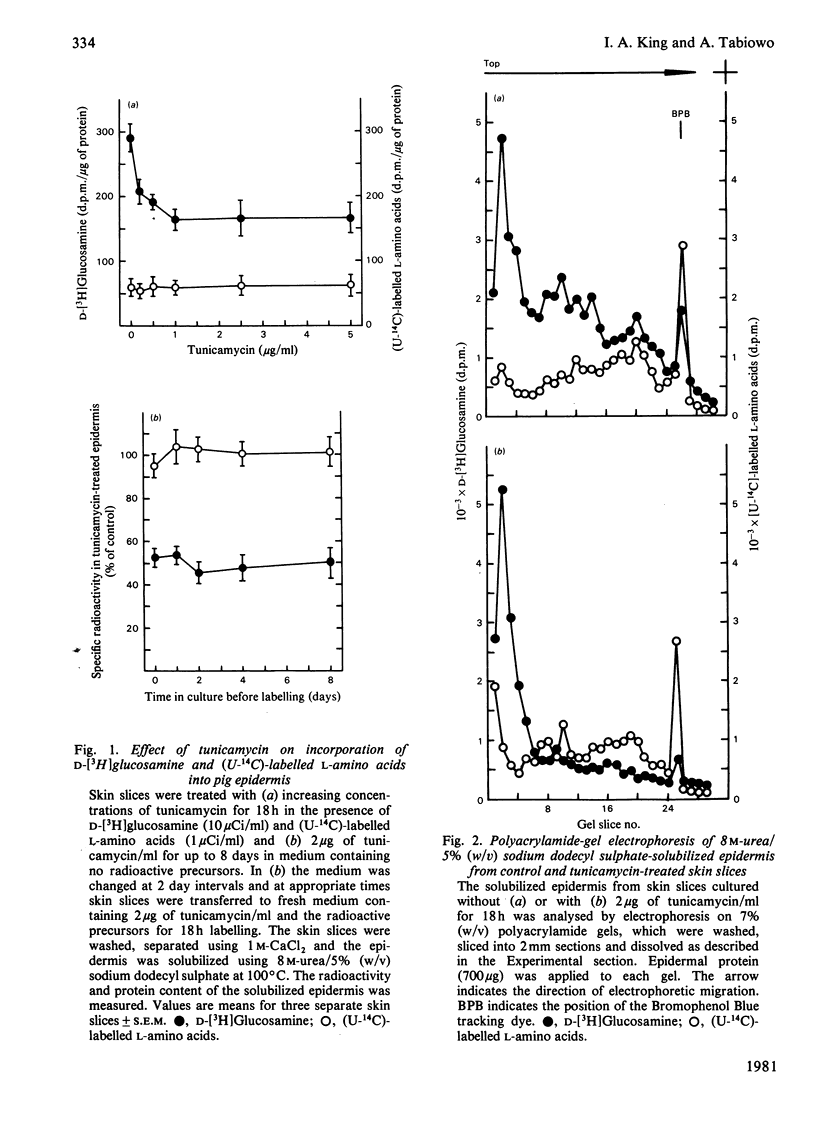
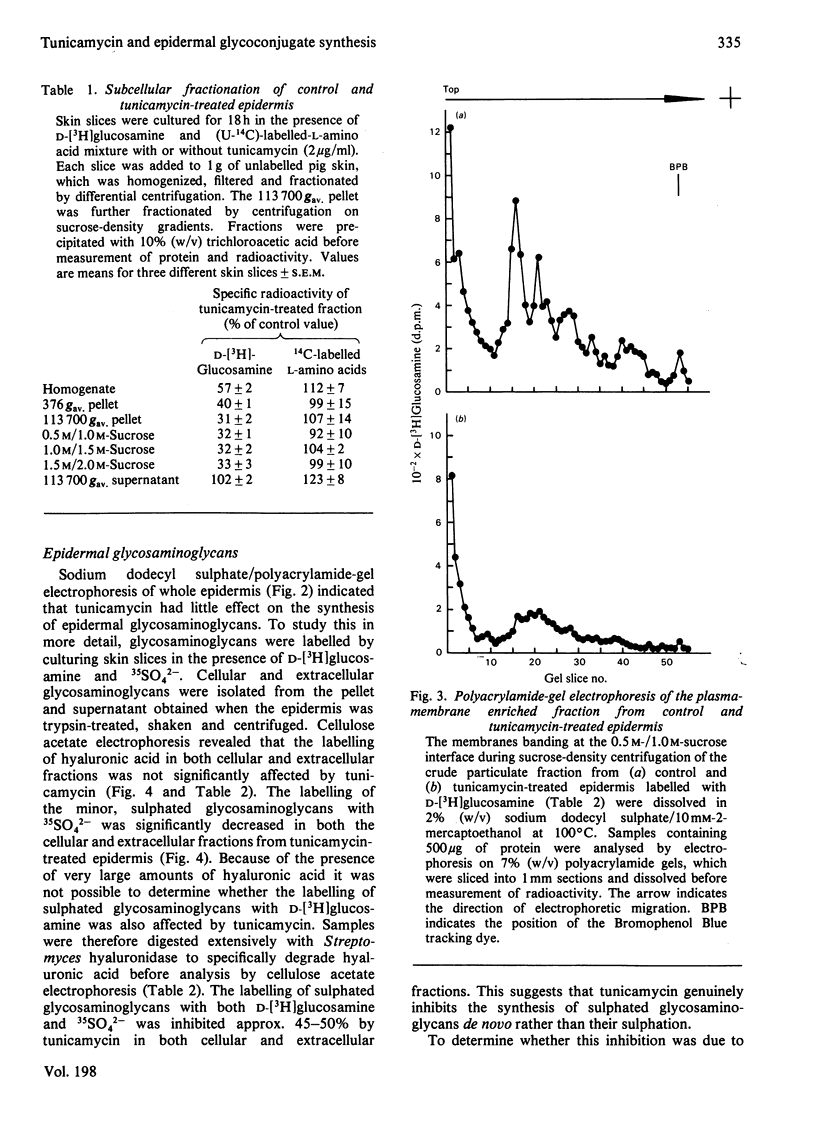
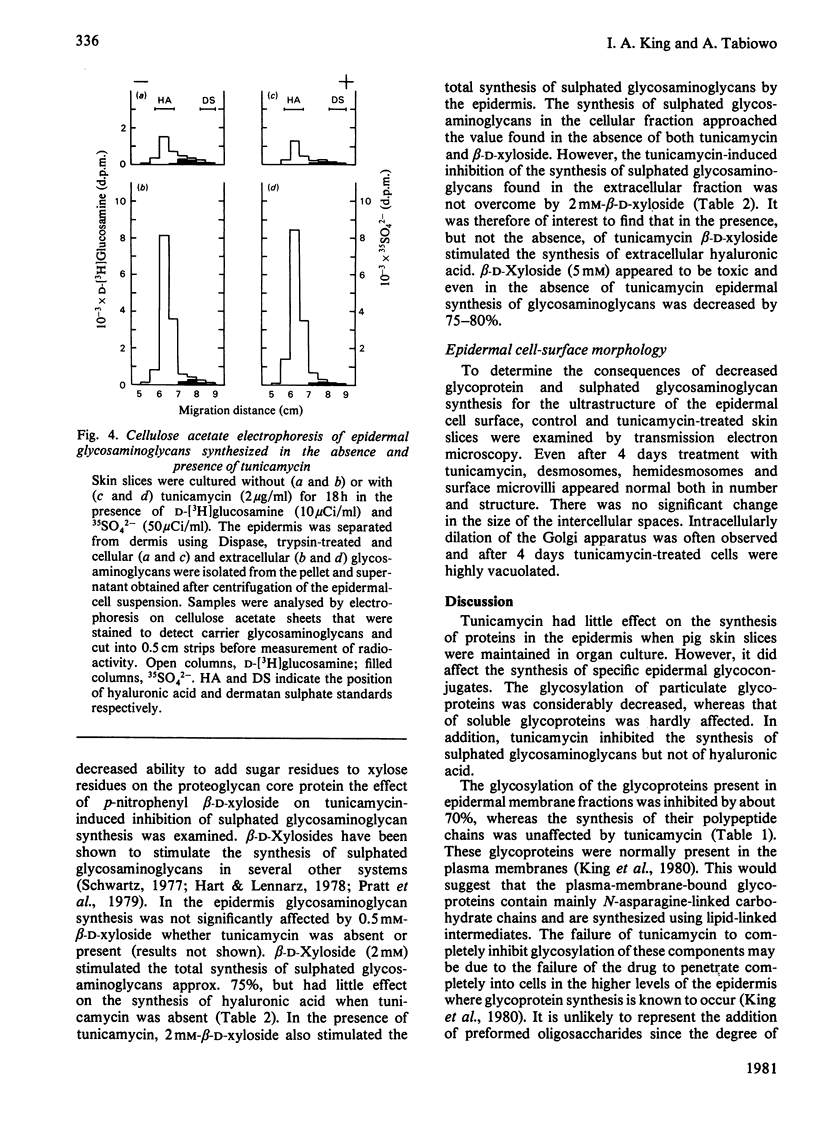
1. When pig ear skin slices were cultured for 18h in the presence of 1μg of tunicamycin/ml the incorporation of d-[3H]glucosamine into the epidermis, solubilized with 8m-urea/5% (w/v) sodium dodecyl sulphate, was inhibited by 45–55%. This degree of inhibition was not increased by using up to 5μg of tunicamycin/ml or by treating the skin slices with tunicamycin for up to 8 days. The incorporation of (U-14C)-labelled l-amino acids under these conditions was not affected by tunicamycin. Polyacrylamide-gel electrophoresis indicated that the labelling of the major glycosaminoglycan peak with d-[3H]glucosamine was unaffected, whereas that of the faster migrating glycoprotein components was considerably decreased in the presence of tunicamycin. 2. Subcellular fractionation indicated that tunicamycin specifically inhibited the incorporation of d-[3H]glucosamine but not of (U-14C)-labelled l-amino acids into particulate (mainly plasma-membrane) glycoproteins by about 70%. The labelling of soluble glycoproteins was hardly affected. Polyacrylamide-gel electrophoresis of the plasma-membrane fraction showed decreased d-[3H]glucosamine incorporation into all glycoprotein components, indicating that the plasma-membrane glycoproteins contained mainly N-asparagine-linked oligosaccharides. 3. Cellulose acetate electrophoresis of both cellular and extracellular glycosaminoglycans showed that tunicamycin had no significant effect on the synthesis of the major component, hyaluronic acid. However, the incorporation of both d-[3H]glucosamine and 35SO42− into sulphated glycosaminoglycans was inhibited by about 50%. This inhibition was partially overcome, at least in the cellular fraction, by 2mm-p-nitrophenyl β-d-xyloside indicating that tunicamycin-treated epidermis retained the ability to synthesize sulphated glycosaminoglycan chains. Tunicamycin may affect the synthesis and/or degradation of proteoglycan core proteins or the xylosyltransferase. 4. Electron-microscopic examination of epidermis treated with tunicamycin for up to 4 days revealed no significant changes in cell-surface morphology or in epidermal-cell adhesion. Either N-asparagine-linked carbohydrates play little role in epidermal-cell adhesion or more probably there is little turnover of these components in epidermal adhesive structures such as desmosomes and hemidesmosomes during organ culture.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butters T. D., Hughes R. C. Effects of tunicamycin on insect cells. Biochem Soc Trans. 1980 Apr;8(2):170–171. doi: 10.1042/bst0080170. [DOI] [PubMed] [Google Scholar]
- Damsky C. H., Levy-Benshimol A., Buck C. A., Warren L. Effect of tunicamycin on the synthesis, intracellular transport and shedding of membrane glycoproteins in BHK cells. Exp Cell Res. 1979 Mar 1;119(1):1–13. doi: 10.1016/0014-4827(79)90329-x. [DOI] [PubMed] [Google Scholar]
- Duksin D., Holbrook K., Williams K., Bornstein P. Cell surface morphology and adhesive properties of normal and virally transformed cells treated with tunicamycin, an inhibitor of protein glycosylation. Exp Cell Res. 1978 Oct 1;116(1):153–165. doi: 10.1016/0014-4827(78)90072-1. [DOI] [PubMed] [Google Scholar]
- Gibson R., Leavitt R., Kornfeld S., Schlesinger S. Synthesis and infectivity of vesicular stomatitis virus containing nonglycosylated G protein. Cell. 1978 Apr;13(4):671–679. doi: 10.1016/0092-8674(78)90217-9. [DOI] [PubMed] [Google Scholar]
- Hart G. W., Lennarz W. J. Effects of tunicamycin on the biosynthesis of glycosaminoglycans by embryonic chick cornea. J Biol Chem. 1978 Aug 25;253(16):5795–5801. [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hickman S., Kornfeld S. Effect of tunicamycin on IgM, IgA, and IgG secretion by mouse plasmacytoma cells. J Immunol. 1978 Sep;121(3):990–996. [PubMed] [Google Scholar]
- Hopwood J. J., Dorfman A. Isolation of lipid glucuronic acid and N-acetylglucosamine derivatives from a rat fibrosarcoma. Biochem Biophys Res Commun. 1977 Mar 21;75(2):472–479. doi: 10.1016/0006-291x(77)91066-x. [DOI] [PubMed] [Google Scholar]
- King I. A. Characterization of epidermal glycosaminoglycans synthesized in organ culture. Biochim Biophys Acta. 1981 Apr 17;674(1):87–95. doi: 10.1016/0304-4165(81)90350-0. [DOI] [PubMed] [Google Scholar]
- King I. A., Tabiowo A. The dermis is required for the synthesis of extracellular glycosaminoglycans in cultured pig epidermis. Biochim Biophys Acta. 1980 Oct 1;632(2):234–243. doi: 10.1016/0304-4165(80)90081-1. [DOI] [PubMed] [Google Scholar]
- King I. A., Tabiowo A. The effect of all-trans-retinoic acid on the synthesis of epidermal cell-surface-associated carbohydrates. Biochem J. 1981 Jan 15;194(1):341–351. doi: 10.1042/bj1940341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King I. A., Tabiowo A., Williams R. H. Incorporation of l-[3H]fucose and d-[3H]glucosamine into cell-surface-associated glycoconjugates in epidermis of cultured pig skin slices. Biochem J. 1980 Jul 15;190(1):65–77. doi: 10.1042/bj1900065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehle L., Tanner W. The specific site of tunicamycin inhibition in the formation of dolichol-bound N-acetylglucosamine derivatives. FEBS Lett. 1976 Nov 15;72(1):167–170. doi: 10.1016/0014-5793(76)80922-2. [DOI] [PubMed] [Google Scholar]
- Olden K., Pratt R. M., Yamada K. M. Role of carbohydrates in protein secretion and turnover: effects of tunicamycin on the major cell surface glycoprotein of chick embryo fibroblasts. Cell. 1978 Mar;13(3):461–473. doi: 10.1016/0092-8674(78)90320-3. [DOI] [PubMed] [Google Scholar]
- Pratt R. M., Yamada K. M., Olden K., Ohanian S. H., Hascall V. C. Tunicamycin-induced alterations in the synthesis of sulfated proteoglycans and cell surface morphology in the chick embryo fibroblast. Exp Cell Res. 1979 Feb;118(2):245–252. doi: 10.1016/0014-4827(79)90149-6. [DOI] [PubMed] [Google Scholar]
- Schwartz N. B., Dorfman A. Purification of rat chondrosarcoma xylosyltransferase. Arch Biochem Biophys. 1975 Nov;171(1):136–144. doi: 10.1016/0003-9861(75)90016-8. [DOI] [PubMed] [Google Scholar]
- Schwartz N. B. Regulation of chondroitin sulfate synthesis. Effect of beta-xylosides on synthesis of chondroitin sulfate proteoglycan, chondroitin sulfate chains, and core protein. J Biol Chem. 1977 Sep 25;252(18):6316–6321. [PubMed] [Google Scholar]
- Singer P. A., Singer H. H., Williamson A. R. Different species of messenger RNA encode receptor and secretory IgM mu chains differing at their carboxy termini. Nature. 1980 May 29;285(5763):294–300. doi: 10.1038/285294a0. [DOI] [PubMed] [Google Scholar]
- Speake B. K., White D. A. The effect of tunicamycin on the glycosylation of lactating-rabbit mammary glycoproteins. Biochem J. 1979 Jun 15;180(3):481–489. doi: 10.1042/bj1800481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck D. K., Siuta P. B., Lane M. D., Lennarz W. J. Effect of tunicamycin on the secretion of serum proteins by primary cultures of rat and chick hepatocytes. Studies on transferrin, very low density lipoprotein, and serum albumin. J Biol Chem. 1978 Aug 10;253(15):5332–5337. [PubMed] [Google Scholar]
- Takatsuki A., Shimizu K. I., Tamura G. Effect of tunicamycin on microorganisms: morphological changes and degradation of RNA and DNA induced by tunicamycin. J Antibiot (Tokyo) 1972 Feb;25(2):75–85. doi: 10.7164/antibiotics.25.75. [DOI] [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]
- Ward J. B. Tunicamycin inhibition of bacterial wall polymer synthesis. FEBS Lett. 1977;78(1):151–154. doi: 10.1016/0014-5793(77)80294-9. [DOI] [PubMed] [Google Scholar]


