Abstract
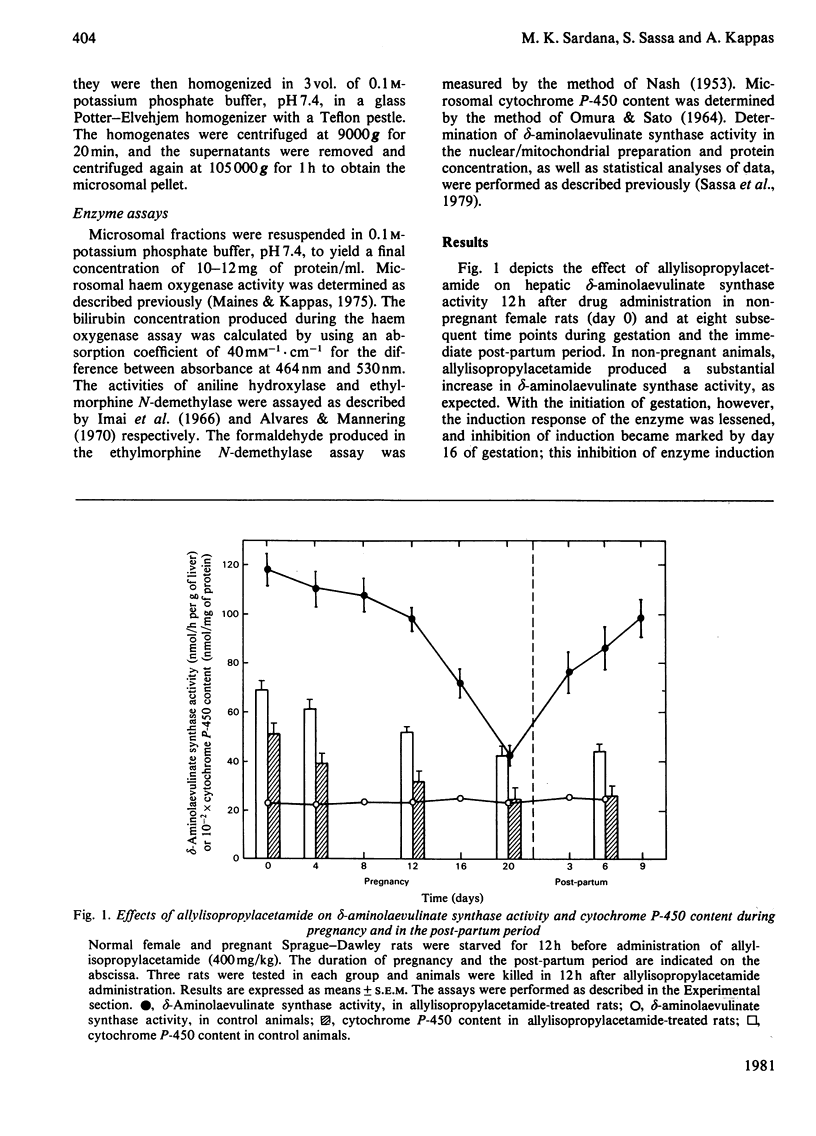
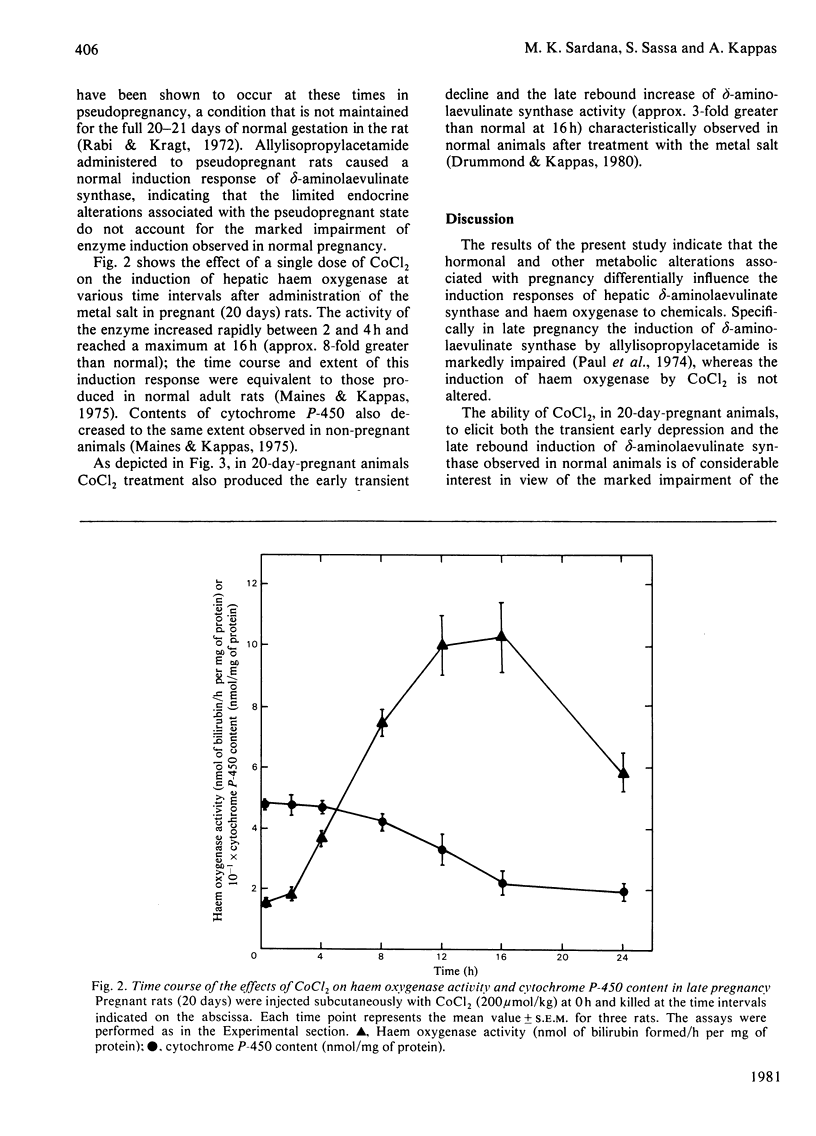
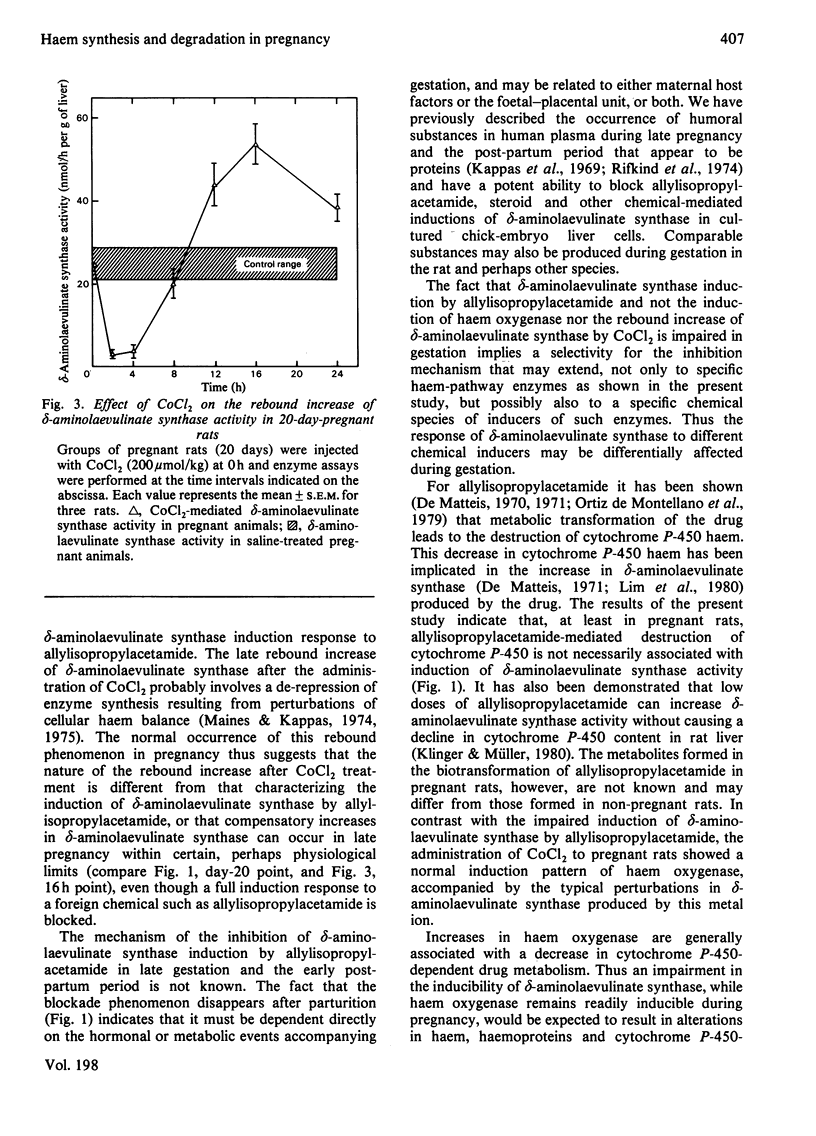
The responses of hepatic delta-aminolaevulinate synthase and microsomal haem oxygenase to inducers were examined in pregnant rats. 2-Allyl-2-isopropylacetamide-mediated induction of delta-aminolaevulinate synthase was greatly decreased during pregnancy and in the early post-partum period. Administration of allylisopropylacetamide to pseudopregnant rats induced delta-aminolaevulinate synthase normally. Treatment of pregnant rats with cortisol failed to restore the drug-mediated induction of delta-aminolaevulinate synthase. Microsomal cytochrome P-450 content and the activities of drug-metabolizing enzymes such as aniline hydroxylase and ethylmorphine. N-demethylase were significantly lowered during pregnancy. In contrast with the greatly impaired induction of delta-aminolaevulinate synthase, the induction of haem oxygenase in response to CoCl2 remained unaltered in pregnant rats. The normal perturbations of delta-aminolaevulinate synthase, consisting of an initial inhibition followed by a rebound increase in the enzyme activity associated with CoCL2 treatment, were observed during pregnancy. These findings indicate that hormones and metabolic factors associated with gestation exert significant but differential controls on the induction patterns of delta-aminolaevulinate synthase and haem oxygenase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvares A. P., Mannering G. J. Two-substrate kinetics of drug-metabolizing enzyme systems of hepatic microsomes. Mol Pharmacol. 1970 May;6(3):206–212. [PubMed] [Google Scholar]
- De Feo V. J. Vaginal-cervical vibration: a simple and effective method for the induction of pseudopregnancy in the rat. Endocrinology. 1966 Aug;79(2):440–442. doi: 10.1210/endo-79-2-440. [DOI] [PubMed] [Google Scholar]
- De Matteis F. Loss of haem in rat liver caused by the porphyrogenic agent 2-allyl-2-isopropylacetamide. Biochem J. 1971 Oct;124(4):767–777. doi: 10.1042/bj1240767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis F. Rapid loss of cytochrome P-450 and haem caused in the liver microsomes by the porphyrogenic agent 2-allyl-2-isopropylacetamide. FEBS Lett. 1970 Feb 25;6(4):343–345. doi: 10.1016/0014-5793(70)80094-1. [DOI] [PubMed] [Google Scholar]
- Drummond G. S., Kappas A. Metal ion interactions in the control of haem oxygenase induction in liver and kidney. Biochem J. 1980 Nov 15;192(2):637–648. doi: 10.1042/bj1920637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y., Ito A., Sato R. Evidence for biochemically different types of vesicles in the hepatic microsomal fraction. J Biochem. 1966 Oct;60(4):417–428. doi: 10.1093/oxfordjournals.jbchem.a128453. [DOI] [PubMed] [Google Scholar]
- Kappas A. Biologic actions of some natural steroids on the liver. N Engl J Med. 1968 Feb 15;278(7):378–384. doi: 10.1056/NEJM196802152780707. [DOI] [PubMed] [Google Scholar]
- Kappas A., Song C. S., Sassa S., Levere R. D., Granick S. The occurrence of substances in human plasma capable of inducing the enzyme delta-aminolevulinate synthetase in liver cells. Proc Natl Acad Sci U S A. 1969 Oct;64(2):557–564. doi: 10.1073/pnas.64.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger W., Müller D. The influence of allyl isopropyl acetamide on d-aminolevulinic acid synthetase and cytochrome P-450. Acta Biol Med Ger. 1980;39(1):107–112. [PubMed] [Google Scholar]
- Lim L. K., Srivastava G., Brooker J. D., May B. K., Elliott W. H. Evidence that in chick embryos destruction of hepatic microsomal cytochrome P-450 haem is a general mechanism of induction of delta-aminolaevulinate synthase by porphyria-causing drugs. Biochem J. 1980 Sep 15;190(3):519–526. doi: 10.1042/bj1900519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt induction of hepatic heme oxygenase; with evidence that cytochrome P-450 is not essential for this enzyme activity. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4293–4297. doi: 10.1073/pnas.71.11.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Cobalt stimulation of heme degradation in the liver. Dissociation of microsomal oxidation of heme from cytochrome P-450. J Biol Chem. 1975 Jun 10;250(11):4171–4177. [PubMed] [Google Scholar]
- Matsuoka T., Yoda B., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of delta-aminolevulinate synthetase in liver mitochondria. 3. Effects of triiodothyronine and hydrocortisone on the induction process. Arch Biochem Biophys. 1968 Aug;126(2):530–538. doi: 10.1016/0003-9861(68)90438-4. [DOI] [PubMed] [Google Scholar]
- NASH T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. Biochem J. 1953 Oct;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale M. G., Parke D. V. Effects of pregnancy on the metabolism of drugs in the rat and rabbit. Biochem Pharmacol. 1973 Jun 15;22(12):1451–1461. doi: 10.1016/0006-2952(73)90323-7. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Yost G. S., Mico B. A., Dinizo S. E., Correia M. A., Kumbara H. Destruction of cytochrome P-450 by 2-isopropyl-4-pentenamide and methyl 2-isopropyl-4-pentenoate: mass spectrometric characterization of prosthetic heme adducts and nonparticipation of epoxide metabolites. Arch Biochem Biophys. 1979 Oct 15;197(2):524–533. doi: 10.1016/0003-9861(79)90276-5. [DOI] [PubMed] [Google Scholar]
- Paul S., Bickers D. R., Levere R. D., Kappas A. Inhibited induction of hepatic delta-aminolevulinate synthetase in pregnancy. FEBS Lett. 1974 May 1;41(2):192–194. doi: 10.1016/0014-5793(74)81209-3. [DOI] [PubMed] [Google Scholar]
- Rabii J., Kragt C. L. Plasma levels of prolactin, FSH and LH in the pseudopregnant rat. Proc Soc Exp Biol Med. 1972 Oct;141(1):359–362. doi: 10.3181/00379727-141-36777. [DOI] [PubMed] [Google Scholar]
- Rifkind A. B., Sassa S., Merkatz I. R., Winchester R., Harber L., Kappas A. Stimulators and inhibitors of hepatic porphyrin formation in human sera. J Clin Invest. 1974 Apr;53(4):1167–1177. doi: 10.1172/JCI107655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardana M. K., Sassa S., Kappas A. Adrenalectomy enhances the induction of heme oxygenase and the degradation of cytochrome P-450 in liver. J Biol Chem. 1980 Dec 10;255(23):11320–11323. [PubMed] [Google Scholar]
- Sassa S., Kappas A., Bernstein S. E., Alvares A. P. Heme biosynthesis and drug metabolism in mice with hereditary hemolytic anemia. Heme oxygenase induction as an adaptive response for maintaining cytochrome P-450 in chronic hemolysis. J Biol Chem. 1979 Feb 10;254(3):729–735. [PubMed] [Google Scholar]
- Sassa S., Kappas A. Induction of aminolevulinate synthase and porphyrins in cultured liver cells maintained in chemically defined medium. Permissive effects of hormones on induction process. J Biol Chem. 1977 Apr 10;252(7):2428–2436. [PubMed] [Google Scholar]
- Schenkman J. B., Frey I., Remmer H., Estabrook R. W. Sex differences in drug metabolism by rat liver microsomes. Mol Pharmacol. 1967 Nov;3(6):516–525. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968 Oct;61(2):748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]



