Abstract
Amplification of human epidermal growth factor 2 receptor (HER2) and overexpression of estrogen receptor (ER) and/or progesterone receptor (PR) are key determinants in the treatment planning for human breast cancer (BC). Currently, targeted therapies for BC are focused mainly on these biomarkers. However, development of resistance to targeted drugs is almost unavoidable, emphasizing the importance of biochemical and pharmaceutical advances to improve treatment outcomes. To the best of our knowledge, the present study is the first to show functional crosstalk in vitro between HER2 and epithelial membrane protein 3 (EMP3), a tetraspan membrane protein, in human BC. EMP3 overexpression significantly promoted BC cell proliferation, invasion and migration by Transwell assays via epithelial-mesenchymal transition and transactivated the HER family, resulting in increased ER and PR expression in vitro. Knocking down EMP3 notably suppressed cell proliferation and migration and was accompanied by decreased expression of HER1-HER3 and p-SRC proteins. Suppression of EMP3 expression enhanced sensitivity of BC cells to trastuzumab in vitro. Xenograft experiments revealed decreased expression of HER1 and HER2 in stable EMP3-knockdown cells, resulting in decreased tumor weight and size. In patients with BC, EMP3 overexpression was detected in 72 of 166 cases (43.4%), with 18 of 43 (41.9%) HER2-amplified BC samples co-expressing EMP3. Co-expression of EMP3 and HER2 was positively associated with ER expression (P=0.028) and tended to be associated with nodal metastasis (P=0.085), however this was not significant. Taken together, the present results supported the potential of targeting EMP3 as a novel therapeutic strategy for human BC via co-expression of HER2 and EMP3.
Keywords: breast carcinoma, EMP3, HER family, estrogen receptor, progesterone receptor, therapy
Introduction
Breast cancer (BC) is the most commonly diagnosed cancer among female patients worldwide and accounted for 666,103 cancer-associated deaths in 2022 (1). BC encompasses multiple heterogeneous subgroups at the molecular, histopathological and clinical levels (2). BC comprises three major tumor subtypes categorized according to presence or absence of estrogen receptor (ER) and progesterone receptor (PR) expression and/or amplification of human epidermal growth factor receptor 2 (HER2) expression. Hormone receptor (HR)-positive/HER2-negative, HER2-positive, and triple-negative (TN) BC (which lacks all three standard molecular markers) represent 70, 15–20 and 15% of BC cases, respectively (3). Surgery and radiation therapy are considered local treatment, whereas systemic treatment includes chemotherapy and hormone and targeted therapy. Recent advancements, including use of adjunct immunotherapy, support the use of a multimodal approach to improve patient prognosis (4).
Current standards of treatment for BC depend on tumor subtype, anatomical cancer staging and patient preference. Hormone therapeutic agents are primary systemic therapy for ER- and PR-positive BC (3,5). HER2-targeted therapy using monoclonal antibodies, antibody-drug conjugates or tyrosine kinase inhibitors is designed for HER2-positive BC, a subtype typically associated with poor prognosis and chemotherapy resistance (3,6). The introduction of anti-HER2 therapy has led to notable improvements in survival of patients with HER2-positive BC in both early and advanced stages (6).
However, acquisition of resistance to targeted drugs is almost unavoidable, emphasizing the importance of biochemical and pharmaceutical advances to improve treatment outcomes (7,8). Moreover, crosstalk between HER2 and ER signaling pathways may contribute to endocrine resistance (9,10). As a result, exploring mechanisms underlying trastuzumab resistance and developing innovative therapy are key for design and implementation of precision medicine for BC.
Epithelial membrane proteins (EMPs) belong to the growth arrest-specific 3 gene family and serve a key role in cell migration, proliferation and differentiation (11). The 4-transmembrane glycoprotein EMP3, an EMP family member, has received increased attention in recent years because of its potential role in pathogenesis of human cancer (11–14). The levels of EMP3 mRNA in BC are significantly higher in cancer than in non-tumor tissue (15). EMP3 expression is associated with the invasive phenotype of mammary carcinoma cell lines (16). Knocking down EMP3 in SK-BR-3 cells inhibits tumor growth in vitro, suggesting that EMP3 may function as an oncogene in human BC (17). In addition, EMP3 is upregulated in association with epithelial-mesenchymal transition (EMT) (18) and is a signature EMT gene in vitro (19). All six cancer stem cell (CSC)-like BC cell lines are the ‘basal B’ subtype and have high EMP3 expression (20). However, contradictory results have reported that EMP3 serves as a tumor suppressor via the negative regulation of DNA replication and damage repair and stem-like properties in BC (21).
Further support for the role of EMP3 in the pathogenesis of BC comes from the observation that EMP3 is significantly upregulated in microarray profiling of HER2 overexpression in immortalized luminal human mammary epithelial cells (22). Expression of EMP3 has a dose-dependent association with HER2 status in vitro (23). Overexpression of EMP3 in primary BC is positively associated with HER2 protein expression, high histological grade and nodal metastasis (15,16). There is also functional crosstalk between HER2 and EMP3 in vitro (24). Co-expression of EMP3 and HER2 is significantly associated with poor disease- and metastasis-free survival of patients with urothelial carcinoma of the upper urinary tract. However, EMP3 positive cases presented significantly better prognosis in HR+HER2+ BC (25), highlighting the need to clarify the clinical role of EMP3 overexpression in BC.
The present study was designed to address the potential role of EMP3 in the pathogenesis of BC and its implications as a novel therapeutic target. In vitro experiments using forward and reverse transfection approaches were performed on cell lines. A SCID mouse model was created to examine the impact of targeting EMP3 on BC in vivo. Finally, expression of EMP3 protein was surveyed in a BC cohort to assess its association with HER2 status and clinical outcome.
Materials and methods
Bioinformatics analysis
The potential use of EMP3 for the treatment of human BC was analyzed in The Human Protein Atlas (proteinatlas.org/ENSG00000142227-EMP3/cancer/breast+ cancer#BRCA_TCGA), cBioPortal (cbioportal.org/results/comparison?cancer_study_list=brca_tcga_gdc&Z_SCORE_THRESHOLD=2.0&RPPA_SCORE_THRESHOLD=2.0&profileFilter=mrna_seq_tpm_Zscores&case_set_id=brca_tcga_gdc_all&gene_list=EMP3&geneset_list=%20&tab_index=tab_visualize&Action=Submit&comparison_selectedGroups=%5B%22Unaltered%20group%22%2C%22Unprofiled%20group%22%2C%22EMP3%22%2C%22Altered%20group%22%5D&comparison_subtab=survival&comparison_overlapStrategy=Exclude&comparison_groupOrder=%5B%22Unprofiled%20group%22%2C%22EMP3%22%2C%22Altered%20group%22%2C%22Unaltered%20group%22%5D), TNBC Database (https://rgcb.res.in/tnbcdb/tnbdbviewer.php?molecule=mRNA&ctype=EMP3), and UALCAN(https://ualcan.path.uab.edu/cgi-bin/TCGA-survival1.pl?genenam=EMP3&ctype=BRCA) and Genetic determinants of cancer patient outcome (https://tcga-survival.com/data-table?view=gene&gene=EMP3&filter=cancer_type&cancer_type=BRCA).
Cell lines and culture
TNBC cell lines (Hs578T and MDA-MB-231) were chosen for EMP3 transfection experiments. MDA-MB-231 cells were provided by Professor P.S. Chen (Department of Medical Laboratory Science and Biotechnology, National Cheng Kung University, Taiwan) and Hs578T cells were obtained from the Institute of Clinical Medicine, National Cheng Kung University, Taiwan. MDA-MB-231 cells were maintained in Leibovitz's L-15 medium (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (HyClone; Cytiva) and 1% penicillin/streptomycin (Cassion Laboratories) in 5% CO2 at 37°C. Hs578T cells were cultured in high-glucose DMEM (HyClone; Cytiva) supplemented with 0.01 mg/ml insulin, 10% FBS and 1% penicillin/streptomycin in 5% CO2 at 37°C. SK-BR-3 (cat. no. ATCC HTB-30) and MDA-MB-453 (cat. no. ATCC HTB 131) BC cell lines from Institute of Clinical Medicine, National Cheng Kung University, Tainan, Taiwan were maintained in high-glucose DMEM (Himsdia Labomlorier) and Leibovitz's L-15 medium (Himsdia Labomlorier) with 10% FBS and 1% penicillin/streptomycin in 5 or 0% CO2, respectively, at 37°C.
DNA constructs and retrovirus preparation
Full-length EMP3 cDNA was amplified from HEK-293 cells using PCR with custom-designed primers (sense, 5′-gcttcgaattcatgtcactcctcttgc-3′ and antisense, 5′-ggtggatcccgctcccgcttccgtag-3′). The PCR was done using a thermal cycler (G-Storm; GMI, Inc., Ramsey, MN). The cycling was as follows: initial denaturation of 1 cycle at 98°C for 2 min, followed by 30 cycles of denaturation at 98°C for 10 sec, annealing at 65°C for 15 sec and extension at 72°C. The final one cycle of extension was set at 72°C for 5 min. Then, full-length EMP3 cDNA was cloned into the pMSCVpuro retroviral vector at 37°C for 14h (Clontech), which was transfected into GP2-293 cells from the Institute of Clinical Medicine, National Cheng Kung University, Tainan, Taiwan via the calcium phosphate method for 24 h at 37°C as previously described (24). Viral supernatant was prepared by collecting GP2-293 culture medium 48 h after transfection and centrifuging at 367 × g) at room temperature for 5 min. Stably infected clones were selected by puromycin (5 mg/ml) and maintained at concentrations of 15 ng/µl. Two overexpressed stable clones, EMP3-1 and EMP3-2, were selected from Hs578T and MDA-MB-231 cell lines, respectively. Successful overexpression of EMP3 was tested on MDA-MB231 cells (HER2-negative) and confirmed by western blotting. The knockdown experiment was performed as described previously (24). Briefly, small interfering (si)RNA was obtained from Invitrogen (cat. no HSS103226; Thermo Fisher Scientific, Inc.). Cells were transfected with 50 nM EMP3 siRNA based on dose-dependent experiments (data not shown) or scramble negative control using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). The constructs of pcDNA6.2-GW/EmGFP-miR-EMP3 were generated using BLOCK-iT Pol II miR RNAi Expression Vector kits (Invitrogen; Thermo Fisher Scientific, Inc.). Linearized vector pcDNA6.2-GW/EmGFP-miR was ligated to the nucleotide sequence of EMP3 (5′-GCAGTAATGTCAGCGAGAATG-3′). The oligonucleotide sequence of negative control was 5′-GAAATGTACTGCGCGTGGAGACGTTTTGGCCACTGACTGACGTCTCCACGCAGTACATTT-3′, which does not target any known vertebrate gene.
Western blotting
Hs578T and MDA-MB-231 cells were lysed in RIPA lysis buffer supplemented with protease inhibitors (Thermo Fisher Scientific, Inc.) to extract protein. Protein concentration was determined using Bradford assay Briefly, a total of 30 µg/lane protein was separated by SDS-PAGE through 6–12% gradient gels and transferred to 0.2–0.45 µm PVDF membrane (Stratagene), which was blocked with 5% non-fat milk in TBST (0.1% Tween-20). The membrane was incubated with primary antibodies (GeneTex, Inc.) overnight at 4°C targeting human EMP3 (1:1,000, clone no. 43972, GeneTex, Inc.), epidermal growth factor receptor (EGFR or HER1, 1:1,000, cat. no. GTX100448), human epidermal growth factor 2 (HER2, 1:1,000, Cat#GTX100509), human epidermal growth factor 3 (HER3, 1:1,000, Cat#GTX50651), human epidermal growth factor 4 (HER4, 1:1,000, Cat#GTX111276), progesterone receptor (PR) (1:1,000, Cat#GTX22765;), estrogen receptor (ER, 1:1,000, Cat. no. GTX127978), focal adhesion kinase (FAK; 1:1,000, Cat#GTX100764), Src proto-oncogene, non-receptor tyrosine kinase (SRC, 1:1,000, Cat#GTX132369), phosphorylated (p-)SRC (1:1,000, Cat#GTX13347), rho associated coiled-coil containing protein kinase 1 (ROCK1, 1:1,000, Cat#GTX629972), ROCK2 (1:1,000, Cat#GTX102619), α-actinin (1:1,000, Cat#GTX103219), JAK2, Cell Signaling Technology, Inc., 1:1,000, Cat#3230), p-JAK2 (1:1,000, Cat#GTX132784), signal transducer and activator of transcription 3 (STAT3, 1:1,000, Cat#GTX104616), p-STAT3 (1:1,000, Cat#GTX118000), RAS Proto-Oncogene, GTPase (RAS, 1:1,000, cat. no. GTX132480), raf-1 proto-oncogene, serine/threonine kinase (Raf-1, 1:1,000, Cat#GTX111588), extracellular signal-regulated kinase 1 and 2 (ERK1/2, 1:1,000, Cat#GTX134462), p-ERK1/2 (1:1,000, Cat#GTX132783), SRY-box transcription factor 2 (SOX2, 1:1,000m Cat#GTX101507), octamer-binding transcription factor 4 (OCT4, 1:1,000, Cat#GTX101497), twist family bhlh transcription factor 1 (Twist1, 1:1,000, Cat#GTX60776), snail family transcriptional repressor 1 (Snail, 1:1,000, Cat#GTX638370), snail family transcriptional repressor 2 (Slug, 1:1,000, Cat#GTX128796), zinc finger e-box binding homeobox 1 (ZEB1, 1:1,000, cat. no. GTX638294;), E-cadherin (1:1,000, Cat#GTX100443), vimentin (1:1,000, Cat#GTX100619) and human β-actin (1:5,000, Cat#GTX109639). Following washing with TBST three times, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (anti-rabbit or anti-mouse IgG; 1:5,000, Cat# GTX213110, GeneTex, Inc.) for 1 h at room temperature. Detection of protein bands was performed using an enhanced chemiluminescence system (cat#GTX400006, GeneTex). The densitometry was performed using ImageJ (1.41o National Institutes of Health).
MTT assay
MTT assay was performed to assess the impact of EMP3 on BC cell proliferation in vitro. A total of 1,000 transfected cells was seeded in 100 µl medium in each well of a 96-well plate and cultured at 37°C for 24, 48, 72 and 96 h. Then, the cells were incubated with 50 µl MTT (Cyrusbioscience, Inc.) reagent/well in the dark at 37°C for 4 h and the formazan crystals were dissolved in DMSO and quantified by measuring absorbance at 590 nm using microplate reader (SpectraMax i3X, Molecular Devices). A total of four independent experiments were performed in all assays. For chemosensitivity, cells were treated with appropriate concentrations of trastuzumab (HERCEPTIN®, 440 mg/20 ml, Genentech, Inc.) and incubated for 3 days at 37°C. MTT assay was performed in quintuplicate to obtain the average.
Cell migration and invasion assay
Migration and invasion experiments were performed using Transwell membrane filter inserts (cat. no. #3422, Corning Costar, Inc.; 8 µm pore size) at 37°C. In brief, 2.5×104 cells (Hs578T or MDA-MB-231) were seeded into DMEM plated in upper chamber of a 24-well Transwell plate with or without Matrigel for invasion and migration assays, respectively. The lower chamber was filled with complete medium (L-15 or DMEM) with 10% FBS. The migration and invasion assays were performed at 37°C for 72 and 48 h, respectively. Migrated cells on the lower surface were stained with 2% crystal violet at room temperature for 10 min, and those that did not penetrate the filter were removed. The number of migrating or invading cells was counted under a light microscope from five randomly selected fields of view/well) in a single chamber (magnification, ×200).
Gap closure assay
To assess cell migration in vitro, culture inserts (Ibidi GmbH) consisting of two wells separated by a 500-µm-thick wall were used. The insert was placed into one well of six-well plates and pressed to ensure tight adhesion. Stable EMP3-overexpressing Hs578T and MDA-MB-231 cells (2×105/100 µl) were seeded into each well and incubated overnight until 90% confluence in DMEM-High glucose (HyClone), supplemented with 0.01 mg/ml insulin, 5% FBS (HyClone) at 37°C. The inserts were removed, and the width between the gaps visible under a light microscope (cat. no. TE300, Nikon) was recorded at 0, 5, 10 and 15 for HS578T and 0, 5, 10, and 20 h for MDA-MB-231 cells (magnification, ×100). The area covered by cells was measured to quantify cell migration in vitro. The closure of the cell-free gap for each treatment was determined by comparing the results to those at 0 h (magnification, ×200).
In vivo xenograft model
A total of 240 Six-week-old male NOD/SCID mice weight, 20 to 25 grams was purchased from the National Cheng Kung University Laboratory Animal Centre and maintained in a pathogen-free facility under isothermal conditions with regular photoperiods. The animals had free access to sterile water and food. The experimental protocol adhered to regulations of the Animal Protection Act of Taiwan (26) and was approved by the NCKU Laboratory Animal Care and User Committee (approval no. 108075). The temperature for mice ranged from 20–24°C, and the humidity maintained between 40–60%. The common light cycle is 12/12-h light/dark cycle. The mice were subcutaneously (s.c.) injected with 1×107 BC cells (SKBR3) in 50 µl normal saline on the left and right flanks. A total of 6 mice (three mice in each group, SKBR3/Vec, and SKBR3/EMP3) (12 tumors) were obtained in each experimental group. Body weight and tumor size were measured every week. If the weight of a single tumor exceeds 10% of body weight, or the average diameter of the tumor in adult mice exceeds 20 mm, the animal needs to be euthanized. The maximum tumor volume and tumor diameter measured were 520 mm3 and 15 mm, respectively. All mice were euthanized by stepwise escalation (30–50% of chamber volume, 0.02 MPa) of carbon dioxide inhalation and cervical dislocation. Death was verified by pupil dilation as well as cessation of breathing and heartbeat. The tumors were resected, photographed and weighed on day 90 post-injection.
Immunohistochemistry (IHC)
IHC was performed to examine expression of HER1, HER2 and EMP3 in xenografts using primary antibodies targeting human EMP3 (GeneTex, Inc., 1:1,000), HER1 (Zeta Corporation, 1:200) and HER2 (Zeta Corporation, 1:200). Briefly, tissue was fixed with 10% neutral formalin at room temperature overnight and paraffin-embedded, Sections of 4 mm thickness were deparaffinized with xylene and pre-treated with Epitope Retrieval Solution 2 (EDTA buffer, pH 9.0, Dako Denmark A/S, Inc.) at 100°C for 30 min. The blocking was carried out by protein blocking buffer (RE7102-CE, Leica Biosystems) at room temperature for 30 min and super blocking buffer (AAA-IFU, ScyTek Laboratories, Inc.) at room temperature for 9 min. Then, sections were reacted with primary antibody at room temperature for 30 min, followed by incubation at room temperature for 8 min using a ready-to-use Bond Polymer Refine Detection kit containing peroxide block for quenching (DS9800, Cat#68086, Leica Biosystems), secondary antibody, polymer reagent and DAB chromogen. The DAB was developed for 10 min. Counterstaining was performed with hematoxylin for 5 min at room temperature. The histology was examined light microscope (Nikon; magnification, ×40 and ×100).
Immunocytochemistry
Cells were plated onto glass coverslips and fixed with 4% paraformaldehyde for 20 min at room temperature, blocked with 5% rabbit serum (Neuromics; Cat# SER003) for 60 min at room temperature, and stained with primary antibody for Ki-67 (1:200 dilution, Cat#sc-23900, Santa Cruz Biotechnology, Inc.) at room temperature for 1 h. A high-sensitivity DAB system (Liquid DAB+ Substrate kit K3468, Dako) was used for immunocytochemical staining. The slides were counterstained with Mayer's hematoxylin at room temperature for 1 min, dehydrated, and then mounted. Specific primary antibody replaced with PBS in tissue sections was used as a negative control. The cytology was examined light microscope (Nikon) (magnification, ×40, ×100, ×200 and ×400).
Expression of EMP3 in primary BC cohort
A retrospective study of a BC cohort, who underwent surgical treatment with modified radical mastectomy or breast-conserving surgery and sentinel lymph node biopsy between April 2005 and October 2014, was recruited under the approval of the Institutional Review Board of the National Cheng Kung University Hospital (approval no. A-ER-107-441). All samples were from female patients recruited from National Cheng Kung University Hospital, Tainan, TAIWAN. The study population comprised 166 patients with median age of 53 years (range, 29–83 years). Clinical demographic data, including TNM status and clinical information, were obtained by chart review. Each case was independently reviewed by two pathologists for diagnosis. The mean follow-up time was 108.28±37.24 months.
IHC staining of EMP3 protein was performed to examine its association with clinicopathological indicators and patient outcomes. The breast tissue was fixed with 10% neutral formalin overnight at room temperature. Then, it was cut for dehydration and paraffin embedding. Immunostaining was performed on formalin-fixed paraffin-embedded tissue using a standard avidin-biotin complex-peroxidase procedure with an automated stainer (BenchMark Ultra Auto-Stainer; Ventana) (24). Briefly, 4-µm tissue sections from each tumor were subjected to heat-induced epitope retrieval using CC1 cell conditioning solution (Ventana). The slides were treated with 3 % hydrogen peroxide in PBS at 4°C for 30 min, and then 10% normal goat serum (Neuromics, Edina, MN; Cat# SER003) at room temperature for 60 min. The slides were incubated with rabbit polyclonal EMP3 antibody (LTK BioLaboratories) or PATHWAY anti-HER2/Neu (4B5) rabbit monoclonal antibody (Ventana) at a dilution of 1:10. The immunostained proteins were visualized using a Roche OptiView DAB IHC Detection kit (Ventana). Anti-rabbit poly-HRP secondary antibody (Leica Biosystems) was added at room temperature for 30 min. Sections were counterstained with hematoxylin at room temperature for 5 min. Samples treated with PBS instead of specific primary antibody were used as a negative control. Both positive reference (BC known to express EMP3) and negative controls (normal liver) were included.
The histology was examined (by light microscope (Nikon) (magnification, ×40 and ×100). HER2 protein expression was scored according to 2018 American Society of Clinical Oncology and the College of American Pathologists for the testing and reporting of biomarkers in cancer HER2 testing in BC guidelines (27). EMP3 staining intensity was graded as negative, +, ++ and +++ by two pathologists using smooth muscle cells of small arteries as the internal reference (28). ++ or +++ was considered to indicate EMP3 upregulation.
Statistical analysis
The data were derived from triplicate experiments. All data are presented as the mean ± SD by GraphPad prism 6 software (GraphPad Software, Inc.; Dotmatics). The differences between categorical variables were analyzed with one-way ANOVA followed by post hoc Tukey's test. Disease-free survival was calculated from the date of surgery to the date of disease recurrence. The significance of various covariates in survival was assessed using univariate analysis with log-rank test. The survival curve was calculated using the Kaplan-Meier method (SPSS 17.0 for Windows (SPSS Inc., All tests were two-sided and P<0.05 was considered to indicate a statistically significant difference.
Results
Bioinformatics analysis
To investigate the potential role of EMP3 in human BC, bioinformatics analysis was performed using The Human Protein Atlas, Gene Expression Profiling Interactive Analysis, cBioPortal, UALCAN and OncoLnc. EMP3 expression tended to be positively associated with poor overall survival in cBioPortal (data not shown) but this was not significant. High EMP3 expression was associated with better survival in The Human Protein Atlas and UALCAN (data not shown) datasets. High EMP3 expression tended to be associated with better overall survival in Gene Expression Profiling Interactive Analysis dataset, but no significant difference was observed for disease-free survival (data not shown). However, analysis of the OncoLnc data did not reveal a prognostic value for EMP3 expression (data not shown).
Biological effects induced by EMP3 overexpression in BC cells
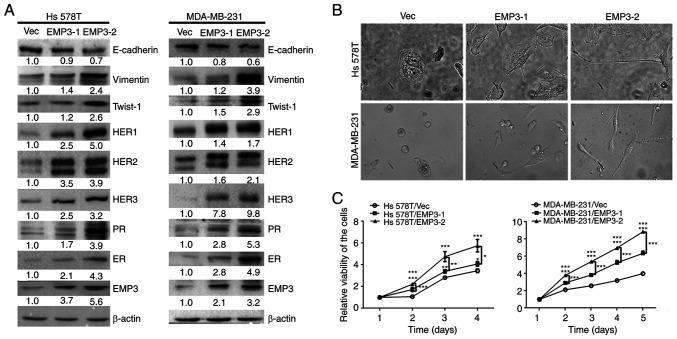
To clarify the biological role of EMP3, stable EMP3-1 and EMP3-2 clones were established in HS578T and MDA-MB-231 BC cell lines, respectively. These stable clones exhibited higher levels of EMP3 protein expression compared with parental cells, as shown by western blotting. Expression of EMP3 protein in stable EMP3-1 and EMP3-2 clones was moderate and high, respectively (Fig. 1A). The protein expression profiles, including those of ER, PR and HER family members and EMT-related markers, were assessed in vector (Vec) control cells and stable EMP3-overexpressing clones (EMP3-1 and EMP3-2; Fig. 1A). The morphology of stable EMP3-overexpressing cells was examined via light microscopy (Fig. 1B). Both Hs578T and MDA-MB-231 cells became slender in shape following transfection with EMP3. The viability was significantly increased in stable EMP3-1- and EMP3-2-overexpressing clones, both in the Hs578T and MDA-MB-231 cell lines (Fig. 1B).
Figure 1.
Protein expression profiles, morphology and proliferation of EMP3-overexpressing BC cells. Stable clones of EMP3-1 and EMP3-2 were established from HS578T and MDA-MB-231 BC cell lines, respectively. (A) Protein expression profiles were examined for EMP3, ER, PR, HER family members and EMT-related markers in stable EMP3-overexpressing cells compared with Vec control cells via western blotting. (B) Both stable EMP3-1 and stable EMP3-2 cells became slender in shape (fibroblastoid) following EMP3 overexpression, particularly stable EMP3-2 clones. (C) EMP3 overexpression significantly increased the viability of tumor cells, with a greater rate observed in stable EMP3-2 clones. *P<0.05, **P<0.01, ***P<0.001 vs. EMP3-1 or −2. EMP, epithelial membrane protein; PR, progesterone receptor; ER, estrogen receptor; HER, human epidermal growth factor receptor; BC, breast cancer; Vec, vector.
Crosstalk of EMP3 with HER family in human BC cells in vitro
Expression of all HER family members (HER1-HER3), PR and ER increased in both Hs578T and MDA-MB-231 cell lines stably overexpressing EMP3 (Fig. 1A). Therefore, overexpression of EMP3 may upregulate expression of HER1-HER3 and HR in human BC in vitro (Fig. 1A). These results indicated that EMP3 can functionally crosstalk with HER superfamily and HR during BC tumorigenesis.
EMP3 overexpression promotes cell migration and invasion
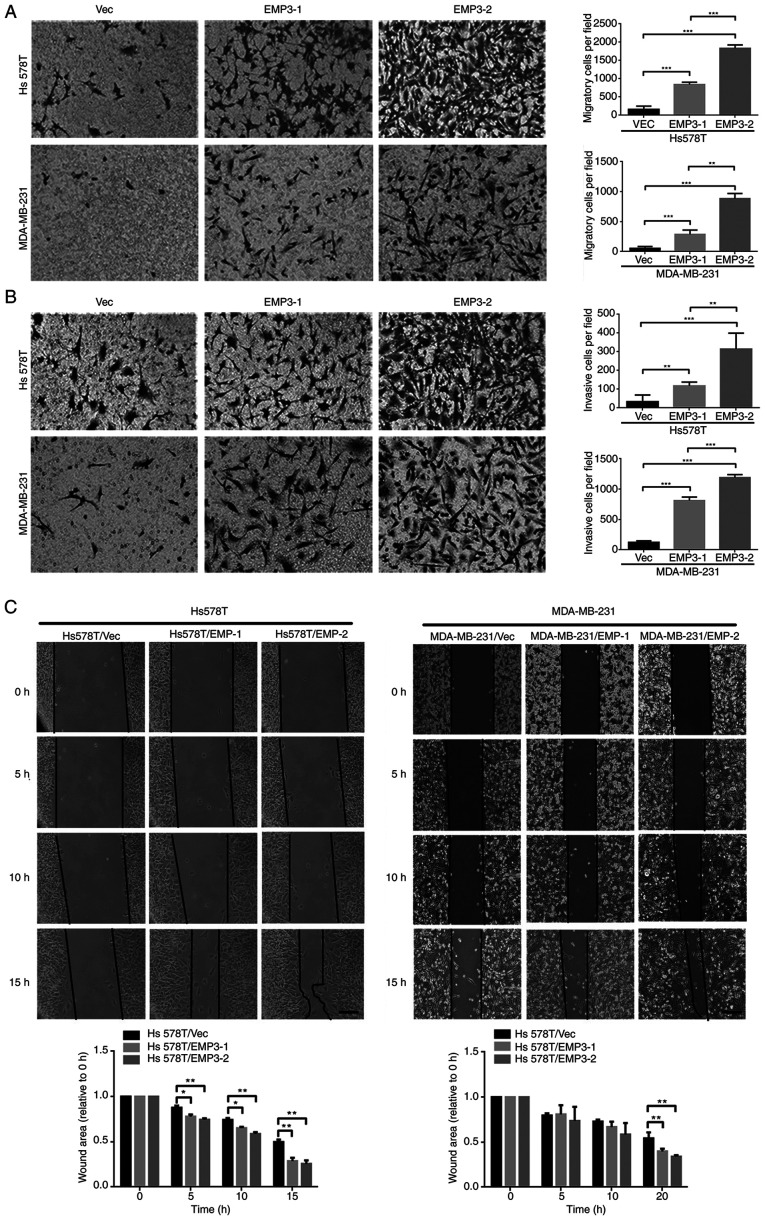
Overexpression of EMP3 upregulated protein expression of fibronectin, twist1/2 and vimentin but suppressed E-cadherin expression in both Hs578T and MDA-MB-231 cell lines (Fig. 1A). However, expression of Snail, Slug and ZEB1 was not upregulated (data not shown). EMP3 overexpression in EMP3 stable cells, defined as two-fold higher expression, compared with vector control may contribute to migration and invasion of TNBC cells in vitro via EMT. Stable clones of EMP3-1 and EMP3-2 were subjected to cell migration, invasion and gap closure assays. Compared with Vec control cells, stable EMP3-overexpressing Hs578T and MDA-MB-231 cells exhibited increased migration (Fig. 2A) and invasion (Fig. 2B). In addition, EMP3-2 stable clones had increased ability to migrate and invade in vitro compared with EMP3-1 stable clones of Hs578T and MDA-MB-231 cell lines, respectively (Fig. 2A).
Figure 2.
Effects of EMP3 on migration and invasion of breast cancer cells in vitro. (A) Migration of EMP3-overexpressing Hs578T stable cell lines was significantly greater than that of Vec control cells (magnification, ×100). (B) Hs578T EMP3-overexpressing cells exhibited increased invasion ability. (C) Gap closure assay. Scale bar represents 100 µm. *P<0.05, **P<0.01, ***P<0.001 represents p value between vector control and EMP3-1 or −2 stable cells or between these two stable cells. EMP, epithelial membrane protein; Vec, vector.
The impact of EMP3 on cell migration was confirmed via gap closure assay. Stable EMP3-1 and EMP3-2 cells showed greater gap closure than Vec control cells (Fig. 2C). This was observed in both Hs578T and MDA-MB-231 cell lines. Together, these findings suggested that EMP3 overexpression exerted pro-oncogenic effects on BC in vitro.
Intracellular signaling pathways upregulated by EMP3 overexpression in human BC
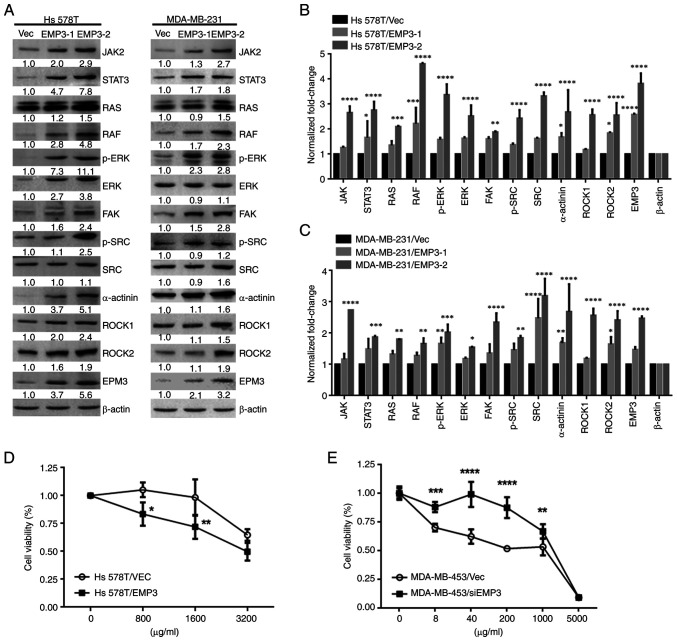
There is crosstalk between EMP2 and integrins αV and β3 in regulation of urothelial cell adhesion and migration during tumorigenesis in vitro (11). Therefore, involvement of this signaling pathway was assessed in BC development. Western blotting showed that components of FAK-c/SRC/RhoA, SRC/JAK2/STAT3, SRC/Ras/Raf-1 and ERK1/2 pathways were upregulated in Hs578T and MDA-MB-231 EMP3 stable cells compared with Vec control cells (Fig. 3A). Protein expression (JAK2, STAT3, RAS, RAF, p-ERK, ERK, SRC, p-SRC, α-actinin ROCK1 and ROCK2) was quantified. All of the signal proteins in Hs578T cells were significantly higher in EMP3-2 stable cells compared with vector control (Fig. 3B and C). Overall, EMP3 upregulated proteins associated with JAK2/STAT3, RAS/RAF/ERK and FAK/SRC/α-actinin/ROCK1/2 signaling pathways in BC in vitro.
Figure 3.
Effects of EMP3 on signaling pathways and chemoresistance in breast cancer cells in vitro. (A) Protein expression of JAK2, STAT3, RAS, RAF, p-ERK, ERK, FAK, p-SRC, SRC, α-actinin, ROCK1, ROCK2, and EMP3. β-actin was used as a loading control in (B) Hs578T and (C) MDA-MB-231 cell lines. Growth curves of (D) trastuzumab-treated Hs578T and (E) EMP3-knockdown MDA-MB-453 cell lines were generated via MTT assay. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 vs. EMP3-1 or −2 stable cells or between these two stable cells. EMP, epithelial membrane antigen; RAF, raf-1 proto-oncogene, serine/threonine kinase; p-, phosphorylated; FAK, focal adhesion kinase; ROCK, rho-associated protein kinase; SRC, Src proto-oncogene, non-receptor tyrosine kinase; Vec, vector; si, small interfering.
EMP3 alters chemosensitivity of BC cells to trastuzumab in vitro
As EMP3 was demonstrated to upregulate expression of HER1-HER3, as well as proteins associated with JAK2/STAT3, RAS/RAF/ERK and FAK/SRC/α-actinin/ROCK1/2 signaling pathways in the Hs578T and MDA-MB-231 BC cell lines, the potential of EMP3 in modulating drug resistance of BC was investigated. Trastuzumab is a monoclonal anti-HER2 antibody for patients with early or metastatic HER2-positive BC (29). Therefore, cell viability was examined in response to trastuzumab in the presence of EMP3 overexpression or knockdown. MTT assay revealed that Hs578T EMP3 stable cells with higher HER2 expression were more sensitive to trastuzumab than Hs578T/Vec control cells (Fig. 3D). By contrast, knocking down EMP3 in MDA-MB-453 cells conferred resistance to trastuzumab compared with MDA-MB-453/Vec control cells (Fig. 3E). Together, these findings indicated that altered EMP3 expression modified the sensitivity of BC cells to trastuzumab treatment in vitro.
EMP3 as a potential novel therapeutic target for human BC in vitro
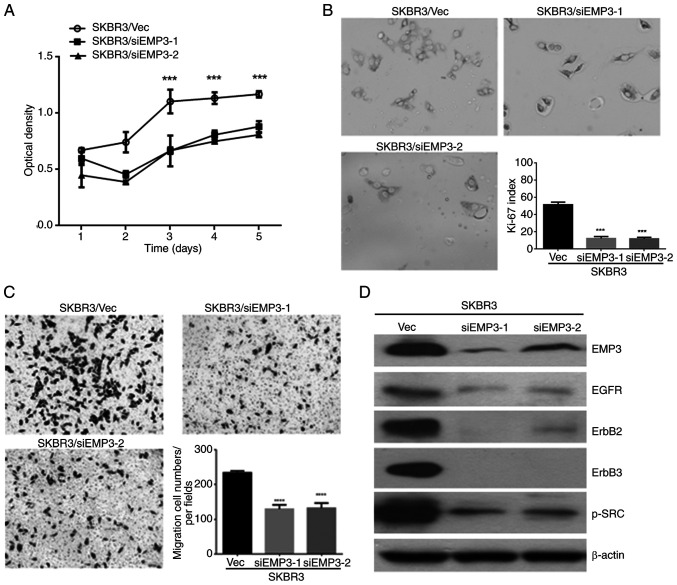
As aforementioned EMP3 was demonstrated to confer pro-tumorigenic effects on human BC in vitro and upregulate HER receptor expression and multiple signaling pathways. To clarify the potential of EMP3 as a therapeutic target for human BC, the SK-BR-3 cell line was assessed because of its endogenous expression of HER1-HER3 and EMP3. Therefore, EMP3-knockdown cell lines, SKBR3/siEMP3-1 and SKBR3/siEMP3-2, and a vector control cell line (SKBR3/Vec) were established from SK-BR-3 BC cells. MTT assay (Fig. 4A) and Ki-67 index measurement (Fig. 4B) revealed that the proliferation of SK-BR-3 cells with EMP3 knockdown was significantly suppressed compared with that of control cells. Compared with SKBR3/Vec control cells, cell migration in vitro was inhibited after EMP3 was knocked down, as demonstrated by Transwell migration assay (Fig. 4C). Furthermore, expression of HER1, HER2, HER3 and p-SRC was downregulated in SKBR3/siEMP3-1 and SKBR3/siEMP3-2 stable cells compared with that in SKBR3/Vec control cells (Fig. 4D). These results suggested EMP3 as a novel therapeutic target for human BC with co-expression of HER2 and EMP3.
Figure 4.
Potential therapeutic targets of EMP3 in breast cancer. Cell proliferation was examined by (A) MTT assay and (B) Ki-67 index after transient transfection. (C) Transwell migration assay was performed to assess migration of EMP3-knockdown cells after 48 h. Magnification, ×100. (D) Expression of HER family members and p-SRC proteins was assessed by western blotting after EMP3 was knocked down in SK-BR-3 cells. ***P<0.001, ****P<0.0001 vs. EMP3-1 or −2 stable cells or between these two stable cells. EMP, epithelial membrane protein; HER, human epidermal growth factor receptor; Vec, vector; p-SRC, phosphorylated Src proto-oncogene, non-receptor tyrosine kinase; EGFR, epidermal growth factor receptor; si, small interfering.
Tumorigenicity of EMP3 in a xenograft NOD/SCID mouse model
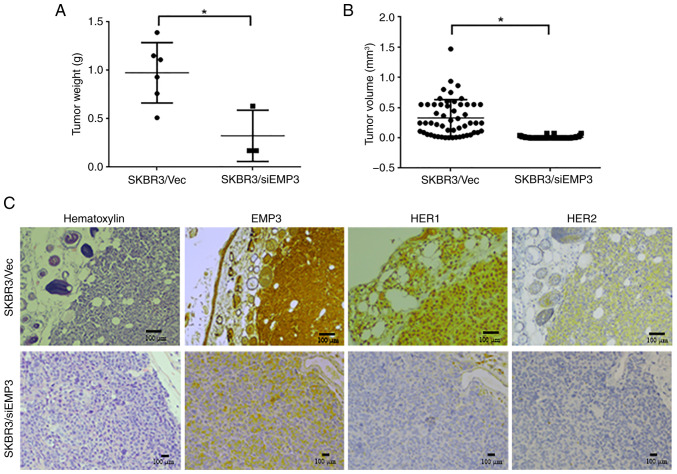
To verify the clinical implications of targeting EMP3 in BC, a xenograft NOD/SCID mouse model was constructed using EMP3-knockdown and vector control SK-BR-3 cell lines. The weights of xenografts derived from SKBR3/siEMP3 stable cells were significantly lower than those derived from SKBR3/Vec control cells (Fig. 5A). The tumor volume derived from SKBR3/siEMP3 cells was also lower than that derived from SKBR3/Vec control cells (Fig. 5B). In terms of biomarker expression in vivo, strong membrane expression of EMP3, HER1 and HER2 was demonstrated in tumors derived from SKBR3/Vec control cells, whereas expression of these proteins was decreased in tumors derived from SKBR3/siEMP3 stable cells (Fig. 5C).
Figure 5.
Tumorigenicity of EMP3 in a xenograft mouse model. SKBR3/Vec and SKBR3/siEMP3 cells were subcutaneously injected into NOD/SCID mice to investigate the tumorigenic potential of EMP3 in breast cancer cells with HER2 amplification. The tumor nodules were measured regularly for 23 days. Compared with those derived from SKBR3/Vec control cells, tumors derived from SKBR3/siEMP3 cells had significantly lower tumor (A) weight and (B) volume. (C) Representative results of hematoxylin and immunostaining of EMP3, HER1, and HER2 in serial sections of SKBR3/Vec and SKBR3/siEMP3 tumors. *P<0.05. EMP, epithelial membrane protein; Vec, vector; si, small interfering; HER, human epidermal growth factor receptor.
Association of EMP3 with clinicopathological indicators of BC
Expression of EMP3 was investigated in BC cases from the NCKUH biobank (Table SI). EMP3 protein was detected in the cytoplasm and/or membrane of cancer cells. In addition, its expression was heterogeneous in tumors, with higher expression usually occurring at the invasive front (Fig. 5C). EMP3 expression was detected in 102 of 166 cases (61.4%) of BC; however, its expression intensity was not associated with tumor stage, nodal metastasis, ER, PR or HER2 status or clinical outcome. EMP3 was upregulated in 72 cases (43.4%) in the BC cohort. Because of crosstalk between EMP3 and HER2 in vitro, co-expression of these biomarkers was analyzed in relation to clinicopathological indicators and patient outcomes (Table I). The incidence of EMP3(−)/HER2(−), EMP3(−)/HER2(+), EMP3(+)/HER2(−), and EMP3(+)/HER2(+) in the BC cohort was 69 (41.6), 25 (15.1), 54 (32.5) and 18 (10.8%) cases, respectively. Co-expression of EMP3 and HER2 was positively associated with ER expression and tended to be associated with nodal metastasis, however this was not significant. In the absence of HER2 amplification, EMP3(+)/ER(+) and EMP3(+)/PR(+) patterns were detected in 46 (27.7) and 30 (18.1%) cases of BC, respectively. Patients with EMP3(+)/HER2(+) BC tended to have a higher risk of recurrence (data not shown) and lower disease-free survival than patients with other expression patterns (Fig. 6), however this was not significant.
Table I.
Association of EMP3 and HER2 expression with clinicopathological indicators and patient outcome (n = 166)
| EMP3/HER2a | |||||
|---|---|---|---|---|---|
|
|
|||||
| Indicator | -/- (n=69) | -/+ (n=25) | +/- (n=54) | +/+ (n=18) | P value |
| Anatomic stage | 0.896 | ||||
| I | 14 (20) | 3 (12) | 12 (22) | 3 (17) | |
| II | 40 (58) | 17 (68) | 33 (61) | 10 (55) | |
| III | 15 (22) | 5 (20) | 9 (17) | 5 (28) | |
| pT status | 0.742 | ||||
| pT1 | 22 (32) | 5 (20) | 14 (26) | 7 (39) | |
| pT2 | 43 (62) | 18 (72) | 35 (65) | 11 (61) | |
| pT3 or pT4 | 4 (6) | 2 (8) | 5 (9) | 0 (0) | |
| Nodal status | 0.085 | ||||
| Negative | 32 (46) | 16 (64) | 37 (69) | 10 (56) | |
| Positive | 37 (54) | 9 (36) | 17 (31) | 8 (44) | |
| ER status | 0.028 | ||||
| Negative | 18 (26) | 10 (40) | 8 (15) | 8 (44) | |
| Positive | 51 (74) | 15 (60) | 46 (85) | 10 (56) | |
| PR status | 0.568 | ||||
| Negative | 33 (48) | 14 (56) | 24 (44) | 11 (61) | |
| Positive | 36 (52) | 11 (44) | 30 (56) | 7 (39) | |
| Recurrence | 0.432 | ||||
| Negative | 57 (83) | 21 (84) | 45 (83) | 12 (67) | |
| Positive | 12 (17) | 4 (16) | 9 (17) | 6 (33) | |
| Survival | |||||
| Alive | 57 (83) | 21 (84) | 45 (83) | 13 (72) | 0.716 |
| Dead | 12 (17) | 4 (16) | 9 (17) | 5 (28) | |
EMP3 (−): - or + intensity; EMP3 (+): ++ or +++ intensity.
Figure 6.
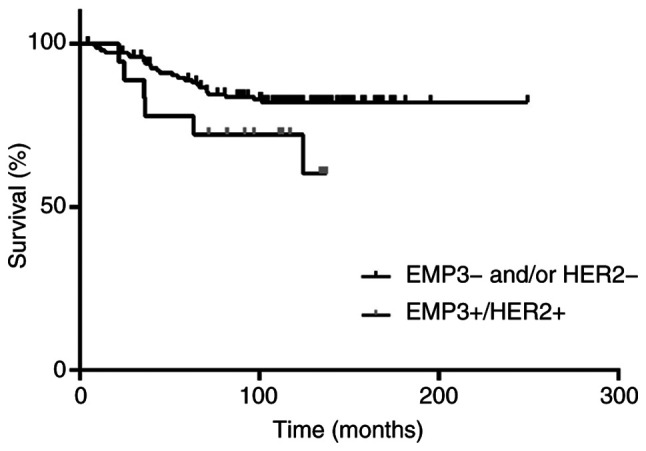
Association of EMP3 overexpression in patients with HER2-amplified BC with disease-specific survival. Patients with EMP3(+)/HER2(+) BC tend to have a lower disease-free survival rate than patients with other expression patterns (P=0.087). EMP, epithelial membrane protein; HER, human epidermal growth factor receptor; BC, breast cancer.
Discussion
EMP3 is a transmembrane signaling protein that serves a key role in the regulation of apoptosis, differentiation and invasion of cancer cells (11). The present showed that EMP3 exerted regulatory effects on the oncogenic HER family and HR in BC in vitro. Moreover, EMP3 activated the expression of proteins associated with JAK2/STAT3, RAS/RAF/ERK and FAK/SRC/α-actinin/ROCK1/2 pathways in BC in vitro, consistent with our previous report and other studies (24,30,31). In addition to roles in proliferation, migration and invasion in vitro, these pathways have also been reported to be involved in EMT and/or trastuzumab resistance in BC (32–34). The present results support the potential role of EMP3-dependent HER1/HER3 regulation in pathogenesis of BC (11,15,17).
Compared with xenografts derived from SK-BR-3/Vec control cells, levels of HER1, HER2, HER3, and p-SRC expression in xenografts derived from SK-BR-3/siEMP3 stable cells were notably lower, in conjunction with lower tumor weight and smaller size. To the best of our knowledge, the present study is the first to demonstrate the potential of EMP3 as a novel therapeutic target for human BC.
As a membrane scaffolding protein, EMP3 forms a network with other transmembrane proteins on the cell surface to promote signaling cascades (35). In human sarcoma, melanoma and embryonic kidney cell line models (11–17), EMP3 serves a key role in organizing signaling complexes at the membrane and is required for maintaining elevated levels of mitogenic signaling as well as cell survival. Using isocitrate dehydrogenase-wild glioblastoma as a model, Martija et al (36) proposed that EMP3 facilitates TBC1D5 recruitment into maturing endosomes, where the complex inactivates member RAS oncogene family (RAB7) and restricts progression of internalized EGFR cargoes toward lysosomal degradation. EMP3-dependent stabilization of EGFR sustains its downstream signaling via CDK2. These mechanisms ensure sustained proliferation, apoptosis resistance and decreased susceptibility to targeted kinase inhibitors. Here, immunoprecipitation (data not shown) revealed that EMP3 interacted strongly with HER2 and weakly with EGFR at the cell membrane of Hs578T/EMP3 stable cells. The present results concur with the mechanism proposed by Martija et al (36).
Although EMP3 serves as an oncogene in BC, previous studies report a tumor-suppressive function (21) and lack of prognostic significance for BC (25). This discrepancy might be explained by unique experimental conditions and dissimilar scoring of immunohistochemistry using different antibodies on limited tissue microarrays.
The present study demonstrated that targeting EMP3 could simultaneously downregulate multiple oncogenes and pleiotropic signaling pathways. This strategy is simple compared with current combination therapies or dual-target inhibitors in the context of immunotherapy and molecular-targeted therapy focused on HER family members (37). Whether dual targeting of EMP3 and HER2 exerts synergistic effects requires clarification. EMP3 is enriched in macrophages and specialized macrophages, such as Kupffer cells in the liver and Hofbauer cells in the placenta (28). Using mouse-derived macrophages, Kusumoto et al (38) demonstrated that EMP3-overexpressing macrophages inhibit induction of alloreactive cytotoxic T lymphocytes, suggesting a role for EMP3 in inhibition of T cell-mediated immunity. Whether a systemic EMP3-targeted approach for BC also affects the tumor microenvironment, particularly in terms of immune cell infiltration or stromal interactions (39–44), requires clarification.
A clinical cohort study revealed a significant association of EMP3 and HER2 co-expression with ER-positive status in BC (17,20), supporting the present in vitro observations. Conversely, ~40% of HER2-amplified BC cases also demonstrated EMP3 upregulation and should be considered for EMP3-targeted therapy. The present data suggested patients with BC with co-expressed EMP3 and HER2, ER or PR may be good candidates for EMP3-targeted therapy. In the present BC cohort, co-expression of EMP3 and HER2 tended to be associated with nodal metastasis (15,16), suggesting the potential role of EMP3 in metastatic progression of BC.
However, EMP3 expression alone was not significantly associated with patient outcome. Further investigation in a larger cohort using monoclonal antibodies and an optimized scoring system is key to clarify the potential of EMP3 as an independent prognostic biomarker, whether for all types or specific subsets of BC. The reason for the difference in the prognostic value of EMP3 in BC dataset analysis is unclear. Potential explanations may be variation in co-expressed biomarkers in the BC cohort and antibody selection with interpretation criteria.
The present study confirmed the role of EMP3 in regulation of HER family signaling in BC. Overexpression of HER1 (45) or HER3 (46,47) is associated with primary resistance to trastuzumab (44–46). The successful in vitro modulation of chemosensitivity to trastuzumab by targeting EMP3 supports its role as a potential co-targeting candidate in the design of HER2-based cancer therapy. A multi-institutional cohort study reported the significance of combined HER3 and HER1 expression score in prediction of resistance to adjuvant chemotherapy in TNBC (48). In addition, 12 of the present patients with TNBC had negative (1+) to equivocal (2+) HER2 expression in the absence of amplification (data not shown). Accordingly, EMP3 warrants exploration as a potential drug target for TNBC. Moreover, expression of HER3 confers HER2-mediated tamoxifen resistance (49). EMP3-targeted agents may have potential as HER2- or HR-targeting follow-up therapy for patients who fail to respond to contemporary regimens.
In conclusion, EMP3 served multiple roles in regulation of the HER family and HR expression via activation of mitogenic signaling pathways in BC in vitro. The potential of EMP3-targeted therapy for BC was supported by chemosensitivity assays and in vitro and in vivo experiments. Further investigations are key for determining the benefit of EMP3-targeted therapy for patients with BC with co-expressed EMP3 and HER2.
Supplementary Material
Acknowledgements
Not applicable.
Glossary
Abbreviations
- BC
breast cancer
- HER2
human epidermal growth factor 2
- HR
hormone receptor
- PR
progesterone receptor
- EMP
epithelial membrane protein
Funding Statement
The present study was supported by Taiwan National Science and Technology Council (grant nos. NSC 105-2320-B-006-029-MY3 and NSC-108-2320-B-006-050-MY3) and China Medical University Hospital (grant nos. C1130502012 and C1130528047).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
YWW, CTL and NHC conceived and designed the project. YLT, YLC and CAC performed experiments. YWW acquired the data. HYC, YLC, HWC and NHC provided technical support. HYC, JYW, CLH and NHC analyzed and interpreted the data. HWC interpreted data. HYC, YWW, NHC wrote the manuscript. All authors read and approved the final manuscript. NHC and CTL confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The present study was approved by the National Cheng Kung University Hospital Laboratory Animal Care and User Committee (IACUC Approval No. 108075).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Testa U, Castelli G, Pelosi E. Breast cancer: A molecularly heterogenous disease needing subtype-specific treatments. Med Sci (Basel) 2020;8:18. doi: 10.3390/medsci8010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waks AG, Winer EP. Breast cancer treatment: A review. JAMA. 2019;321:288–300. doi: 10.1001/jama.2018.20751. [DOI] [PubMed] [Google Scholar]
- 4.Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J, Cardoso F. Breast cancer. Nat Rev Dis Primers. 2019;5:66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 5.Spring LM, Gupta A, Reynolds KL, Gadd MA, Ellisen LW, Isakoff SJ, Moy B, Bardia A. Neoadjuvant endocrine therapy for estrogen receptor-positive breast cancer: A systematic review and meta-analysis. JAMA Oncol. 2016;2:1477–1486. doi: 10.1001/jamaoncol.2016.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swain SM, Shastry M, Hamilton E. Targeting HER2-positive breast cancer: Advances and future directions. Nat Rev Drug Discov. 2023;22:101–126. doi: 10.1038/s41573-022-00579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tesch ME, Gelmon KA. Targeting HER2 in breast cancer: Latest developments on treatment sequencing and the introduction of biosimilars. Drugs. 2020;80:1811–1830. doi: 10.1007/s40265-020-01411-y. [DOI] [PubMed] [Google Scholar]
- 8.Hanker AB, Sudhan DR, Arteaga CL. Overcoming endocrine resistance in breast cancer. Cancer Cell. 2020;37:496–513. doi: 10.1016/j.ccell.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nayar U, Cohen O, Kapstad C, Cuoco MS, Waks AG, Wander SA, Painter C, Freeman S, Persky NS, Marini L, et al. Acquired HER2 mutations in ER+ metastatic breast cancer confer resistance to estrogen receptor-directed therapies. Nat Genet. 2019;51:207–216. doi: 10.1038/s41588-018-0287-5. [DOI] [PubMed] [Google Scholar]
- 10.Pegram M, Jackisch C, Johnston SRD. Estrogen/HER2 receptor crosstalk in breast cancer: Combination therapies to improve outcomes for patients with hormone receptor-positive/HER2-positive breast cancer. NPJ Breast Cancer. 2023;9:45. doi: 10.1038/s41523-023-00533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang YW, Cheng HL, Ding YR, Chou LH, Chow NH. EMP1, EMP 2, and EMP3 as novel therapeutic targets in human cancer. Biochim Biophys Acta Rev Cancer. 2017;1868:199–211. doi: 10.1016/j.bbcan.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Xia S, Zhao Z, Deng L, Wang H, Yang D, Hu Y, Ji J, Huang D, Xin T. EMP3 as a prognostic biomarker correlates with EMT in GBM. BMC Cancer. 2024;24:89. doi: 10.1186/s12885-023-11796-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Q, Zhang Y, Liang H, Zhang F, Liu F, Chen S, Hu Y, Jiang L, Hao Y, Li M, Liu Y. EMP3 as a key downstream target of miR-663a regulation interferes with MAPK/ERK signaling pathway to inhibit gallbladder cancer progression. Cancer Lett. 2023;575:216398. doi: 10.1016/j.canlet.2023.216398. [DOI] [PubMed] [Google Scholar]
- 14.Kim EK, Koo JS. Expression of epithelial membrane protein (EMP) 1, EMP 2, and EMP 3 in thyroid cancer. Histol Histopathol. 2022;37:51–61. doi: 10.14670/HH-18-378. [DOI] [PubMed] [Google Scholar]
- 15.Zhou W, Jiang Z, Li X, Xu F, Liu Y, Wen P, Kong L, Hou M, Yu J. EMP3 overexpression in primary breast carcinomas is not associated with epigenetic aberrations. J Korean Med Sci. 2009;24:97–103. doi: 10.3346/jkms.2009.24.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evtimova V, Zeillinger R, Weidle UH. Identification of genes associated with the invasive status of human mammary carcinoma cell lines by transcriptional profiling. Tumour Biol. 2003;24:189–198. doi: 10.1159/000074429. [DOI] [PubMed] [Google Scholar]
- 17.Hong XC, Fen YJ, Yan GC, Hong H, Yan CH, Bing LW, Zhong YH. Epithelial membrane protein 3 functions as an oncogene and is regulated by microRNA-765 in primary breast carcinoma. Mol Med Rep. 2015;12:6445–6450. doi: 10.3892/mmr.2015.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blick T, Hugo H, Widodo E, Waltham M, Pinto C, Mani SA, Weinberg RA, Neve RM, Lenburg ME, Thompson EW. Epithelial mesenchymal transition traits in human breast cancer cell lines parallel the CD44(hi/)CD24 (lo/-) stem cell phenotype in human breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:235–252. doi: 10.1007/s10911-010-9175-z. [DOI] [PubMed] [Google Scholar]
- 19.Kohn KW, Zeeberg BM, Reinhold WC, Pommier Y. Gene expression correlations in human cancer cell lines define molecular interaction networks for epithelial phenotype. PLoS One. 2014;9:e99269. doi: 10.1371/journal.pone.0099269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isbilen M, Mert Senses K, Osmay Gure A. Predicting chemotherapy sensitivity profiles for breast cancer cell lines with and without stem cell-like features. Curr Signal Transduct Ther. 2013;8:268–273. doi: 10.2174/1574362409666140206222115. [DOI] [Google Scholar]
- 21.Zhou K, Sun Y, Dong D, Zhao C, Wang W. EMP3 negatively modulates breast cancer cell DNA replication, DNA damage repair, and stem-like properties. Cell Death Dis. 2021;12:844. doi: 10.1038/s41419-021-04140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackay A, Jones C, Dexter T, Silva RL, Bulmer K, Jones A, Simpson P, Harris RA, Jat PS, Neville AM, et al. cDNA microarray analysis of genes associated with ERBB2 (HER2/neu) overexpression in human mammary luminal epithelial cells. Oncogene. 2003;22:2680–2688. doi: 10.1038/sj.onc.1206349. [DOI] [PubMed] [Google Scholar]
- 23.Chakrabarty A, Bhola NE, Sutton C, Ghosh R, Kuba MG, Dave B, Chang JC, Arteaga CL. Trastuzumab-resistant cells rely on a HER2-PI3K-FoxO-survivin axis and are sensitive to PI3K inhibitors. Cancer Res. 2013;73:1190–1200. doi: 10.1158/0008-5472.CAN-12-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang YW, Li WM, Wu WJ, Chai CY, Liu HS, Lai MD, Chow NH. Potential significance of EMP3 in patients with upper urinary tract urothelial carcinoma: Crosstalk with ErbB2-PI3K-Akt pathway. J Urol. 2014;192:242–251. doi: 10.1016/j.juro.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Cha YJ, Koo JS. Expression and role of epithelial membrane proteins in tumorigenesis of hormone receptor-positive breast cancer. J Breast Cancer. 2020;23:385–397. doi: 10.4048/jbc.2020.23.e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Animal Protection Act. Ministry of Agriculture, ROC (Taiwan), corp-author https://law.moj.gov.tw/ENG/LawClass/LawAll.aspx?pcode=M0060027 2021 [Google Scholar]
- 27.Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline focused update. Arch Pathol Lab Med. 2018;142:1364–1382. doi: 10.5858/arpa.2018-0902-SA. [DOI] [PubMed] [Google Scholar]
- 28.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C, Sjöstedt E, Asplund A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 29.Gemmete JJ, Mukherji SK. Trastuzumab (herceptin) AJNR Am J Neuroradiol. 2011;32:1373–1374. doi: 10.3174/ajnr.A2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsieh YH, Hsieh SC, Lee CH, Yang SF, Cheng CW, Tang MJ, Lin CL, Lin CL, Chou RH. Targeting EMP3 suppresses proliferation and invasion of hepatocellular carcinoma cells through inactivation of PI3K/Akt pathway. Oncotarget. 2015;6:34859–34874. doi: 10.18632/oncotarget.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christians A, Poisel E, Hartmann C, von Deimling A, Pusch S. Characterization of the epithelial membrane protein 3 interaction network reveals a potential functional link to mitogenic signal transduction regulation. Int J Cancer. 2019;145:461–473. doi: 10.1002/ijc.32107. [DOI] [PubMed] [Google Scholar]
- 32.Kar R, Jha NK, Jha SK, Sharma A, Dholpuria S, Asthana N, Chaurasiya K, Singh VK, Burgee S, Nand P. A ‘NOTCH’ deeper into the epithelial-to-mesenchymal transition (EMT) program in breast cancer. Genes (Basel) 2019;10:961. doi: 10.3390/genes10120961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee KL, Kuo YC, Ho YS, Huang YH. Triple-negative breast cancer: Current understanding and future therapeutic breakthrough targeting cancer stemness. Cancers (Basel) 2019;11:1334. doi: 10.3390/cancers11091334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Derakhshani A, Rezaei Z, Safarpour H, Sabri M, Mir A, Sanati MA, Vahidian F, Gholamiyan Moghadam A, Aghadoukht A, Hajiasgharzadeh K, Baradaran B. Overcoming trastuzumab resistance in HER2-positive breast cancer using combination therapy. J Cell Physiol. 2020;235:3142–3156. doi: 10.1002/jcp.29216. [DOI] [PubMed] [Google Scholar]
- 35.Martija AA, Pusch S. The multifunctional role of EMP3 in the regulation of membrane receptors associated with IDH-wild-type glioblastoma. Int J Mol Sci. 2021;22:5261. doi: 10.3390/ijms22105261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martija AA, Krauß A, Bächle N, Doth L, Christians A, Krunic D, Schneider M, Helm D, Will R, Hartmann C, et al. EMP3 sustains oncogenic EGFR/CDK2 signaling by restricting receptor degradation in glioblastoma. Acta Neuropathol Commun. 2023;11:177. doi: 10.1186/s40478-023-01673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumagai S, Koyama S, Nishikawa H. Antitumour immunity regulated by aberrant ERBB family signalling. Nat Rev Cancer. 2021;21:181–197. doi: 10.1038/s41568-020-00322-0. [DOI] [PubMed] [Google Scholar]
- 38.Kusumoto Y, Okuyama H, Shibata T, Konno K, Takemoto Y, Maekawa D, Kononaga T, Ishii T, Akashi-Takamura S, Saitoh SI, et al. Epithelial membrane protein 3 (Emp3) downregulates induction and function of cytotoxic T lymphocytes by macrophages via TNF-α production. Cell Immunol. 2018;324:33–41. doi: 10.1016/j.cellimm.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Sun M, Jiang H, Liu T, Tan X, Jiang Q, Sun B, Zheng Y, Wang G, Wang Y, Cheng M, et al. Structurally defined tandem-responsive nanoassemblies composed of dipeptide-based photosensitive derivatives and hypoxia-activated camptothecin prodrugs against primary and metastatic breast tumors. Acta Pharm Sin B. 2022;12:952–966. doi: 10.1016/j.apsb.2021.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng R, Li Y, Li Y, Wan Q, Huang Z, Qiu Z, Tang D. Smartphone-based photoelectrochemical immunoassay with Co9S8@ZnIn2S4 for point-of-care diagnosis of breast cancer biomarker. Research (Wash D C) 2022;2022:9831521. doi: 10.34133/2022/9831521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen H, Li H, Shi W, Qin H, Zheng L. The roles of m6A RNA methylation modification in cancer stem cells: New opportunities for cancer suppression. Cancer Insight. 2022;1:1–18. [Google Scholar]
- 42.Yin Y, Yan Y, Fan B, Huang W, Zhang J, Hu HY, Li X, Xiong D, Chou SL, Xiao Y, Wang H. Novel combination therapy for triple-negative breast cancer based on an intelligent hollow carbon sphere. Research (Wash DC) 2023;6:0098. doi: 10.34133/research.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ji X, Tian X, Feng S, Zhang L, Wang J, Guo R, Zhu Y, Yu X, Zhang Y, Du H, et al. Intermittent F-actin perturbations by magnetic fields inhibit breast cancer metastasis. Research (Wash DC) 2023;6:0080. doi: 10.34133/research.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng C, Zhang D, Kong Y, Niu M, Zhao H, Song Q, Feng Q, Li X, Wang L. Dynamic regulation of drug biodistribution by turning tumors into decoys for biomimetic nanoplatform to enhance the chemotherapeutic efficacy of breast cancer with bone metastasis. Exploration (Beijing) 2023;3:20220124. doi: 10.1002/EXP.20220124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheng H, Ballman K, Vassilakopoulou M, Dueck AC, Reinholz MM, Tenner K, Gralow J, Hudis C, Davidson NE, Fountzilas G, et al. EGFR expression is associated with decreased benefit from trastuzumab in the NCCTG N9831 (alliance) trial. Br J Cancer. 2014;111:1065–1071. doi: 10.1038/bjc.2014.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang L, Li Y, Shen E, Cao F, Li L, Li X, Wang X, Kariminia S, Chang B, Li H, Li Q. NRG1-dependent activation of HER3 induces primary resistance to trastuzumab in HER2-overexpressing breast cancer cells. Int J Oncol. 2017;51:1553–1562. doi: 10.3892/ijo.2017.4130. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe S, Yonesaka K, Tanizaki J, Nonagase Y, Takegawa N, Haratani K, Kawakami H, Hayashi H, Takeda M, Tsurutani J, Nakagawa K. Targeting of the HER2/HER3 signaling axis overcomes ligand-mediated resistance to trastuzumab in HER2-positive breast cancer. Cancer Med. 2019;8:1258–1268. doi: 10.1002/cam4.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogden A, Bhattarai S, Sahoo B, Mongan NP, Alsaleem M, Green AR, Aleskandarany M, Ellis IO, Pattni S, Li XB, et al. Combined HER3-EGFR score in triple-negative breast cancer provides prognostic and predictive significance superior to individual biomarkers. Sci Rep. 2020;10:3009. doi: 10.1038/s41598-020-59514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu B, Ordonez-Ercan D, Fan Z, Edgerton SM, Yang X, Thor AD. Downregulation of erbB3 abrogates erbB2-mediated tamoxifen resistance in breast cancer cells. Int J Cancer. 2007;120:1874–1882. doi: 10.1002/ijc.22423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.