Abstract
Background
Liver cirrhosis is not included in surgical risk prediction models despite being a significant risk factor associated with high periprocedural morbidity and mortality in patients undergoing cardiac surgery. Limited contemporary data exists assessing the outcomes of transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) in patients with cirrhosis.
Methods
Patients with cirrhosis who underwent TAVR or SAVR were identified from the Nationwide Readmissions Database. Propensity-score matched analysis was performed to compare the clinical characteristics, in-hospital, and 30-day outcomes between the two groups.
Results
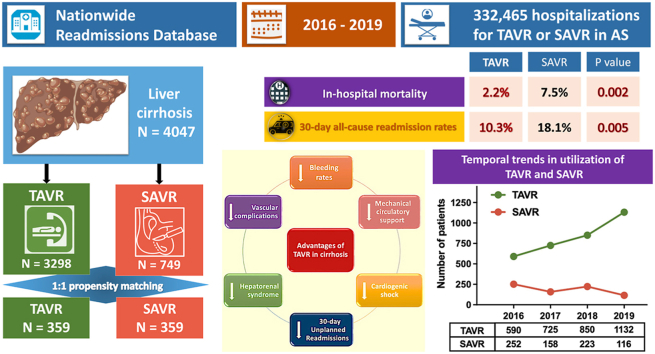
Between 2016 and 2019, 4047 patients with cirrhosis underwent TAVR (n = 3298) or SAVR (n = 749). TAVR adoption sharply rose, doubling the number of cases performed during the study period. Following propensity matching among 718 patients, the TAVR group consistently exhibited significantly lower rates of in-hospital mortality (2.2 vs. 7.5%; p = 0.002), bleeding (14.5 vs. 52.9%; p < 0.001), vascular complications (1.4 vs. 5%; p = 0.011), hepatorenal syndrome (3.3 vs. 8.9%; p = 0.003), cardiogenic shock (2.8 vs. 7%; p = 0.015), mechanical circulatory support utilization (0.6 vs. 4.7%; p = 0.001), 30-day all-cause readmission rates (10.3 vs. 18.1%; p = 0.005), and 30-day unplanned readmission rates (10 vs. 16.6%; p = 0.015) compared to the SAVR group. The TAVR group had significantly shorter median hospital stays, lower non-home disposition rates, and reduced hospital costs.
Conclusions
TAVR is associated with significantly lower rates of in-hospital mortality, bleeding, vascular complications, hepatorenal syndrome, cardiogenic shock, mechanical circulatory support utilization, and 30-day readmission rates compared to SAVR and represents a safe therapeutic option for aortic valve replacement in patients with cirrhosis.
Keywords: Aortic stenosis, Cirrhosis, Liver disease, Readmissions, Structural heart interventions, Valvular heart diseases
Graphical abstract
Highlights
-
•
Transcatheter aortic valve replacement (TAVR) usage surged twofold among cirrhotic patients from 2016 to 2019, despite their advanced age and increased comorbidities compared to surgical aortic valve replacement (SAVR) recipients.
-
•
TAVR as a strategy for aortic valve replacement in patients with cirrhosis is a viable and safe therapeutic option.
-
•
In-hospital mortality, 30-day all-cause, and unplanned readmission rates are significantly lower with TAVR compared to SAVR.
-
•
TAVR presents clear advantages over SAVR with reduced rates of bleeding, vascular complications, and hepatorenal syndrome.
-
•
Notably, TAVR is associated with diminished rates of cardiogenic shock and decreased utilization of mechanical circulatory support compared to SAVR.
-
•
Cirrhosis should not preclude access to TAVR.
Introduction
Liver cirrhosis is a significant medical condition that can heighten the susceptibility of patients to surgical complications.1 The pathophysiological alterations secondary to liver cirrhosis result in coagulation abnormalities and multi-organ dysfunction causing an increased risk of bleeding, infection, organ failure, and mortality during and after surgery.2 Such patients may experience an increased incidence of anesthesia-related complications, such as respiratory or cardiovascular morbidity. Although liver cirrhosis is widely regarded as a high-risk condition for open-heart surgery, including surgical aortic valve replacement (SAVR), it is not currently incorporated as a prognostic factor in the most commonly used surgical risk scoring systems, and the current preoperative risk stratification remains imprecise.3, 4, 5
Transcatheter aortic valve replacement (TAVR) has emerged as the standard of care for the treatment of symptomatic aortic stenosis (AS) in patients who are deemed high-risk for surgery. The advent and widespread utilization of TAVR have enabled a broader population of patients with severe AS to access definitive treatment. The comparison of TAVR and SAVR in patients with cirrhosis is inadequately studied due to their exclusion from prominent clinical trials and registries.6, 7, 8, 9, 10, 11, 12 Although administrative and national databases have been employed to assess the associated risks of cirrhosis, these studies have been limited by small sample sizes, precluding definitive conclusions.13, 14, 15 Here, we utilized the Nationwide Readmissions Database (NRD) to compare mortality, in-hospital outcomes, hospitalization costs, and 30-day readmission rates in patients with cirrhosis undergoing TAVR and isolated SAVR. Additionally, we aimed to compare these outcomes with propensity score pair-matched control groups between TAVR and SAVR.
Methods
Data Source
We examined the NRD for all hospital discharges in the United States between January 2016 and November 2019. The NRD data elements include hospital characteristics, patient demographics, chronic comorbidities, procedures, primary and secondary discharge diagnoses, length of stay, and payment source. The NRD is part of the Healthcare Cost and Utilization Project created by the Agency for Healthcare Research and Quality.16 The 2016 to 2019 NRD includes hospital discharge data from 30 states and accounts for 56.6% to 60.4% of all hospitalizations in the United States. Each patient record contains de-identified data on diagnoses and procedures performed during the hospitalization, which are based on the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) and Procedure Coding System (ICD-10-PCS) using validated codes as outlined in the summary statistics of the NRD. This study was exempt from the institutional review board and informed consent was not required for the current study as the NRD is a publicly available database with de-identified patient information.
Study Design and Population
Hospitalizations between January 2016 and November 2019 of patients with a discharge diagnosis of AS (ICD-10-CM code: “I35.0”) and liver cirrhosis (diagnosis of cirrhosis or complications of cirrhosis) (ICD-10-CM codes: "K70.30," "K70.31," "K74.xx,” "I85.01," "I85.00," "I85.10," "I85.11," "K65.2," "K70.30," "K76.7," "K72.90," "K72.91") were identified. Patients who underwent TAVR (ICD-10-PCS codes: “02RF37H," "02RF37Z," "02RF38H," "02RF38Z," "02RF3JH," "02RF3JZ," "02RF3KH," "02RF3KZ”) or SAVR (ICD-10-PCS codes: "02RF07Z," "02RF08Z," "02RF0JZ," "02RF0KZ") during the index hospitalization were included for comparative analysis. Other relevant ICD-10 codes used in the study are summarized in Supplementary Table 1. Baseline patient comorbidities and procedural characteristics were described. To identify the cases of isolated aortic valve replacement (AVR), we excluded hospitalizations with concomitant repair or replacement of other valves, coronary artery bypass grafting, infundibulectomy, creation of septal defect, endocardial cushion defect repair, ventricular or atrial repair, congenital heart defect repair, transmyocardial revascularization, ventricular restoration procedures, heart transplantation, previous valve surgeries, previous surgeries to heart and great vessels, and previous heart transplantation. We also excluded hospitalizations with a diagnosis of aortic regurgitation without a diagnosis of AS. To ensure a consistent follow-up period, we included only patients who had at least 30 days of postdischarge monitoring. Using verified patient linkages, we then tracked all readmissions within 30 days of hospital discharge for any reason.
Study Outcomes
The primary study outcomes were in-hospital mortality and 30-day all-cause readmission rates. The secondary outcomes included vascular complications, pacemaker implantation, bleeding rates, renal replacement therapy (RRT), hepatorenal syndrome, acute ischemic stroke, 30-day readmission-related mortality rates, 30-day unplanned readmissions, 30-day readmissions for heart failure, 30-day readmissions for bleeding, median length of stay, and hospital charges.
Statistical Analysis
All statistical analyses were performed using R software version 4.3.2 (www.R-project.org, assessed on October 31, 2023). All analyses used a survey methodology, specifically an R package “survey,” for the design of stratified, cluster sampling of NRD. Discharge weights and strata provided by NRD were used for all analyses; thus, all reported numbers are weighted U.S. national estimates. We compared baseline patient and hospital-level characteristics for patients who underwent TAVR vs. SAVR for management of AS in patients with liver cirrhosis. Quantitative variables are presented as mean ± standard error or median and interquartile range using survey-specific linear regression test or Mann-Whitney-Wilcoxon test. Categorical variables are provided as absolute numbers and corresponding percentages. Chi-square test or Fisher’s exact, as appropriate, were used for categorical variables. We conducted a propensity score-matched analysis comparing patients who underwent TAVR with those who underwent SAVR. The propensity score was estimated using a logistic regression model. The dependent variable was the treatment group (TAVR or SAVR), and independent variables were covariates including demographic, clinical, and hospital characteristics considered to be clinically relevant. The final covariables used for propensity matching were: age group, sex, congestive heart failure, smoking, hypertension, diabetes, dyslipidemia, family history of coronary artery disease, coronary artery disease, peripheral vascular disease, chronic pulmonary disease, chronic kidney disease, anemia, atrial fibrillation, mitral stenosis, coagulopathy, obesity, hypothyroidism, dementia, depression, cancer, household incomes, medical insurance, weekend admission, teaching/nonteaching status, urban/rural, hospital bed size, fiscal year, and discharge weight. A propensity score-matched cohort was obtained with a 1:1 ratio of TAVR and SAVR patients using a nearest neighbor match without replacement and a caliper width of 0.2 of the standard deviation of the logistic score. The balance of the matching was evaluated using graphical density plots of the logistic score and love plot. Comparison of continuous and categorical variables were performed with the same methods as the unmatched cohort with no weighting. In addition to the propensity score matching analysis, we also performed multivariable generalized linear regression analyses for primary and secondary outcomes in the unmatched cohort to adjust for potential confounders and to estimate the adjusted odds ratio and 95% CIs for the evaluation of primary and secondary outcomes. Covariates with p < 0.1 in univariable analyses were initially chosen for multivariable analyses. Final parsimonious models were created by backward removal of each covariate based on the Akaike information criterion while ensuring each removal did not result in >10% change in the measure of association for the primary predictor variable. The estimated costs for each hospitalization were calculated by using the cost-to-charge ratio validated by Healthcare Cost and Utilization Project. Cumulative costs or charges were defined as the cost or charge of index hospitalization plus cost or charge of readmission in 30 days. A two-sided p value <0.05 was considered statistically significant.
Results
Baseline Characteristics of Unmatched and Propensity-Matched Cohort
During the study period, a total of 332,465 eligible hospitalizations among patients aged 18 years or older undergoing TAVR or SAVR for AS were considered. Among these, 4874 unique hospitalizations were identified for patients with liver cirrhosis. After excluding hospitalizations involving both TAVR and SAVR, cases related to aortic insufficiency, and other cardiac surgeries, the final analysis included a total of 4047 hospitalizations: 3298 in the TAVR group and 749 in the SAVR group (Supplementary Figure 1). The mean age of the study population was 70.8 years (standard error 0.2), with 60.3% being men. Table 1 summarizes the baseline patient-level characteristics of the study population. While gender distribution was similar, patients who underwent TAVR were significantly older and had a higher number of comorbid conditions compared to those who underwent SAVR. A substantial proportion of both TAVR and SAVR procedures were performed at teaching institutions (91.6% for TAVR and 81.4% for SAVR), which were more commonly located in urban locations (65.6% for TAVR and 55.9% for SAVR). The propensity score-matched analysis yielded a total of 718 patients (359 in each group) with well-matched baseline demographic and clinical characteristics, as indicated by standardized mean differences of less than 10%. Table 1 provides an overview of the patient-level characteristics used for propensity score matching in the TAVR and SAVR groups.
Table 1.
Baseline demographics, comorbidities, and hospital characteristics among patients with cirrhosis who underwent TAVR or SAVR for unmatched and propensity-matched cohorts
| Characteristics | Baseline unmatched cohort |
Propensity-matched cohort |
SMD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | TAVR | SAVR | p value | Overall | TAVR | SAVR | p value | ||
| Number of patients, n (%)∗ | 4047 | 3298 (81.5%) | 749 (18.5) | 718 | 359 (50%) | 359 (50%) | |||
| Patient characteristics | |||||||||
| Age, mean (SE/SD)†, y | 70.8 (0.2) | 72.2 (0.2) | 65.0 (0.5) | <0.001‡ | 66.7 (8.3) | 67.3 (7.8) | 66.2 (8.8) | 0.079 | |
| Age group, y | <0.001§ | 0.344 | |||||||
| <50 y | 53 (1.3) | 17 (0.5) | 36 (4.8) | 18 (2.5) | 7 (1.9) | 11 (3.1) | 0.054 | ||
| 50-59 y | 359 (8.9) | 217 (6.6) | 142 (19.0) | 103 (14.3) | 48 (13.4) | 55 (15.3) | 0.049 | ||
| 60-69 y | 1377 (34.0) | 1044 (31.7) | 333 (44.5) | 331 (46.1) | 167 (46.5) | 164 (45.7) | 0.016 | ||
| 70-79 y | 1502 (37.1) | 1296 (39.3) | 206 (27.5) | 234 (32.6) | 125 (34.8) | 109 (30.4) | 0.100 | ||
| ≥80 y | 756 (18.7) | 724 (22.0) | 32 (4.3) | 32 (4.5) | 12 (3.3) | 20 (5.6) | 0.103 | ||
| Gender | 0.375 | 0.699 | |||||||
| Male | 2440 (60.3) | 1971 (59.8) | 469 (62.6) | 452 (63.0) | 223 (62.1) | 229 (63.8) | 0.035 | ||
| Female | 1607 (39.7) | 1327 (40.2) | 280 (37.4) | 266 (37.0) | 136 (37.9) | 130 (36.2) | 0.035 | ||
| Comorbidities | |||||||||
| Hypertension | 1442 (35.6) | 1066 (32.3) | 376 (50.2) | <0.001 | 340 (47.4) | 171 (47.6) | 169 (47.1) | 0.940 | 0.011 |
| Diabetes mellitus | 2077 (51.3) | 1763 (53.5) | 314 (41.9) | <0.001 | 309 (43.0) | 157 (43.7) | 152 (42.3) | 0.763 | 0.028 |
| Dyslipidemia | 2008 (49.6) | 1677 (50.9) | 331 (44.2) | 0.028 | 311 (43.3) | 154 (42.9) | 157 (43.7) | 0.880 | 0.017 |
| Obesity | 1094 (27.0) | 813 (24.7) | 281 (37.5) | <0.001 | 232 (32.3) | 112 (31.2) | 120 (33.4) | 0.576 | 0.047 |
| Smoking | 1472 (36.4) | 1239 (37.6) | 233 (31.1) | 0.022 | 243 (33.8) | 121 (33.7) | 122 (34.0) | 1.000 | 0.006 |
| Coronary artery disease | 2083 (51.5) | 1819 (55.2) | 264 (35.2) | <0.001 | 275 (38.3) | 144 (40.1) | 131 (36.5) | 0.357 | 0.076 |
| Congestive heart failure | 341 (8.4) | 276 (8.4) | 65 (8.7) | 0.849 | 66 (9.2) | 32 (8.9) | 34 (9.5) | 0.897 | 0.019 |
| Atrial fibrillation | 1362 (33.7) | 1004 (30.5) | 358 (47.8) | <0.001 | 332 (46.2) | 163 (45.4) | 169 (47.1) | 0.708 | 0.034 |
| Chronic kidney disease | 1353 (33.4) | 1181 (35.8) | 172 (23.0) | <0.001 | 158 (22.0) | 79 (22.0) | 79 (22.0) | 1.000 | <0.001 |
| Peripheral vascular disease | 836 (20.7) | 691 (21.0) | 145 (19.4) | 0.491 | 148 (20.6) | 78 (21.7) | 70 (19.5) | 0.518 | 0.056 |
| Family history of CAD | 311 (7.7) | 248 (7.5) | 63 (8.4) | 0.640 | 55 (7.7) | 27 (7.5) | 28 (7.8) | 1.000 | 0.011 |
| Chronic pulmonary disease | 1258 (31.1) | 1040 (31.5) | 218 (29.1) | 0.404 | 198 (27.6) | 103 (28.7) | 95 (26.5) | 0.559 | 0.050 |
| Anemia | 1138 (28.1) | 977 (29.6) | 161 (21.5) | 0.003 | 172 (24.0) | 86 (24.0) | 86 (24.0) | 1.000 | <0.001 |
| Coagulopathy | 1982 (49.0) | 1545 (46.8) | 437 (58.4) | <0.001 | 399 (55.6) | 197 (54.9) | 202 (56.3) | 0.764 | 0.028 |
| Hypothyroidism | 773 (19.1) | 656 (19.9) | 117 (15.6) | 0.081 | 100 (13.9) | 45 (12.5) | 55 (15.3) | 0.332 | 0.077 |
| Cancer | 271 (6.7) | 240 (7.3) | 31 (4.1) | 0.056 | 25 (3.5) | 10 (2.8) | 15 (4.2) | 0.416 | 0.072 |
| Household income quartile | <0.001 | 0.734 | |||||||
| First quartile | 943 (23.3) | 691 (21.0) | 252 (33.7) | 214 (29.8) | 104 (29.0) | 110 (30.6) | 0.036 | ||
| Second quartile | 1139 (28.2) | 933 (28.3) | 206 (27.5) | 203 (28.3) | 108 (30.1) | 95 (26.5) | 0.082 | ||
| Third quartile | 1106 (27.3) | 938 (28.4) | 168 (22.5) | 169 (23.5) | 84 (23.4) | 85 (23.7) | 0.007 | ||
| Fourth quartile | 858 (21.2) | 736 (22.3) | 122 (16.3) | 132 (18.4) | 63 (17.5) | 69 (19.2) | 0.043 | ||
| Primary payer | <0.001 | 0.451 | |||||||
| Medicare | 3063 (75.7) | 2641 (80.1) | 422 (56.3) | 451 (62.8) | 234 (65.2) | 217 (60.4) | 0.096 | ||
| Medicaid | 222 (5.5) | 120 (3.6) | 102 (13.6) | 76 (10.6) | 35 (9.7) | 41 (11.4) | 0.048 | ||
| Private including HMO | 587 (14.5) | 385 (11.7) | 202 (27.0) | 162 (22.6) | 74 (20.6) | 88 (24.5) | 0.089 | ||
| Self-pay/no charge/other | 174 (4.3) | 151 (4.6) | 23 (3.1) | 29 (4.0) | 16 (4.5) | 13 (3.6) | 0.048 | ||
| Weekend admission | 179 (4.4) | 133 (4.0) | 46 (6.1) | 0.065 | 46 (6.4) | 25 (7.0) | 21 (5.8) | 0.648 | 0.046 |
| Hospital characteristics | |||||||||
| Hospital teaching status | <0.001 | 0.431 | |||||||
| Teaching | 3631 (89.7) | 3021 (91.6) | 610 (81.4) | 593 (82.6) | 292 (81.3) | 301 (83.8) | 0.064 | ||
| Nonteaching | 416 (10.3) | 277 (8.4) | 139 (18.6) | 125 (17.4) | 67 (18.7) | 58 (16.2) | |||
| Hospital location | 0.002 | 1.000 | 0.064 | ||||||
| Rural | 1465 (36.2) | 1135 (34.4) | 330 (44.1) | 285 (39.7) | 143 (39.8) | 142 (39.6) | 0.006 | ||
| Urban | 2581 (63.8) | 2162 (65.6) | 419 (55.9) | 433 (60.3) | 216 (60.2) | 217 (60.4) | 0.006 | ||
| Hospital bed size | 0.010 | 0.986 | |||||||
| Small | 169 (4.2) | 117 (3.5) | 52 (6.9) | 46 (6.4) | 23 (6.4) | 23 (6.4) | <0.001 | ||
| Medium | 923 (22.8) | 728 (22.1) | 195 (26.0) | 194 (27.0) | 98 (27.3) | 96 (26.7) | 0.013 | ||
| Large | 2955 (73.0) | 2453 (74.4) | 502 (67.0) | 478 (66.6) | 238 (66.3) | 240 (66.9) | 0.012 | ||
Abbreviations: CAD, coronary artery disease; HMO, health maintenance organization; SAVR, surgical aortic valve replacement; SD, standard deviation; SE, standard error; SMD, standardized mean difference; TAVR, transcatheter aortic valve replacement.
Values are presented as the number (percentage) of patients, unless otherwise indicated.
Age presented as mean ± standard error for baseline cohort and as mean ± standard deviation for the propensity matched cohort.
Survey-specific linear regression was performed.
Rao-Scott χ2 test or Fisher exact test were used for all statistical tests unless stated otherwise.
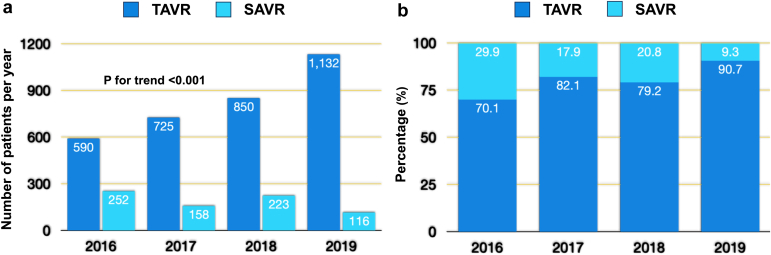
From 2016 to 2019, there was a temporal trend of a steady increase in the utilization of TAVR in this patient population (p for trends <0.001) (Figure 1). Specifically, the proportion of patients with cirrhosis undergoing TAVR consistently rose each year, from approximately 70% in 2016 to over 90% in 2019.
Figure 1.
Temporal trends in annual utilization of TAVR and SAVR among patients with cirrhosis in the United States, 2016 to 2019.
Abbreviations: SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
In-Hospital and 30-Day Postdischarge Outcomes
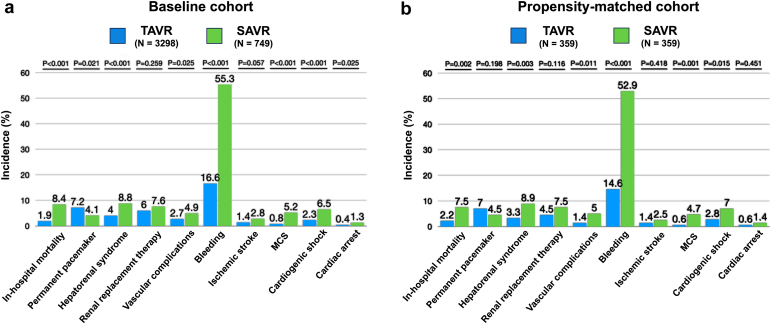
Figure 2 and Supplementary Table 2 summarize the in-hospital outcomes for both the baseline unmatched and propensity-score matched populations. After propensity matching, the TAVR group exhibited a significantly lower in-hospital mortality rate compared to the SAVR group (2.2 vs. 7.5%, p = 0.002). Additionally, the TAVR group demonstrated significantly lower rates of bleeding (14.6 vs. 52.9%; p < 0.001), vascular complications (1.4 vs. 5%; p = 0.011), and hepatorenal syndrome (3.3 vs. 8.9%; p = 0.003) in comparison to the SAVR group. Remarkably, the TAVR group was associated with significantly lower rates of cardiogenic shock (2.8 vs. 7.0%; p = 0.015), need for mechanical ventilation (4.5 vs. 14.5%; p < 0.001), and utilization rates of mechanical circulatory support (MCS) (0.6 vs. 4.7%; p = 0.001) when compared to the SAVR group. No statistically significant differences were observed in the rates of permanent pacemaker implantation (7.0 vs. 4.5%; p = 0.198), acute kidney injury requiring RRT (4.5 vs. 7.5%; p = 0.116), cardiac arrest (0.6 vs. 1.4%; p = 0.451), and acute ischemic stroke (1.4 vs. 2.5%; p = 0.418) between the two interventions. Furthermore, patients undergoing TAVR had a higher likelihood of being discharged home (66.1 vs. 30.1%; p < 0.001), experienced a shorter hospital stay (3 vs. 9 days; p < 0.001), and incurred lower overall hospital charges and hospital costs compared to those undergoing SAVR.
Figure 2.
In-hospital outcomes of patients with cirrhosis undergoing TAVR or SAVR from 2016 to 2019. (a) Baseline unmatched cohort. (b) Propensity-matched cohort.
Abbreviations: MCS, mechanical circulatory support; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
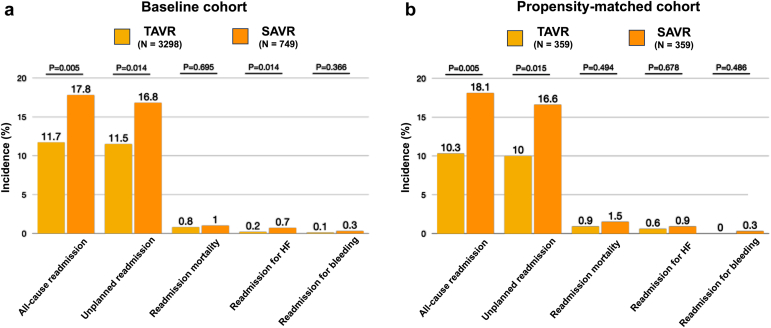
The 30-day postdischarge outcomes for both the baseline unmatched and propensity score-matched populations are summarized in Figure 3 and Supplementary Table 3. Notably, following propensity matching, patients who underwent TAVR demonstrated significantly lower all-cause readmission rates compared to those undergoing SAVR (10.3 vs. 18.1%; p = 0.005). Correspondingly, TAVR patients also showed lower rates of 30-day unplanned readmissions (10 vs. 16.6%; p = 0.015). No statistically significant differences were observed between the two groups concerning 30-day readmission mortality rates (0.9 vs. 1.5%; p = 0.494), readmissions for heart failure (0.6 vs. 0.9%; p = 0.678), and readmissions for bleeding (0 vs. 0.3%; p = 0.486).
Figure 3.
Thirty-day outcomes (excluding in-hospital deaths) of patients with cirrhosis undergoing TAVR or SAVR from 2016 to 2019. (a) Baseline unmatched cohort. (b) Propensity-matched cohort.
Abbreviations: HF, heart failure; SAVR, surgical aortic valve replacement; TAVR, transcatheter aortic valve replacement.
In the baseline unmatched cohort, a multivariable regression analysis was also conducted to assess primary and secondary outcomes (Supplementary Table 4). Following multivariable adjustment, the SAVR group continued to exhibit elevated adjusted rates of in-hospital mortality, bleeding, vascular complications, hepatorenal syndrome, MCS utilization, and cardiogenic shock in comparison to the TAVR group within the overall population. No significant associations were observed with other variables examined in the study.
Discussion
In this large national database observational analysis including more than 4000 contemporaneous hospitalizations in patients with AS and a concomitant diagnosis of liver cirrhosis, we compared the in-hospital and 30-day outcomes of TAVR with SAVR. Our study conducted the most extensive propensity score-matched analysis to date, comparing outcomes in a population of more than 700 hospitalizations. In the propensity score-matched cohorts, we found significantly lower rates of in-hospital mortality, vascular complications, bleeding rates, rates of hepatorenal syndrome, and median length of hospital stay in patients receiving TAVR compared to patients undergoing SAVR. The results assume heightened significance due to the younger age of the propensity-matched cirrhotic patients, noted to be a decade younger from the conventional noncirrhotic cohort undergoing AVR within the entire database, while demonstrating comparable in-hospital outcome rates observed in noncirrhotic patients. Given the periprocedural risk difference between TAVR and SAVR that may narrow beyond the in-hospital period,17 outcomes beyond this period help provide insights into the trends in the relative safety of the complementary approaches. This study also revealed a significant reduction in 30-day all-cause readmission rates and unplanned readmission rates among patients undergoing TAVR compared to those who underwent SAVR (Figure 4). We found no significant difference in the rates of in-hospital acute kidney injury requiring RRT, pacemaker implantation rates, and acute ischemic stroke between the two interventions. We also did not find any significant difference in 30-day rates of readmission mortality and readmissions for heart failure and bleeding suggesting a good long-term prognosis if patients survive the initial postprocedural hospital stay. The propensity score matching approach effectively addressed confounding variables, enhancing the reliability of the study results and enabling a robust comparison of subgroups with comparable comorbidities and demographics.
Our study also demonstrated a consistent rise in the total number of AVR procedures performed in patients with underlying cirrhosis throughout the duration of the study, despite the heightened risks associated with this comorbidity. Furthermore, we observed a progressive upward trend in the utilization of TAVR among patients with cirrhosis. Notably, within this high-risk patient subgroup, the population of cirrhosis patients undergoing TAVR experienced more than twofold increase in absolute numbers from 2016 to 2019, despite the fact that these patients had advanced age and more significant comorbidities at baseline compared to patients undergoing SAVR. Studies highlighting temporal trends have consistently shown significant expansion in utilization of TAVR across all age groups,18,19 especially since the rapid expansion of the indications for TAVR to patients with intermediate-risk and low-risk populations by the Food and Drug Administration.8,20 The temporal scope of our study encompassed the years 2016 through 2019, during which the majority of non-high-risk patients underwent the procedures under investigation. Therefore, our findings are reflective of the current state of clinical practice with respect to cirrhotic patients and provide insight into contemporary procedural outcomes accounting for recent advances in TAVR procedures.21
Although TAVR was initially proposed for high-risk and nonoperable patients, current research has notably overlooked individuals with liver cirrhosis.7,8,17 Furthermore, evidence from multiple studies has consistently demonstrated worse clinical outcomes in cirrhotic patients undergoing cardiac surgery compared to those predicted by conventional risk scoring systems, resulting in higher in-hospital mortality, increased incidence of major complications, and prolonged hospital stays.22, 23, 24 The reported 30-day mortality rate among cirrhotic patients undergoing cardiac surgery with cardiopulmonary bypass or extracorporeal perfusion ranges from 10% to 50%.25, 26, 27 In contrast, our study revealed lower overall in-hospital mortality rates (<10%) and 30-day readmission mortality rates (1.5%) in the surgical group, indicating advancements in managing this high-risk patient subgroup and leading to improved outcomes. The inherent difficulty in executing randomized trials in patients with cirrhosis underscores a substantial challenge in research endeavors.28,29 Therefore, observational studies, like the present one, assume a crucial role in providing valuable insights that can guide informed treatment recommendations for AS patients with cirrhosis, facilitating patient-centered heart team discussions regarding benefits and risks.
Limited comparative data exists regarding the outcomes of TAVR vs. SAVR in patients suffering from chronic liver disease. Initial small single-center series in 18 cirrhotic patients suggested excellent short-term in-hospital outcomes in the TAVR group.30 Other small case studies reported feasibility and safety of TAVR in low- to moderate-risk patients with chronic liver disease, along with favorable outcomes and high procedural success rates with low rates of complications and mortality compared to SAVR.31,32 A recent study including all-comer cirrhosis patients aged over 65 found significantly lower in-hospital mortality rates in TAVR compared to SAVR patients.13 A 2:1 propensity analysis conducted on patients with end-stage liver disease revealed a 3.5-fold higher in-hospital mortality rates among the SAVR subgroup.33 Conversely, two separate propensity-matched analyses found no significant difference among the in-hospital mortality rates between the two groups.14,15 One study revealed no significant disparities in 30-day readmission rates between the two groups in a nonpropensity-matched analysis. It is important to note that the aforementioned studies included patients from 2005 to 2015, when older generations of valves were used, and they share common limitations of small sample sizes and inclusion of patients who underwent TAVR during the early stages of its commercialization. Our study overcomes these limitations by representing the largest and most contemporary analysis of this specific patient population. It incorporates more recent data from 2016 to 2019, a larger sample size of more than 4000 patients, and includes both in-hospital and 30-day outcomes, enhancing the robustness of the study findings.
Importantly, our analysis revealed a higher requirement for RRT in the overall propensity cohort group, with numerically higher rates observed in the SAVR group compared to the TAVR group. Most TAVR studies consistently report dialysis rates after the procedure to be below 5%.34,35 Additionally, our study demonstrated higher rates of hepatorenal syndrome in the SAVR group compared to the TAVR group, even after propensity matching. Through an additional analysis, we observed RRT rates of 2.2% in TAVR patients and 3.2% in SAVR patients without cirrhosis. This observation suggests that the increased dialysis rates seen in patients with cirrhosis may be attributed to an underlying hepatorenal syndrome. Given that end-stage liver disease renders individuals more susceptible to hepatorenal syndrome, the less invasive nature of TAVR, coupled with the increasing use of local anesthesia and conscious sedation, effectively obviates the requirement for cardiopulmonary bypass. Consequently, TAVR may help mitigate the risk of acute kidney injury and potentially lead to improved outcomes in this high-risk patient population. Additionally, we showed a significantly greater proportion of patients undergoing TAVR were discharged home compared to those undergoing SAVR. Notably, it is widely recognized that patients who cannot be discharged home have poorer long-term outcomes.36,37 Furthermore, our study found that TAVR was associated with lower average hospital costs compared to SAVR in this specific population subset, indicating its potential economic advantage over SAVR in the current era.38,39 This contrasts with previous analyses, which did not detect any significant cost differences between the two treatments.40 Overall, these findings confirm the safety and efficacy of TAVR in patients with cirrhosis and suggest that TAVR should be offered to patients who are anatomically suitable candidates. Moreover, TAVR has emerged as a cost-effective treatment option for patients with higher risk profiles, facilitating earlier discharge and ultimately yielding better overall outcomes.
Limitations
The use of administrative data sets in analysis may present certain limitations, as they rely on diagnostic codes rather than actual clinical data. Firstly, certain limitations of our study are intrinsic to the examination of a vast administrative database, which may contain coding inaccuracies and misrepresented data regarding the frequency of procedures. As a result, we relied on ICD-10 diagnoses and procedure codes within the NRD datasets. However, these administrative datasets have been used in several published manuscripts, and the quality of coding data has been deemed appropriate.41,42 Secondly, the estimation of liver disease severity using scoring systems such as model for end-stage liver disease or Child-Pugh score may be hindered by the absence of physiological parameters in administrative databases. However, despite this limitation, previous studies have reported high accuracy in identifying patients with confirmed liver cirrhosis using administrative databases.43,44 Thirdly, it is important to acknowledge the presence of inherent selection bias in our study, as decompensated cirrhotic patients may have been excluded from undergoing any procedure. Fourthly, it is essential to recognize that the cross-sectional and observational design of this study, along with the lack of long-term follow-up data and procedural details in the database, imposes limitations on drawing definitive conclusions about the temporal association between outcomes. However, it is important to highlight that the database still provides valuable insights into the associations between various variables and outcomes in our sample population.43 Lastly, despite utilizing a propensity-matched design to address selection bias and achieve covariate balance through our matching algorithm, it is crucial to note that certain key covariates that could serve as predictors of outcomes may be absent from our analysis, potentially impacting our findings.
Conclusions
This large observational analysis among patients with liver cirrhosis and symptomatic AS demonstrates that TAVR was associated with lower rates of in-hospital mortality, 30-day all-cause and unplanned readmissions, bleeding rates, rates of vascular complications, hepatorenal syndrome, cardiogenic shock, MCS utilization, shorter length of hospital stay, and reduced hospitalization costs compared to SAVR. Despite patients being older and with more comorbidities, this analysis indicates a lower overall mortality rate, which highlights the significant advancements in technology and current clinical practices in managing this high-risk subgroup of patients. Given contemporary outcomes for TAVR and the poor outcomes for untreated AS, TAVR in this subgroup of patients appears to be a reasonable therapeutic option. Further research involving larger cohorts and longer follow-up periods is imperative to strengthen the validity and generalizability of our findings.
Ethics Statement
The research reported in this manuscript has adhered to the relevant ethical guidelines. This study was exempt from the institutional review board and informed consent was not required for the current study as the NRD is a publicly available database with de-identified patient information.
Consent Statement
The authors have no funding to report for this particular analysis. This is an analysis of a publicly available, fully de-identified national database. This study was therefore exempt from institutional review board and informed consent was not required.
Funding
The authors have no funding to report.
Disclosure Statement
S. Elmariah reports a relationship with Medtronic Inc, Abbott, and Edwards Lifesciences Corporation that includes consulting or advisory. J. K. Forrest reports a relationship with Medtronic Inc and Edwards Lifesciences Corporation that includes consulting or advisory. A. N. Vora reports a relationship with Medtronic Inc that includes consulting or advisory.
The other authors had no conflicts to declare.
Acknowledgments
MV was supported by the Argonne Leadership Computing Facility, which is a U.S. Department of Energy Office of Science User Facility operated under contract DE-AC02-06CH11357
Footnotes
Supplemental data for this article can be accessed on the publisher’s website
Supplementary Material
References
- 1.Friedman L.S. The risk of surgery in patients with liver disease. Hepatology. 1999;29(6):1617–1623. doi: 10.1002/hep.510290639. [DOI] [PubMed] [Google Scholar]
- 2.Gundling F., Seidl H., Gansera L., et al. Early and late outcomes of cardiac operations in patients with cirrhosis: a retrospective survival-rate analysis of 47 patients over 8 years. Eur J Gastroenterol Hepatol. 2010;22(12):1466–1473. doi: 10.1097/MEG.0b013e32834059b6. [DOI] [PubMed] [Google Scholar]
- 3.Nashef S.A., Roques F., Michel P., Gauducheau E., Lemeshow S., Salamon R. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardio Thorac Surg. 1999;16(1):9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 4.STS. STS Risk Score The Society of Thoracic surgery score Website: the Society of Thoracic surgery. 1999. http://riskcalc.sts.org/stswebriskcalc/#/ 2016.
- 5.Thielmann M., Mechmet A., Neuhäuser M., et al. Risk prediction and outcomes in patients with liver cirrhosis undergoing open-heart surgery. Eur J Cardio Thorac Surg. 2010;38(5):592–599. doi: 10.1016/j.ejcts.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 6.Kodali S.K., Williams M.R., Smith C.R., et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366(18):1686–1695. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 7.Leon M.B., Smith C.R., Mack M., et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 8.Leon M.B., Smith C.R., Mack M.J., et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 9.Adams D.H., Popma J.J., Reardon M.J., et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370(19):1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 10.Popma J.J., Adams D.H., Reardon M.J., et al. Transcatheter aortic valve replacement using a self-expanding bioprosthesis in patients with severe aortic stenosis at extreme risk for surgery. J Am Coll Cardiol. 2014;63(19):1972–1981. doi: 10.1016/j.jacc.2014.02.556. [DOI] [PubMed] [Google Scholar]
- 11.Grover F.L., Vemulapalli S., Carroll J.D., et al. 2016 annual report of the Society of Thoracic Surgeons/American College of Cardiology transcatheter valve therapy Registry. J Am Coll Cardiol. 2017;69(10):1215–1230. doi: 10.1016/j.jacc.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 12.Wendler O., Schymik G., Treede H., et al. SOURCE 3 Registry: design and 30-day results of the European post approval Registry of the Latest generation of the SAPIEN 3 transcatheter heart valve. Circulation. 2017;135(12):1123–1132. doi: 10.1161/CIRCULATIONAHA.116.025103. [DOI] [PubMed] [Google Scholar]
- 13.Alqahtani F., Aljohani S., Ghabra A., et al. Outcomes of transcatheter versus surgical aortic valve implantation for aortic stenosis in patients with hepatic cirrhosis. Am J Cardiol. 2017;120(7):1193–1197. doi: 10.1016/j.amjcard.2017.06.067. [DOI] [PubMed] [Google Scholar]
- 14.Thakkar B., Patel A., Mohamad B., et al. Transcatheter aortic valve replacement versus surgical aortic valve replacement in patients with cirrhosis. Catheter Cardiovasc Interv. 2016;87(5):955–962. doi: 10.1002/ccd.26345. [DOI] [PubMed] [Google Scholar]
- 15.Dhoble A., Bhise V., Nevah M.I., et al. Outcomes and readmissions after transcatheter and surgical aortic valve replacement in patients with cirrhosis: a propensity matched analysis. Catheter Cardiovasc Interv. 2018;91(1):90–96. doi: 10.1002/ccd.27232. [DOI] [PubMed] [Google Scholar]
- 16.Agency for Healthcare Research. Quality R. NRD description of data elements. "Healthcare cost and utilization Project (HCUP)". 2021. https://www.hcup-us.ahrq.gov/db/nation/nrd/nrddde.jsp Available at:
- 17.Mack M.J., Leon M.B., Thourani V.H., et al. Transcatheter aortic-valve replacement with a Balloon-Expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695–1705. doi: 10.1056/NEJMoa1814052. [DOI] [PubMed] [Google Scholar]
- 18.Sharma T., Krishnan A.M., Lahoud R., Polomsky M., Dauerman H.L. National trends in TAVR and SAVR for patients with severe isolated aortic stenosis. J Am Coll Cardiol. 2022;80(21):2054–2056. doi: 10.1016/j.jacc.2022.08.787. [DOI] [PubMed] [Google Scholar]
- 19.Mori M., Gupta A., Wang Y., et al. Trends in transcatheter and surgical aortic valve replacement among older Adults in the United States. J Am Coll Cardiol. 2021;78(22):2161–2172. doi: 10.1016/j.jacc.2021.09.855. [DOI] [PubMed] [Google Scholar]
- 20.Smith C.R., Leon M.B., Mack M.J., et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 21.Ando T., Briasoulis A., Panaich S. Advances in transcatheter aortic valve replacement. J Geriatr Cardiol. 2019;16(9):724–732. doi: 10.11909/j.issn.1671-5411.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steffen R.J., Bakaeen F.G., Vargo P.R., et al. Impact of cirrhosis in patients who underwent surgical aortic valve replacement. Am J Cardiol. 2017;120(4):648–654. doi: 10.1016/j.amjcard.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 23.Murata M., Kato T.S., Kuwaki K., Yamamoto T., Dohi S., Amano A. Preoperative hepatic dysfunction could predict postoperative mortality and morbidity in patients undergoing cardiac surgery: utilization of the MELD scoring system. Int J Cardiol. 2016;203:682–689. doi: 10.1016/j.ijcard.2015.10.181. [DOI] [PubMed] [Google Scholar]
- 24.Wallwork K., Ali J.M., Abu-Omar Y., De Silva R. Does liver cirrhosis lead to inferior outcomes following cardiac surgery? Interact Cardiovasc Thorac Surg. 2019;28(1):102–107. doi: 10.1093/icvts/ivy221. [DOI] [PubMed] [Google Scholar]
- 25.Downing S.W., Edmunds L.H., Jr. Release of vasoactive substances during cardiopulmonary bypass. Ann Thorac Surg. 1992;54(6):1236–1243. doi: 10.1016/0003-4975(92)90113-i. [DOI] [PubMed] [Google Scholar]
- 26.Casey L.C. Role of cytokines in the pathogenesis of cardiopulmonary-induced multisystem organ failure. Ann Thorac Surg. 1993;56(5 Suppl):S92–S96. doi: 10.1016/0003-4975(93)91143-b. [DOI] [PubMed] [Google Scholar]
- 27.Okano N., Miyoshi S., Owada R., et al. Impairment of hepatosplanchnic oxygenation and increase of serum hyaluronate during normothermic and mild hypothermic cardiopulmonary bypass. Anesth Analg. 2002;95(2):278–286. doi: 10.1097/00000539-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Forrest J.K., Deeb G.M., Yakubov S.J., et al. Low risk trial Investigators 3-Year outcomes after transcatheter or surgical aortic valve replacement in low-risk patients with aortic stenosis. J Am Coll Cardiol. 2023;81(17):1663–1674. doi: 10.1016/j.jacc.2023.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Toff W.D., Hildick-Smith D., Kovac J., et al. UK TAVI trial Investigators Effect of transcatheter aortic valve implantation vs surgical aortic valve replacement on all-cause mortality in patients with aortic stenosis: a randomized clinical trial. JAMA. 2022;327(19):1875–1887. doi: 10.1001/jama.2022.5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greason K.L., Mathew V., Wiesner R.H., Suri R.M., Rihal C.S. Transcatheter aortic valve replacement in patients with cirrhosis. J Card Surg. 2013;28(5):492–495. doi: 10.1111/jocs.12177. [DOI] [PubMed] [Google Scholar]
- 31.Pascual I., Muñoz-García A.J., López-Otero D., Avanzas P., Alonso-Briales J.H., Morís C. Long-term outcome of cirrhotic patients with severe aortic stenosis treated with transcatheter aortic valve implantation. Rev Esp Cardiol. 2015;68(4):353–354. doi: 10.1016/j.rec.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 32.Shah A.M., Ogbara J., Herrmann H.C., et al. Outcomes of transcatheter aortic valve replacement in patients with chronic liver disease. Catheter Cardiovasc Interv. 2015;86(5):888–894. doi: 10.1002/ccd.25994. [DOI] [PubMed] [Google Scholar]
- 33.Khan M.Z., Khan M.U., Munir M.B., Khan S.U., Osman M., Balla S. Contemporary trends and outcomes in aortic valve replacement in patients with end-stage liver disease. Catheter Cardiovasc Interv. 2020;96(4):947–955. doi: 10.1002/ccd.28834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Julien H.M., Stebbins A., Vemulapalli S., et al. Incidence, predictors, and outcomes of acute kidney injury in patients undergoing transcatheter aortic valve replacement: insights from the Society of Thoracic Surgeons/American College of Cardiology national cardiovascular data Registry-transcatheter valve therapy Registry. Circ Cardiovasc Interv. 2021;14(4) doi: 10.1161/CIRCINTERVENTIONS.120.010032. [DOI] [PubMed] [Google Scholar]
- 35.Ferro C.J., Law J.P., Doshi S.N., et al. UK TAVI Steering group and the national Institute for cardiovascular outcomes research. Dialysis following transcatheter aortic valve replacement: risk factors and outcomes: an analysis from the UK TAVI (transcatheter aortic valve implantation) Registry. JACC Cardiovasc Interv. 2017;10(20):2040–2047. doi: 10.1016/j.jcin.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 36.Henry L., Halpin L., Hunt S., Holmes S.D., Ad N. Patient disposition and long-term outcomes after valve surgery in octogenarians. Ann Thorac Surg. 2012;94(3):744–750. doi: 10.1016/j.athoracsur.2012.04.073. [DOI] [PubMed] [Google Scholar]
- 37.Hua M., Gong M.N., Brady J., Wunsch H. Early and late unplanned rehospitalizations for survivors of critical illness. Crit Care Med. 2015;43(2):430–438. doi: 10.1097/CCM.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baron S.J., Wang K., House J.A., et al. Cost-Effectiveness of transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at intermediate risk. Circulation. 2019;139(7):877–888. doi: 10.1161/CIRCULATIONAHA.118.035236. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds M.R., Magnuson E.A., Lei Y., et al. PARTNER Investigators Cost-effectiveness of transcatheter aortic valve replacement compared with surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results of the PARTNER (Placement of Aortic Transcatheter Valves) trial (Cohort A) J Am Coll Cardiol. 2012;60(25):2683–2692. doi: 10.1016/j.jacc.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 40.Osnabrugge R.L., Head S.J., Genders T.S., et al. Costs of transcatheter versus surgical aortic valve replacement in intermediate-risk patients. Ann Thorac Surg. 2012;94(6):1954–1960. doi: 10.1016/j.athoracsur.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Goldsweig A.M., Tak H.J., Chen L.W., et al. The Evolving management of aortic valve disease: 5-year trends in SAVR, TAVR, and medical therapy. Am J Cardiol. 2019;124(5):763–771. doi: 10.1016/j.amjcard.2019.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jang S.J., Webber F.M., Alam M.M., et al. Early clinical outcomes of patients with Stress-induced Cardiomyopathy receiving acute mechanical support in the US. J Soc Card Ang & Interv. 2023;2(6) doi: 10.1016/j.jscai.2023.101185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nehra M.S., Ma Y., Clark C., Amarasingham R., Rockey D.C., Singal A.G. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol. 2013;47(5):e50–e54. doi: 10.1097/MCG.0b013e3182688d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garg S.K., Goyal H., Obaitan I., et al. Incidence and predictors of 30-day hospital readmissions for liver cirrhosis: insights from the United States National Readmissions Database. Ann Transl Med. 2021;9(13):1052. doi: 10.21037/atm-20-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.