Abstract
Background: Bupropion has been available in the United States since 1989. Initially a thrice-daily immediate-release formulation, a twice-daily sustained-release formulation followed in 1996, and, in August 2003, a once-daily extended-release formulation was introduced. On the 15th anniversary of its introduction, we undertook a review of the background/history, mechanism of action, formulations, and clinical profile of bupropion.
Data Sources: Major efficacy trials and other reports were obtained and reviewed from MEDLINE searches, review of abstracts from professional meetings, and the bupropion SR manufacturer's databases. Searches of English-language articles were conducted from June 2003 through August 2004. No time limit was specified in the searches, which were conducted using the search terms bupropion, bupropion SR, and bupropion XL.
Data Synthesis: Bupropion inhibits the re-uptake of norepinephrine and dopamine neurotransmission without any significant direct effects on serotonin neurotransmission. Bupropion is an effective antidepressant with efficacy comparable to selective serotonin reuptake inhibitors and other antidepressants. It is well tolerated in short-and longer-term treatment. Headache, dry mouth, nausea, insomnia, constipation, and dizziness are the most common adverse events. Seizure and allergic reactions are medically important adverse events associated with bupropion and are reported rarely. Among all the newer antidepressants in the United States, bupropion appears to have among the lowest incidence of sexual dysfunction, weight gain, and somnolence. Although not U.S. Food and Drug Administration approved for these indications, bupropion has also been used as an adjunctive treatment to reverse antidepressant-induced sexual dysfunction and to augment anti-depressant efficacy in partial responders and non-responders to other agents.
Conclusion: Bupropion has played and will continue to play an important role as a treatment for major depressive disorder in adults, as well as for other related disorders.
Bupropion HCl was initially developed to improve on the safety and tolerability of existing antidepressants.1 It is the only antidepressant available with a dual effect on norepinephrine (NE) and dopamine (DA) neuro-transmitter systems.2 Bupropion was initially marketed as an immediate-release (IR) product; a sustained-release (SR) formulation was approved in 1996, and in August 2003, a once-daily extended-release (XL) formulation became available.3 Bupropion is now approved for use in over 50 countries for the treatment of major depressive disorder and/or as an aid to smoking cessation, with over 40 million patients receiving treatment (data on file, GlaxoSmithKline, Research Triangle Park, N.C.). On the 15th anniversary of the introduction of bupropion, this review was developed to provide an overview of bupropion HCl that encompasses the compound's background and history, mechanism of action, distinct formulations, and clinical profile. Major efficacy trials and other reports were obtained and reviewed from MEDLINE searches, review of abstracts from professional meetings, and the bupropion SR manufacturer's databases. Searches of English-language articles were conducted from June 2003 through August 2004. No time limit was specified in the searches, which were conducted using the search terms bupropion, bupropion SR, and bupropion XL.
BACKGROUND AND HISTORY
The development of bupropion resulted from a targeted program designed to produce an antidepressant with a unique pharmacologic profile that offered safety and tolerability advantages over existing agents.1 Bupropion's safety and efficacy were established in several clinical trials, and with U.S. Food and Drug Administration (FDA) approval in 1989, it was introduced as a treatment for major depressive disorder. The formulation was an IR tablet, which required thrice-daily dosing. Although clinically effective, the dosing schedule was inconvenient for patients. Because of this inconvenience, as well as evidence implicating a relationship between peak plasma concentrations and some adverse events, development of an SR formulation was initiated.4,5
Bupropion SR was developed and marketed in 1996 based on the demonstration of bioequivalence (both rate and extent of absorption) to the IR formulation. This formulation allowed for twice-daily dosing up to 400 mg/day. Although the SR formulation was bioequivalent to the IR formulation, the time to maximum plasma concentration was prolonged, and the peak plasma concentrations of bupropion were somewhat (15%) lower (data on file, GlaxoSmithKline, Research Triangle Park, N.C.). With the convenience of a twice-daily dosing schedule and the potential for an improved tolerability/safety profile, bupropion SR quickly became the most frequently prescribed formulation of bupropion.2 However, twice-daily dosing remained a clinical issue, especially relative to other antidepressants that allowed for once-daily dosing. In a study on attitudes and behavior in depressed patients, bupropion SR–treated patients, compared to selective serotonin reuptake inhibitor (SSRI)–treated patients, reported a greater frequency of noncompliance, which they attributed to both having to take the medication too frequently and difficulty in remembering to take the medication.6 This promulgated the development of an XL formulation. Bupropion XL, which allows for once-daily dosing up to 450 mg, was approved for the treatment of major depressive disorder in August 2003. Once-daily bupropion XL is bioequivalent to both twice-daily bupropion SR and thrice-daily bupropion as evidenced by similar peak plasma concentrations (Cmax), area under the curve (AUC), and plasma concentration versus time profiles (data on file, GlaxoSmithKline, Research Triangle Park, N.C.). The unique drug delivery matrix provides a 24-hour plasma concentration of bupropion following a single therapeutic dose, while avoiding a peak plasma level of bupropion at bedtime and offering increased convenience.
MECHANISM OF ACTION
Considerable effort was spent on understanding the mechanism of action of bupropion.7 Preclinical data demonstrate that bupropion does not inhibit monoamine oxidase, nor does bupropion bind to postsynaptic receptors including histamine, α- or β-adrenergic, serotonin, DA, or acetylcholine receptors.8–10 Preclinical evidence also indicates bupropion does not affect release of neurotransmitters, although 1 report suggests that bupropion may enhance NE release.8–11 Over time, understanding of the neuropharmacology of bupropion has evolved, and the mechanism of action is attributed by most researchers to its ability to enhance monoaminergic neurotransmission via its effects on NE and DA by reuptake inhibition.9,10 This mechanistic profile, which some researchers have defined as a NE/DA reuptake inhibitor (NDRI), is distinctly different from the profiles of other antidepressants. Bupropion is the only new generation antidepressant available in the United States with no appreciable serotonergic activity.9,10,12,13
Bupropion appears to enhance monoaminergic neurotransmission via its effects on both the NE and DA transporters, with subsequent reuptake inhibition of these 2 neurotransmitters. Bupropion and 2 of its metabolites, hydroxybupropion and threohydrobupropion, were shown to decrease the uptake of NE and DA into rat and mouse synaptosomes.9 Bupropion's activity as a DA transporter inhibitor is further supported by a recent study showing approximately a 3-fold increase in expression of the immediate early gene c-fos in human DA transporter cells, and, if coincubated with DA, an attenuation of the DA-induced c-fos in human DA transporter cells.14 Acute administration of bupropion reduces firing of brain stem NE and DA neurons (data on file, GlaxoSmithKline, Research Triangle Park, N.C.). Additionally, firing rates of NE neurons in the locus ceruleus are inhibited after acute bupropion administration.9 Microdialysis studies following acute administration of bupropion demonstrate that extra-cellular NE and DA concentrations are increased in the nucleus accumbens (data on file, GlaxoSmithKline, Research Triangle Park, N.C.). Further evidence of the NDRI action of bupropion was found when administration of NE- or DA-blocking drugs was shown to reduce the efficacy of bupropion, and hydroxybupropion, in animal models of depression.15
The therapeutic effect of bupropion is presumed to be, in part, due to the antidepressant activity of 3 metabolites: hydroxybupropion, threohydrobupropion, and erythrohydrobupropion.3 Using cells expressing human transporters for DA, NE, and serotonin, it has been shown in vitro that bupropion, together with the active metabolites, inhibits both the NE and DA human transporters (data on file, GlaxoSmithKline, Research Triangle Park, N.C.). Inhibition of serotonin reuptake was negligible even at the highest concentrations tested.
Studies assessing DA transporter occupancy following administration of bupropion in humans have demonstrated measurable occupancy.16–18 The initial study enrolled healthy volunteers, with the subsequent 2 studies enrolling depressed patients. Taken together, all of the above studies provide strong evidence for a dual NE/DA mechanism of action of bupropion.
PHARMACOKINETIC PROFILES
Plots of the steady-state plasma concentrations of bupropion over a 24-hour period for the IR, SR, and XL formulations of bupropion are presented in Figure 1 (data on file, GlaxoSmithKline, Research Triangle Park, N.C.). With steady-state dosing at 300 mg/day, both the SR and XL formulations, relative to the IR formulation, deliver an equivalent amount of bupropion (AUC) and do so while producing Cmax similar to or somewhat less than those seen with the IR formulation. Since some adverse events are believed to be associated with Cmax, it is worth noting that the SR and XL formulations decrease the number of daily exposures of peak plasma levels to twice and once daily, respectively. Importantly, time to Cmax (Tmax) is prolonged with the SR (3 hours) and XL formulations (5 hours), relative to the IR formulation (2 hours). Both the prolongation of Tmax and the decrease in the number of peak plasma levels may result in better tolerability, although this has not been formally evaluated. With once-daily dosing of the XL formulation in the morning, plasma bupropion concentration levels are lower during evening hours compared to the SR formulation (Figure 2) (data on file, GlaxoSmithKline, Research Triangle Park, N.C.). This may result in a lower rate of insomnia, an adverse event that has been reported to be correlated with plasma concentrations.5 The extended release of bupropion seen with the XL formulation is achieved by a moisture-barrier coating and a control-releasing coating surrounding a tablet core containing bupropion. The integrity of the delivery matrix is essential to providing an extended-release rate; chewing, cutting, or crushing bupropion XL tablets would disrupt the delivery matrix, resulting in immediate release of bupropion, which could cause significant adverse events.
Figure 1.
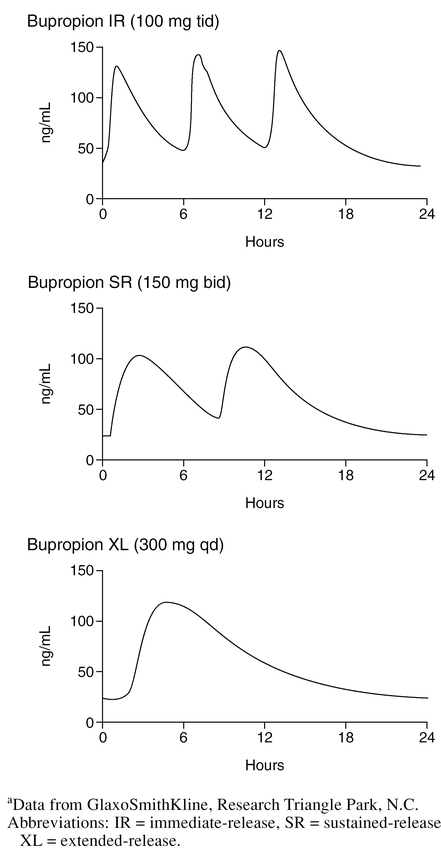
Steady-State Plasma Level Concentrations for Bupropion 300 mg/day for IR, SR, and XL Formulationsa
Figure 2.
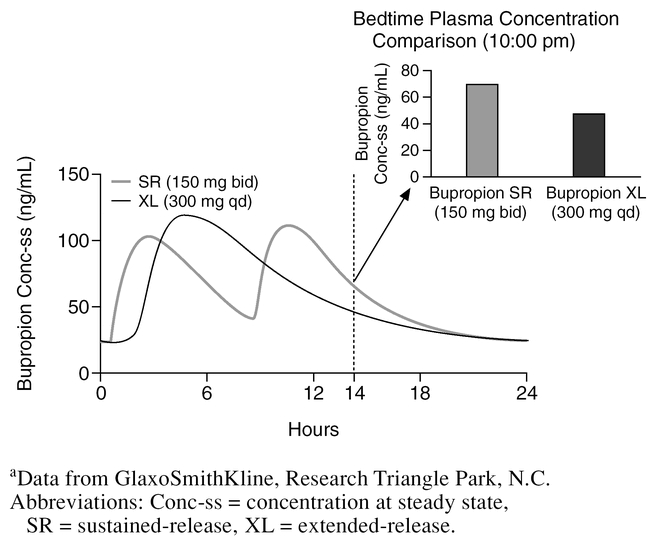
Bedtime Steady-State Plasma Levels for Bupropion XL Compared to Bupropion SRa
CLINICAL PROFILE
Efficacy in Major Depressive Disorder
Bupropion has been shown to be effective in the treatment of major depressive disorder in numerous double-blind controlled trials. Efficacy has been demonstrated relative to placebo in both inpatients and out-patients.19,20 Additionally, in comparative studies, bupropion has been found to have efficacy comparable to other antidepressants (references 21–31 and data on file, GlaxoSmithKline, Research Triangle Park, N.C.). Seven studies employed an SSRI as the active control (references 21–26 and data on file, GlaxoSmithKline, Research Triangle Park, N.C.), and 4 of these also included a placebo control (references 21, 22, 25, and data on file, GlaxoSmithKline, Research Triangle Park, N.C.). Thase et al.32 conducted a pooled analysis using the original data from all 7 SSRI studies and found that bupropion and SSRIs produced identical remission rates, and both treatments were statistically superior to placebo. Clinical trial data show bupropion is an appropriate selection for depressed patients with comorbid anxiety. Bupropion is efficacious in decreasing symptoms of depression-related anxiety relative to placebo and does so at a magnitude and time course similar to that seen for other antidepressants, including SSRIs.19,26,33 However, bupropion has not been extensively evaluated for specific anxiety disorders, and use for these disorders would be considered off-label.
Longer-term efficacy was demonstrated in a 1-year relapse-prevention study in which patients who had responded to 8 weeks of therapy with bupropion SR (150 mg b.i.d.) were randomly assigned in a double-blind manner to either continue therapy with active medication or switch to placebo.34 Using survival analysis, bupropion separated statistically from placebo, demonstrating that maintenance treatment with bupropion prevents relapse. In fact, by study end, the odds of relapsing for subjects in the placebo group were almost twice that seen in subjects in the bupropion group. Median time to relapse was greater than 44 weeks for bupropion compared to 24 weeks for placebo.
There are several small studies in which bupropion was added to the treatment regimen of patients with major depressive disorder who were partially or not responding to SSRIs.35–38 In addition, bupropion has also been used alone in patients who were considered nonresponders to other antidepressants, with encouraging results reported.39,40 In a recently published study,41 26 patients with major depressive disorder refractory to an 8- to 12-week open trial of fluoxetine were switched to open-label treatment with bupropion SR for 8 weeks. A modified intent-to-treat analysis resulted in approximately 65% of patients experiencing a full (34.6%) or partial (30.8%) response to bupropion SR.41 In the soon to be completed STAR*D (Sequenced Treatment Alternatives to Relieve Depression), a large study of major depressive disorder funded by the National Institute of Mental Health, bupropion augmentation and a switch to bupropion are among the options being tested for citalopram nonresponders.42
There are also data regarding the use of bupropion for seasonal affective disorder. The initial report was a small open uncontrolled study.43 Recently, 2 multicenter studies evaluating bupropion XL versus placebo for the prevention of seasonal depressive episodes were completed (data on file, GlaxoSmithKline, Research Triangle Park, N.C.). In the pooled analysis of both studies, bupropion XL was found to have a significant effect preventing the onset of a seasonal depressive episode.
Efficacy for Other Indications
Bupropion has been evaluated for use in a number of on- and off-label indications either as a result of clinical observations or based on theoretical reasons given the NDRI mechanism. Foremost of these is tobacco dependence, for which bupropion was initially noted to have an “anti-smoking” effect in the depression clinical trial program.44 Bupropion SR has been shown to be effective and well tolerated in relatively healthy adult smokers and a medically ill smoking population (cardiovascular disease and chronic obstructive pulmonary disease).45–48 In a longer-term study, bupropion prevented relapse to smoking across 1 year.49
Early in the development of bupropion for the treatment of major depressive disorder, it was observed that bupropion-treated patients appeared less likely to switch to mania.50,51 Based on these reports, a small desipramine-comparator study with bupropion was conducted in patients with bipolar I depressive episodes.52 Bupropion was as effective as desipramine for bipolar depression, but was associated with a statistically lower rate of switch into mania over a 1-year period. A subsequent report from the same group expanding the sample size of the original report confirmed the comparable efficacy in the treatment of bipolar depression between desipramine and bupropion, but the switch rate for bupropion was not significantly different from desipramine, although there was a trend toward a lower rate for bupropion.53 The use of bupropion in the treatment of bipolar depression has not been approved by the FDA.
A number of studies have evaluated bupropion for the treatment of attention-deficit/hyperactivity disorder (ADHD).54–57 Following initial open-label studies, a methylphenidate crossover study and 2 placebo-controlled studies, 1 in children and 1 in adults, were conducted.55–57 Both placebo-controlled studies provide evidence of efficacy for ADHD, while the crossover study indicated that the efficacy of bupropion was similar in magnitude to that of methylphenidate. Recently, a placebo-controlled trial of bupropion XL in adults demonstrated efficacy for this disorder (data on file, GlaxoSmithKline, Research Triangle Park, N.C.). Bupropion was generally well tolerated in these studies. The use of bupropion in the treatment of ADHD has not been approved by the FDA.
Lastly, bupropion has been studied for its effects on weight loss. During major depressive disorder clinical trials, patients receiving bupropion did not have the weight gain commonly seen with other antidepressants, especially tricyclic antidepressants, and compared to placebo, there was a mild weight loss.58 Three placebo-controlled studies (8 weeks to 6 months in duration) have been conducted in adults evaluating bupropion as a treatment for obesity.59–61 In these studies, bupropion was demonstrated to be superior to placebo in helping patients lose weight (2%–10% of initial body weight depending on type of diet, dose of bupropion, and duration of treatment). One of the adult studies was conducted in patients with mild depressive symptomatology, and in the subgroup of patients with a prior history of major depressive disorder, bupropion was also beneficial relative to placebo in relieving depressive symptomatology.60 Recently, results from a 16-week trial enrolling overweight adolescents were reported, which also demonstrated the superiority of bupropion SR to placebo.62 The use of bupropion in the treatment of obesity has not been approved by the FDA.
Tolerability
The safety databases for bupropion are extensive, comprising thousands of clinical trial subjects and over 40 million patients who have received bupropion clinically (data on file, GlaxoSmithKline, Research Triangle Park, N.C.). The most commonly reported adverse events (occurring more than 5% and more than placebo) during bupropion SR placebo-controlled trials (300-mg and 400-mg daily dosing) include headache, dry mouth, nausea, insomnia, constipation, and dizziness.63 Of these, dry mouth, nausea, and insomnia occurred at a statistically higher incidence with bupropion SR relative to placebo. Adverse events resulting in discontinuation occurred at a relatively low rate: 7% across all dose groups (9% and 11% for the 300- and 400-mg/day bupropion SR dose groups, respectively), compared to 4% for placebo.3,63 Rash, nausea, agitation, and migraine were the most frequently cited adverse events leading to discontinuation in the bupropion SR clinical trials. Bupropion SR is also well tolerated with long-term use.34 In the 52-week relapse-prevention study,34 adverse events were lower in the randomized portion of the study, suggesting that adverse events may diminish with continued treatment.
An important adverse event associated with bupropion use is seizure. With the IR formulation, the rate is 0.4% (4/1000) at doses of 300 to 450 mg/day; however, the rate increases substantially at doses above this level. With the SR formulation, the rate is 0.1% (1/1000) at the target dose of 300 mg/day.4 The incidence of seizure with bupropion XL has not been evaluated. Bupropion is not the only antidepressant associated with seizures. SSRI antidepressants are associated with seizure at a similar rate of approximately 0.1%.4 Certain factors may increase the risk of seizure; therefore, prior to the prescription of bupropion, patients should be screened for the presence of medical comorbidities, clinical situations, or concomitant medications that may lower seizure threshold.
Allergic/anaphylactic reactions have been reported rarely with bupropion. Patients should be advised to stop treatment and consult with their doctor if symptoms arise. Although there have been spontaneous reports of hypertension in clinical practice, bupropion alone has not been associated with effects on blood pressure relative to placebo in clinical trials.63,64 In a clinical trial designed to look at treatment-emergent hypertension, bupropion did not differ from placebo in volunteers with preexisting untreated mild hypertension.65 However, in a study in which bupropion was combined with a nicotine patch, there was an increased incidence of treatment-emergent hypertension in the combination group compared to the placebo group or the bupropion alone group.46 Blood pressure should be monitored in patients receiving bupropion in combination with a nicotine patch.
The adverse experience profile is often predictable on the basis of the neurochemical effects of a particular antidepressant, which allows us to predict not only associated adverse events but also the lack of such events relative to other antidepressants that have a different neurochemical profile.66 Insomnia, for example, might be expected with a drug such as bupropion, affecting both NE and DA neurotransmitter systems and is, in fact, a common adverse event associated with its treatment. The rate of insomnia for bupropion (11%–20%), while greater than that seen for placebo (4%–7%), is similar to the rate associated with SSRIs (10%–19%).21–26
In bupropion clinical trials, the prevalence of somnolence in subjects taking bupropion was similar to that of placebo subjects and less than that seen with the tricyclic antidepressants and trazodone, which may be partly mediated by the latter drugs' effect on histamine and 5-HT2 receptors.28–31 Moreover, the somnolence rates were higher in SSRI-treated patients relative to bupropion (Figure 3).22,24–26
Figure 3.
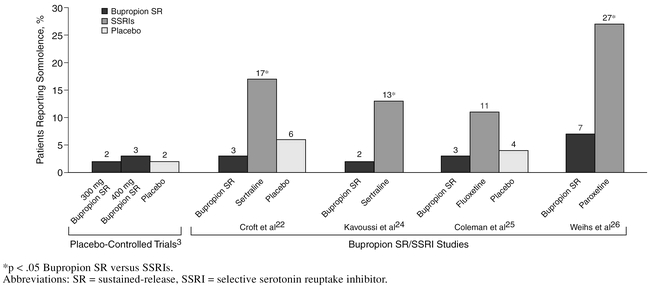
Prevalence of Somnolence in Bupropion SR Clinical Trials
Sexual dysfunction is frequently associated with SSRI treatment and, across studies using direct questioning, rates of 40% to 50% are reported.67 Importantly, in the SSRI/bupropion comparator studies, bupropion has consistently been associated with statistically significantly less sexual dysfunction than SSRIs, including orgasmic dysfunction. In the pooled SSRI comparator trial analysis by Thase et al.,32 bupropion SR was associated with less orgasmic dysfunction, sexual arousal disorder, and sexual desire disorder relative to SSRIs. It is noteworthy that, in this large data set, the risk of sexual dysfunction during bupropion therapy was virtually identical to that of placebo. In an observational study enrolling 6297 patients,68 bupropion was associated with the lowest incidence of sexual dysfunction among the newer antidepressants available at the time of the study: citalopram, fluoxetine, mirtazapine, nefazodone, paroxetine, sertraline, venlafaxine, and venlafaxine XR. For patients without other known causes of sexual dysfunction, the odds of developing sexual dysfunction were 4 to 6 times greater with SSRIs or venlafaxine XR compared to bupropion.
Based on the low risk of sexual dysfunction and, importantly, the lack of significant serotonergic activity, bupropion has been added to existing SSRI treatment regimens for the management of treatment-induced sexual dysfunction. Initial open, uncontrolled studies were encouraging, and recently a small double-blind study69 found that bupropion, relative to placebo, increases desire to engage in sexual activity and frequency of engaging in sexual activity.67,69,70 In all of these studies, bupropion has been added to the SSRI or serotonin-norepinephrine reuptake inhibitor as a daily regimen. There are no data to support p.r.n. use of bupropion, and given the pharmacology and pharmacokinetics/dynamics of bupropion, p.r.n. dosing would be considered inappropriate. The use of bupropion in the treatment of antidepressant-induced sexual dysfunction has not been approved by the FDA.
Weight gain would be an expected consequence of antidepressants with affinity for histamine and/or 5-HT2C receptors. Bupropion has no relevant affinity for these receptors and is not associated with weight gain. It is typically associated with a slight weight loss (e.g., approximately equal to 1.5 kg with initial treatment). In the long-term relapse prevention study, a greater weight loss was observed in patients with a greater body mass index at baseline.71
CONCLUSION
Bupropion is an NDRI antidepressant that has been available for over 15 years for major depressive disorder and over 6 years as an aid to smoking cessation. Data indicate that bupropion may be effective as a treatment for ADHD and in helping obese patients lose weight, although the use of bupropion in these conditions is not approved by the FDA, and additional studies are needed in both areas. For bipolar disorder, encouraging evidence suggests that not only is bupropion effective for depressive episodes, but it also appears to have a smaller risk of inducing a manic switch; these studies are rather small and further investigation is required. Among all the newer antidepressants in the United States, bupropion appears to have among the lowest incidence of sexual dysfunction, weight gain, and somnolence. Recently, a once-daily formulation, bupropion XL, was developed with the goal of further improving tolerability and compliance. Future studies should investigate the tolerability advantages of this formulation. Due to its unique profile, bupropion has played and will continue to play an important role in the treatment of major depressive disorder.
Drug names: bupropion (Wellbutrin, Zyban, and others), citalopram (Celexa and others), desipramine (Norpramin and others), fluoxetine (Prozac and others), methylphenidate (Ritalin, Concerta, and others), mirtazapine (Remeron and others), nefazodone (Serzone and others), paroxetine (Paxil and others), sertraline (Zoloft), trazodone (Desyrel and others), venlafaxine (Effexor).
Financial disclosure: Dr. Fava has received research support from Abbott, Lichtwer Pharma GmbH, and Lorex; has received honoraria from Bayer, Compellis, Cypress, Dov Pharmaceuticals, Janssen, Knoll, Lundbeck, and Somerset; and has received both research support and honoraria from Aspect, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Forest, GlaxoSmithKline, J & J Pharmaceuticals, Novartis, Organon, Pfizer, Pharmavite, Roche, Sanofi-Synthelabo, Solvay, and Wyeth-Ayerst. Dr. Rush has received grant/research support from the National Institute of Mental Health, the Robert Wood Johnson Foundation, and the Stanley Foundation; has been a consultant/advisor for Bristol-Myers Squibb, Cyberonics, Eli Lilly, Forest, GlaxoSmithKline, Organon, and the Urban Institute; and has participated in speakers bureaus for Cyberonics, Eli Lilly, Forest, and GlaxoSmithKline. Dr. Thase has been a consultant for AstraZeneca, Bristol-Myers Squibb, Cephalon, Cyberonics, Eli Lilly, Forest, GlaxoSmithKline, Janssen, Novartis, Organon, Pfizer, and Wyeth and has participated in speakers bureaus for AstraZeneca, Eli Lilly, GlaxoSmithKline, Organon, and Wyeth. Dr. Clayton has received grant support from Bayer, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Merck, Neuronetics, Organon, Pfizer, and Pherin; has been a consultant for and participated in advisory boards of Bayer, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Organon, Pfizer, Vela, and Wyeth; and has received honoraria from and participated in speakers bureaus for Eli Lilly, GlaxoSmithKline, Organon, Pfizer, and Wyeth. Dr. Stahl has received grant/research support from Abbott, Asahi, AstraZeneca, Bristol-Myers Squibb, Cephalon, Cypress Bioscience, Eli Lilly, GlaxoSmithKline, Pfizer, Pierre Fabre, and Wyeth and has received honoraria from and been a consultant for Abbott, Asahi, AstraZeneca, Bristol-Myers Squibb, Cephalon, Cypress Bioscience, Eli Lilly, GlaxoSmithKline, Organon, Pfizer, Pierre Fabre, Sanofi-Synthelabo, and Wyeth. Dr. Pradko has been a consultant for GlaxoSmithKline and has participated in speakers/advisory boards for GlaxoSmithKline, Pfizer, and Wyeth. Dr. Johnston is retired from and has been a consultant for GlaxoSmithKline.
Acknowledgments
The authors acknowledge GlaxoSmithKline in providing support for the manuscript preparation of this article.
Footnotes
Financial disclosure appears at the end of the article.
REFERENCES
- Soroko FE, Maxwell RA. The pharmacologic basis for therapeutic interest in bupropion. J Clin Psychiatry. 1983 44(sec 2). 67–73. [PubMed] [Google Scholar]
- Stahl SM. Essential Psychopharmacology. 2nd ed. New York, NY: Cambridge University Press. 2000 [Google Scholar]
- Wellbutrin (bupropion) tablets, Wellbutrin SR (bupropion), and Wellbutrin XL (bupropion). Physician's Desk Reference. Montvale, NJ: Thomson PDR. 2005 1655–1659. 1659,–1663. 1663–1668. [Google Scholar]
- Dunner DL, Zisook S, and Billow AA. et al. A prospective safety surveillance study for bupropion sustained release in the treatment of depression. J Clin Psychiatry. 1998 59:366–373. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Fiedler-Kelly J, and Glover ED. et al. Relationship between drug exposure and the efficacy and safety of bupropion sustained-release for smoking cessation. Nicotine Tob Res. 2001 3:131–140. [DOI] [PubMed] [Google Scholar]
- Depression Patient Study. New Hanover, NJ: NOP World Health. 2002. [Google Scholar]
- Ferris RM, Cooper BR, and Maxwell RA. Studies of bupropion's mechanism of antidepressant activity. J Clin Psychiatry. 1983 44(sec 2). 74–78. [PubMed] [Google Scholar]
- Baldessarini RJ. Drugs and the treatment of psychiatric disorders: depression and anxiety disorders. In: Hardman JG, Limbird LE, eds. Goodman & Gilman's The Pharmacological Basis of Therapeutics. New York, NY: McGraw-Hill. 2001 447–483. [Google Scholar]
- Ascher JA, Cole JO, and Colin J-N. et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995 56:395–401. [PubMed] [Google Scholar]
- Stahl SM, Pradko JF, Haight BR. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6:159–166. doi: 10.4088/pcc.v06n0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Blier P. Modification of norepinephrine and serotonin, but not dopamine, neuron firing by sustained bupropion treatment. Psychopharmacology (Berl) 2001;155:52–57. doi: 10.1007/s002130000665. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Essential Psychopharmacology, First Edition. New York, NY: Cambridge University Press. 1996 [Google Scholar]
- Richelson E. Synaptic effects of antidepressants. J Clin Psychopharmacol. 1996;16(3 suppl 2):1S–9S. doi: 10.1097/00004714-199606002-00001. [DOI] [PubMed] [Google Scholar]
- Yatin SM, Miller GM, and Norton C. et al. Dopamine transporter-dependent induction of c-fos in HEK cells. Synapse. 2002 45:52–65. [DOI] [PubMed] [Google Scholar]
- Cooper BR, Hester TJ, Maxwell RA. Behavioral and biochemical effects of the antidepressant bupropion (Wellbutrin): evidence for selective blockade of dopamine uptake in vivo. J Pharmacol Exp Ther. 1980;215:127–134. [PubMed] [Google Scholar]
- Learned-Coughlin SM, Bergstrom M, and Savitcheva I. et al. In vivo activity of bupropion at the human dopamine transporter as measured by positron emission tomography. Biol Psychiatry. 2003 54:800–805. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Goulding VS, and Wilson AA. et al. Bupropion occupancy of the dopamine transporter is low during clinical treatment. Psychopharmacology (Berl). 2002 163:102–105. [DOI] [PubMed] [Google Scholar]
- Szabó Z, Àrgyelán M, and Kanyó B. et al. The effect of bupropion on the activity of dopamine transporter in depression: preliminary results. Eur Neuropsychopharmacol. 2003 13suppl 4. S210. Abstract No P.1.085. [Google Scholar]
- Fabre LF, Brodie HK, and Garver D. et al. A multicenter evaluation of bupropion versus placebo in hospitalized depressed patients. J Clin Psychiatry. 1983 44(sec 2). 88–94. [PubMed] [Google Scholar]
- Lineberry CG, Johnston JA, and Raymond RN. et al. A fixed-dose (300 mg) efficacy study of bupropion and placebo in depressed outpatients. J Clin Psychiatry. 1990 51:194–199. [PubMed] [Google Scholar]
- Coleman CC, Cunningham LA, and Foster VJ. et al. Sexual dysfunction associated with the treatment of depression: a placebo-controlled comparison of bupropion sustained-release and sertraline treatment. Ann Clin Psychiatry. 1999 11:205–215. [DOI] [PubMed] [Google Scholar]
- Croft H, Settle E, and Houser T. et al. A placebo-controlled comparison of the antidepressant efficacy and effects on sexual functioning of sustained-release bupropion and sertraline. Clin Ther. 1999 21:643–658. [DOI] [PubMed] [Google Scholar]
- Feighner JP, Gardner EA, and Johnston JA. et al. Double-blind comparison of bupropion and fluoxetine in depressed outpatients. J Clin Psychiatry. 1991 52:329–335. [PubMed] [Google Scholar]
- Kavoussi RJ, Segraves RT, and Hughes AR. et al. Double-blind comparison of bupropion sustained release and sertraline in depressed outpatients. J Clin Psychiatry. 1997 58:532–537. [DOI] [PubMed] [Google Scholar]
- Coleman CC, King BR, and Bolden-Watson C. et al. A placebo-controlled comparison on sexual functioning of bupropion SR and fluoxetine. Clin Ther. 2001 23:1040–1058. [DOI] [PubMed] [Google Scholar]
- Weihs KL, Settle EC, and Batey SR. et al. Bupropion sustained-release versus paroxetine for the treatment of depression in the elderly. J Clin Psychiatry. 2000 61:196–202. [DOI] [PubMed] [Google Scholar]
- Mendels J, Amin MM, and Chouinard G. et al. A comparative study of bupropion and amitriptyline in depressed outpatients. J Clin Psychiatry. 1983 44(sec 2). 118–120. [PubMed] [Google Scholar]
- Branconnier RJ, Cole JO, and Ghazvinian S. et al. Clinical pharmacology of bupropion and imipramine in elderly depressives. J Clin Psychiatry. 1983 44(sec 2). 130–133. [PubMed] [Google Scholar]
- Masco HL, Kiev A, and Holloman LC. et al. Safety and efficacy of bupropion and nortriptyline in outpatients with depression. Curr Ther Res. 1994 55:851–863. [Google Scholar]
- Feighner J, Hendrickson G, and Miller L. et al. Double-blind comparison of doxepin versus bupropion in outpatients with a major depressive disorder. J Clin Psychopharmacol. 1986 6:27–32. [PubMed] [Google Scholar]
- Weisler RH, Johnston JA, and Lineberry CG. et al. Comparison of bupropion and trazodone for the treatment of major depression. J Clin Psychopharmacol. 1994 14:170–179. [PubMed] [Google Scholar]
- Thase ME, Haight BR, and Richard N. et al. Remission rates following antidepressant therapy with bupropion or selective serotonin reuptake inhibitors: a meta-analysis of original data from 7 randomized controlled trials. J Clin Psychiatry. In press. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, and Carmody TJ. et al. Response in relation to baseline anxiety levels in major depressive disorder treated with bupropion sustained release or sertraline. Neuropsychopharmacology. 2002 25:131–138. [DOI] [PubMed] [Google Scholar]
- Weihs L, Houser T, and Batey S. et al. Continuation phase treatment with bupropion SR decreases risk for relapse of depression. Biol Psychiatry. 2002 51:753–761. [DOI] [PubMed] [Google Scholar]
- Bodkin JA, Lasser RA, and Wines JD Jr. et al. Combining serotonin reuptake inhibitors and bupropion in partial responders to antidepressant monotherapy. J Clin Psychiatry. 1997 58:137–145. [DOI] [PubMed] [Google Scholar]
- Spier SA. Use of bupropion with SSRIs and venlafaxine. Depress Anxiety. 1998;7:73–75. [PubMed] [Google Scholar]
- DeBattista C, Solvason HB, and Poirier J. et al. A prospective trial of bupropion SR augmentation of partial and non-responders to serotonergic antidepressants. J Clin Psychopharmacol. 2003 23:27–30. [DOI] [PubMed] [Google Scholar]
- Lam RW, Hossie H, and Solomons K. et al. Citalopram and bupropion-SR: combining versus switching in patients with treatment-resistant depression. J Clin Psychiatry. 2004 65:337–340. [PubMed] [Google Scholar]
- Ferguson J, Cunningham L, and Meridith C. et al. Bupropion in tricyclic nonresponders with unipolar major depressive disorder. Ann Clin Psychiatry. 1994 6:153–169. [DOI] [PubMed] [Google Scholar]
- Stern WC, Harto-Truax N, and Bauer N. Efficacy of bupropion in tricyclic-resistant or intolerant patients. J Clin Psychiatry. 1983 44(sec 2). 148–152. [PubMed] [Google Scholar]
- Fava M, Papakostas GI, and Petersen T. et al. Switching to bupropion in fluoxetine-resistant major depressive disorder. Ann Clin Psychiatry. 2003 15:17–22. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, and Trivedi MH. et al. Background and rationale for the sequenced treatment alternatives to relieve depression (STAR*D) study. Psychiatr Clin North Am. 2003 26:457–494. [DOI] [PubMed] [Google Scholar]
- Dilsaver SC, Qamar AB, Del Medico VJ. The efficacy of bupropion in winter depression: results of an open trial. J Clin Psychiatry. 1992;53:252–255. [PubMed] [Google Scholar]
- Ferry L, Johnston JA. Efficacy and safety of bupropion SR for smoking cessation: data from clinical trials and 5 years of postmarketing experience. Int J Clin Pract. 2003;57:224–230. [PubMed] [Google Scholar]
- Hurt RD, Sachs DP, and Glover ED. et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997 227:1195–1202. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Leischow SJ, and Nides MA. et al. A controlled trial of sustained-release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med. 1999 340:685–691. [DOI] [PubMed] [Google Scholar]
- Tonstad S, Farsang C, and Klaene G. et al. Bupropion SR for smoking cessation in smokers with cardiovascular disease: a multicenter, randomized study. Eur Heart J. 2003 24:946–955. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Kanner RK, and Bailey W. et al. Smoking cessation in patients with chronic obstructive pulmonary disease: a double-blind, placebo-controlled, randomized trial. Lancet. 2001 357:1571–1575. [DOI] [PubMed] [Google Scholar]
- Hays JT, Hurt RD, and Rigotti NA. et al. Sustained-release bupropion for pharmacologic relapse prevention after smoking cessation. Ann Intern Med. 2000 135:423–433. [DOI] [PubMed] [Google Scholar]
- Shopsin B. Bupropion's prophylactic efficacy in bipolar affective illness. J Clin Psychiatry. 1983 44(sec 2). 163–169. [PubMed] [Google Scholar]
- Wright G, Galloway L, and Kim J. et al. Bupropion in the long-term treatment of cyclic mood disorders: mood stabilizing effects. J Clin Psychiatry. 1985 46:22–25. [PubMed] [Google Scholar]
- Sachs GS, Lafer B, and Stoll AL. et al. A double-blind trial of bupropion versus desipramine for bipolar depression. J Clin Psychiatry. 1994 55:391–393. [PubMed] [Google Scholar]
- Guille C, Demopulos C, and Sachs G. Bupropion vs desipramine in the treatment of bipolar depression [poster]. Presented at the 151st annual meeting of the American Psychiatric Association; May 30–June 4, 1998; Toronto, Ontario, Canada. [Google Scholar]
- Wender PH, Reimherr FW. Bupropion treatment of attention-deficit hyperactivity disorder in adults. Am J Psychiatry. 1990;147:1018–1020. doi: 10.1176/ajp.147.8.1018. [DOI] [PubMed] [Google Scholar]
- Barrickman LL, Perry PJ, and Allen AJ. et al. Bupropion versus methylphenidate in the treatment of ADHD. J Am Acad Child Adolesc Psychiatry. 1995 35:649–657. [DOI] [PubMed] [Google Scholar]
- Conners CK, Casat CD, and Gualtieri CT. et al. Bupropion in attention deficit disorder with hyperactivity. J Am Acad Child Adolesc Psychiatry. 1996 34:1314–1321. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Spencer TJ, and Biederman J. et al. A controlled clinical trial of bupropion for attention deficit hyperactivity disorder in adults. Am J Psychiatry. 2001 158:282–288. [DOI] [PubMed] [Google Scholar]
- Gardner EA. Effects of bupropion on weight in patients intolerant to previous antidepressants. Curr Ther Res. 1985;35:188–199. [Google Scholar]
- Gadde KM, Parker CB, and Maner LG. et al. Bupropion for weight loss: investigation of efficacy and tolerability in overweight and obese women. Obes Res. 2001 9:544–551. [DOI] [PubMed] [Google Scholar]
- Jain AK, Kaplan RA, and Gadde KM. et al. Bupropion SR vs placebo for weight loss in obese patients with depressive symptoms. Obes Res. 2002 10:1049–1056. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Greenway FL, and Fujioka K. et al. Bupropion SR enhances weight loss: a 48-week double-blind, placebo-controlled trial. Obes Res. 2002 10:633–641. [DOI] [PubMed] [Google Scholar]
- Anderson JW, Greenway FL. The effect of bupropion sustained release on weight loss in obese adolescents [poster]. Presented at the 157th annual meeting of the American Psychiatric Association; May 1–6, 2004; New York, NY. [Google Scholar]
- Settle EC, Stahl SM, and Batey SR. et al. Safety of sustained-release bupropion in depression: results of three clinical trials. Clin Ther. 1999 21:454–463. [DOI] [PubMed] [Google Scholar]
- Settle EC. Bupropion sustained release: side effect profile. J Clin Psychiatry. 1998 59suppl 4. 32–36. [PubMed] [Google Scholar]
- Thase ME, Haight B, and Hunt T. et al. Bupropion SR does not affect blood pressure of hypertensive patients [poster]. Presented at the 156th annual meeting of the American Psychiatric Association; May 17–22, 2003; San Francisco, Calif. [Google Scholar]
- Stahl SM. Selecting an antidepressant by using mechanism of action to enhance efficacy and avoid side effects. J Clin Psychiatry. 1998 59suppl 18. 23–29. [PubMed] [Google Scholar]
- Fava M, Rankin M. Sexual functioning and SSRIs. J Clin Psychiatry. 2002 63suppl 5. 13–16. [PubMed] [Google Scholar]
- Clayton AH, Pradko JF, and Croft HA. et al. Prevalence of sexual dysfunction among newer antidepressants. J Clin Psychiatry. 2002 63:357–366. [DOI] [PubMed] [Google Scholar]
- Clayton AH, Warnock JK, and Kornstein SG. et al. A placebo-controlled trial of bupropion SR as an antidote for selective serotonin reuptake inhibitor–induced sexual dysfunction. J Clin Psychiatry. 2004 65:62–67. [DOI] [PubMed] [Google Scholar]
- Clayton AH, McGarvey EL, and Abouesh AI. et al. Substitution of an SSRI with bupropion sustained release following SSRI-induced sexual dysfunction. J Clin Psychiatry. 2001 62:185–190. [DOI] [PubMed] [Google Scholar]
- Croft H, Houser TL, and Jamerson B. et al. Effect on body weight of bupropion SR in patients with major depression treated for 52 weeks. Clin Ther. 2002 24:662–672. [DOI] [PubMed] [Google Scholar]


