Abstract
Background and aims:
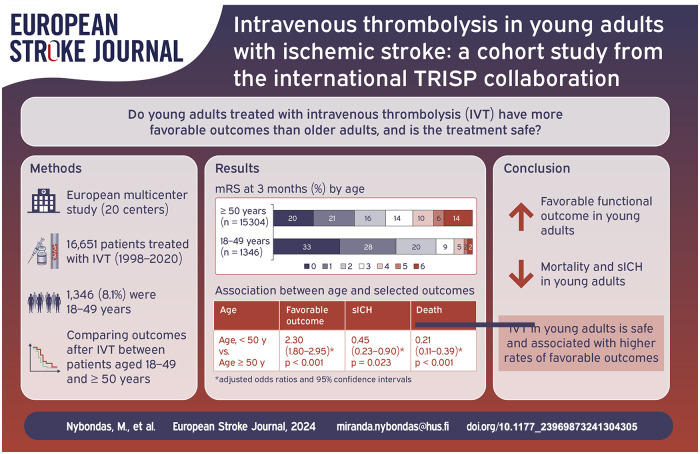
Previous observational data indicate that young adults treated with intravenous thrombolysis (IVT) for acute ischemic stroke have more favorable outcomes and less complications when compared to older adults. Given the limited data on this topic, we aimed to provide more evidence on clinical outcomes and safety in such patients, using a large international thrombolysis registry.
Methods:
In this prospective multicenter study, we used data from the Thrombolysis in Ischemic Stroke Patients (TRISP) registry from 1998 to 2020. Patients who received endovascular treatment (EVT), as only treatment or in addition to IVT, were not included in this cohort. Using multivariable regression models, we compared thrombolysed young patients aged 18–49 years with those aged ⩾50 years with regards to the following outcomes: favorable outcome in stroke survivors (modified Rankin Scale ⩽2), symptomatic intracranial hemorrhage (sICH) according to European Cooperative Acute Stroke Study II (ECASS II) criteria, and three-months all-cause death.
Results:
Of the 16,651 IVT treated patients, 1346 (8.1%) were 18–49 years. Young adults in TRISP were more often male (59.6% vs 54.0%), had a lower median NIHSS score on admission, 7 (4–13) versus 8 (5–15), and had less cardiovascular risk factors except for smoking (42.0% vs 19.0%) when compared to older patients. When compared to thrombolysed patients aged ⩾50 years, a favorable functional outcome was more likely in young adults: 81.9% versus 56.4%, aOR 2.30 (1.80–2.95), whilst sICH 1.6% versus 4.6%, aOR 0.45 (0.23–0.90) and death 2.3% versus 14.2%, aOR 0.21 (0.11–0.39) were less likely.
Conclusions:
Intravenous thrombolysis in young adults is independently associated with higher rates of favorable outcomes and lower rates of complications.
Keywords: Ischemic stroke, outcome, young adults, intravenous thrombolysis
Graphical abstract.
Introduction
Although ischemic stroke (IS) incidence rises with age, stroke in young adults (aged under 50 years by a frequently used definition) is not a rare disease. At least one in 10 ischemic strokes affects younger individuals.1–4 During the last decades the incidence of stroke in young adults has been consistently increasing worldwide regardless of income level, in contrast to the decreasing incidence in older adults.5,6 The reasons for rising stroke incidences in young adults are still unclear.1,7–9
Stroke in young adults has a better prognosis regarding functional outcome than in older patients.10,11 However, young-onset stroke has a major socioeconomic impact because of long-term disability costs and loss of labor, as well as profound effects on patients’ quality of life.7,8,12–14
Compared to older subjects, effective acute treatment may produce substantially more quality-weighted life-years. Current guidelines for management of acute ischemic stroke suggest the same treatment regimen for young adults as well as older patients. Intravenous thrombolysis (IVT) appears to be effective and safe in young adults.15–17 According to recent research, young adults were more likely to achieve functional independence at 3 months compared to older individuals. They had only about half the risk of symptomatic intracranial hemorrhage (sICH) and mortality. However, more evidence in this subject is needed, as earlier studies have been limited by either small sample size or missing data on key outcomes.18,19
The aim of this study was to provide additional data on outcome and safety of IVT in young-onset IS compared to older patients in a large observational international cohort of stroke patients.
Methods
For this cohort study we used prospectively collected data from the Thrombolysis in Ischemic Stroke Patients (TRISP) registry which has been described previously. 20 Altogether 20 centers participated in this study, geographically covering an area from Scandinavia to the Mediterranean and from Eastern to Western Europe. Data was collected locally in each comprehensive stroke center using a standardized form with predefined parameters.21–23 Local study investigators filled in the forms systematically using prospectively ascertained in-hospital stroke databases or thrombolysis registries. Data from the local centers were pooled in the coordinating center Basel University Hospital and analyzed in Helsinki University Hospital. Patients who in addition to IVT received endovascular therapy (EVT), were not included in this cohort.
Patients were excluded from this study if they were younger than 18 years or if data was missing on age, modified Rankin Scale (mRS) at 3 months or sICH. Data included in this study was collected from March 14, 1998 until September 13, 2020. Supplemental Table S1 provides details on number of patients and the study period for each center.
For the present study, the parameters of interest were age, gender, National Institutes of Health Stroke Scale (NIHSS) score, onset-to-treatment time, dependency (mRS ⩾3) before stroke, admission blood pressure and admission glucose level before IVT treatment, admission creatinine level, vascular risk factors according to predefined criteria 24 and a valid prescription or self-reported active use of any antithrombotic agent (antiplatelet or anticoagulant) prior to IVT.
As primary outcome parameter we used favorable functional outcome. Functional outcome was assessed by outpatient visits or telephone calls (with patients or relatives) using the mRS at 3 months. Favorable functional outcome was defined as 3 months mRS 0–2.
As safety outcome parameters we used sICH by European Cooperative Acute Stroke Study II (ECASS II) criteria and all-cause death. ECASS II defines sICH as any intracranial hemorrhage associated with a clinical deterioration leading to an increase of ⩾4 points on the NIHSS. 25 In all centers, intracranial hemorrhage was routinely monitored with a computed tomography or magnetic resonance imaging scan, 24 h ± 12 h from IVT, as well as with additional scans in case of clinical deterioration. 22
Ethics statement
Each center has received necessary official approval from their respective local authorities and/or ethical committees according to national and local rules. This manuscript conforms to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting guideline and checklist.
Statistical analysis
For the main analysis, patients were divided into two age groups: those aged 18–49 years and those aged ⩾50 years. Patients aged ⩾50 years served as a reference group. For the categorical variables we used χ2-test or Fishers exact test when appropriate. For continuous variables we used Mann–Whitney U test. For continuous variables, data was summarized as median and interquartile range (IQR). The association of age (as a categorical variable as defined above) with chosen outcomes was estimated by calculating the odds ratios (ORs) with 95% confidence intervals (CIs) using binary logistic regression models. All covariates with an association to the selected outcomes with a p-value ⩽0.05 in the univariate analysis were included in the multivariate analyses. Statistical analyses were performed using SPSS Statistics (IBM version 29.0.0.0 (241)).
For a secondary analysis, we divided young adults into two subgroups aged 18–30 and 31–49 years and performed the same statistical analysis as described above.
Furthermore, we examined stroke etiology by modified Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification 26 in those aged 18–49 years compared to those aged ⩾50 years. In the young adult patient population, we also examined the association of TOAST-classification to functional outcome.
Results
Study population
Data was available for 17,919 IVT-treated patients, of which 16,651 were eligible for the analysis. Pediatric and adolescent patients aged <18 years (n = 28) were excluded from this study. Other reasons for exclusion were missing data on mRS score at 3 months (n = 864) and missing data on sICH (n = 376). The total number of excluded patients was 1268 (7.1%) (Supplemental Figure S1).
Baseline characteristics
In the included study population, 1346 (8.1%) patients were 18–49 years and 15,305 (91.9%) patients were ⩾50 years. The median age in the group of younger patients was 43 (37–47) years, versus 74 (65–82) years in the group of patients aged ⩾50 years. Patients in the younger group were more often male (59.6% vs 54.0% p < 0.001), had a lower median NIHSS score on admission (7 [4–13] vs 8 [5–15], p < 0.001), and were less often dependent (defined as pre-stroke mRS ⩾3) before the index stroke (1.2% vs 10.6% p < 0.001). Their median onset-to-treatment time was comparable (140 [100–190] min vs 148 [105–195] min, p = 0.001). They had lower systolic blood pressure, glucose, and creatinine levels on admission. They also had less cardiovascular risk factors, that is, hypertension, hypercholesterolemia, atrial fibrillation, diabetes mellitus, prior ischemic stroke, and coronary artery disease. They were also less likely to be using any prior antithrombotic medication. However, the patients in the younger group were more likely to be either current smokers or having stopped within 2 years (42.0% vs 19.0% p < 0.001) (Table 1).
Table 1.
Comparison of selected baseline characteristics and primary outcomes between younger and older patients.
| Age 18–49 years (n = 1346) | Age ⩾50 years (n = 15305) | Total (n = 16651) | p-Value | |
|---|---|---|---|---|
| Age (years) | 43 (37–47) | 74 (65–82) | 73 (62–81) | <0.001 |
| Men | 802 (59.6) | 8268 (54.0) | 9070 (54.5) | <0.001 |
| Stroke severity (NIHSS) | 7 (4–13) | 8 (5–15) | 8 (5–15) | <0.001 |
| Dependent before stroke (pre-mRS score 3–5) | 14 (1.2) | 1435 (10.6) | 1449 (9.8) | <0.001 |
| Onset-to-treatment time (min) | 140 (100–190) | 148 (105–195) | 147 (105–195) | 0.002 |
| Admission systolic blood pressure (mmHg) | 140 (125–152) | 157 (140–173) | 155 (140–172) | <0.001 |
| Admission glucose (mmol/L) | 6.0 (5.4–6.9) | 6.6 (5.8–8.0) | 6.6 (5.7–8.0) | <0.001 |
| Admission creatinine (µmol/L) | 74 (64–86) | 82 (69–99) | 81 (68–98) | <0.001 |
| Atrial fibrillation | 40 (3.5) | 3896 (28.2) | 3936 (26.3) | <0.001 |
| Hypertension | 325 (24.2) | 11,139 (72.9) | 11,464 (69.0) | <0.001 |
| Current (or stopped <2 years) smoking | 534 (42.0) | 2664 (19.0) | 3198 (20.9) | <0.001 |
| Hypercholesterolemia | 320 (23.8) | 7088 (46.5) | 7408 (44.7) | <0.001 |
| Diabetes | 67 (5.0) | 3158 (20.7) | 3225 (19.4) | <0.001 |
| Coronary artery disease | 72 (5.4) | 2939 (19.3) | 3011 (18.1) | <0.001 |
| Prior ischemic stroke | 93 (6.9) | 2418 (15.8) | 2511 (15.1) | <0.001 |
| Prior antithrombotics, any | 147 (11.2) | 7215 (48.3) | 7362 (45.3) | <0.001 |
| sICH (ECASS2) | 22 (1.6) | 700 (4.6) | 722 (4.3) | <0.001 |
| Death | 31 (2.3) | 2167 (14.2) | 2198 (13.2) | <0.001 |
| Favorable functional outcome* | 1102 (81.9) | 8626 (56.4) | 9728 (58.4) | <0.001 |
| Poor functional outcome† | 190 (16.1) | 3612 (30.9) | 3802 (29.5) | <0.001 |
ECASS indicates European Cooperative Acute Stroke Study; IVT: intravenous thrombolysis; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; and sICH: symptomatic intracerebral hemorrhage (ECASS II definition).
Data expressed as either n (%) or median (interquartile range). Cohort of 16,651 IVT treated patients aged 18–105 years. Multicenter data collected in the years of 1998–2020.
Favorable functional outcome = mRS 3 months score ⩽2.
Poor functional outcome = mRS 3 months score 3–5 for patients with prestroke mRS score ⩽2 and mRS score 4–5 for patients with prestroke mRS score >2.
Age 18–49 years versus age ⩾50 years
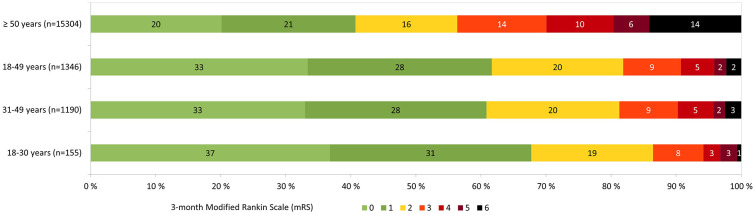
In the group of patients aged 18–49 years a favorable functional outcome occurred more often, 81.9% versus 56.4%, whilst sICH 1.6% versus 4.6% and death 2.3% versus 14.2% were rare when compared to the reference group of patients ⩾50 years (Table 1 and Supplemental Table S2). After adjusting for potential confounders, including NIHSS on admission, dependency before stroke, onset-to-treatment-time, systolic blood pressure on admission, creatinine on admission, glucose on admission, atrial fibrillation, diabetes, current smoking or stopped within 2 years, prior antithrombotics and coronary artery disease, the probability of a favorable functional outcome remained significantly higher, aOR 2.30 (1.80–2.95). Probability of sICH remained significantly lower, aOR 0.45 (0.23–0.90). Probability of death was significantly lower, aOR 0.21 (0.11–0.39) when compared to patients ⩾50 years (Tables 2 and Supplemental Table S3). The distribution of mRS at 3 months in different age groups is presented in Figure 1 and Supplemental Tables S4 and S5.
Table 2.
Multivariate analysis on association between age and selected outcome variables.
| Age | Favorable functional outcome* | sICH | Death |
|---|---|---|---|
| Age, <50 years versus age ⩾50 years | 2.30 (1.80–2.95) p < 0.001† | 0.45 (0.23–0.90) p = 0.023‡ | 0.21 (0.11–0.39) p < 0.001† |
| Age, 18–29 years versus age 30–49 years | 1.82 (0.69–4.77) p = 0.224† | 0.38 (0.05–2.89) p = 0.278§ | 0.57 (0.07–4.36) p = 0.586ǁ |
ECASS indicates European Cooperative Acute Stroke Study; IVT: intravenous thrombolysis; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; and sICH: symptomatic intracerebral hemorrhage (ECASS II definition).
Results are expressed as odds ratio (OR) and 95 % confidence interval (CI). Results are adjusted for all covariates that had a p-value of 0.05 or less in the univariate analysis. Cohort of 16,651 IVT treated patients aged 18–105 years. Multicenter data collected in the years of 1998–2020.
Favorable functional outcome = mRS 3 months score ⩽2.
Adjusted for: sex, NIHSS on admission, dependent before stroke (mRS 3–5), onset-to-treatment-time, systolic blood pressure on admission, creatinine on admission, glucose on admission, atrial fibrillation, diabetes, hypercholesterolemia, current smoking or stopped within 2 years, prior ischemic stroke, prior antithrombotics, coronary artery disease.
Adjusted for: NIHSS on admission, systolic blood pressure on admission, creatinine on admission, glucose on admission, atrial fibrillation, diabetes, current smoking or stopped within 2 years, prior antithrombotics, coronary artery disease.
Adjusted for: NIHSS on admission, prior antithrombotics.
Adjusted for: NIHSS on admission, prior antithrombotics, atrial fibrillation.
Figure 1.
Distribution of modified Rankin Scale (mRS) at 3 months in intravenous thrombolysis treated stroke patients aged for peer review ⩾50, 18–49, 31–49 and 18–30 years.
18–29 years versus 30–49 years
We divided young patients into two subgroups: early adulthood, 18–29 years (n = 155) and middle adulthood, 30–49 years (n = 1191). The median age in the early adulthood group was 26 (23–28) years and 44 (39–47) years in the middle adulthood group. The patients in the early adulthood group were more often female (51.0% vs 38.9%) compared to the middle adulthood group. They had lower systolic blood pressure, glucose, and creatinine levels on admission. They had significantly less hypertension and hypercholesterolemia. They also had a lower frequency of other risk factors such as atrial fibrillation, smoking, and diabetes, but the difference between groups was not significant. The onset-to-treatment time between groups was comparable (146 vs 140 min). The patients in the early adulthood group were non-significantly more likely to have a favorable functional outcome (86.5% vs 81.3%), whereas sICH (0.6% vs 1.8%), and death (0.6% vs 2.5%) were less likely when compared to the middle adulthood group (Supplemental Table S6). In the adjusted analysis, very young age was not significantly associated with any of the outcomes when compared to the group of patients aged 30–49 years: favorable functional outcome, aOR 1.82 (0.69–4.77), sICH, aOR 0.38 (0.05–2.89), or death, aOR 0.57 (0.07–4.36) (Table 2).
Stroke etiology
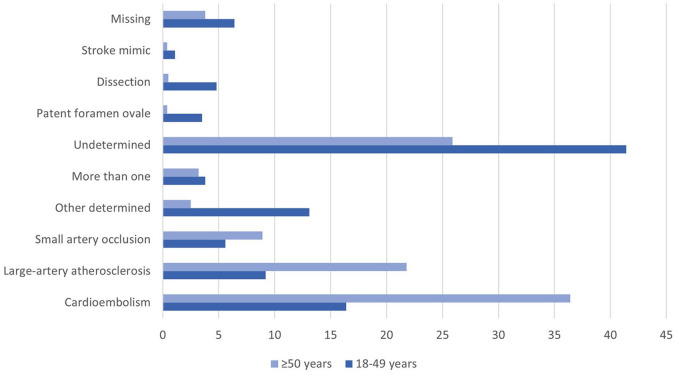
Cardioembolism (CE) was less common in young patients compared to older patients (16.4% vs 36.4%, p < 0.001). Likewise large-artery atherosclerosis (LAA) and small-artery occlusion (SAO) were less frequent in young adults versus older patients (9.2% vs 21.8%, p < 0.001 and 5.6% vs 8.9%, p < 0.001, respectively). Stroke of other or undetermined origin, cervical artery dissection, and stroke associated with patent foramen ovale (PFO) were more prevalent etiologies in young adults when compared to older adults. The results are presented in Supplemental Table S7 and Figure 2.
Figure 2.
Etiology in fractions by age group, modified TOAST classification.
Treatment before and after January 1, 2015
Finally, we did a subgroup analysis of patients treated before or after January 1, 2015, when EVT became a more common practice. The patients treated after January 1, 2015 had a lower median NIHSS score on admission 6 (4–11) versus 9 (5–16) and their outcomes were better: favorable outcome (62.4% vs 56.5%), sICH (3.4% vs 4.8%) and mortality (11.1% vs 14.1%) compared to those treated before 2015. In the multivariate analysis the difference between young adults and older patients was somewhat more evident for those treated after January 1, 2015 regarding favorable outcome, aOR 3.05 (1.66–5.59) versus 2.12 (1.62–2.80) compared to those treated before 2015. The results are presented in Supplemental Table S8 and S9.
Discussion
Our study showed that younger patients treated with IVT for acute IS had a higher chance of a favorable functional outcome at 3 months and a lower risk of sICH and death, when compared to older individuals also treated with IVT. These results remained robust after adjustment for several potential confounders.
Patients aged 18–49 years were 2.3 times more likely to achieve favorable functional outcome. This association is complementary when weighed against previous evidence. A large study from the Safe Implementation of Thrombolysis in Stroke–International Stroke Thrombolysis Register (SITS-ISTR) analyzed 3246 young patients aged between 18–50 years treated with IVT compared to patients aged 51–80 years. They reported that young adults were 1.6 times more likely to reach functional independence (mRS 3 months 0–2) compared to older patients. 18 The more favorable results in our study are likely explained by the difference in median admission NIHSS score in young patients, 7 in our study versus 10 in SITS-ISTR. The exclusion of patients treated with EVT is a probable reason for milder symptoms on admission in our cohort. Also, this might reflect that we currently treat patients with minor IS more frequently than in the past, the TRISP registry containing more recent data compared to the SITS-ISTR (collected 1998–2020 vs 2002–2010). Additionally, the centers participating in the registry are tertiary centers specialized in acute stroke care, with highly optimized IVT admission protocols.
A previous observational study compared IVT treated patients with age matched controls not treated with IVT. They analyzed 4140 young adult ischemic stroke patients of which 340 (8.2%) received IVT. Their results provided evidence that IVT is at least equally effective in young adults compared to older individuals and the highest benefit was observed in very young patients aged 18–30 years. 17 A recent study from The get with the guidelines – stroke registry (GWTG-Stroke registry) enrolled 30,448 young-onset IS patients, of which 3806 (12.5%) were treated with IVT within 4.5 h. Since their definition of young adults was different (18–40 years), numbers are not directly comparable to ours. They showed that young patients treated with IVT more frequently had a mRS score 0–2 at discharge when compared to older patients, 69.3% versus 41.1% respectively. 19 However, both studies were limited by not having data on mRS at 3 months.
Our study showed that risk of sICH was only about half as high compared to older adults, 1.6% and 4.6%, respectively. The proportion of sICH by ECASS II definition in young adults in previous studies has been similar, 1.7% versus 4.5% (GWTG-Stroke registry study, 18–40 vs >40 years) and 2.2% versus 5.4% (SITS-ISTR study, 18–50 vs 51–80 years). 19 Indeed, the incidence of sICH has been reported to increase with every 10-year age band from <30 to 80 years, potentially plateauing after that.18,21
Mortality rates have been previously found to be significantly lower in young adults with ischemic stroke treated with IVT. In our analysis the mortality of patients aged 18–49 was 2.3% versus 14.2% in patients aged 50 years or more. In comparison, results from SITS-ISTR showed a higher (4.9%) three-month mortality for young adults while the mortality rate for older patients was similar (14.4%). 18 The lower mortality in our study might be explained by the more recent data and difference in admission NIHSS score as discussed above.
Compared to older adults, young patients had slightly lower median NIHSS score on admission, and they were less often dependent prior to stroke. As expected, they had less cardiovascular risk factors, except for smoking. Smoking rate in young adults was high (42%). This might in part reflect high smoking prevalence in the population, especially during earlier years of data collection. However, previous research shows that the relative risk of stroke among smokers is highest in middle age, declining with advancing years.27,28 Similar to our results, a recent Japanese study found a very high rate of smoking in young adult stroke patients (64%). 29 Smoking had an unexpected favorable effect on all outcomes. However, this is not to be interpreted as an actual protecting effect of smoking, since this might be explained by a significantly lower median age in smokers (61.8 vs 73.2 years, Supplemental Table S10 and S11). A diagnosis of hypercholesterolemia on admission also had a surprising favorable effect on mortality and poor functional outcome. This could be due to statin use prior to stroke (51.2% vs 9.7%, Supplemental Table S12), or it could be speculated that they had otherwise better primary prevention related to the patient having to visit healthcare to receive the diagnosis.
A larger variety of etiologies and risk factors are to be considered in young stroke patients.1,8 As expected, we found that CE, LAA, and SAO were less frequent etiologies in patients aged 18–49 years. In contrast, arterial dissection, PFO, stroke of other determined etiology, and cryptogenic strokes were more common in young adults. It can be speculated, that differences in stroke etiology could in part explain better outcomes in young adults. However, we did not find evidence to support this, as stroke of other determined etiology in young adults was significantly associated with a lower probability of a favorable outcome and SAO was associated with the highest chance of a favorable outcome (Supplemental Table S13).
In the subgroup analysis, patients treated after January 1, 2015 (when EVT became more common practice), had more favorable outcomes, compared to those treated before 2015. This was probably largely due to significantly lower median NIHSS score in the patients treated after January 1, 2015.
In summary, the current set of findings are reassuring and complementary to previous studies. The results are corroborative regarding higher rates of favorable functional outcomes and good safety of IVT in young-onset IS.
The strengths of this study are: (1) a large sample size of 16,651 consecutive IVT treated patients of which 1346 were 18–49 years, which allowed for adjustment of confounders, (2) the small number of excluded patients, only 6.9% of the whole study population, (3) low frequencies of missing data on key variables, (4) participating centers being tertiary centers with high stroke expertise and optimized protocols for IVT treatment, and (5) having 3 months mRS outcome.
However, this study also has limitations. All patients were treated with IVT, so a comparison with non-IVT treated patients was not possible, and hence no treatment effect could be calculated. Consequently, it is difficult to assess how much of the favorable effect on outcomes is due to treatment effect, and how much to young age itself. However, a randomized trial with this setting would be hard to justify ethically. Furthermore, since patients treated with EVT were not included in this cohort, it could have led to a degree of selection bias. Still, it is not likely that this would have significantly changed the difference in outcomes between young and older adults. Preference of modality for the first imaging on admission (CT vs MR) varies between centers and according to patient’s age. However, this information was not available in our database, so we were not able to stratify for this. Finally, although data was entered prospectively following certain criteria, the retrospective design of the study may have caused selection bias and data under-report.
In conclusion, our results showed that young adults receiving IVT have a significantly higher chance of favorable functional outcome, compared to those aged ⩾50 years receiving IVT, even after adjustment for multiple confounders. IVT is safe in young-onset IS and the risk of sICH should be of minor concern.
Supplemental Material
Supplemental material, sj-docx-1-eso-10.1177_23969873241304305 for Intravenous thrombolysis in young adults with ischemic stroke: A cohort study from the international TRISP collaboration by Miranda Nybondas, Nicolas Martinez-Majander, Peter Ringleb, Matthias Ungerer, Christoph Gumbinger, Simon Trüssel, Valerian Altersberger, Jan F Scheitz, Regina von Rennenberg, Christoph Riegler, Charlotte Cordonnier, Andrea Zini, Guido Bigliardi, Francesca Rosafio, Patrik Michel, Nabila Wali, Paul J Nederkoorn, Mirjam Heldner, Marialuisa Zedde, Rosario Pascarella, Visnja Padjen, Ivana Berisavac, Yannick Béjot, Jukka Putaala, Gerli Sibolt, Marjaana Tiainen, Laura Mannismäki, Tuomas Mertsalmi, Elina Myller, Alessandro Pezzini, Ronen R Leker, Georg Kägi, Susanne Wegener, Carlo W Cereda, Annika Nordanstig, George Ntaios, Christian H Nolte, Henrik Gensicke, Stefan T Engelter and Sami Curtze in European Stroke Journal
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Nicolas Martinez-Majander reports funding from the Finnish Medical Foundation.
Jukka Putaala reports personal fees from Bayer, Boehringer-Ingelheim, BMS-Pfizer, Abbott; Advisory board: Portola, Novo Nordisk, and Herantis Pharma, outside the submitted work.
Yannick Béjot reports personal fees from BMS, Pfizer, Medtronic, Amgen, Boehringer-Ingelheim, Servier, NovoNordisk, Novartis, outside the submitted work.
Peter Ringleb received speaker and advisory board honoraria from Boehringer Ingelheim, Bayer, BMS and Pfizer paid to the institution.
Christoph Gumbinger received Research support from the Gesellschaft für Internationale Zusammenarbeit (GIZ), European Stroke Research Foundation (paid to the institution) and Study compensation from AstraZeneca/Alexion/ (paid to the institution); all outside the submitted work.
Matthias Ungerer has received research support from the European Stroke Research Foundation (paid to the institution), outside the submitted work.
Marialuisa Zedde reports personal fees from Amicus, Sanofi Genzyme, Takeda, outside the submitted work.
Christian H Nolte reports personal fees from AstraZeneca/Abbot, Alexion,/ (paid to the institution);, Astra-Zeneca, Bristol-Myers Squibb, Daiichi Sankyo, Novartis, Pfizer, Portola and Takeda, all outside the submitted work.
Ronen R Leker reports personal fees from IschemaView, Filterlex, Bayer, Biogen, Boeringer-Ingelheim, Abott, all outside of the submitted work.
Visnja Padjen reports travel or speaker honoraria from Boehringer Ingelheim; honoraria from scientific advisory board from Medtronic, outside the submitted work.
Andrea Zini reports speaker honoraria from CSL Behring, Alexion-Astra Zeneca and Daiichi Sankyo and advisory board honoraria from Bayer, Astra Zeneca, all outside of the submitted work.
Susanne Wegener reports speaker honoraria from Amgen, Springer, Teva Pharma, ADVISIS-AG, FOMF, Astra Zeneca, and a consultancy fee from Bayer and Novartis; all outside this work.
Miriam Heldner reports grants from SITEM Research Support Funds and Swiss National Science Foundation, Swiss Heart Foundation, not directly related to this manuscript.
Ivana Berisavac reports speaker honoraria from Medtronic.
George Ntaios reports advisory boards/research support/speaker fees from Abbott, Amgen, AstraZeneca, Boehringer Ingelheim, Javelin Medical, Novartis, and Sanofi; Clinical trial steering/executive committees for Janssen and Javelin Medical. All paid directly to the University of Thessaly. All outside of the submitted work.
The other authors report nothing to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: HUS Helsinki University Hospital.
Ethical approval and informed consent: Each center has received necessary official approval from their respective local authorities and/or ethical committees according to national and local rules. This manuscript conforms to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) reporting guideline and checklist.
Guarantor: Sami Curtze
Contributorship: MN, NM and SC researched literature and conceived the study. The multicenter data was pooled by HG and SE. MN performed data analysis and wrote the first draft of the manuscript. All authors participated in collecting the data and reviewed and edited the manuscript and approved the final version of the manuscript.
ORCID iDs: Miranda Nybondas  https://orcid.org/0009-0001-2030-7283
https://orcid.org/0009-0001-2030-7283
Nicolas Martinez-Majander  https://orcid.org/0000-0001-8489-7051
https://orcid.org/0000-0001-8489-7051
Jan F Scheitz  https://orcid.org/0000-0001-5835-4627
https://orcid.org/0000-0001-5835-4627
Christoph Riegler  https://orcid.org/0000-0002-2478-3500
https://orcid.org/0000-0002-2478-3500
Charlotte Cordonnier  https://orcid.org/0000-0002-5697-6892
https://orcid.org/0000-0002-5697-6892
Nabila Wali  https://orcid.org/0009-0000-5410-7805
https://orcid.org/0009-0000-5410-7805
Visnja Padjen  https://orcid.org/0000-0002-6126-8305
https://orcid.org/0000-0002-6126-8305
Yannick Béjot  https://orcid.org/0000-0001-7848-7072
https://orcid.org/0000-0001-7848-7072
Marjaana Tiainen  https://orcid.org/0000-0001-5107-1990
https://orcid.org/0000-0001-5107-1990
Laura Mannismäki  https://orcid.org/0000-0002-3696-7219
https://orcid.org/0000-0002-3696-7219
Alessandro Pezzini  https://orcid.org/0000-0001-8629-3315
https://orcid.org/0000-0001-8629-3315
Ronen R Leker  https://orcid.org/0000-0003-4794-0334
https://orcid.org/0000-0003-4794-0334
Susanne Wegener  https://orcid.org/0000-0003-4369-7023
https://orcid.org/0000-0003-4369-7023
George Ntaios  https://orcid.org/0000-0002-0629-9248
https://orcid.org/0000-0002-0629-9248
Christian H Nolte  https://orcid.org/0000-0001-5577-1775
https://orcid.org/0000-0001-5577-1775
Supplemental material: Supplemental material for this article is available online.
References
- 1. Ekker MS, Boot EM, Singhal AB, et al. Epidemiology, aetiology, and management of ischaemic stroke in young adults. Lancet Neurol 2018; 17: 790–801. [DOI] [PubMed] [Google Scholar]
- 2. Boot E, Ekker MS, Putaala J, et al. Ischaemic stroke in young adults: a global perspective. J Neurol Neurosurg Psychiatry 2020; 91: 411–417. [DOI] [PubMed] [Google Scholar]
- 3. Marini C, Russo T, Felzani G. Incidence of stroke in young adults: a review. Stroke Res Treat. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3010685/ (2011, accessed 18 October 2023). [DOI] [PMC free article] [PubMed]
- 4. Sarfo FS, Ovbiagele B, Gebregziabher M, et al. Stroke among young West Africans: evidence from the SIREN large multi-site case-control study. Stroke 2018; 49: 1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Béjot Y, Delpont B, Giroud M. Rising stroke incidence in young adults: more epidemiological evidence, more questions to be answered. J Am Heart Assoc Cardiovasc Cerebrovasc Dis 2016; 5: e003661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol 2021; 20: 795–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Putaala J. Ischemic stroke in young adults. Contin Lifelong Learn Neurol 2020; 26: 386. [DOI] [PubMed] [Google Scholar]
- 8. Maaijwee NAMM, Rutten-Jacobs LCA, Schaapsmeerders P, et al. Ischaemic stroke in young adults: risk factors and long-term consequences. Nat Rev Neurol 2014; 10: 315–325. [DOI] [PubMed] [Google Scholar]
- 9. Li L, Scott CA, Rothwell PM. Association of younger vs older ages with changes in incidence of stroke and other vascular events, 2002–2018. J Am Med Assoc 2022; 328: 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goeggel Simonetti B, Mono ML, Huynh-Do U, et al. Risk factors, aetiology and outcome of ischaemic stroke in young adults: the Swiss Young Stroke Study (SYSS). J Neurol 2015; 262: 2025–2032. [DOI] [PubMed] [Google Scholar]
- 11. Putaala J, Curtze S, Hiltunen S, et al. Causes of death and predictors of 5-year mortality in young adults after first-ever ischemic stroke. Stroke 2009; 40: 2698–2703. [DOI] [PubMed] [Google Scholar]
- 12. Maaijwee NAMM, Rutten-Jacobs LCA, Arntz RM, et al. Long-term increased risk of unemployment after young stroke: a long-term follow-up study. Neurology 2014; 83: 1132–1138. [DOI] [PubMed] [Google Scholar]
- 13. Andersen G, Christensen D, Kirkevold M, et al. Post-stroke fatigue and return to work: a 2-year follow-up. Acta Neurol Scand 2012; 125: 248–253. [DOI] [PubMed] [Google Scholar]
- 14. Hannerz H, Holbæk Pedersen B, Poulsen OM, et al. A nationwide prospective cohort study on return to gainful occupation after stroke in Denmark 1996–2006. BMJ Open 2011; 1: e000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Putaala J, Metso TM, Metso AJ, et al. Thrombolysis in young adults with ischemic stroke. Stroke 2009; 40: 2085–2091. [DOI] [PubMed] [Google Scholar]
- 16. Poppe AY, Buchan AM, Hill MD. Intravenous thrombolysis for acute ischaemic stroke in young adult patients. Can J Neurol Sci J Can Sci Neurol 2009; 36: 161–167. [DOI] [PubMed] [Google Scholar]
- 17. Reuter B, Gumbinger C, Sauer T, et al. Intravenous thrombolysis is effective in young adults: results from the Baden-Wuerttemberg stroke registry. Front Neurol 2015; 6: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Toni D, Ahmed N, Anzini A, et al. Intravenous thrombolysis in young stroke patients: results from the SITS-ISTR. Neurology 2012; 78: 880–887. [DOI] [PubMed] [Google Scholar]
- 19. Dodds JA, Xian Y, Sheng S, et al. Thrombolysis in young adults with stroke: findings from get with the guidelines-stroke. Neurology 2019; 92: e2784–e2792. [DOI] [PubMed] [Google Scholar]
- 20. Scheitz JF, Gensicke H, Zinkstok SM, et al. Cohort profile: thrombolysis in Ischemic Stroke Patients (TRISP): a multicentre research collaboration. BMJ Open 2018; 8: e023265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Altersberger VL, Rusche N, Martinez-Majander N, et al. Intravenous thrombolysis in patients with ischemic stroke aged ⩾90 years: a cohort study from the TRISP collaboration. Stroke 2022; 53: 3557–3563. [DOI] [PubMed] [Google Scholar]
- 22. Engelter ST, Soinne L, Ringleb P, et al. IV thrombolysis and statins. Neurology 2011; 77: 888–895. [DOI] [PubMed] [Google Scholar]
- 23. Engelter ST, Rutgers MP, Hatz F, et al. Intravenous thrombolysis in stroke attributable to cervical artery dissection. Stroke 2009; 40: 3772–3776. [DOI] [PubMed] [Google Scholar]
- 24. Fluri F, Hatz F, Voss B, et al. Restenosis after carotid endarterectomy: significance of newly acquired risk factors. Eur J Neurol 2010; 17: 493–498. [DOI] [PubMed] [Google Scholar]
- 25. Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian acute stroke study investigators. Lancet Lond Engl 1998; 352: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 26. Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke 1993; 24: 35–41. [DOI] [PubMed] [Google Scholar]
- 27. Girot M. Smoking and stroke. Presse Medicale Paris Fr 1983 2009; 38: 1120–1125. [DOI] [PubMed] [Google Scholar]
- 28. Hankey GJ. Smoking and risk of stroke. J Cardiovasc Risk 1999; 6: 207–211. [DOI] [PubMed] [Google Scholar]
- 29. Ohya Y, Matsuo R, Sato N, et al. Causes of ischemic stroke in young adults versus non-young adults: A multicenter hospital-based observational study. PLoS One 2022; 17:e0268481. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-eso-10.1177_23969873241304305 for Intravenous thrombolysis in young adults with ischemic stroke: A cohort study from the international TRISP collaboration by Miranda Nybondas, Nicolas Martinez-Majander, Peter Ringleb, Matthias Ungerer, Christoph Gumbinger, Simon Trüssel, Valerian Altersberger, Jan F Scheitz, Regina von Rennenberg, Christoph Riegler, Charlotte Cordonnier, Andrea Zini, Guido Bigliardi, Francesca Rosafio, Patrik Michel, Nabila Wali, Paul J Nederkoorn, Mirjam Heldner, Marialuisa Zedde, Rosario Pascarella, Visnja Padjen, Ivana Berisavac, Yannick Béjot, Jukka Putaala, Gerli Sibolt, Marjaana Tiainen, Laura Mannismäki, Tuomas Mertsalmi, Elina Myller, Alessandro Pezzini, Ronen R Leker, Georg Kägi, Susanne Wegener, Carlo W Cereda, Annika Nordanstig, George Ntaios, Christian H Nolte, Henrik Gensicke, Stefan T Engelter and Sami Curtze in European Stroke Journal