Abstract
Pain and alcohol use disorder (AUD) frequently co‐occur, but the underlying neurobiology is not well‐understood. Although many studies have reported disruptions in stress and reward cue‐elicited neural reactivity and heightened alcohol craving in individuals with AUD, little is known about these constructs among patients who experience pain. Here, individuals with pain (Pain+, n = 31) and without pain (Pain−, n = 37) completed a well‐validated functional magnetic resonance imaging (fMRI) paradigm involving stress (S), alcohol (A) and neutral (N) cue exposure with repeated alcohol craving assessments. Using whole‐brain, voxel‐based analyses (p < 0.001, whole‐brain cluster correction at α < .05), the Pain+ versus Pain− group evidenced greater dorsal anterior cingulate cortex and left amygdala hyperactivation during N, but hypoactivation during the S‐N contrast. Additionally, Pain+ exhibited blunted right anterior insular cortex (AIC) during S‐N and blunted anteromedial thalamus and left AIC with hyperactive orbitofrontal cortex (OFC) during A‐N. Exploratory analyses further revealed that individuals with pain and AUD (n = 17) relative to pain alone (n = 14) showed hyperactive bilateral AIC and hypoactive right dorsal caudate during A‐N. Alcohol cue‐induced craving, significantly higher in Pain+ (p = 0.03), correlated with blunted right AIC and OFC responses during A‐N. In sum, these results provide first evidence of heightened alcohol cue‐elicited craving and disrupted stress‐ and alcohol cue‐reactivity within corticostriatal‐limbic regions implicated in negative affect and preoccupation/anticipation stages of AUD in those with pain and with comorbid pain and AUD. Future investigations of pain‐AUD interaction are needed that include systematic pain assessment and longitudinal designs with larger sample sizes.
Keywords: alcohol craving, AUD, fMRI, insula, pain, stress and alcohol cue reactivity
Pain and alcohol use disorder (AUD) frequently co‐occur, yet their shared brain processes remain understudied. This functional brain imaging study identified disrupted responses to stress and alcohol cues in stress and reward processing regions among individuals with pain, particularly those with co‐occurring AUD. Heightened alcohol craving in the pain group was associated with altered activity in interoceptive (anterior insula) and choice and decision‐making (orbitofrontal cortex) regions, underscoring the need for further research to explore these interactions in larger, longitudinal studies.

1. INTRODUCTION
Pain and alcohol use disorder (AUD) are frequently comorbid conditions that pose significant public health and socioeconomic burden. 1 In the United States alone, the estimated prevalence of recurring pain among treatment‐seeking patients with AUD is between 30% and 50%. 2 , 3 , 4 In addition, treatment‐seeking pain patients tend to misuse alcohol (partly due to its analgesic properties) and report greater incidences of AUD than the general population. 5 , 6 , 7 However, excessive alcohol use may also cause hyperalgesia, and attempts to alleviate this heightened pain state could further contribute to alcohol misuse and AUD risk. 8 It therefore appears that alcohol and pain are mutually reinforcing wherein alcohol intake modulates pain and pain influences alcohol‐related behaviours. Improving our understanding of the underlying neural mechanisms linking pain and AUD would be an important first step towards advancement of prevention and treatment efforts for pain and AUD comorbidity.
Numerous studies have independently associated AUD with disrupted neural circuitry implicated in reward and stress responding, resulting in diminished impact of rewarding stimulation and reduced ability to control stress response (i.e., reward deficit and stress surfeit). 9 , 10 , 11 For example, significant maladaptations in the medial prefrontal cortex (PFC), striatal circuits as well as amygdala and hippocampal limbic regions have been observed 9 , 10 , 11 , 12 , 13 and these maladaptations were further associated with high stress‐ and cue‐related alcohol craving, thereby predicting greater relapse and AUD treatment failure risk. 12 , 13 , 14 Although many studies have reported heightened alcohol craving and cue reactivity in individuals with AUD when exposed to stress and alcohol cues in a laboratory or their environment, 12 , 13 little is known about this phenomenon among patients who experience pain.
One theory suggests that pain may similarly impact reward and stress systems such that, over time, the rewarding properties of pain relief may diminish and pain‐related negative affect and stress may become more overwhelming. 15 This may be in part due to substantial overlap between the neural substrates of pain, stress and reward processing, particularly in the regulatory prefrontal cortices (e.g., ventromedial PFC/vmPFC), salience (e.g., insula, dorsal anterior cingulate cortex/dACC) and related emotion processing striatal‐limbic regions (e.g., amygdala, ventral and dorsal striatum). 8 , 16 , 17 , 18 , 19 Furthermore, regions involved in pain processing resemble those involved in the withdrawal/negative affect and preoccupation/anticipation stages of AUD, suggesting the importance of incentive salience and stress/emotion dysfunction in both disorders and a potential rationale for their comorbidity. 5 , 20 In particular, the interoceptive insular regions involved in pain sensing may drive pain‐specific negative reinforcement, such as pain avoidance, cue‐related craving and pain‐related heavy drinking. However, no prior research has assessed chronic alcohol effects on these circuits, such as the insula, separate from the common stress and pain regulation PFC‐striatal circuits, to determine how distinct maladaptations of the reward and stress systems may contribute to the development of pain‐AUD comorbidity.
Thus, the present study had two main goals. Using our well‐validated functional magnetic resonance imaging (fMRI) task contrasting stress (S), alcohol (A) and neutral (N) control visual stimulus conditions presented in a block design with repeated alcohol craving assessment, 12 , 13 we first examined whether subjective alcohol craving and stress‐ and cue‐elicited neural responses within corticostriatal‐limbic network were altered in adults who experienced pain versus those who did not. Second, we explored whether individuals with pain who had AUD (Pain + AUD) responded differently to these cues compared with those without AUD (Pain only). We then conducted an exploratory whole‐brain correlation analysis to identify possible associations between alcohol craving and pain‐specific alterations in the cue‐elicited neural responses. We hypothesized that adults with pain would report greater subjective alcohol craving relative to those without pain, particularly during alcohol cue exposure. Based on prior work, we further expected that pain individuals may experience blunted corticostriatal‐limbic functioning and that, among these individuals, blunted insular activity would be associated with higher provoked alcohol craving during alcohol relative to the neutral condition. 12 , 21 , 22 , 23 Furthermore, we hypothesized that pain patients with AUD versus those without AUD would exhibit hyperactive insular responses consistent with hyperalgesia, but lower PFC/striatal responses suggestive of blunted self‐regulation of pain and alcohol intake. 13 , 24 , 25
2. METHODS
2.1. Participants
Participants (total N = 68) were recruited via advertisements and flyers posted in the Greater New Haven community and were required to either have no personal history of AUD (i.e., non‐binging, social drinkers/SD) or meet criteria for moderate to severe AUD, as determined by the Structured Clinical Interview for DSM‐5 (SCID‐5), 26 and be treatment‐seeking to cut back on their alcohol misuse. Additional inclusion criteria for AUD participants included a positive alcohol urine toxicology screen at study intake or other evidence of recent heavy drinking such as previous AUD treatment admission for corroboration of current AUD. All participants were additionally assessed for pain symptoms across organ systems (e.g., pain in the eye, heart/chest, stomach, joints, back, arms/legs/feet) using the Cornell Medical Index (CMI) 27 and categorized into individuals with pain symptoms (17 AUD, 14 SD) if they endorsed pain on at least one of the questions (for more detail, please see Supporting information) and without pain symptoms (23 AUD, 14 SD) if they reported no pain on CMI.
Eligible participants were 21–60 years of age, able to read and speak in English and provide written informed consent. General exclusion criteria included a history of severe psychiatric disorders requiring specific medication or hospitalization (including psychotic or Axis I disorder), other current DSM‐5 diagnoses for Substance Use Disorder, except for cannabis, nicotine and caffeine and taking medications for such illnesses, acute untreated medical condition requiring immediate attention (including cerebral, renal, thyroid, hepatic, cardiac pathology or pregnancy), a history of loss of consciousness for longer than 30 min and contraindications for fMRI. Individuals requiring medical detoxification from alcohol were also excluded. Breath alcohol and urine toxicology screens were used to confirm drug and alcohol abstinence for baseline and scanning sessions. The study protocol was approved by the Yale University School of Medicine's Human Investigation Committee.
2.2. Cue provocation paradigm
The fMRI cue provocation task used in this study is well‐validated in prior stress and alcohol studies. 12 , 25 , 28 , 29 It utilized a block design, consisting of three condition blocks with each block presenting visual stimuli from one of stress, alcohol cue and neutral‐relaxing conditions (Figure 1). Each block consists of nine runs total: Three baseline runs of blank grey fixation images followed by six task/provocation runs of the same type (i.e., alcohol, stress or neutral‐relaxing images). Each run includes 11 trials, where each trial consists of 5‐s of visual image presentation (e.g., grey fixation, alcohol, stress or neural‐relaxing) with a 1‐s interstimulus interval. Visual stimuli were presented using E‐prime software (Psychology Software Tools, Inc., PA, USA). There were 66 alcohol‐cue, 66 stressful and 66 neutral‐relaxing images, which were either selected from International Affective Picture System (IAPS) 30 or developed and validated by the Yale Stress Center as described previously. 12 , 25 , 28 To ensure equivalent levels of emotional intensity of visual stimuli across runs, there were no statistical differences in valence and arousal ratings of pictures or in visual content across six provocation runs in each condition. Each alcohol cue, stress and neutral condition was counterbalanced across participants and presented in randomized order to prevent order effects in the responses. Outside the scanner, participants completed a practice task consisting of exposure to 10 trials using stimuli that were not used for the in‐scan fMRI task. Afterwards, they underwent fMRI scanning.
FIGURE 1.
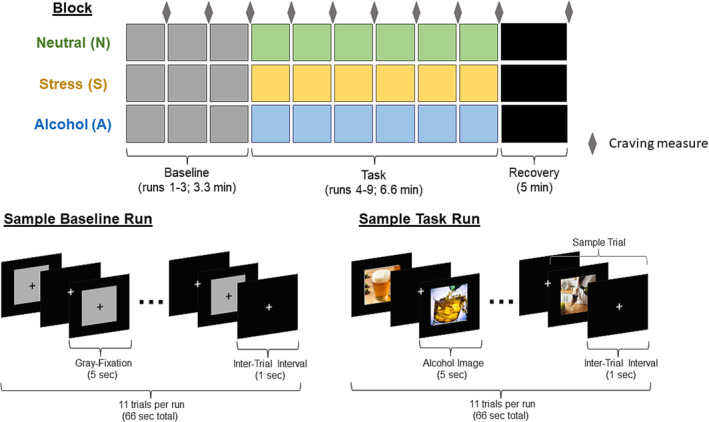
Functional magnetic resonance imaging (fMRI) cue provocation task involves sustained exposures to stress, alcohol cue and neutral pictures in a block design. Each block consists of three baseline grey‐fixation runs of 1 min length for comparison with the subsequent six consecutive runs of 1 min in length. Each run includes 11 trials, where each trial consists of 5‐s of visual image presentation with a 1‐s interstimulus interval for each of the alcohol, stress or neutral conditions. Each alcohol cue, stress and neutral condition was counterbalanced across participants and presented in randomized order to prevent order effects in the responses. Subjective alcohol craving (ratings ranges from 1 to 9) was assessed after each baseline and provocation run in each condition block. There was a 5‐min post‐provocation recovery period after each condition (not included in the analyses), during which participants were asked to relax without viewing any cues. During that time, they viewed a black background with a white crosshair on the screen.
2.3. fMRI acquisition
Scanning was performed in a 3 T multiband Siemens Trio or Prisma MRI system equipped with a standard quadrature head coil, using the T1 magnetization‐prepared rapid acquisition gradient‐echo (MPRAGE) sequence for structural scanning and T2*‐sensitive gradient‐recalled single‐shot echo‐planar pulse sequence for functional scans. BOLD Signals for the functional MRI scans were acquired with a 64‐channel head coil with a multiband accelerated echo planar imaging sequence. Seventy‐five axial slices parallel to the anterior commissure–posterior commissure line covering the whole brain were acquired (TR = 1000 ms, TE = 30 ms, bandwidth = 1895 Hz/pixel, flip angle = 55°, field of view = 220 × 220 mm, slice thickness = 2 mm, no gap).
2.4. fMRI data preprocessing
Images were spatially realigned to correct for head motion using SPM12. Trials with linear displacement greater than 1.5 mm and rotation greater than 2° were discarded. Brain extraction was done using BET tool in FSL. To account for individual anatomical differences, three sequential registrations were performed using BioImage Suite: linear registration of raw data into two‐dimensional anatomical images, the two‐dimensional to three‐dimensional linear registration and a nonlinear registration to a reference three‐dimensional MNI image. The AFNI programme 3dREMLfit was used to fit a generalized linear model (GLM) to the data using a restricted maximum likelihood approach to estimate the temporal autocorrelation structure with an autoregressive moving average (ARMA) as a ‘pre‐whitening’ strategy to address any potential noise due to autocorrelations. Temporal filtering was carried out by including drift correction in the GLM. Each image was spatially smoothed using a 6‐mm Gaussian kernel and individually normalized to generate beta maps.
2.5. Data analytic approach
2.5.1. Demographic, clinical and Behavioural analyses
Group differences in baseline demographics and clinical characteristics were assessed using a series of two‐sample t‐tests and Pearson chi‐square tests (parametric) or Mann–Whitney's and Fisher tests (nonparametric) as appropriate. Linear mixed‐effects (LME) model with a random intercept was conducted to assess main and interaction effects for group (between‐subject factor: pain and no pain) and condition (within‐subject factor: neutral, stress and drug cues) on alcohol craving responses during imagery runs corrected for baseline grey runs during fMRI, while controlling for age, sex and AUD diagnosis. All tests were two‐tailed and were deemed statistically significant at α < 0.05. Analyses were performed, and results were graphed using R (Version 4.2.1 31 ).
2.5.2. Group‐level neuroimaging analyses
The first‐level general linear model regressors of interest included task runs relative to the baseline grey fixation period, resulting in a task‐baseline contrast for each condition (stress, alcohol cue, neutral), respectively. These contrast maps were then entered into 3 s‐level whole‐brain voxelwise fMRI analyses, which were conducted with AFNI (Version 22.1.14). First, to examine differences in whole‐brain cue‐reactivity responses between pain and no pain groups, we performed an LME analysis using 3dLME (Version 2.0.9), where subject was treated as the random effect while condition (stress, alcohol, neutral imagery runs relative to baseline runs) was a within‐subject fixed‐effects factor, group (pain vs. no pain) a between‐subject fixed‐effects factor, and age, sex and AUD diagnosis were covariates. Next, to examine how AUD might alter whole‐brain cue‐reactivity in the pain and no‐pain groups separately, we ran 2 s LME analyses, where subject was treated as the random effect while condition was a within‐subject fixed‐effects factor, group (AUD vs. SD) a between‐subject fixed‐effects factor, and age and sex were covariates. The neutral condition served as an active control condition because it does not increase alcohol craving above basal levels and serves as a control for the non‐specific effects of the experimental manipulation. Thus, all group differences are presented in the form of alcohol cue‐neutral (A‐N) and stress‐neutral (S‐N) second‐level contrasts and responses in the neutral (N) control condition alone. To correct for multiple comparisons, we used familywise error (FWE) correction determined by Monte Carlo simulation using AFNI's 3dClustSim (Version 22.6.24). We used the autocorrelation values of the 3dFWHMx using the residuals of the 3dLME as input into 3dClustSim. In all second‐level analyses, we considered activations that survived FWE whole‐brain correction at α < 0.05 achieved with a voxel threshold of p < 0.001 as statistically significant.
3. RESULTS
3.1. Baseline demographic and clinical characteristics
Individuals with pain and without pain were not significantly different on age, IQ, years of education, race, sex, proportion of smokers and lifetime diagnoses of mood, anxiety and PTSD, years or alcohol use, percentage of drinking and heavy drinking days (all p's < 0.05; Table 1). However, individuals without pain reported more average drinks per drinking day relative to the pain group (W = 343.5, p = 0.02). In the pain group alone, AUD and SD individuals were matched on all demographic characteristics (all p's < 0.05). With regard to clinical characteristics, more SD than AUD with pain met criteria for lifetime PTSD diagnosis (Fisher's exact, p < 0.001). In contrast, AUD with pain reported greater number of years of alcohol use (W = 202, p < 0.001), average drinks per drinking day (t = 3.98, p < 0.001), percentage of drinking days (W = 210, p < 0.001) and heavy drinking days (W = 190, p < 0.001) as well as higher proportion of smokers (Fisher's exact, p < 0.001) relative to SD with pain. However, individuals with AUD with and without pain had equivalent levels of drinking.
TABLE 1.
Participant demographics and baseline clinical characteristics.
| All participants (N = 68) | Pain participants only (N = 31) | |||
|---|---|---|---|---|
| Pain (n = 31) | No pain (n = 37) | AUD (n = 17) | SD (n = 14) | |
| Demographics | ||||
| Age (years) | 34.5 (11.5) | 33.2 (10.3) | 34.6 (12.3) | 34.3 (10.9) |
| Sex (% female) | 21 (68%) | 20 (54%) | 10 (59%) | 11 (79%) |
| Education level (years) | 14.8 (2.6) | 14.4 (2.3) | 14.1 (2.2) | 15.7 (2.8) |
| Shipley IQ Estimate | 110.2 (9.0) | 107.5 (10.6) | 108.3 (9.9) | 112.6 (7.2) |
| Race | ||||
| Caucasian | 16 (52%) | 21 (57%) | 7 (41%) | 9 (64%) |
| African American | 11 (35%) | 12 (32%) | 9 (53%) | 2 (14%) |
| Other | 4 (13%) | 4 (11%) | 1 (5.9%) | 3 (21%) |
| SCID diagnoses | ||||
| Lifetime mood disorder | 12 (39%) | 9 (24%) | 4 (24%) | 8 (57%) |
| Lifetime anxiety disorder | 7 (23%) | 6 (16%) | 3 (18%) | 4 (29%) |
| Lifetime PTSD | 6 (19%) | 2 (5.4%) | 0 (0%)c | 6 (43%)d |
| Substance use | ||||
| Smoker | 10 (32%) | 12 (32%) | 9 (53%)c | 1 (7.1%)d |
| Years of alcohol use | 8.6 (10.0) | 9.4 (9.2) | 13.8 (10.6)c | 2.6 (4.7)d |
| Average drinks per drinking day | 3.6 (2.8)a | 5.4 (3.2)b | 5.2 (2.8)c | 1.9 (1.4)d |
| Percentage of drinking days | 35.1 (39.3) | 33.9 (34.3) | 67.6 (27.3)c | 0.3 (0.3)d |
| Percentage of heavy drinking days | 24.8 (34.6) | 24.8 (27.6) | 47.8 (34.8)c | 0.1 (0.1)d |
Note: Means (and standard deviations) or percentages with different subscripts across rows were significantly different in pairwise comparisons (p < 0.05, two‐sided, chi‐square/Fisher test for categorical variables and Tukey's honestly significant test/Mann–Whitney's test for continuous variables).
Abbreviations: AUD, alcohol use disorder; PTSD, posttraumatic stress disorder; SD, social drinker; SCID, Structured Clinical Interview for DSM‐5.
3.2. Pain group differences in alcohol craving during fMRI
There was a significant main effect of condition (F = 129.5, df = 2,1117, p < 0.0001) stemming from significant increases in craving induced during stress‐ and alcohol cues relative to neutral cues and during alcohol relative to stress cues (p's < 0.0001, Figure 2). In addition, there was a significant condition‐by‐group interaction (F = 11.7, df = 2,1117, p < 0.0001) arising from the pain versus no pain groups showing significantly higher alcohol cue‐elicited alcohol craving (p = 0.03; Figure 2).
FIGURE 2.
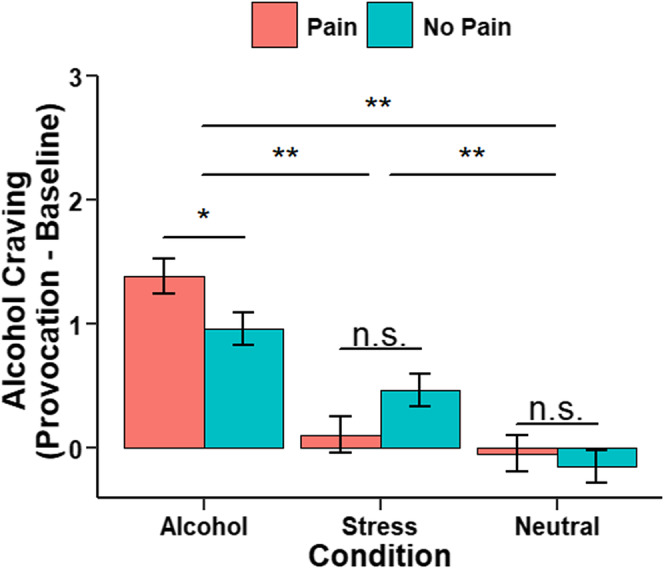
Group differences (pain/no pain) in subjective alcohol craving responses during functional magnetic resonance imaging (fMRI). Mean alcohol craving ratings in response to neutral‐relaxing (N), stress cue (S) and alcohol cue (A) imagery relative to baseline runs (imagery minus baseline for each task run, then averaged per condition block). Alcohol craving was significantly higher in response to stress and alcohol cue compared to neutral imagery (a > N: p < 0.001; S > N: p < 0.001, a > S: p < 0.001). Individuals with pain relative to those without pain reported greater alcohol craving during alcohol condition (p = 0.03), but not during stress (p = 0.06) or neutral condition (p = 0.59). All data are displayed as mean ± S.E.M. *p < 0.05, **p < 0.001, n.s., not significantly different.
3.3. Pain group differences in neural correlates of stress and alcohol cue reactivity
Whole‐brain voxelwise 3dLME analyses revealed a significant pain group × condition interaction effect (Table 2). Specifically, pain individuals evidenced greater dACC and left amygdala activation in response to neutral‐relaxing images, but hypoactivation during S‐N contrast. In addition, pain group exhibited blunted right anterior insular cortex (AIC) during S‐N, blunted anteromedial thalamus and left AIC during A‐N and hyperactive orbitofrontal cortex (OFC) during A‐N (Figure 3).
TABLE 2.
Regional localization of significant clusters for CONDITION × GROUP (pain/no pain) interaction within corticostriatal‐limbic circuit (N = 68).
| Region | MNI coordinates | Cluster (voxels) | Volume (mm3) | F score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Dorsal anterior cingulate cortex | −8 | 8 | 40 | 1248 | 9984 | 17.2 |
| Anteromedial thalamus | 4 | −8 | 10 | 335 | 2,680 | 37.7 |
| L anterior insula | −36 | 8 | −6 | 113 | 904 | 18.1 |
| R orbitofrontal cortex | 16 | 20 | −18 | 79 | 632 | 25.8 |
| L amygdala | −28 | −8 | −20 | 54 | 432 | 16.1 |
| R anterior insula | 42 | 14 | −4 | 40 | 320 | 13.8 |
Note: Reporting of all significant peak voxels at p < 0.05, familywise error corrected (FWE), with a cluster size of >38 contiguous voxels.
Abbreviations: L, Left; MNI, Montreal Neurologic Institute; R, Right.
FIGURE 3.
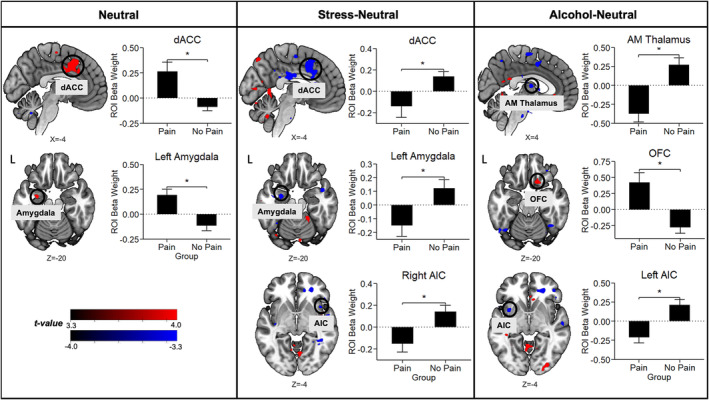
Whole‐brain voxelwise post hoc linear mixed‐effects (LME) analyses controlling for age and sex show disruptions in corticostriatal‐limbic activation patterns in response to stress (S) and alcohol cue (A) relative to neutral (N). Horizontal brain slices show the difference in activation during S‐N and A‐N between the pain and no pain groups, with activations in red indicating pain > no pain and activations in blue indicating pain < no pain. All differences were significant after whole‐brain familywise error [FWE] correction at p < 0.001 and cluster correction at α < 0.05. The bar plots show the average change in region‐of‐interest (ROI) beta weights for the pain and no pain groups. Abbreviations: AIC, anterior insular cortex; AM thalamus, anteromedial thalamus; dACC, dorsal anterior cingulate cortex; L, left; OFC, orbitofrontal cortex.
3.4. Group differences in neural correlates of stress and alcohol cue reactivity between AUD and SD individuals with pain
Whole‐brain voxelwise 3dLME analyses in pain individuals, revealed a significant group (AUD vs. SD) × condition interaction effect (Table 3). Specifically, AUD individuals with pain evidenced greater bilateral AIC and blunted right dorsal caudate activation during A‐N (Figure 4). No other significant group differences were found during S‐N or N condition.
TABLE 3.
Regional localization of significant clusters for CONDITION × GROUP (AUD/SD) interaction within corticostriatal‐limbic circuit in the pain participants (N = 31).
| Region | MNI coordinates | Cluster (voxels) | Volume (mm3) | F score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| L anterior insula | −32 | 32 | −2 | 979 | 7832 | 40.6 |
| R dorsal caudate | 8 | 18 | 2 | 103 | 824 | 18.2 |
| R anterior insula | 48 | 18 | 2 | 91 | 728 | 12.2 |
Note: Reporting of all significant peak voxels at p < 0.05, familywise error corrected (FWE), with a cluster size of >38 contiguous voxels.
Abbreviations: AUD, alcohol use disorder; L, left; MNI, Montreal Neurologic Institute; R, right; SD, social drinker
FIGURE 4.
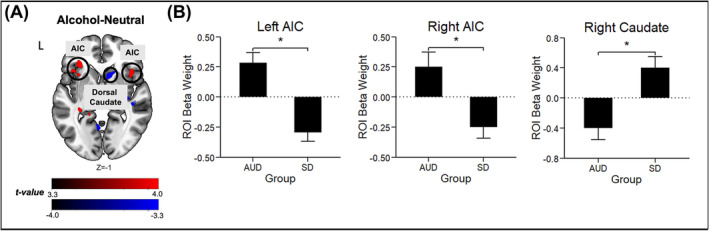
Whole‐brain voxel‐wise post‐hoc LME analyses controlling for age and sex show disruptions in cortico‐striatal‐limbic activation patterns in response to alcohol cue (A) relative to neutral (N). (A) Horizontal brain slice shows the difference in activation during A‐N between the alcohol use disorder (AUD) patients with pain and social drinkers (SD) with pain, with activations in red indicating AUD>SD and activations in blue indicating AUD<SD. All differences were significant after whole‐brain family‐wise error [FWE] correction at p<.001 and cluster correction at α<.05. No significant group differences were found during S‐N contrast and N condition. (B) The bar plots show the average change in region‐of‐interest (ROI) beta weights for the AUD and SD groups. Abbreviations. AIC = anterior insula, L = left.
3.5. Group differences in neural correlates of stress and alcohol cue reactivity between AUD and SD individuals without pain
Whole‐brain voxelwise 3dLME analyses in no pain individuals, revealed a significant group (AUD vs. SD) × condition interaction effect (Table S1). Specifically, AUD individuals without pain evidenced hypoactive dorsomedial and dorsolateral PFC (dm/dlPFC), vmPFC and hyperactive bilateral hippocampus during S‐N relative to SD without pain (Figure S1). In addition, AUD individuals exhibited hypoactive dmPFC and hyperactive left putamen during A‐N compared to SD individuals without pain. For reference, Figure S2 includes the neural responses to stress, alcohol and neutral cues in SD participants without pain.
3.6. Associations between whole‐brain A‐N reactivity and alcohol craving in pain individuals
As the pain group showed significantly higher alcohol cue‐induced craving than the no pain group, we conducted a whole‐brain correlation analysis (p < 0.005, uncorrected) in pain individuals between alcohol cue‐induced craving responses and neural response in the A‐N contrast as an exploratory aim. We found that activation in two regions (right AIC and OFC) in the A‐N contrast was negatively related to provoked alcohol craving, such that a more blunted response in these areas was associated with greater alcohol cue‐induced craving among individuals with pain (Figure 5).
FIGURE 5.
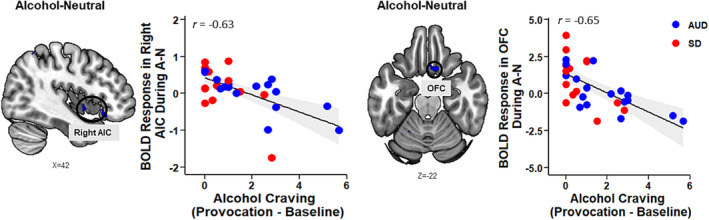
Whole‐brain correlation analysis (p < 0.005, uncorrected) between neural response to alcohol cue (A) relative to neutral (N) and average alcohol craving rating during alcohol runs relative to baseline runs in pain individuals. Right anterior insular cortex (AIC; x = 42, y = 2, z = −6, k = 20, z = 3.4) and orbitofrontal cortex (OFC; x = 10, y = 18, z = −24, k = 45, z = 3.6) hypoactivity (shown here in blue) was significantly associated with higher alcohol craving. AUD, alcohol use disorder; SD, social drinker.
4. DISCUSSION
Prior work has shown that individuals with AUD report heightened alcohol craving and cue‐reactivity when exposed to stress and alcohol cues in their environment. 12 , 13 However, studies examining stress and alcohol cue‐elicited craving and neural activation among individuals with pain and comorbid pain and AUD are critically lacking. The current investigation contributes novel insights to the limited body of research on the frequently co‐occurring conditions of pain and AUD by (1) identifying the maladaptive effect of pain on alcohol craving and neural reactivity to stress and alcohol cues and (2) examining how AUD relative to social drinking may distinctively alter these neural substrates of cue reactivity among individuals with pain. An additional goal was to conduct an exploratory whole‐brain correlation analysis to investigate associations between alcohol craving and neural correlates of cue reactivity in the pain group.
As hypothesized, our results showed that individuals with pain versus those without pain self‐reported higher alcohol craving, but only during alcohol and not in response to stress cue exposure. Although our study is the first to report on cue‐elicited alcohol craving in individuals with pain, a small number of other studies using experimental pain paradigms (e.g., capsaicin‐heat model, cold‐pressor task) found a relationship between acute pain provocation and increased urge to drink alcohol 32 or smoke 33 to alleviate pain‐related stress/hyperkatifeia. Overall, our preliminary results suggest that there is increased salience of alcohol cues and associated alcohol craving among pain individuals who may be more sensitized to rewarding alcohol incentives in their environment, thereby making them more susceptible to heavy drinking.
Following up on current significant differences in alcohol cue‐elicited alcohol craving reports between adults with and without pain, we also observed differences in neural responses to alcohol cues between these groups. Specifically, we found that pain individuals exhibited hyperactive OFC and blunted left AIC and anteromedial thalamus during alcohol relative to neutral cue exposure. All of these regions are involved in the sensory discriminative and affective motivational components of pain and, thus, play an important role in pain processing and modulation. 34 , 35 , 36 In the present context, activation of this network of regions may underlie the processes by which passive viewing of pleasurable appetitive alcohol cues may help mitigate pain, as would be expected with rewarding stimuli. It is plausible that these individuals may therefore develop increased alcohol cue salience attribution, making them more vulnerable to future coping‐motivated alcohol use. Relatedly, prior work has found that pleasure‐elicited pain inhibition was associated with increased activation in the OFC, which is important for integration of bodily signals to guide behaviour. 37 , 38 Anatomically, OFC is well connected with the AIC, involved in receiving and integrating interoceptive and somatosensory information, 39 , 40 and the anteromedial thalamus, which acts not only as a relay centre for somatosensory and visceral information but also plays an important role in the processing of salient internal and external stimuli to redirect attention and behaviour. 41 Results of our exploratory analysis further revealed that blunted AIC and OFC activation was associated with increased alcohol craving in pain individuals. These results contrast somewhat with the findings above describing hyperactive OFC during alcohol cues in pain relative to no pain groups, although these differences may stem from different processes by which OFC may consciously or subconsciously affect cue‐related drinking. 42 Together, our findings point to disrupted, sensitized alcohol cue processing in the brain associated with altered pain perception that may in turn increase susceptibility to alcohol‐related behaviours via increase in alcohol craving. However, further studies are warranted, particularly those investigating potentially separable differences in the OFC subregion activation by cues and associated alcohol craving.
Although we did not see significant pain group differences in craving during stress and neutral cue conditions, we found that pain individuals evidenced hyperactive amygdala and dACC activation during exposure to neutral stimuli, but hypoactive amygdala, dACC and right AIC during exposure to stressful relative to neutral cues. This is perhaps not surprising given that these regions are known to be involved in the processing of aversive stressful stimuli and also pain processing. 43 , 44 , 45 Although increased and decreased activation in these regions in relation to pain processing has been previously observed, 46 , 47 our study is the first to elucidate the relationship between pain and hypoactivity in the amygdala and salience network (dACC and AIC) during stress provocation. Importantly, however, in prior research ACC hypoactivity during stress cue exposure has been posited to confer risk for relapse in a population of recovering AUD patients. 13 Our preliminary findings therefore also point to a potential overlap in the neural circuitry of these two disorders that are characterized by maladaptive stress processing and potential susceptibility to stress and pain associated alcohol relapse in addition to heightened cue reactivity.
Relative to social drinkers with pain, we found that AUD patients with pain showed hyperactive bilateral AIC and hypoactive right dorsal caudate during alcohol relative to neutral cue exposure. One hypothesis is that hyperactive insular responses in AUD patients with pain might be consistent with hyperalgesia, 36 wherein heightened sensitivity to pain also amplifies interoceptive awareness. 48 This could drive emotional and physiological responses to salient alcohol cues, which could influence craving in complex ways, and where alcohol use may become a maladaptive coping strategy to alleviate pain or stress. At the same time, the blunted dorsal caudate response may reflect impaired self‐regulation of pain, reducing the individual's ability to manage pain or stress adaptively and fostering coping‐motivated alcohol use. These preliminary findings also contribute to prior work that has found that pain catastrophizing, reflecting pain hypervigilance and a belief that experienced pain will confer harm, was associated with greater alcohol cue‐induced activation in brain regions involved in habit formation and compulsive alcohol use in individuals with AUD. 49 Experiencing pain can therefore have a powerful influence on the reward mechanisms, possibly facilitating the transition to or maintenance of AUD. 50 , 51 , 52 Targeting dysregulated alcohol cue‐elicited activation, particularly within the insular cortex, may represent valuable therapeutic strategy for treatment of pain in the context of AUD.
Although this study is novel in that it assesses alcohol craving and cue‐reactivity in pain and comorbid pain and AUD, there are some limitations. The sample sizes of the four individual comparison groups (AUD ± Pain; SD ± Pain) were relatively small, thus limiting the generalizability of our results and precluding the use of a 2 × 2 full factorial analytic approach given the lack of power to detect significant interactions. Related, we were underpowered to assess sex differences. In addition, the pain group was defined by having recently experienced pain, and detailed information regarding the onset, frequency, duration and/or intensity of participants' pain was not obtained. Consequently, we could not account for potential confounding effects of pain variability in our sample, which could have implications for the underlying neurocircuitry (e.g., whether participants experienced acute or chronic pain). Because we matched individuals with and without pain on age, education and gender, participants with AUD in the pain and no pain groups had higher levels of education than typically observed in the AUD population, potentially limiting the generalizability of our findings to AUD groups in the general population. Although our groups were not different on prevalence of co‐occurring mood and anxiety disorders, increased distress symptoms, even at subclinical levels, may influence pain perception and neural responses to stress and alcohol cues. Therefore, future studies should assess and control for anxiety and depressive symptoms. Finally, as this was cross‐sectional study, we also could not determine whether pain worsened or preceded AUD. Future investigations of pain‐AUD interaction should include systematic pain assessment and longitudinal designs with larger sample sizes. This approach could potentially identify additional brain regions beyond the corticostriatal‐limbic network that may contribute to the pain‐stress neurocircuit and play a role in AUD.
In summary, our study provides first evidence of heightened alcohol craving and disrupted cue‐reactivity within corticostriatal‐limbic circuit in individuals with pain and comorbid pain and AUD. Despite the preliminary nature of our findings, the present study provides initial insights on the neural mechanisms linking pain and AUD. While the association between pain and AUD is complex, our findings suggest that the study of alcohol craving and stress and drug cue reactivity in comorbid pain and AUD may be a promising area of research that could lead to development of targeted prevention and treatment efforts.
AUTHOR CONTRIBUTIONS
Milena Radoman was responsible for data analyses and interpretation of the results and was a major contributor to manuscript preparation. Cheryl Lacadie assisted with data analyses. Colleen McGowan and Emily Heilner assisted in data collection and manuscript preparation. Rajita Sinha designed the study, developed study hypotheses, implemented and oversaw all study procedures, contributed to data analysis, manuscript preparation and revisions and secured funding for data collection.
CONFLICT OF INTEREST STATEMENT
All authors declare no financial relationships with commercial interests.
Supporting information
Table S1. Regional localization of significant clusters for CONDITION x GROUP (AUD/SD) interaction within cortico‐striatal‐limbic circuit in the no pain participants (N = 37).
Figure S1. Whole‐brain voxel‐wise post‐hoc LME analyses controlling for age and sex show disruptions in cortico‐striatal‐limbic activation patterns in response to stress (S) and alcohol cue (A) relative to neutral (N). Horizontal brain slices show the difference in activation during S‐N and A‐N between the alcohol use disorder (AUD) group and the social drinker (SD) group, with activations in red indicating AUD > SD and activations in blue indicating AUD < SD. All differences were significant after whole‐brain family‐wise error [FWE] correction at p < .001 and cluster correction at α < .05. No significant group differences were found during N condition. The bar plots show the average change in region‐of‐interest (ROI) beta weights for the AUD and SD groups. Abbreviations. dm/dlPFC = dorsomedial/dorsolateral prefrontal cortex, vmPFC = ventromedial prefrontal cortex, L = left.
Figure S2. Whole‐brain voxel‐wise post‐hoc LME analyses controlling for age and sex show disruptions in cortico‐striatal‐limbic activation patterns in response to stress (S) and alcohol cue (A) relative to neutral (N). Horizontal brain slices show the activation during S‐N and A‐N in the social drinker (SD) group, with activations in red indicating S > N (or A > N) and activations in blue indicating S < N (or A < N). All activations were significant after whole‐brain family‐wise error [FWE] correction at p < .001 and cluster correction at α < .05. No significant activations were found during N condition. Abbreviations. dmPFC = dorsomedial/dorsolateral prefrontal cortex, dACC = dorsal anterior cingulate cortex, vmPFC = ventromedial prefrontal cortex, Hypothal. = hypothalamus, SMA = supplementary motor area.
ACKNOWLEDGEMENTS
This work was funded by grants R01‐AA013892 and R01‐AA026514 (PI: Rajita Sinha) from the National Institutes of Health (NIH) and National Institute on Alcohol Abuse and Alcoholism (NIAAA).
Radoman M, McGowan C, Heilner E, Lacadie C, Sinha R. Neural responses to stress and alcohol cues in individuals with pain with and without alcohol use disorder. Addiction Biology. 2024;29(12):e70010. doi: 10.1111/adb.70010
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Edwards S, Vendruscolo LF, Gilpin NW, Wojnar M, Witkiewitz K. Alcohol and pain: a translational review of preclinical and clinical findings to inform future treatment strategies. Alcohol Clin Exp Res. 2020;44(2):368‐383. doi: 10.1111/acer.14260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Larson MJ, Paasche‐Orlow M, Cheng DM, Lloyd‐Travaglini C, Saitz R, Samet JH. Persistent pain is associated with substance use after detoxification: a prospective cohort analysis. Addiction. 2007;102(5):752‐760. doi: 10.1111/j.1360-0443.2007.01759.x [DOI] [PubMed] [Google Scholar]
- 3. Boissoneault J, Lewis B, Nixon S. Characterizing chronic pain and alcohol use trajectory among treatment‐seeking alcoholics. Alcohol. 2019;75:47‐54. doi: 10.1016/j.alcohol.2018.05.009 [DOI] [PubMed] [Google Scholar]
- 4. Sheu R, Lussier D, Rosenblum A, et al. Prevalence and characteristics of chronic pain in patients admitted to an outpatient drug and alcohol treatment program. Pain Med. 2008;9(7):911‐917. doi: 10.1111/j.1526-4637.2008.00420.x [DOI] [PubMed] [Google Scholar]
- 5. Vowles KE, Witkiewitz K, Pielech M, et al. Alcohol and opioid use in chronic pain: a cross‐sectional examination of differences in functioning based on misuse status. J Pain. 2018;19(10):1181‐1188. doi: 10.1016/j.jpain.2018.04.013 [DOI] [PubMed] [Google Scholar]
- 6. Caldeiro RM, Malte CA, Calsyn DA, et al. The association of persistent pain with out‐patient addiction treatment outcomes and service utilization. Addiction. 2008;103(12):1996‐2005. doi: 10.1111/j.1360-0443.2008.02358.x [DOI] [PubMed] [Google Scholar]
- 7. Alford DP, German JS, Samet JH, Cheng DM, Lloyd‐Travaglini CA, Saitz R. Primary care patients with drug use report chronic pain and self‐medicate with alcohol and other drugs. J Gen Intern Med. 2016;31(5):486‐491. doi: 10.1007/s11606-016-3586-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Egli M, Koob GF, Edwards S. Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev. 2012;36(10):2179‐2192. doi: 10.1016/j.neubiorev.2012.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141(1):105‐130. doi: 10.1196/annals.1441.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koob G. Drug addiction: Hyperkatifeia/negative reinforcement as a framework for medications development. J Pharmacol Rev. 2021;73(1):163‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blaine SK, Seo D, Sinha R. Peripheral and prefrontal stress system markers and risk of relapse in alcoholism. Addict Biol. 2017;22(2):468‐478. doi: 10.1111/adb.12320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Blaine SK, Wemm S, Fogelman N, et al. Association of prefrontal‐striatal functional pathology with alcohol abstinence days at treatment initiation and heavy drinking after treatment initiation. Am J Psychiatry. 2020;177(11):1048‐1059. doi: 10.1176/appi.ajp.2020.19070703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry. 2013;70(7):727‐739. doi: 10.1001/jamapsychiatry.2013.762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martins JS, Fogelman N, Wemm S, Hwang S, Sinha R. Alcohol withdrawal and craving at treatment entry prospectively predict alcohol use outcomes during outpatient treatment. Drug Alcohol Depend. 2021;231:109253. doi: 10.1016/j.drugalcdep.2021.109253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron. 2016;89(1):11‐36. doi: 10.1016/j.neuron.2015.11.027 [DOI] [PubMed] [Google Scholar]
- 16. Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI‐based neurologic signature of physical pain. N Engl J Med. 2013;368(15):1388‐1397. doi: 10.1056/NEJMoa1204471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Atlas LY, Lindquist MA, Bolger N, Wager TD. Brain mediators of the effects of noxious heat on pain. Pain. 2014;155(8):1632‐1648. doi: 10.1016/j.pain.2014.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee M, Manders TR, Eberle SE, et al. Activation of corticostriatal circuitry relieves chronic neuropathic pain. J Neurosci. 2015;35(13):5247‐5259. doi: 10.1523/JNEUROSCI.3494-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee JJ, Kim HJ, Čeko M, et al. A neuroimaging biomarker for sustained experimental and clinical pain. Nat Med. 2021;27(1):174‐182. doi: 10.1038/s41591-020-1142-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robins MT, Heinricher MM, Ryabinin AE. From pleasure to pain, and Back again: the intricate relationship between alcohol and nociception. Alcohol Alcohol. 2019;54(6):625‐638. doi: 10.1093/alcalc/agz067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pahng A, Edwards S. The convergent neuroscience of affective pain and substance use disorder. Alcohol Res. 2021;41(1):14. doi: 10.35946/arcr.v41.1.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Naqvi N, Gaznick N, Tranel D, Bechara A. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci. 2014;1316(1):17‐70. doi: 10.1111/nyas.12415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zeng J, Yu S, Cao H, Su Y, Dong Z, Yang X. Neurobiological correlates of cue‐reactivity in alcohol‐use disorders: a voxel‐wise meta‐analysis of fMRI studies. Neurosci Biobehav Rev. 2021;128:294‐310. doi: 10.1016/j.neubiorev.2021.06.031 [DOI] [PubMed] [Google Scholar]
- 24. Woo CW, Schmidt L, Krishnan A, et al. Quantifying cerebral contributions to pain beyond nociception. Nat Commun. 2017;8(1):1‐14. doi: 10.1038/ncomms14211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sinha R, Lacadie CM, Constable RT, Seo D. Dynamic neural activity during stress signals resilient coping. Proc Natl Acad Sci U S A. 2016;113(31):8837‐8842. doi: 10.1073/pnas.1600965113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Karg FMBWJ, RS, Spitzer RL. Structured clinical interview for DSM‐5‐research version (SCID‐5 for DSM‐5, research version; SCID‐5‐RV). American Psychiatric Association; 2015. [Google Scholar]
- 27. Brodman K, Erdmann AJ. The Cornell medical index; a adjunct to medical interview. JAMA. 1949;140(6):530‐534. doi: 10.1001/jama.1949.02900410026007 [DOI] [PubMed] [Google Scholar]
- 28. Hwang S, Martins JS, Douglas RJ, Choi JJ, Sinha R, Seo D. Irregular autonomic modulation predicts risky drinking and altered ventromedial prefrontal cortex response to stress in alcohol use disorder. Alcohol Alcohol. 2022;57(4):437‐444. doi: 10.1093/alcalc/agab064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feola B, Flook EA, Seo DJ, et al. Altered brain and physiological stress responses in early psychosis. Schizophr Res. 2024;271:112‐119. doi: 10.1016/j.schres.2024.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lang PJ, Bradley MM, Cuthbert BN, others . International affective picture system (IAPS): Instruction manual and affective ratings. In: The Center for Research in Psychophysiology. University of Florida; 1999. [Google Scholar]
- 31. R Development Core Team V Austria . R: a language and environment for statistical computing. R Foundation for Statistical Computing. 2004. https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing
- 32. Moskal D, Maisto SA, De Vita M, Ditre JW. Effects of experimental pain induction on alcohol urge, intention to consume alcohol, and alcohol demand. Exp Clin Psychopharmacol. 2018;26(1):65‐76. doi: 10.1037/pha0000170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ditre JW, Brandon TH. Pain as a motivator of smoking: effects of pain induction on smoking urge and behavior. J Abnorm Psychol. 2008;117(2):467‐472. doi: 10.1037/0021-843X.117.2.467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shirvalkar P, Prosky J, Chin G, et al. First‐in‐human prediction of chronic pain state using intracranial neural biomarkers. Nat Neurosci. 2023;26(6):1090‐1099. doi: 10.1038/s41593-023-01338-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ab Aziz CB, Ahmad AH. The role of the thalamus in modulating pain. Malaysian J Med Sci. 2006;13(2):11‐18. [PMC free article] [PubMed] [Google Scholar]
- 36. Labrakakis C. The role of the insular cortex in pain. Int J Mol Sci. 2023;24(6):5736. doi: 10.3390/ijms24065736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Younger J, Aron A, Parke S, Chatterjee N, Mackey S. Viewing pictures of a romantic partner reduces experimental pain: involvement of neural reward systems. PLoS ONE. 2010;5(10):e13309. doi: 10.1371/journal.pone.0013309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Becker S, Gandhi W, Pomares F, Wager TD, Schweinhardt P. Orbitofrontal cortex mediates pain inhibition by monetary reward. Soc Cogn Affect Neurosci. 2017;12(4):651‐661. doi: 10.1093/scan/nsw173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59‐70. doi: 10.1038/nrn2555 [DOI] [PubMed] [Google Scholar]
- 40. Craig ADB. Significance of the insula for the evolution of human awareness of feelings from the body. Ann N Y Acad Sci. 2011;1225(1):72‐82. doi: 10.1111/j.1749-6632.2011.05990.x [DOI] [PubMed] [Google Scholar]
- 41. Sherman SM. The thalamus is more than just a relay. Curr Opin Neurobiol. 2007;17(4):417‐422. doi: 10.1016/j.conb.2007.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moorman DE. The role of the orbitofrontal cortex in alcohol use, abuse, and dependence. Prog Neuropsychopharmacol Biol Psychiatry. 2018;87(Pt A):85‐107. doi: 10.1016/j.pnpbp.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Seeley WW. The salience network: a neural system for perceiving and responding to homeostatic demands. J Neurosci. 2019;39(50):9878‐9882. doi: 10.1523/JNEUROSCI.1138-17.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Abdallah CG, Geha P. Chronic pain and chronic stress: two sides of the same coin? Chronic Stress (Thousand Oaks). 2017;1:2470547017704763. doi: 10.1177/2470547017704763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schaffer J, Fogelman N, Seo D, Sinha R. Chronic pain, chronic stress and substance use: overlapping mechanisms and implications. Front Pain Res (Lausanne). 2023;4:1145934. doi: 10.3389/fpain.2023.1145934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D. The human amygdala and pain: evidence from neuroimaging. Hum Brain Mapp. 2012;35(2):527‐538. doi: 10.1002/hbm.22199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10(3):221‐234. doi: 10.1177/1073858403261077 [DOI] [PubMed] [Google Scholar]
- 48. Horsburgh A, Summers SJ, Lewis A, Keegan RJ, Flood A. The relationship between pain and Interoception: a systematic review and meta‐analysis. J Pain. 2024;25(7):104476. doi: 10.1016/j.jpain.2024.01.341 [DOI] [PubMed] [Google Scholar]
- 49. Nieto SJ, Grodin EN, Burnette EM, Cahill CM, Ray LA. Pain catastrophizing is associated with increased alcohol Cue‐elicited neural activity among individuals with alcohol use disorder. Alcohol Alcohol. 2022;57(6):727‐733. doi: 10.1093/alcalc/agac029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Roltsch Hellard EA, Impastato RA, Gilpin NW. Intra‐cerebral and intra‐nasal melanocortin‐4 receptor antagonist blocks withdrawal hyperalgesia in alcohol‐dependent rats. Addict Biol. 2017;22(3):692‐701. doi: 10.1111/adb.12360 [DOI] [PubMed] [Google Scholar]
- 51. Witkiewitz K, Vowles KE, McCallion E, Frohe T, Kirouac M, Maisto SA. Pain as a predictor of heavy drinking and any drinking lapses in the COMBINE study and the UK alcohol treatment trial. Addict Biol. 2015;110(8):1262‐1271. doi: 10.1111/add.12964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McDermott KA, Joyner KJ, Hakes JK, Okey SA, Cougle JR. Pain interference and alcohol, nicotine, and cannabis use disorder in a national sample of substance users. Drug Alcohol Depend. 2018;186:53‐59. doi: 10.1016/j.drugalcdep.2018.01.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Regional localization of significant clusters for CONDITION x GROUP (AUD/SD) interaction within cortico‐striatal‐limbic circuit in the no pain participants (N = 37).
Figure S1. Whole‐brain voxel‐wise post‐hoc LME analyses controlling for age and sex show disruptions in cortico‐striatal‐limbic activation patterns in response to stress (S) and alcohol cue (A) relative to neutral (N). Horizontal brain slices show the difference in activation during S‐N and A‐N between the alcohol use disorder (AUD) group and the social drinker (SD) group, with activations in red indicating AUD > SD and activations in blue indicating AUD < SD. All differences were significant after whole‐brain family‐wise error [FWE] correction at p < .001 and cluster correction at α < .05. No significant group differences were found during N condition. The bar plots show the average change in region‐of‐interest (ROI) beta weights for the AUD and SD groups. Abbreviations. dm/dlPFC = dorsomedial/dorsolateral prefrontal cortex, vmPFC = ventromedial prefrontal cortex, L = left.
Figure S2. Whole‐brain voxel‐wise post‐hoc LME analyses controlling for age and sex show disruptions in cortico‐striatal‐limbic activation patterns in response to stress (S) and alcohol cue (A) relative to neutral (N). Horizontal brain slices show the activation during S‐N and A‐N in the social drinker (SD) group, with activations in red indicating S > N (or A > N) and activations in blue indicating S < N (or A < N). All activations were significant after whole‐brain family‐wise error [FWE] correction at p < .001 and cluster correction at α < .05. No significant activations were found during N condition. Abbreviations. dmPFC = dorsomedial/dorsolateral prefrontal cortex, dACC = dorsal anterior cingulate cortex, vmPFC = ventromedial prefrontal cortex, Hypothal. = hypothalamus, SMA = supplementary motor area.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


