ABSTRACT
rVSVΔG-ZEBOV-GP and Ad26.ZEBOV, MVA-BN-Filo are WHO-prequalified vaccination regimens against Ebola virus disease (EVD). Challenges associated with measuring long-term clinical protection warrant the evaluation of immune response kinetics after vaccination. Data from a large phase 2 randomized double-blind clinical trial (PREVAC) were used to evaluate waning of anti-Ebola virus (EBOV) glycoprotein (GP1,2) antibody concentrations after rVSVΔG-ZEBOV-GP or Ad26.ZEBOV, MVA-BN-Filo vaccination with linear mixed-effect regression models. After a post-vaccination peak, each vaccination strategy was associated with a decrease of anti-EBOV GP1,2 antibody concentrations with distinct kinetics, highlighting a less-rapid decline in antibody levels after vaccination by rVSVΔG-ZEBOV-GP. One year after administration of the vaccine, antibody concentrations were higher in children compared to adults for both vaccines, although with different effect sizes: 1.74-fold higher concentrations (95% confidence interval [CI] [1.48; 2.02]) for children 12–17 years old to 3.10-fold higher concentrations (95% CI [2.58; 3.69]) for those 1–4 years old compared to adults for Ad26.ZEBOV, MVA-BN-Filo versus 1.36-fold (95% CI [1.12; 1.61]) to 1.41-fold (95% CI [1.21; 1.62]) higher than these values for adults, with relatively small changes from one age category of children to another, for rVSVΔG-ZEBOV-GP. Antibody concentrations also differed according to geographical location, pre-vaccination antibody concentration, and sex. In combination with knowledge on memory response, characterization of the major determinants of immune response durability of both vaccinations may guide future EVD control protocols.
Trial registration: ClinicalTrials.gov identifier: NCT02876328.
KEYWORDS: Ebola virus disease, vaccine, immunogenicity, antibody, Western Africa, modelling
Introduction
Ebola virus (EBOV) has caused recurrent outbreaks of Ebola virus disease (EVD) for more than four decades. The Western African outbreak (2013–2016) prompted a global research response that included the rapid acceleration of vaccine clinical trials [1,2].
Two vaccines – rVSVΔG-ZEBOV-GP (based on a recombinant vesicular stomatitis Indiana virus) and Ad26.ZEBOV, MVA-BN-Filo (dose 1 based on a recombinant human adenovirus type 26, followed by dose 2 of modified vaccinia virus Ankara) – have been prequalified by the World Health Organization (WHO) and have received marketing authorization by the European Medicines Agency (EMA) [3]. Also, rVSVΔG-ZEBOV-GP has been licensed by the US Food and Drug Administration (FDA). Both vaccines have been shown immunogenic and safe in numerous clinical trials [4–11]. However, the sporadic nature of EVD outbreaks has limited the ability to conduct large phase 3 efficacy trials, with the notable exception of rVSVΔG-ZEBOV-GP having been shown to provide protection in an immediate-versus-deferred ring vaccination trial, conducted during the 2013–2016 outbreak in Guinea [12]. Efficacy was recently reaffirmed by a study conducted during the 2018–2020 outbreak in the Democratic Republic of the Congo [13]. Although definitive correlates of protection have not yet been demonstrated, previous studies have suggested that immunoglobulin G (IgG) antibodies targeting EBOV glycoprotein (GP1,2) are associated with protection against EVD, albeit without a validated protective threshold [14]. The marketing authorization for Ad26.ZEBOV, MVA-BN-Filo has therefore been primarily based on extrapolation from experimental nonhuman primate efficacy studies and vaccine immunogenicity assessed by antibody responses in human clinical trials. However, the exact level of protection remains unknown.
Both rVSVΔG-ZEBOV-GP and Ad26.ZEBOV, MVA-BN-Filo vaccination strategies have been used widely in recent EVD outbreaks [15–17]. In the absence of trials to assess longer-term efficacy, the characterization of the durability of antibody responses provides an important, albeit partial, indicator of the durability of protection. Additionally, it is important to characterize the host factors that may be associated with the magnitude, kinetics, and durability of the antibody responses to each vaccine. The Partnership for Research on Ebola Vaccinations (PREVAC) [18] has recently published results of its randomized trial evaluating the immunogenicity and safety of antibody responses in adults and children from several Western African countries over a 12-month period following vaccination with either rVSVΔG-ZEBOV-GP or Ad26.ZEBOV, MVA-BN-Filo [19]. Here, we report the results of a modelling study, based on the PREVAC data, that evaluated the kinetics of antibody responses among participants with different demographic characteristics throughout the 12-month period after vaccination.
Materials and methods
Study design and population
The PREVAC trial (NCT02876328) was an international, randomized, double-blinded, placebo-controlled clinical trial that assessed three vaccination strategies in healthy adults and children older than 1 year in Guinea, Liberia, Mali, and Sierra Leone. Vaccine strategies included: (1) one dose of rVSVΔG-ZEBOV-GP, with a second dose at day 56; (2) one dose of rVSVΔG-ZEBOV-GP, followed by a placebo dose at day 56 (referred to as rVSVΔG-ZEBOV-GP-placebo arm); and (3) a dose of Ad26.ZEBOV, followed by a dose of MVA-FN-Filo at day 56. Because rVSVΔG-ZEBOV-GP is approved as a one-dose vaccine regimen, the experimental two-dose arm was not included in this modelling study; rather, modelling was based on data from participants of rVSVΔG-ZEBOV-GP–placebo; Ad26.ZEBOV, MVA-BN-Filo; and pooled “placebo–placebo” arms as defined by the protocol. The primary endpoint of the trial was the anti-EBOV GP1,2 IgG antibody concentrations measured 12 months after vaccination [18]. After the initial dose of vaccine or placebo, participants were scheduled for follow-up visits at day 7 (±3 days), day 14 (±3 days), and day 28 (±7 days). All participants were administered a second dose of a vaccine or placebo on day 56 (53–66 days), followed by visits at day 63 (7 ± 3 days after the second dose), month 3 (±14 days), month 6 (±1 month), and month 12 (±1 month).
Antibody assay
Serum concentrations of IgG binding antibodies against EBOV GP1,2 were measured before vaccination and at each follow-up visit by the Filovirus Animal Non-Clinical Group (FANG) enzyme-linked immunosorbent assay (ELISA). Details and results from the formal validation of the FANG assay were previously described [20] and discussed in supplementary section S3.6.1 of the PREVAC trial primary publication [19]. The standard operating procedure that contains the FANG assay for the PREVAC trial is in the Supplementary Material. Analyses were performed by two separate laboratories according to the country of participant origin: the Liberian Institute for Biomedical Research (LIBR) in Liberia analyzed samples from Guinea and Sierra Leone, and the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID) Integrated Research Facility at Fort Detrick (IRF-Frederick) in Maryland, USA, analyzed samples from Liberia and Mali.
Statistical analysis
Linear mixed-effect regression models were used to investigate anti-EBOV GP1,2 IgG dynamics after the post-vaccination peak and to determine any association with participant characteristics. Two models were used to independently evaluate the two active vaccination strategies: model A for rVSVΔG-ZEBOV-GP–placebo and pooled placebo arms; model B for Ad26.ZEBOV, MVA-BN-Filo and pooled placebo arms. The dependent variable was the log10-transformed anti-EBOV GP1,2 IgG concentrations over time. Modelling began at the peak of anti-EBOV GP1,2 IgG concentrations (day 28 for model A and month 3 for model B) and ended at month 12. Time was modelled as a linear trend with one slope for model A (rVSVΔG-ZEBOV-GP) and two slopes for model B (Ad26.ZEBOV, MVA-BN-Filo), with a transition at month 6 to better consider the specific dynamics. The following variables were analyzed as fixed-effect covariates (single-effect and interaction with time): vaccine arm (vaccine versus pooled placebo arms, with placebo as reference), age category (1–4 years, 5–11 years, 12–17 years, ≥18 years [reference]), sex (women, men [reference]), country (Guinea, Liberia, Sierra Leone, Mali [reference]), laboratory for FANG assay analyses, anti-EBOV GP1,2 IgG concentrations prior to vaccination, vaccination time (hour in the day) for dose 1 and dose 2, body mass index (BMI; for adults) or Z score (for children), malaria status (defined as a known clinical malaria case within a month prior to the boost), HIV-1 infection status, lymphopenia, neutropenia, and eosinophilia. The analyses were performed on available data; only 5.2% of antibody response measurement data were missing (all time points considered) and considered missing at random. To build the multivariate model and to find the best trade-off between the goodness of fit and model simplicity, the final selection among the multivariable models was guided by the Akaike Information Criterion (AIC). An effect was considered significant if the p-value was less than 0.05 using a Wald test. Both models included a random effect on the intercept, and model A included a random effect on the slope. Both models were fitted using restricted maximum-likelihood estimation (REML). The fixed-effect covariates in the final model were determined to be age category, sex (only for the rVSVΔG-ZEBOV-GP–placebo arm), country, and pre-vaccination anti-EBOV GP1,2 IgG concentrations. The performance of the models in predicting the anti-EBOV GP1,2 IgG concentrations at month 12 was evaluated by a leave-one-out cross-validation.
For both models, the effect of each covariate is presented as a ratio of the geometric mean concentrations (GMCs) in a natural scale between the category of interest and its reference. In addition, tables were created for the probability of reaching a concentration threshold (200; 600; and 1000 EU/mL, corresponding to empirical thresholds used in previous clinicals trials [19,21] with unknown clinical significance) at the end of the study and the time required after the beginning of the vaccination strategy for 50% of the population to drop below the anti-EBOV GP1,2 IgG GMC threshold, according to the age category (and sex for the rVSVΔG-ZEBOV-GP arm). Additional details regarding the analyses are provided in the Supplementary Material. All analyses were performed with R, version 4.1.3 (R Foundation for Statistical Computing).
Results
Study population
The PREVAC randomized trial enrolled 1400 adults and 1401 children. Participants with administration errors, missing covariate data, and total missing data on the antibody levels within the model timeframe were removed from the analyses. Thus, we utilized data from 781 (out of 802) participants randomized to the single-dose rVSVΔG-ZEBOV-GP arm (administered as rVSVΔG-ZEBOV-GP–placebo), 779 (out of 799) participants randomized to the Ad26.ZEBOV, MVA-BN-Filo arm, and 791 and 786 (out of 801) participants randomized to the pooled placebo arm in models A and B, respectively. The age, sex, country, and an anti-EBOV IgG concentration of >200 EU/mL prior to vaccination were well-balanced among arms [19].
Anti-EBOV GP1,2 IgG dynamics
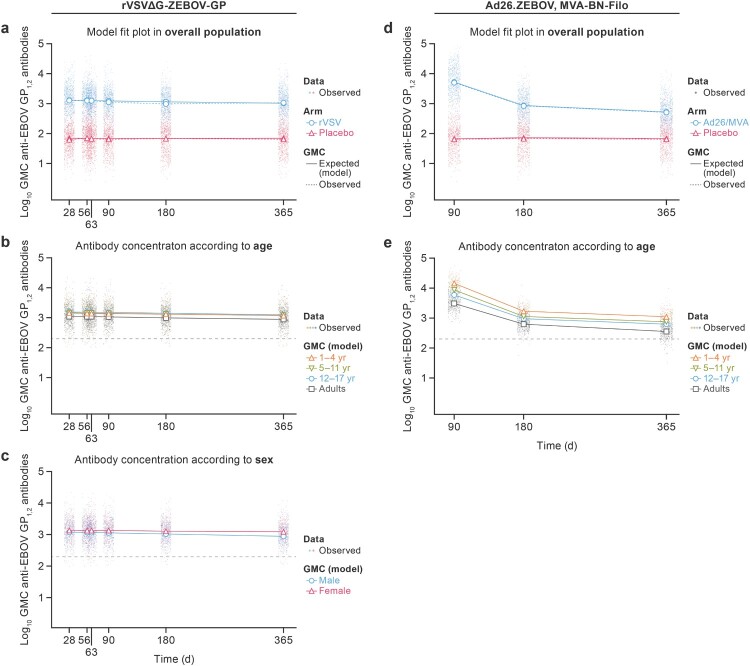
Both vaccination strategies resulted in robust peak IgG responses when measured approximately 28–34 days after completion of the respective vaccination regimen. Graphical representations of the multivariable models’ outputs are presented for the overall population, by age, sex (Figure 1), country, and pre-vaccination antibody concentrations (see Supplementary Material). Very slow waning anti-EBOV GP1,2 IgG (GMCs) were observed after rVSVΔG-ZEBOV-GP vaccination from the peak (28 days) to month 12. Although the early post-vaccination peak was higher after Ad26.ZEBOV, MVA-BN-Filo vaccination, anti-EBOV GP1,2 IgG GMCs decreased at two different rates from the peak (month 3, after dose 1), with rapid decline to month 6 and slower decline from month 6 to month 12.
Figure 1.
Anti-EBOV GP1,2 IgG response (EU/mL) from the post-vaccination peak to 12 months after the first dose of rVSVΔG-ZEBOV-GP or Ad26.ZEBOV, MVA-BN-Filo, respectively. (a) (rVSVΔG-ZEBOV-GP) and (d) (Ad26.ZEBOV, MVA-BN-Filo): Observed and modelled (multivariable models) antibody GMCs over time in vaccine arm and pooled placebo arms in overall population. (b) (rVSVΔG-ZEBOV-GP) and (e) (Ad26.ZEBOV, MVA-BN-Filo): modelled (multivariable models) IgG GMCs over time in active vaccine arm according to age category. (c) (rVSVΔG-ZEBOV-GP): modelled (multivariable models) IgG GMCs over time in active vaccine arm according to sex. IgG GMCs over time after (Ad26.ZEBOV, MVA-BN-Filo) vaccination is not presented according to sex, as sex was not included in the model (not significantly associated). Dots represent individually observed serum samples. The dashed horizontal line in each panel indicates 200 EU/mL anti-EBOV GP1,2 IgG concentration. GMC = geometric mean concentration. GP1,2 = glycoprotein. EBOV = Ebola virus.
Determinants of rVSVΔG-ZEBOV-GP single-dose vaccination and antibody concentration
The anti-EBOV GP1,2 IgG response after a single dose of rVSVΔG-ZEBOV-GP varied according to age, sex, country, and IgG titer prior to vaccination. At peak and month 12, higher GMCs were associated with a younger age, female sex, and higher GMCs prior to vaccination. All age categories of children had higher IgG GMCs than adults: the model outputs, expressed as GMC ratios, estimated that GMCs for children were 1.32-fold higher (95% confidence interval [CI] [1.10; 1.57]) to 1.41-fold higher (95% CI [1.22; 1.64]) at peak and 1.36-fold (95% CI [1.12; 1.61]) to 1.41-fold higher (95% CI [1.21; 1.62]) at month 12 than these values for adults, with relatively small changes from one age category of children to another. Women had estimated 1.15-fold higher (95% CI [1.01; 1.30]) GMCs than men at peak, with an increase to 1.40-fold higher (95% CI [1.22; 1.59]) at the end of the study. Compared to participants from Mali, GMCs of participants from Sierra Leone were lower with a ratio of 0.80 (95% CI [0.67; 0.95] at peak and 0.69 (95% CI [0.58; 0.81]) at month 12, and GMCs of participants from Guinea were lower with a ratio of 0.73 (95% CI [0.62; 0.85]) at peak and of 0.74 (95% CI [0.63; 0.86]) at month 12. No difference was seen in GMCs from Liberia and Mali. Antibody concentrations higher than 200 EU/mL prior to vaccination were associated with a 1.31-fold higher (95% CI [1.07; 1.57]) response at peak and up to a 1.19-fold higher (95% CI [0.99, 1.41]) response at the end of the study. All GMC ratios for rVSVΔG-ZEBOV-GP are presented in Table 1.
Table 1.
Mixed-model analysis of variables associated with anti-EBOV GP1,2 IgG antibody concentrations after rVSVΔG-ZEBOV-GP vaccination.
| Variable | Anti-EBOV GP1,2 antibody | Anti-EBOV GP1,2 antibody |
|---|---|---|
| Peaka | End of studya | |
| Ratio of geometric mean concentration [95% CI] | Ratio of geometric mean concentration [95% CI] | |
| Age category | ||
| Adults | Reference | Reference |
| Children 1–4 yr | 1.32 [1.10; 1.57] | 1.36 [1.12; 1.61] |
| Children 5–11 yr | 1.35 [1.14; 1.57] | 1.37 [1.16; 1.61] |
| Children 12–17 yr | 1.41 [1.22; 1.64] | 1.41 [1.21; 1.62] |
| Sex | ||
| Males | Reference | Reference |
| Females | 1.15 [1.01; 1.30] | 1.40 [1.22; 1.59] |
| Country (laboratory) | ||
| Mali (IRF-Frederick) | Reference | Reference |
| Guinea (LIBR) | 0.73 [0.62; 0.85] | 0.74 [0.63; 0.86] |
| Sierra Leone (LIBR) | 0.80 [0.67; 0.95] | 0.69 [0.58; 0.81] |
| Liberia (IRF-Frederick) | 1.07 [0.87; 1.30] | 1.01 [0.81; 1.24] |
| Pre-vaccination antibody level | ||
| <200 EU/mL | Reference | Reference |
| >200 EU/mL | 1.31 [1.07; 1.57] | 1.19 [0.99; 1.41] |
The peak and the end of the study were defined as 90 days and 12 months after receipt of the prime vaccination, respectively. EBOV = Ebola virus. IRF-Frederick = Integrated Research Facility at Fort Detrick. GP1,2 = glycoprotein. LIBR: Liberian Institute for Biomedical Research. yr = years. EU/mL = enzyme-linked immunosorbent assay units per milliliter. CI = confidence interval.
The model allowed estimation of the percentage of the IgG GMCs at month 12 remaining above different empirical thresholds (>200, >600, and >1000 EU/mL) (Table 2). For rVSVΔG-ZEBOV-GP, there was a very high and homogenous percentage of GMCs above 200 EU/mL at the end of study for children (99% for females and 97% for males) and for adults (97% for females and 94% for males). A 600 EU/mL threshold increased the difference in percentage for adults, diminishing to 75% for females and to 61% for males at end of the study; in contrast, at the 600 EU/mL threshold, the percentage for children decreased less than adults and remained homogenous within each age category compared to adults. At the 1000 EU/mL threshold, the percentage dropped to 54% for female adults, 38% for male adults, 66% for female children, and 53% for male children. Overall, a higher estimated percentage of females reached the designated threshold compared to males, regardless of age category. Additional probability explorations with a continuous threshold scale and time after vaccination required for 50% of the population to drop below the same three anti-EBOV GP1,2 GMC thresholds are shown in the Supplementary Material.
Table 2.
Probability of having an antibody concentration above threshold 12 months after rVSVΔG-ZEBOV-GP vaccination (one dose).
| Probability of reaching anti-EBOV GP1,2 antibody threshold (EU/mL) at day 365 [95% CI] | |||
|---|---|---|---|
| >200 | >600 | >1000 | |
| Females | |||
| Children 1–4 yr | 0.99 [0.97; 1.00] | 0.84 [0.78; 0.90] | 0.67 [0.59; 0.74] |
| Children 5–11 yr | 0.99 [0.97; 1.00] | 0.84 [0.78; 0.90] | 0.66 [0.58; 0.74] |
| Children 12–17 yr | 0.99 [0.98; 1.00] | 0.85 [0.79; 0.90] | 0.68 [0.60; 0.76] |
| Adults | 0.97 [0.96; 0.99] | 0.75 [0.71; 0.80] | 0.54 [0.49; 0.60] |
| Males | |||
| Children 1–4 yr | 0.97 [0.95; 0.99] | 0.73 [0.66; 0.80] | 0.51 [0.43; 0.60] |
| Children 5–11 yr | 0.97 [0.95; 0.99] | 0.74 [0.67; 0.80] | 0.53 [0.45; 0.60] |
| Children 12–17 yr | 0.97 [0.95; 0.99] | 0.76 [0.69; 0.82] | 0.55 [0.47; 0.63] |
| Adults | 0.94 [0.91; 0.96] | 0.61 [0.56; 0.66] | 0.38 [0.33; 0.43] |
Note: EBOV = Ebola virus. GP1,2 = glycoprotein. yr = years. EU/mL = enzyme-linked immunosorbent assay units per milliliter. CI = confidence interval.
Determinants of Ad26.ZEBOV, MVA-BN-Filo vaccination and antibody concentration
The anti-EBOV GP1,2 IgG GMCs after vaccination with Ad26.ZEBOV, MVA-BN-Filo differed according to age, country, and IgG titer prior to vaccination. At peak and month 12, significantly higher IgG GMCs were associated with a younger age and location in Mali. Higher IgG GMCs prior to vaccination were associated with higher antibody concentrations at month 12. In contrast to the rVSVΔG-ZEBOV-GP model, sex was not associated with the antibody response after Ad26.ZEBOV, MVA-BN-Filo vaccination.
Age had a major effect on the IgG GMCs at peak (90 days after dose 1), with 4.68-fold higher (95% CI [3.82; 5.64]) IgG GMCs for children 1–4 years old compared to adults. With increased age, the difference in peak GMCs compared to adults was diminished to 2.82-fold higher (95% CI [2.32; 3.38]) for children 5–11 years old and 1.93-fold higher (95% CI [1.57; 2.35]) for those 12–17 years old. Similar trends were found at the end of the study, with 1.74-fold higher (95% CI [1.48; 2.02]) concentrations for children 12–17 years old and 3.10-fold higher (95% CI [2.58; 3.69]) concentrations for those 1–4 years old compared to adults. Participants from Guinea and Sierra Leone had a lower peak IgG GMCs than those from Mali with ratios of 0.59 (95% CI [0.49; 0.72]) and 0.38 (95% CI [0.30; 0.47]), respectively. These differences were maintained at month 12 with ratios of 0.80 (95% CI [0.67; 0.95]) and 0.61 (95% CI [0.49; 0.74]), respectively, compared to Mali. The IgG GMCs were also lower in Liberia compared to Mali with a ratio of 0.57 (at both peak and at month 12, 95% CI [0.44; 0.73] and [0.45; 0.72], respectively). Furthermore, pre-vaccination IgG GMCs higher than 200 EU/mL were associated with 1.38-fold higher (95% CI [1.12; 1.68]) IgG GMCs at the end of the study. All GMC ratios for Ad26.ZEBOV, MVA-BN-Filo are presented in Table 3.
Table 3.
Mixed-model analysis of variables associated with anti-EBOV GP1,2 antibody concentrations after Ad26.ZEBOV, MVA-BN-Filo vaccination.
| Anti-EBOV GP1,2 antibody | Anti-EBOV GP1,2 antibody | |
|---|---|---|
| Peaka | End of studya | |
| Ratio of geometric mean concentration [95% CI] | Ratio of geometric mean concentration [95% CI] | |
| Age category | ||
| Adults | Reference | Reference |
| Children 1–4 yr | 4.68 [3.82; 5.64] | 3.10 [2.58; 3.69] |
| Children 5–11 yr | 2.82 [2.32; 3.38] | 2.09 [1.78; 2.44] |
| Children 12–17 yr | 1.93 [1.57; 2.35] | 1.74 [1.48; 2.02] |
| Country (laboratory) | ||
| Mali (IRF-Frederick) | Reference | Reference |
| Guinea (LIBR) | 0.59 [0.49; 0.72] | 0.80 [0.67; 0.95] |
| Sierra Leone (LIBR) | 0.38 [0.30; 0.47] | 0.61 [0.49; 0.74] |
| Liberia (IRF-Frederick) | 0.57 [0.44; 0.73] | 0.57 [0.45; 0.72] |
| Pre-vaccine antibody concentration | ||
| <200 EU/mL | Reference | Reference |
| >200 EU/mL | 0.93 [0.75; 1.13] | 1.38 [1.12; 1.68] |
The peak and the end of the study were defined as 90 days and 12 months after receipt of the prime vaccination, respectively. EBOV = Ebola virus. IRF-Frederick = Integrated Research Facility at Fort Detrick. GP1,2: glycoprotein. LIBR: Liberian Institute for Biomedical Research. yr = years. EU/mL = enzyme-linked immunosorbent assay units per milliliter. CI = confidence interval.
There was a much higher percentage of minimum IgG GMCs of 200 EU/mL at month 12 for children compared to adults (76%), a difference which slightly decreased with increased age (from 98% for children 1–4 years old to 91% for those 12–17 years old). In addition, the percentage for adults dropped to 28% for the 600 EU/mL GMC threshold and to 12% for the 1000 EU/mL GMC threshold. With higher thresholds, the effect of age on the estimated percentage in children was even clearer. For the threshold 600 EU/mL, the percentages were 53% (12–17 years) and 77% (1–4 years); for the 1000 EU/mL threshold, the percentages were 30% (12–17 years) and 56% (1–4 years). The percentage table is shown in Table 4. Additional probability explorations with a continuous threshold scale and time after vaccination required for 50% of the population to drop below the same three anti-EBOV GP1,2 GMC thresholds are available in the Supplementary Material.
Table 4.
Probability of having an antibody concentration above threshold 12 months after Ad26.ZEBOV/MVA-BN-Filo vaccination.
| Probability of reaching anti-EBOV GP1,2 antibody threshold (EU/mL) at day 365 [95% CI] | |||
|---|---|---|---|
| >200 | >600 | >1000 | |
| Children 1–4 yr | 0.98 [0.96; 0.99] | 0.77 [0.70; 0.82] | 0.56 [0.48; 0.63] |
| Children 5–11 yr | 0.94 [0.91; 0.97] | 0.61 [0.54; 0.68] | 0.38 [0.30; 0.45] |
| Children 12–17 yr | 0.91 [0.88; 0.94] | 0.53 [0.45; 0.60] | 0.30 [0.24; 0.36] |
| Adults | 0.76 [0.72; 0.79] | 0.28 [0.24; 0.32] | 0.12 [0.09; 0.14] |
Note: EBOV = Ebola virus. GP1,2 = glycoprotein. yr = years. EU/mL = enzyme-linked immunosorbent assay units per milliliter. CI = confidence interval.
Discussion
This modelling study, based on a large international randomized clinical trial, provides new information on the longitudinal dynamics of immune responses for the two currently approved EVD vaccination strategies. A more rapid decline in antibody levels after vaccination by Ad26.ZEBOV, MVA-BN-Filo was observed. Higher IgG responses were observed in children than adults, with a more prominent difference after Ad26.ZEBOV, MVA-BN-Filo vaccination. Higher IgG responses were seen in participants with higher pre-vaccination antibody concentrations for both vaccination strategies and in women for rVSVΔG-ZEBOV-GP vaccine only.
The observed differences in antibody response kinetics between the two vaccines may be explained by their distinct features, including different platforms and vectors, the surface GP1,2 used in the vaccine, and the dosage. Our study confirms that vaccine-specific features are not the only variables responsible for differential response profiles, but host demographic characteristics also have a major impact on the immune response after vaccination. This impact differed between the two vaccination strategies, with greater variability for the Ad26.ZEBOV, MVA-BN-Filo vaccine.
Age was particularly associated with the humoral response to Ad26.ZEBOV, MVA-BN-Filo, with IgG GMCs at 12 months three times higher in the youngest children (1–4 years old) than in adults. The overall trend of higher immune responses in younger children is consistent with similar findings reported previously [22]. It has been well-documented in the context of other vaccines that age at vaccine receipt greatly influences subsequent vaccine-specific immune responses that are generally lower with increased age and extremely low in the elderly [23]. In this study, factors contributing to IgG response differences between adults and children include the lack of dose adaptation for participant age or BMI. Additionally, pre-existing memory responses to environmental adenoviruses may impair immune responses to adenovirus-based vaccines [24,25]. However, pre-existing immunity to Ad26 did not have an effect on vaccine-induced immune response in EVD or coronavirus disease 2019 (COVID-19) studies [26].
Sex was found to influence the humoral response to the rVSVΔG-ZEBOV-GP vaccination strategy, with women exhibiting higher IgG GMCs than men across age categories, in line with what had already been described [27,28]. Although this difference was significant but relatively small at peak, it reached 40% at 1 year after vaccination, putting it at the same order of magnitude as the effect of age on this vaccination strategy.
Pre-vaccination IgG concentrations influenced responses for both vaccination strategies, with higher humoral response at month 12 among participants with higher baseline concentrations. It should be noted that a history of EVD or previous vaccination against EVD were both exclusion criteria for the PREVAC trial but were self-reported. The unexpectedly notable baseline antibody concentrations were also found in previous clinical studies [21,29] and prompted use of centralized analysis of the samples conducted in an independent laboratory (Q2 Solutions, which is validated for regulatory purposes) [30]. Pre-vaccination IgG may derive from prior unrecognized, unrecalled, or subclinical (or asymptomatic) EBOV infection, as suggested in previous studies [31,32]. This phenomenon aligns with the trial’s geographic context, conducted in the three Western African countries most severely affected by the extensive 2013–2016 EVD outbreak. Notably, pre-existing antibodies were more common in participants from these countries than those from Mali and rare in the youngest children born post-epidemic. Alternatively, though scored as “positive,” detection of low concentrations of IgG in the assay may represent prior exposure to cross-reacting antigens.
Since the FANG antibody assay was performed in two different laboratories, it is difficult to discriminate between country-specific and laboratory-related variation in the PREVAC trial. The FANG assay performance is associated with some degree of variability between laboratories despite use of similar method protocols and reagents [33]. The FANG assay has been commonly used in multiple vaccine trials [4,6,8,11], and its limitations have largely been addressed in the annex of a previous PREVAC paper [19]. However, a difference was observed between Malian and Liberian participants after the Ad26.ZEBOV, MVA-BN-Filo vaccine; notably, this geographic variation was observed despite analysis in the same central laboratory and confirmed in a second centralized analysis of the same data [29]. This geographic variation, unexplained by levels of baseline antibody concentrations, might be resultant of unmeasured genetic or exposure factors.
While this modelling is drawn from a single study, one of its main strengths is the design, as it relies on data from a multi-country randomized double-blind clinical trial that incorporated two authorized vaccination strategies, was based on a large recruitment population from several Western African countries and included rural and urban participants with balance across sex and age strata.
Our results provide important insight to the longitudinal dynamics of the anti-EBOV GP1,2 IgG response, which, while not a validated correlate of protection, can be considered the best immunological marker associated with vaccine efficacy against EVD [34]. The probability of having an IgG titer above the empirical threshold of 200 EU/mL IgG at 1 year remained above 90% in most cases, except in adults vaccinated with Ad26.ZEBOV, MVA-BN-Filo. However, at higher thresholds, the probability decreased, raising questions about the level of immune response necessary for optimal protection. It remains to be determined whether the same host characteristics also impact the memory immune response in previously vaccinated individuals. Current data suggest that vaccinated individuals are likely protected one-year post-vaccination, but it is still too early to recommend changes to the EVD vaccination program, including adaptations for specific populations. However, it is crucial to closely monitor the waning of immune responses over time to ensure sustained vaccine efficacy longitudinally across different populations.
Recent EVD outbreaks are sporadic and rapidly brought under control due to a well-coordinated response that includes vaccination campaigns. Thus, it is challenging to detect infectious breakthroughs, cases that could prompt the need for additional injections. Our modelling data highlight the need for research on future EVD vaccination strategies, especially evaluating the value of follow-up doses with the same or different vaccine. The ongoing PREVAC trial (PREVAC-UP) will characterize the durability of immune response up to 5 years post-vaccination and will not be published before 2025; results from this trial will be particularly useful in assessing the antibody level dynamics after 1 year, estimating the durability of humoral response over time and if, when, and for whom follow-up vaccination might be appropriate.
Supplementary Material
Acknowledgements
We are grateful to the Ministries of Health of Guinea, Liberia, Sierra Leone, and Mali, which permitted the conduct of the trial. We furthermore thank The Alliance for International Medical Action and all site collaborators for their contribution in the implementation of the trial. The authors and study team thank the participants who consented to the trial. We also thank Anya Crane (Integrated Research Facility at Fort Detrick, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Fort Detrick, Frederick, MD, USA) for critically editing the manuscript and Jiro Wada (Integrated Research Facility at Fort Detrick, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Fort Detrick, Frederick, MD, USA) for preparing figures. Part of the experiments presented in this paper were carried out using the PlaFRIM experimental testbed, supported by Inria; Centre National de la Recherche Scientifique; Laboratoire Bordelais de Recherche en Informatique and Institut de Mathématique de Bordeaux, Université de Bordeaux; Bordeaux INP; and Conseil Régional d’Aquitaine (see https://www.plafrim.fr). YY and YL conceptualized the study. AHB, SD, MK, BG, BL, SOS, LR, and EL contributed to the study design. SV, MPo, and MA accessed and verified the data, did the data linkage, and conducted the formal analysis. EL, LR, and MPr supervised the analyses and verified the data. All authors contributed to data interpretation. SV created the first drafts of the figures and wrote the first draft of the manuscript. All authors critically revised and edited the manuscript and approved the final version for submission. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. PREVAC study team: Jamila Aboulhab2 MD, Michelle Aguirre-MacKenzie12 BS, Pauline Akoo3 MB, ChB, Esther Akpa2 MSN, MPH, RN, Robert Akpata1 MD, Sara Albert17 BA, MPH, Boni Maxime Ale4 MD, MSc, MPH, Serry Alimamy-Bangura11 MB, ChB, Pierre Andong1 MSt, Benetta C. Andrews10 MD, Stephane Anoma6 MD, Negin Atri2 MPH, CPH, Augustin Augier6 MCom, Ken Awuondo3 MSc, Ahidjo Ayouba30 PhD, Moses Badio10 MSc, Aminata Bagayoko1 MD, Abby Balde17 MPH, Joséphine Balssa1,8 PharmD, Lamin Molecule Bangura11 BSc, Kesha Barrington17 MPA, Eric Barte de Saint Fare6 BA, Beth Baseler17 MS, Ali Bauder12 BA, PMP, Claire Bauduin4 MSc, Luke Bawo10 MSc, Abdoul Habib Beavogui9 MD, PhD, Michael Belson2 BS, Safaa Ben-Farhat4 MEng, Marion Bererd6 BS, Nicolas Bernaud1,18 MSc, Teedoh Beyslow10 PharmD, Neirade Biai30 MSc, Jeanne Billioux2 MD, Shere Billouin-Frazier17 MSc, Blandine Binachon4 MD, MPH, Julie Blie10 MSc, Viki Bockstal13 PhD, Patricia Boison17 MS, Fatorma Bolay10 PhD, Aliou Boly6 MM, Rachael Elizabeth Bonawitz12 MD, MS, Anne-Gaëlle Borg6 MCM, Samuel Bosompem2 PharmD, MSc, Courtney Bozman28 MSc, Tyler Brady2 MPH, Sarah Browne10 RN, BSN, Ryan Bullis12 PhD, Barbara Cagniard1 PhD, Kelly Cahill2 RN, MSc, CCRC, RAC, Yingyun Cai28 PhD, Aissata Abdoulaye Camara6 MSc, Aboubacar Keira Camara1 MD, Alseny Modet Camara6 MD, Antoine Campagne1 PhD, Cécilia Campion4 MSc, Alexandre Cantan4,29 BSc, Jennifer Cash17 BS, Siew Pin Chai13 BBio-MedSc, Francois Chambelin1 MHist, Michael Chea10 BSc, Geneviève Chêne4 MD, PhD, Edouard Choi3 PhD, Michelle Chouinard6 MSW, Florence Chung1 PhD, Lucy Chung2 PharmD, Séverine Ciancia1 MJ, Papa Ndiaga Cisse14 PhD, Elfrida Cline-Cole17 MA, Céline Colin4 MSc, Beth-Ann Coller12 PhD, Djélikan Siaka Conde1 MD, Katherine Cone2 MSW, LCSW-C, C-SWHC, Laurie Connor12 MS, Nicholas Connor3 MSc, Joseph Boye Cooper10 MSc, Sandrine Couffin-Cardiergues1 PhD, Fatoumata Coulibaly1 BS, Mariam Coulibaly20 PharmD, MSc, Page Crew2 PharmD, MPH, BCPS, Sandrine Dabakuyo-Yonli4 PharmD, PhD, Djeneba Dabitao20 PharmD, PhD, Thierry Damerval1 PhD, Bionca Davis5 MPH, Gibrilla Fadlu Deen11 MD, MSc, Eline Dekeyster13 PhD, Jean-François Delfraissy1 MD, PhD, Christelle Delmas1 MSc, Mahamadou Diakite20 PharmD, DPhil, Alpha Diallo1,8 MD, MPH, Fatoumata Abdoulaye Diallo6, MD, Mamadou Saliou Diallo6 MD, MPH, Ayouba Diarra20 MSc, Samba Diarra20 MSc, PhD, Oualy Diawara19 BS, Bonnie Dighero-Kemp28 BSc, Samba Diop21 MSc, PhD, Waly Diouf14 PhD, Saurabh Dixit2 PhD, Barry Djenabou6 MSc, Laurie Doepel2 BA, Eric D’Ortenzio1,7,8 MD, MPH, Seydou Doumbia20 MD, PhD, Moussa Moise Doumbia19 MD, Macaya Douoguih13 MD, MPH, Nelson Dozier28 MSc, Natasha Dubois Cauwelaert1,8 PhD, Alain DuChêne5 BS, Michael Duvenhage17 NDIPIT, Risa Eckes2 RN, Elizabeth Elliott2 MSc, Luisa Enria3 PhD, Hélène Espérou1 MD, Cécile Etienne1 MSc, Allison Eyler17 HSD, Lawrence Fakoli10 MSc, Mosoka Fallah10 PhD, Marie-Alix Fauvel1 MSc, Sylvain Faye14 PhD, John Fayiah10 MSc, Suzanne Fleck3 PhD, Vemy Fofana6 BComp, Karine Fouth Tchos2 MD, MPH, Kokulo Franklin10 MPhil, MSc, Daniela Fusco1 PhD, Auguste Gaddah13 PhD, Marylène Gaignet1 MSc, Katherine Gallagher3 PhD, Julie Gardner12, BS, Harrison Gichini28 MSc, Julia Garcia Gozalbes1 MD, Greg Grandits5 MS, Maima Gray10 BPharm, Brian Greenwood3 MD, Nico Grobler13 PhD, Robin Gross28 MSc, Louis Grue17 RN, BS, BSN, Birgit Grund27 PhD, Oumar Guindo20 MSc, PharmD, Swati Gupta12 DrPH, MPH, Fadima Haidara19 MD, Benjamin Hamzé1 PharmD, Emma Hancox3 MSc, Jean-Christophe Hébert1 MSL, Jenny Hendriks13 PhD, Patricia Hensley3 MPh, Lisa E Hensley28 PhD, MSPH, Betsey Herpin2, MSN, Elisabeth Higgs2 MD, DTMH, MIA, Trudi Hilton3 BPharm, MSc, Mickael Hneino1, PhD, Tracey-Ann Höeltermann28, BSc, MPH, Horace Preston Holley17 MD, Marie Hoover16 PhD, Natasha Howard3 PhD, Melissa Hughes12 BA, MBA, CPM, PMP, Dicko Ilo20 MD, MPH, Skip Irvine12 BS, David Ishola3 MD, PhD, Yvonne Jato2 MPH, Madison Joe10 MSc, Melvin Johnson10 MSc, Aboubacar Sidiki Kaba6 MD, Jonathan Kagan2 PhD, Kade Kallon17 MSc, Michael Kamara3 MBChB, MSc, Myriam Kante4 BS, Judith Katoudi6 MD, MPH, Cheick Mohamed Keita6 MD, Sakoba Keita15 MD, Seykou Keita22 MD, Stephen B. Kennedy10 MD, MSc, Babajide Keshinro13 MBBS, FWACP, Hassan Kiawu10 MSc, Mark Kieh10 MD, MSMHC, Brent Killinger12 BA, Moumouni Kinda6 MD, MBA, Matthew Kirchoff2 PharmD, MSc, MBA, Gregory Kocher28 MSc, Mamoudou Kodio19 PharmD, Brian Kohn3 BSc, Lamine Koivogui23 PharmD, PhD, Richard Kojan6 MD, Cece Francis Kolié6 PharmD, Jacques Seraphin Kolié6 MD, David Kollie10 BSc, Stacy Kopka17 MS, Bockarie Koroma11 BPharm, Dickens Kowuor3 BSc, MSc, PhD, Catherine Kpayieli-Freeman10 MSc, Liane Kwast13 MSc, Christine Lacabaratz1,18 PhD, Boris Lacarra1 MD, Laurie Lambert17 BS, Courtney Lambeth12 BS, Solange Lancrey-javal1,8 PharmD, H. Clifford Lane2 MD, Shadrach Langba10 BSc, Bolarinde Lawal3 MSc, Andrew Wen-Tseng Lee12 MD, Shona Lee3 PhD, Shelley Lees3 PhD, Annabelle Lefevre1 MD, Bailah Leigh11 MD, MSc, Frederic Lemarcis1 PhD, Yves Lévy1,18 MD, PhD, Claire Levy-Marchal1 MD, Maarten Leyssen13 MD, PhD, Edouard Lhomme26 MD, PhD, Janie Liang28 MSc, Mameni Linga10 MSc, Ken Liu12 PhD, Brett Lowe3 MPhil, Julia Lysander10 MSc, Ibrah Mahamadou6 PharmD, Irina Maljkovic-Berry28 PhD, Marvington Mambiah10 ASc, Daniela Manno3 MD, PhD, Jonathan Marchand2,17 MS, Lindsay Marron28 MSc, Moses B.F. Massaquoi10 MD, MSc, Laure Masson1 MIBL, Charly Matard4 BS, Steven Mazur28 BS, John McCullough16 BS, Katherine McFadyen13 MPH, Chelsea McLean13 PhD, Noémie Mercier1 PharmD, Pauline Michavila6 BBus, Tracey Miller17 RN, BSN, Niouma Pascal Millimouno6 MD, Alejandra Miranda17 MS, Soumaya Mohamed6 BJ, Tom Mooney3 BA, Dally Muamba6 MD, James Mulbah11 BPharm, Rita Lukoo Ndamenyaa6 MD, MSc, James Neaton5 PhD, Désiré Neboua1 MD, Micki Nelson12 BSN, MS, Kevin Newell17 MPH, MEd, Vinh-kim Nguyen24 MD, Yusupha Njie3 BSc, Wissedi Njoh17 MSN, Anna Novotney-Barry13 MSC, Matthew Onorato12 BS, Uma Onwuchekwa22 BSc, Susan Orsega2 MSN, FNP-BC, Inmaculada Ortega-Perez1,8 PhD, MPH, Cynthia Osborne17 BS, Tuda Otieno3 MSC, Davy Oulaï4 MD, Sushma Patel12 MS, PMP, Danielle Peart2 BS, Martine Peeters30 PhD, James Pettitt28 MSc, Nathan Peiffer-Smadja1 MD, PhD, Robert Phillips3 MSc, Jerome Pierson2 PhD, Peter Piot3 MD, PhD, Micheal Piziali2 JD, MSc, Stéphany Pong1,8 PharmD, Elena Postnikova2 PhD, Calvin Proffitt17 MA, Alexandre Quach1 MD, Sinead Quigley1 MSSc, Nadeeka Randunu2,17 BSc, MBA, Laura Richert26 MD, PhD, Priscille Rivière1 MSc, Cynthia Robinson13 MD, Céline Roy4,29, PhD, Amy Falk Russell12 MS, Philip Sahr10 MD, Katy Saliba2 MSc, PhD, Mohamed Samai11 MBBS, PhD, Sibiry Samake20 PharmD, MSc, Jen Sandrus17 AA, Ibrahim Sanogo20 MsP, MD, Yeya Sadio Sarro20 PharmD, PhD, Serge Sawadogo6 MD, MSc, Sani Sayadi6 MD, MPH, Maxime Schvartz1 MD, Christine Schwimmer4 PhD, Fatou Secka3 BSc, MSc, MBChB, Heema Sharma28 MSc, Denise Shelley17 MS, Bode Shobayo10 MSc, Sophia Siddiqui2 MD, MPH, Jakub Simon12 MD, Shelly Simpson17 MS, Billy Muyisa Sivahera6 MD, Karen Slater3, Mary Smolskis2 BSN, MA, Elizabeth Smout3 MD, MSc, Emily Snowden3 MA, Anne-Aygline Soutthiphong4,29 MSc, Amadou Sow6 MSc, Samba O. Sow22 MD, MSc, Ydrissa Sow2 MD, MPH, Michael Stirratt25 PhD, Jeroen Stoop13 PhD, Guna Subramaniam13 MSc, Léa Surugue1 MJ, Nathalie Swales13 MSc, Sienneh Tamba10 RN, BSN, Chan Tang13 BSc, Cheick Tangara20 MSc, Milagritos D. Tapia22 MD, Julius Teahton10 MSc, Jemee Tegli10 MSc, Monique Termote4 MSc, Guillaume Thaurignac30 MSC, Rodolphe Thiebaut4 MD, PhD, Greg Thompson5 BS, John Tierney2 BSN, MPM, Daniel Tindanbil3 MSc, Abdoulaye Touré23 PharmD, MPH, PhD, Elvis Towalid10 BPharm, Stacey Traina12 BS, Awa Traore19 PharmD, Tijili Tyee10 PharmD, David Vallée1 PharmD, Renaud Vatrinet1 PhD, Corine Vincent4 MSc, Susan Vogel2 RN, BSN, Cedrick Wallet4 MSc, Travis Warren2 PhD, Deborah Watson-Jones3 MD, PhD, Wade Weaver28 MSc, Deborah Wentworth5 MPH, Cecelia Wesseh10 BSc, Hilary Whitworth3 PhD, Jimmy Whitworth3 PhD, Aurelie Wiedemann1,18 PhD, Wouter Willems13 PhD, Barthalomew Wilson10 MSc, Jayanthi Wolf12 PhD, Alie Wurie11 MD, MSc, Delphine Yamadjako17 MS, Marcel Yaradouno6 MSc, Quiawiah Yarmie10 MSc, Yazdan Yazdanpanah1,7,8 MD, PhD, Shuiqing Yu28 BS, Zara Zeggani6 MSc, Huanying Zhou28 BS.
Affiliations
1French Institute for Health and Medical Research (Inserm), 75013 Paris, France
2National Institute of Allergy and Infectious Diseases, Bethesda, MD, USA or under contract/subcontract to NIAID
3London School of Hygiene & Tropical Medicine, London, UK
4Univ. Bordeaux, INSERM, Institut Bergonié, CHU de Bordeaux, CIC-EC 1401, Euclid/F-CRIN clinical trials platform, F-33000 Bordeaux, France
5School of Public Health, University of Minnesota, Minneapolis, MN, USA
6The Alliance for International Medical Action, Alima, B.P.15530 Dakar, Sénégal
7AP-HP, Hôpital Bichat-Claude Bernard, Service de Maladies Infectieuses et Tropicales, Paris F-75018, France
8ANRS Emerging Infectious Diseases, Paris, France
9Centre National de Formation et de Recherche en Santé Rurale de Maferinyah, Maferinyah, Guinea
10Partnership for Research on Ebola Virus in Liberia (PREVAIL), Monrovia, Liberia
11College of Medicine and Allied Health Sciences (COMAHS), University of Sierra Leone, Freetown, Sierra Leone
12Merck Sharp & Dohme Corp, Inc., Kenilworth, NJ, USA
13Janssen Vaccines and Prevention BV Leiden, The Netherlands
14Département de Sociologie, FLSH, Université Cheikh Anta DIOP, Dakar, Sénégal
15Agence Nationale de Sécurité Sanitaire, Conakry, Guinea
16Advanced BioMedical Laboratories, L.L.C., 1605 Industrial Hwy, Cinnaminson, NJ, USA
17Leidos Biomedical Research, Inc. Frederick, MD 21704, USA
18Vaccine Research Institute, Univ. Paris Est Créteil, Henri Mondor Hospital, Créteil, France
19Centre pour le Développement des Vaccins, Ministère de la Santé, Bamako, Mali
20University Clinical Research Center (UCRC), University of Sciences, Techniques and Technologies of Bamako (USTTB), Bamako, Mali
21Liberia Institute for Biomedical Research Ethics Committee/National, Monrovia, Liberia
22Center for Vaccine Development and Global Health, University of Maryland School of Medicine, 685 West Baltimore Street Baltimore, MD 21201-1509, USA
23INSP (Institut Nationale de Santé Publique), Conakry, Guinea
24École de santé publique de l’Université de Montréal, Montréal, Canada
25National Institute of Mental Health, Bethesda, MD, USA
26Univ. Bordeaux, INSERM, Institut Bergonié, CHU de Bordeaux, CIC-EC 1401, Euclid/F-CRIN clinical trials platform and U1219 BPH Inria Sistm, F-33000 Bordeaux, France
27School of Statistics, University of Minnesota, Minneapolis, MN, USA
28Integrated Research Facility at Fort Detrick (IRF-Frederick), National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), Fort Detrick, Frederick, MD, USA
29Univ. Bordeaux, INSERM, MART, UMS 54, F-33000 Bordeaux, France
30Recherche Translationnelle Appliquée au VIH et aux Maladies Infectieuses, Institut de Recherche pour le Développement, University of Montpellier, INSERM, 34090, Montpellier, France.
Funding Statement
This work was supported by the Innovative Medicines Initiative 2 Joint Undertaking (IMI2JU) under grant number 115854, EBOVAC1. This project has also received support from the European Union’s Horizon 2020 research and innovation programme and EFPIA. The dissemination represents only the authors’ views and IMI2JU is not responsible for any use of the information contained in the dissemination. This work was also supported by a dedicated Institut national de la santé et de la recherche médicale (Inserm) allocation on behalf the French Research Ministry. This work was supported in part by the National Institutes of Health (NIH), by Inserm, and by the London School of Hygiene and Tropical Medicine (LSHTM). The clinical trial was conducted with the support of Janssen, Bavarian Nordic, and Merck Sharp & Dohme Corp., which provided the vaccines. This work was also supported in part by the National Cancer Institute contract number HHSN261201500003I through the Frederick National Laboratory for Cancer Research. This work was supported in part through the Laulima Government Solutions, LLC, prime contract with NIH National Institute of Allergy and Infectious Diseases (NIAID) under contract number HHSN272201800013C. IMB performed this work as an employee of Laulima Government Solutions, LLC. JHK performed this work as an employee of Tunnell Government Services (TGS), a subcontractor of Laulima Government Solutions, LLC, under contract number HHSN272201800013C. The views and conclusions contained in this document are those of the authors and should not be interpreted as necessarily representing the official policies, either expressed or implied, of the US Department of Health and Human Services or of the institutions and companies affiliated with the authors, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. PREVAC-UP trial was part of the EDCTP2 programme supported by the European Union (grant number RIA2017S-2014 – PREVAC-UP) and by the US National Institute of Allergy and Infectious Diseases of the National Institutes of Health.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data used are from the recently published PREVAC trial [19]. Data from this study can be accessed by submitting a request to Professor Yazdan Yazdanpanah (yazdan.yazdanpanah@aphp.fr). The data availability begins one year after completion of the 5-year follow-up of the PREVAC trial.
Code availability
The code to reproduce the analyses can be accessed via https://github.com/sistm/PREVAC_UP_WP_Modeling.git.
References
- 1.Jacob ST, Crozier I, Fischer WA II, et al. Ebola virus disease. Nat Rev Dis Primers. 2020 Feb 20;6(1):13. doi: 10.1038/s41572-020-0147-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuhn JH, Amarasinghe GK, Perry DL.. Filoviridae. In: Howley PM, Knipe DM, Whelan SPJ, editors. Fields virology. Vol. 1, Emerging viruses. 7th ed. Philadelphia (PA: ): Wolters Kluwer/Lippincott Williams & Wilkins; 2020. p. 449–503. [Google Scholar]
- 3.European Medicines Agency . Ebola; 2022. Available from: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/ebola
- 4.Bolay FK, Grandits G, Lane HC, et al. PREVAIL I cluster vaccination study with rVSVΔG-ZEBOV-GP as part of a public health response in Liberia. J Infect Dis. 2019 Apr 19;219(10):1634–1641. doi: 10.1093/infdis/jiy698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahlke C, Kasonta R, Lunemann S, et al. Dose-dependent T-cell dynamics and cytokine cascade following rVSV-ZEBOV immunization. EBioMedicine. 2017 May;19:107–118. doi: 10.1016/j.ebiom.2017.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halperin SA, Das R, Onorato MT, et al. Immunogenicity, lot consistency, and extended safety of rVSVΔG-ZEBOV-GP vaccine: a phase 3 randomized, double-blind, placebo-controlled study in healthy adults. J Infect Dis. 2019 Aug 30;220(7):1127–1135. doi: 10.1093/infdis/jiz241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henao-Restrepo AM, Longini IM, Egger M, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet. 2015 Aug 29;386(9996):857–866. doi: 10.1016/S0140-6736(15)61117-5 [DOI] [PubMed] [Google Scholar]
- 8.Huttner A, Agnandji ST, Combescure C, et al. Determinants of antibody persistence across doses and continents after single-dose rVSV-ZEBOV vaccination for Ebola virus disease: an observational cohort study. Lancet Infect Dis. 2018 Jul;18(7):738–748. doi: 10.1016/S1473-3099(18)30165-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishola D, Manno D, MO A, et al. Safety and long-term immunogenicity of the two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Sierra Leone: a combined open-label, non-randomised stage 1, and a randomised, double-blind, controlled stage 2 trial. Lancet Infect Dis. 2022 Jan;22(1):97–109. doi: 10.1016/S1473-3099(21)00125-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milligan ID, Gibani MM, Sewell R, et al. Safety and immunogenicity of novel adenovirus type 26- and modified vaccinia Ankara-vectored Ebola vaccines: a randomized clinical trial. JAMA. 2016 Apr 19;315(15):1610–1623. doi: 10.1001/jama.2016.4218 [DOI] [PubMed] [Google Scholar]
- 11.Pollard AJ, Launay O, Lelievre J-D, et al. Safety and immunogenicity of a two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in adults in Europe (EBOVAC2): a randomised, observer-blind, participant-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2021 Apr;21(4):493–506. doi: 10.1016/S1473-3099(20)30476-X [DOI] [PubMed] [Google Scholar]
- 12.Henao-Restrepo AM, Camacho A, Longini IM, et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet. 2017 Feb 4;389(10068):505–518. doi: 10.1016/S0140-6736(16)32621-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meakin S, Nsio J, Camacho A, et al. Effectiveness of rVSV-ZEBOV vaccination during the 2018–20 Ebola virus disease epidemic in the Democratic Republic of the Congo: a retrospective test-negative study. Lancet Infect Dis. 2024 Aug 20;S1473-3099(24)00419-5. doi: 10.1016/S1473-3099(24)00419-5 [DOI] [PubMed] [Google Scholar]
- 14.Medaglini D, Santoro F, Siegrist C-A.. Correlates of vaccine-induced protective immunity against Ebola virus disease. Semin Immunol. 2018 Oct;39:65–72. doi: 10.1016/j.smim.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization . WHO adapts Ebola vaccination strategy in the Democratic Republic of the Congo to account for insecurity and community feedback; 2019. Available from: https://www.who.int/news/item/07-05-2019-who-adapts-ebola-vaccination-strategy-in-the-democratic-republic-of-the-congo-to-account-for-insecurity-and-community-feedback
- 16.World Health Organization . Strategic Advisory Group of Experts (SAGE) on immunization. Interim recommendations on vaccination against Ebola virus disease (EVD); 2019 May 7. Available from: https://cdn.who.int/media/docs/default-source/immunization/ebola/interim-ebola-recommendations-may-2019.pdf?sfvrsn=c54ce264_9
- 17.World Health Organization . Ebola virus disease – Democratic Republic of the Congo. Wkly Epidemiol Rec. 2020;95(27):301–306. [Google Scholar]
- 18.Badio M, Lhomme E, Kieh M, et al. Partnership for Research on Ebola VACcination (PREVAC): protocol of a randomized, double-blind, placebo-controlled phase 2 clinical trial evaluating three vaccine strategies against Ebola in healthy volunteers in four West African countries. Trials. 2021 Jan 23;22(1):86. doi: 10.1186/s13063-021-05035-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.PREVAC Study Team , Kieh M, Richert L, et al. Randomized trial of vaccines for Zaire Ebola virus disease. N Engl J Med. 2022 Dec 29;387(26):2411–2424. doi: 10.1056/NEJMoa2200072 [DOI] [PubMed] [Google Scholar]
- 20.Rudge TL Jr, Sankovich KA, Niemuth NA, et al. Development, qualification, and validation of the Filovirus Animal Nonclinical Group anti-Ebola virus glycoprotein immunoglobulin G enzyme-linked immunosorbent assay for human serum samples. PLoS One. 2019;14(4):e0215457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kennedy SB, Bolay F, Kieh M, et al. Phase 2 placebo-controlled trial of two vaccines to prevent Ebola in Liberia. N Engl J Med. 2017 Oct 12;377(15):1438–1447. doi: 10.1056/NEJMoa1614067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Afolabi MO, Ishola D, Manno D, et al. Safety and immunogenicity of the two-dose heterologous Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in children in Sierra Leone: a randomised, double-blind, controlled trial. Lancet Infect Dis. 2022 Jan;22(1):110–122. doi: 10.1016/S1473-3099(21)00128-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siegrist C-A, Aspinall R.. B-cell responses to vaccination at the extremes of age. Nat Rev Immunol. 2009 Mar;9(3):185–194. doi: 10.1038/nri2508 [DOI] [PubMed] [Google Scholar]
- 24.Barouch DH, Pau MG, Custers JHHV, et al. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004 May 15;172(10):6290–6297. doi: 10.4049/jimmunol.172.10.6290 [DOI] [PubMed] [Google Scholar]
- 25.Shiver JW, Emini EA.. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu Rev Med. 2004;55:355–372. doi: 10.1146/annurev.med.55.091902.104344 [DOI] [PubMed] [Google Scholar]
- 26.Le Gars M, Sadoff J, Struyf F, et al. Impact of preexisting anti-adenovirus 26 humoral immunity on immunogenicity of the Ad26.COV2.S coronavirus disease 2019 vaccine. J Infect Dis. 2022 Sep 21;226(6):979–982. doi: 10.1093/infdis/jiac142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simon JK, Kennedy SB, Mahon BE, et al. Immunogenicity of rVSVΔG-ZEBOV-GP Ebola vaccine (ERVEBO®) in African clinical trial participants by age, sex, and baseline GP-ELISA titer: a post hoc analysis of three Phase 2/3 trials. Vaccine. 2022 Nov 2;40(46):6599–6606. doi: 10.1016/j.vaccine.2022.09.037 [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann P, Curtis N.. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019 Mar 20;32(2):e00084-18. doi: 10.1128/CMR.00084-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLean C, Barry H, Kieh M, et al. Immune response of a two-dose heterologous Ebola vaccine regimen: summary of three African clinical trials using a single validated Filovirus Animal Nonclinical Group enzyme-linked immunosorbent assay in a single accredited laboratory. EBioMedicine. 2023 Apr 24;91:104562. doi: 10.1016/j.ebiom.2023.104562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee AW, Liu K, Lhomme E, et al. Immunogenicity and vaccine shedding after 1 or 2 doses of rVSVΔG-ZEBOV-GP Ebola vaccine (ERVEBO®): results from a phase 2, randomized, placebo-controlled trial in children and adults. Clin Infect Dis. 2024 Apr 10;78(4):870–879. doi: 10.1093/cid/ciad693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glynn JR, Bower H, Johnson S, et al. Asymptomatic infection and unrecognised Ebola virus disease in Ebola-affected households in Sierra Leone: a cross-sectional study using a new non-invasive assay for antibodies to Ebola virus. Lancet Infect Dis. 2017 Jun;17(6):645–653. doi: 10.1016/S1473-3099(17)30111-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thom R, Tipton T, Strecker T, et al. Longitudinal antibody and T cell responses in Ebola virus disease survivors and contacts: an observational cohort study. Lancet Infect Dis. 2021 Apr;21(4):507–516. doi: 10.1016/S1473-3099(20)30736-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson MS, Niemuth NA, Sabourin CL, et al. Interlaboratory comparison for the Filovirus Animal Nonclinical Group (FANG) anti-Ebola virus glycoprotein immunoglobulin G enzyme-linked immunosorbent assay. PLoS One. 2020;15(8):e0238196. doi: 10.1371/journal.pone.0238196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grais RF, Kennedy SB, Mahon BE, et al. Estimation of the correlates of protection of the rVSVΔG-ZEBOV-GP Zaire ebolavirus vaccine: a post-hoc analysis of data from phase 2/3 clinical trials. Lancet Microbe. 2021 Feb;2(2):e70–e78. doi: 10.1016/S2666-5247(20)30198-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used are from the recently published PREVAC trial [19]. Data from this study can be accessed by submitting a request to Professor Yazdan Yazdanpanah (yazdan.yazdanpanah@aphp.fr). The data availability begins one year after completion of the 5-year follow-up of the PREVAC trial.