Abstract
BACKGROUND
The insula is a central node in network models of compassion and empathy. Because of this, resection of the insula for the treatment of drug-resistant epilepsy can change an individual’s level of compassion.
OBSERVATIONS
Here, the authors present the clinical case of a woman with drug-resistant epilepsy localized to the nondominant insula. Because of the widespread literature implicating insular function in empathy and compassion, including lesion studies, her primary concern was changes in her compassion level after insular resection. In this case, the authors performed a novel compassion mapping paradigm before resection, using 30-second video clips to elicit compassion. This showed no changes in compassion with electrical stimulation of sites spanning the anterior insula, providing some reassurance that resection would not affect her compassion. Consistent with this, pre- and postresection testing, along with informal subjective reports by the patient, demonstrated no change in compassion or subcomponents of compassion (sadness and empathy) after right insular resection.
LESSONS
While resection of the nondominant insular cortex warrants caution, this case illustrates a compassion mapping paradigm that reassured the clinical team and the patient that her compassion would not be affected and formal postoperative testing that was consistent with this.
Keywords: insula, insular epilepsy, compassion mapping, empathy mapping, sadness, epilepsy surgery
ABBREVIATIONS: EEG = electroencephalography, IRI = Interpersonal Reactivity Index, MCA = middle cerebral artery, MRI = magnetic resonance imaging.
The insula is the fifth and perhaps least well-understood lobe in the human brain. It lies deep within the sylvian fissure, flanked by the frontal, parietal, and temporal opercula. During insular seizures, patients usually retain awareness and have a wide range of symptoms, including pain, heat, difficulty breathing, or visceral sensorimotor symptoms (abdominal sensations).1
In line with the semiology, the insula serves several important functions, including pain and temperature sensation, gustation, internal organ sensation, and sensation of the body’s internal physiological state (interoception). Consistent with the insula’s role in representing how we feel,2, 3 many studies have implicated the insula as a significant node in the generation and perception of compassion and empathy.4–21 There is also evidence for functional lateralization in which the nondominant insula is specific for the affective-perceptual form of empathy.4–9 Because of the volume of literature implicating the insula in these emotions, some clinicians have concerns that insular resection could lead to changes in emotional temperament or personality. Indeed, lesion studies have shown changes in empathy10 or impaired subcomponents of these emotions, such as recognition of emotion in others.11, 12
Here, we report on a patient with drug-resistant insular epilepsy whose primary concern was a loss of compassion after the resection of her nondominant insula. Compassion is a complex emotion that involves several steps: 1) detection of sadness or suffering, 2) mirroring of emotional experience (empathy), 3) assessing the deservedness of the sufferer, and 4) feeling warmth/affection and the motivation to help (Fig. 1).13 Thus, compassion is a complex prosocial emotion that can involve sadness and empathy early in the compassion pathway.
FIG. 1.
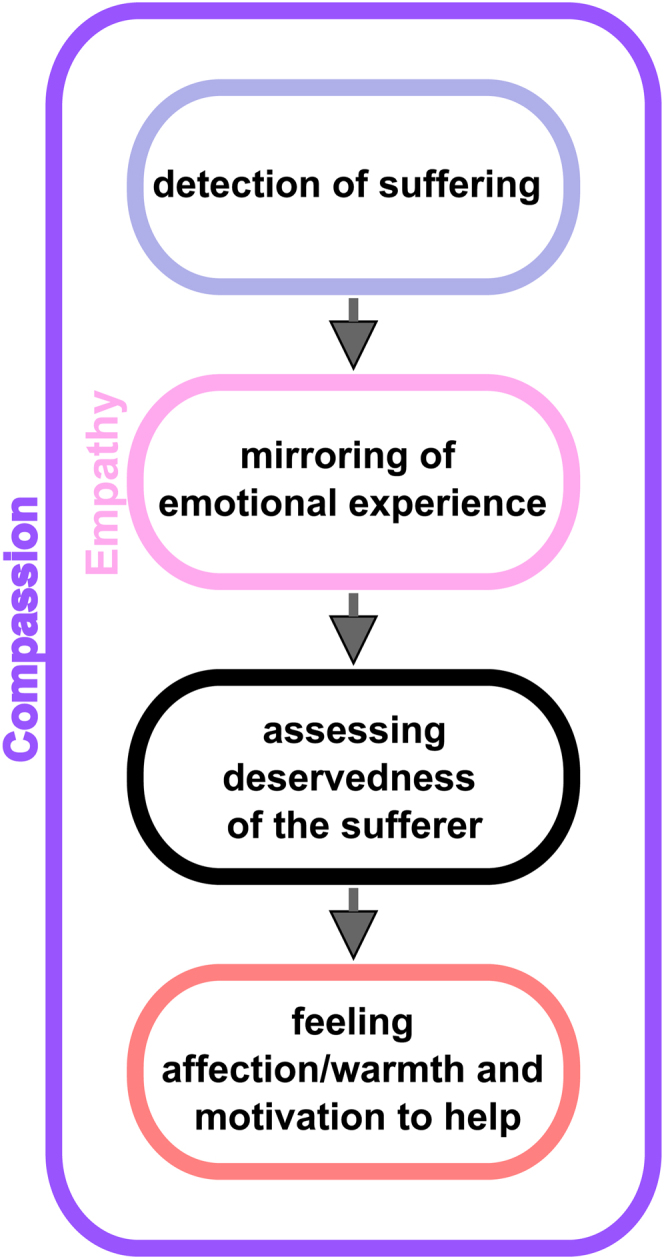
Diagram showing the cognitive and emotional steps that are involved along the compassion pathway. As shown, empathy is a subcomponent of compassion. By assessing for intact compassion with stimulation in the anterior insula, we are also evaluating for intact empathy.
To assess the risk of compassion changes prior to resection, a novel compassion mapping task was generated and performed, showing no change in compassion from stimulation at several sites spanning the right anterior insula. The patient then underwent further pre- and postoperative measurements of compassion, empathy, and sadness reactivity with no significant changes after total right insular resection, thus reinforcing the mapping paradigm findings.
Illustrative Case
Presentation and Workup
A 33-year-old right-handed woman presented for evaluation of drug-resistant focal epilepsy. The patient had an onset of seizures when she was 15 years old, and, despite numerous antiseizure medication trials, her seizure frequency increased over time. Her only epilepsy risk factor was severe traumatic brain injury in the setting of cardiogenic syncope with a right-sided skull fracture at age 14 years. At age 18 years, she underwent a right anterior temporal lobectomy for treatment of her drug-resistant epilepsy but only experienced a reduction in her seizure frequency for 4 months. She continued medication trials up through 2022 and then presented to our center for evaluation. At that time, she had been having four seizures a month.
Her seizure semiology involves déjà vu, metallic dysgeusia, and “butterflies” in her stomach, followed by behavioral arrest, facial grimacing, hypersalivation, palpitations, and right-hand automatisms with left-hand dystonic posturing. She often has postictal anxiety or panic attacks. She does not secondarily generalize but occasionally has rapid falls with significant associated morbidity.
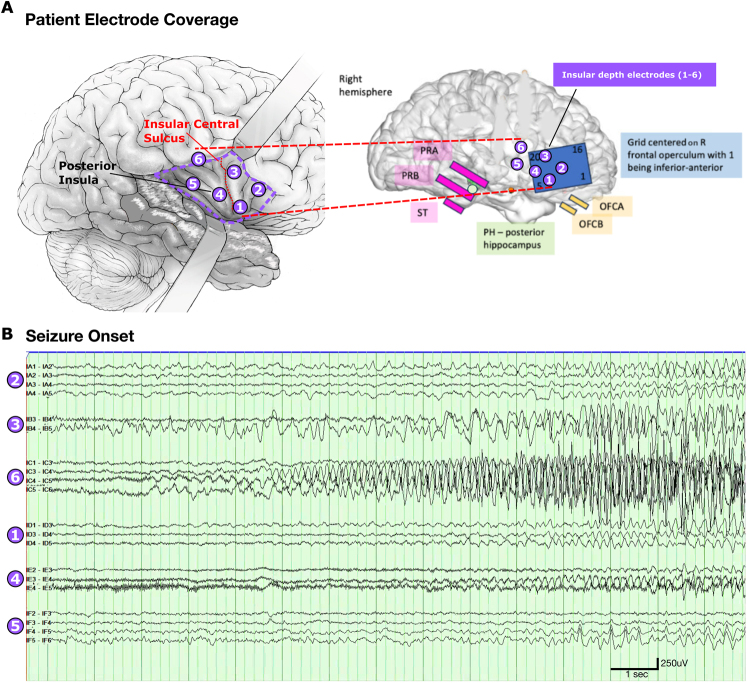
After establishing epilepsy care at our center, we admitted the patient for scalp video-electroencephalography (EEG) monitoring. Video-EEG showed multiple right-lateralized, poorly localized electroclinical seizures with an ictal pattern over the right posterior temporal region. Notably, during the onset of one seizure, she had ictal bradycardia on the rhythm strip. Magnetic resonance imaging (MRI) showed a residual hippocampal tail from the prior anterior temporal lobectomy and a resection margin of 5.8 cm from the temporal pole. Magnetoencephalography showed focal slowing and epileptiform discharges from the right frontotemporal region, with source modeling localizing this activity to regions anterior and superior to the resection cavity. Neuropsychological evaluation was intact except for isolated spatial memory impairment, consistent with the prior nondominant anterior temporal lobe resection. During intracranial monitoring with stereo-EEG, the seizure onset zone was localized to the right insula (Fig. 2).
FIG. 2.
A: Intracranial electrode coverage. The insular targets of six stereo-depth electrodes (left) evenly sample anterior and posterior insula. Noninsular coverage (right) along the prior temporal lobe resection margin, residual hippocampus, right inferior frontal lobe, and orbital frontal cortex. B: Typical seizure ictal onset recorded by stereo depth electrodes. There was no ictal correlate in noninsular coverage. As shown, the seizure starts in insular depth electrode 6 and spreads to insular depth electrodes 3, 4, and 5 at ictal onset. OFCA = orbital frontal cortex A; OFCB = orbital frontal cortex B; PH = posterior hippocampus; PRA = peri-resection A; PRB = peri-resection B; ST = subtemporal.
Given the right insular localization and abundant literature implicating the right insula in compassion and empathy (found during online searches by the patient), she was concerned about the potential loss of empathy and, more specifically, loss of compassion for others after surgery. To provide information on the risk of reduced compassion after right insular resection, we developed and performed a novel stimulation mapping paradigm designed to identify sites in the right insula involved in compassion. To do this, we selected three stimulation sites spanning the anterior insula, the insular area that has been implicated in mediating compassion (Fig. 3B). The posterior insula is considered to be primarily relevant for interoception rather than compassion, so this region was not included in stimulation.3, 14–17 Testing was also restricted to sites that span the anterior insula because generating a large enough set of compassion video clips for compassion mapping is a major limiting factor. Thus, we aimed for a video clip library with sufficient power to primarily test the cortex (anterior insula) with moderate to high suspicion of being necessary for the subjective experience of compassion.
FIG. 3.
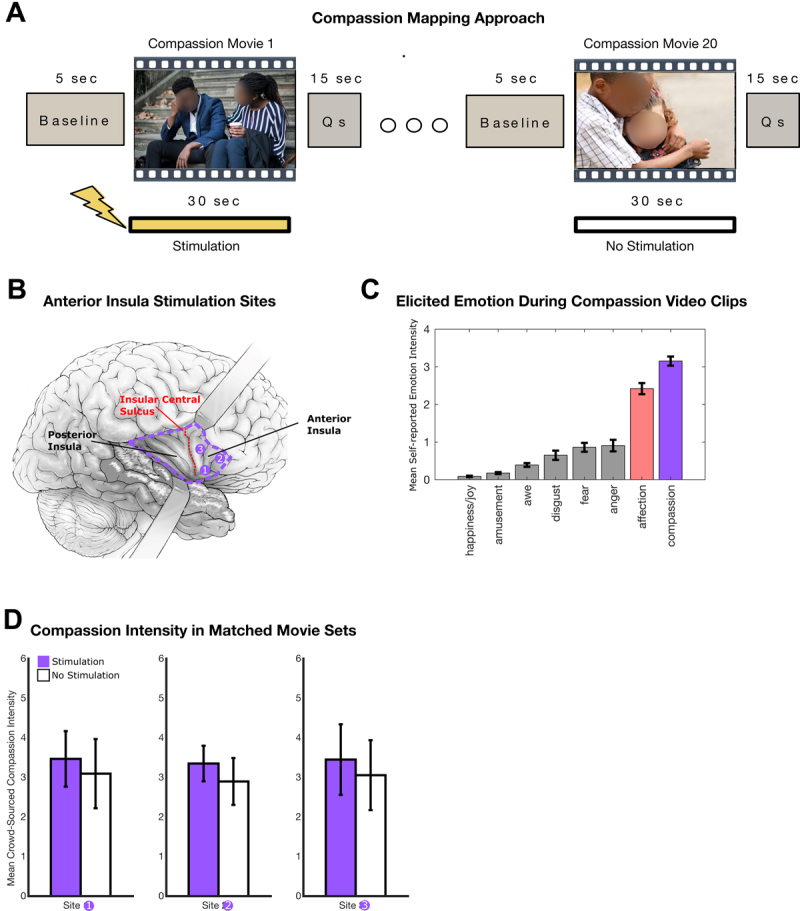
Approach to compassion mapping. A: The mapping approach involves the presentation of 30-second video clips that elicit compassion while stimulation is delivered on every other clip for the duration of the video. At the end of each video clip, the participant reports whether they felt compassion and, if so, its overall intensity. B: Planned anterior insula stimulation sites for compassion mapping. C: Compassion video clip validation data. As expected, the compassion video clips elicit moderate to high levels of compassion, as measured through direct self-report ratings (3.4 ± 0.15 standard error of the mean). Consistent with compassion elicitation, a moderate level of affection is elicited because the last step in the compassion pathway comprises a feeling of affection/warmth and a motivation to help. As expected, there is minimal to no elicitation of noncompassion-related emotions such as fear, anger, amusement, awe, disgust, and joy. D: Video clip set quantification for each stimulation site showing that the mean compassion intensity is the same for videos paired with stimulation and videos without stimulation. Given the same level of elicited compassion in both video clip sets, any measured difference in the experience of compassion will be attributable to anterior insula stimulation. Note that each set of 20 video clips for each site (60 video clips in total) is unique to prevent emotion elicitation decrements, which would occur if the same clip was presented more than once during the mapping procedure. Qs = questions.
To build a compassion video clip library, we estimated that a set of 60 unique compassion video clips would have adequate power to test the anterior insula (3 insular sites, 20 unique compassion clips per site, 60 unique video clips in total). During the compassion mapping task, each video clip was presented no more than once, since repetition of the same video clip leads to emotional desensitization.18 For example, viewing the same compassion clip within the same day leads to lower elicited compassion on the second viewing. Therefore, sets of unique videos are needed for each site, so decrements in mean compassion over a set can be attributed to stimulation rather than desensitization from video repetition.
To measure the level of subjectively experienced compassion elicited by each clip in the library, videos were crowdsourced online to obtain self-report ratings of the level of compassion elicited. This was done through Gorilla Experiment Builder, an online platform to create and crowdsource behavioral experiments. All raters were in the United States, male and female (50%), and were age-matched to the distribution of patients seen in the epilepsy monitoring unit at our center. After each video, raters answered a multiple-choice question about the content of the video to ensure that they attended to the video during the presentation (3% error rate, error trials were excluded). To quantify compassion elicitation, raters (total n = 1140) self-reported the level of compassion elicited by each video clip on a six-point Likert scale (0 = no compassion, 6 = most compassion ever felt). Given that the subjective experience of emotion reflects how one feels internally, the most direct insight into a person’s experience of emotion is through self-report measures, as done here.19 While there are other ways to measure emotions, such as autonomic nervous system measurement, facial expressions, vocalization characteristics, and whole body behavior,20 the metric of interest clinically and to the patient is her subjective experience (how much compassion she feels) and is quantified during the mapping procedure by self-reported compassion. As shown in Fig. 3C, the mean intensity of compassion elicited for each video was 3.4 on a six-point Likert scale, indicating a moderate to high degree of compassion elicitation. To further validate the task’s ability to elicit and quantify compassion, we analyzed whether six other non–compassion-related emotions (fear, anger, disgust, happiness/joy, and awe) were elicited by the videos. Consistent with video clips that elicit compassion, there was minimal elicitation of other unrelated emotions such as fear, anger, amusement, awe, disgust, and happiness/joy (Fig. 3C). Finally, given that the last step in the compassion pathway comprises a feeling of warmth/affection and a motivation to help (Fig. 1), these videos also elicited a moderate degree of affection (Fig. 3C). Overall, these data support the validity of a video-based task that quantifies elicited compassion.
To test each anterior insula site, a unique (nonrepeated) set of 20 video clips was presented to the patient, with electrical stimulation on every other clip for the duration of each video (Fig. 3A). Thus, for each site, we presented a set of 10 compassion clips with stimulation and 10 nonrepeated compassion clips without stimulation. Critically, the stimulation and nonstimulation video clip sets for each site were matched (pairwise) in their average emotive power to elicit compassion (Fig. 3D), so any difference in the patient’s mean reported compassion across the set would be attributable to stimulation-induced changes in compassion. Additionally, before the mapping procedure began, 30-second trains of stimulation (task-free, no-compassion video clips) were administered to ensure the absence of stimulation-induced sensory phenomena, pain, or emotion that would indirectly change the experience of compassion. Lastly, current thresholds sufficient to drive motor activations were determined by stimulating the right frontal grid, which overlies the motor cortex (tongue/lip). This helped estimate the current necessary (3.0 mA) to drive clinical effects and served as a positive control for stimulation-based changes sufficient to perturb function.
Using this approach, we performed compassion mapping in the nondominant insula. As shown in Fig. 4, there was no difference in her subjective experience of compassion between movies with stimulation and those without. This result indicates that stimulation at sites spanning the nondominant anterior insula does not cause changes in compassion, helping reassure the patient and clinicians that insular resection would not alter her compassion level.
FIG. 4.
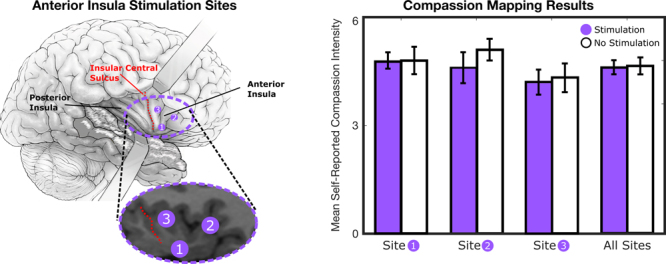
Compassion mapping results. There was no significant change in video-elicited compassion with stimulation at three sites spanning the anterior insula. Ten video clips without stimulation and ten video clips with stimulation were presented at each site. The stimulation parameters for each video clip were: 30-second duration, 3.0-mA current, 100-μsecond pulse duration, and 50-Hz pulse frequency. Before video-based compassion mapping was performed, 30-second trains of stimulation at each site (without an emotion-eliciting video clip) did not elicit pain, subjective experience, or positive sensory phenomena that would have indirectly changed the subjective experience of compassion.
To assess whether there were any changes in her ability to experience compassion or subcomponents of compassion (empathy and sadness) after right insular resection, she underwent preoperative testing to establish her baseline compassion, empathy, and sadness reactivity. Sadness and empathy were tested so that, in case of a change in overall compassion levels, we could further investigate the relative contributions of subcomponents of this complex prosocial emotion. Prior to surgery, a set of 20 video clips that elicit sadness were presented to her, with a sadness rating on a Likert-type scale reported after each video clip. Sadness was chosen because it is among the most common emotions that evoke compassion. Additionally, her baseline level of empathy and overall compassion were measured using the Interpersonal Reactivity Index (IRI),21, 22 a well-validated and standard multidimensional tool used in affective science to measure cognitive empathy, affective-perceptual empathy, and compassion (empathic concern).
Operation, Postoperative Course, and Testing
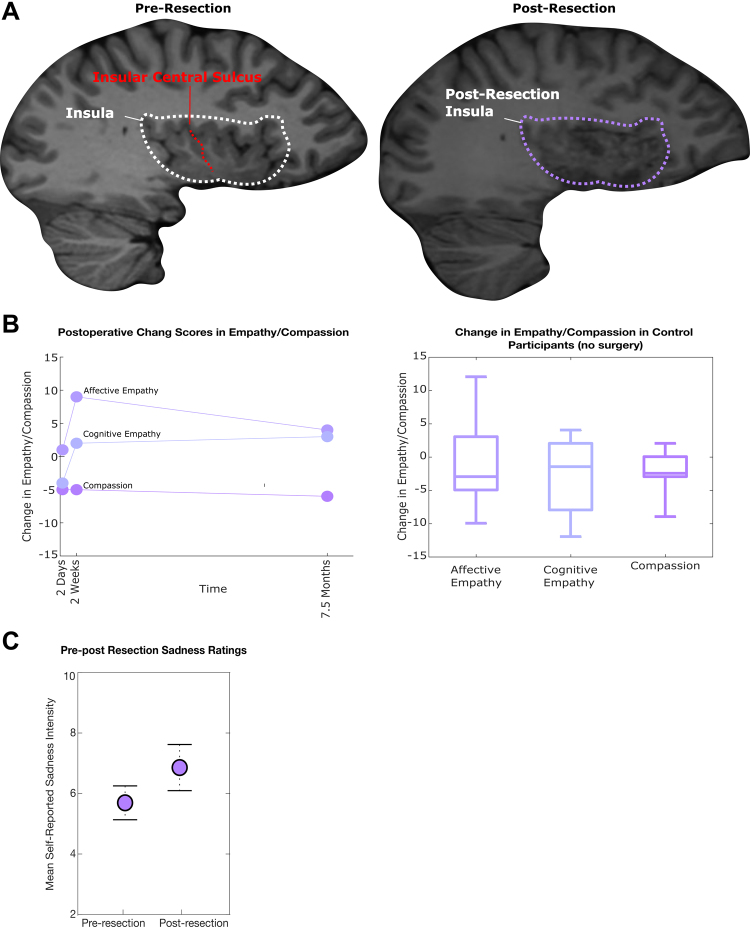
After risk-benefit counseling, the patient underwent complete right insular resection (Fig. 5A). No further functional testing was performed intraoperatively. Aided by the prior right anterior temporal lobectomy, the insular surface was exposed with good visualization of the middle cerebral artery (MCA) vessels traversing the area. The insula was resected through the MCA candelabra with care to protect the vessels. The vast majority of the insula was removed except for a small area in the posterior-superior zone that was inaccessible because of vascular anatomy. Care was taken to protect the underlying white matter, and no motor or speech complications were present postoperatively. On postoperative day 1, the patient subjectively felt she was at her cognitive and emotional baseline, apart from 2/10 pain at the incision site.
FIG. 5.
A: Pre- (left) and postresection (right) sagittal T1-weighted MRI to visualize the right insula. B: Empathy and compassion change scores relative to the preoperative baseline (left). There were no significant changes from baseline in cognitive empathy, affective empathy, or compassion (empathic concern) after right insular resection at 2 days, 2 weeks, and 7.5 months after resection (two-sided Wilcoxon signed rank-test, p > 0.05 at each time point). Because of normal fluctuation in empathy and compassion scores on repeat interval testing, the distribution of change scores in a control group (n = 20, no resection) were collected to determine if the patient’s change scores were within normal limits of fluctuation on repeat testing (right). The control distributions (right) overlap with the change scores in the patient (left), indicating no significant change in empathy or compassion. C: Acute postresection sadness reactivity. There was no change in the median intensity of sadness elicited by sadness video clips when compared to the preoperative baseline (Wilcoxon rank-sum test, p > 0.05; 80%–90% of videos elicited sadness on the pre- and postresection presentation of the video clip set).
Three days after insular resection, the same 20 sadness video clips were presented and rated. Overall, the proportion of videos she rated as sad was unchanged (80% preoperatively versus 85% postoperatively, not plotted), and the median sadness level for those videos was not significantly different (Fig. 5C; 5.8 versus 6.4, p > 0.05, Wilcoxon rank-sum test). To address the primary outcome of changes in compassion or empathy, she was reassessed with the IRI questionnaire 2 days, 2 weeks, and 7.5 months after insular resection. The IRI measures compassion (empathic concern) and the two main components of empathy (cognitive and affective empathy). As shown in Fig. 5B, there were no significant changes in compassion, cognitive empathy, or affective empathy at 2 days, 2 weeks, and 7.5 months after right insular resection. The patient’s small postoperative changes (near zero change in IRI) were in the range of normal variation of repeat testing in age-matched individuals with no resection 0.5–7.5 months apart (Fig. 5B). Overall, these data show no significant change in this patient’s compassion or subcomponents of compassion after nondominant insular resection. The patient remains seizure-free at 7.5 months postresection.
Informed Consent
The necessary informed consent was obtained in this study.
Discussion
Observations
In this case study, we employed a novel compassion mapping task to determine if the anterior insula is involved in compassion. Just as mapping of speech is critical for guiding resection in drug-resistant epilepsy, preservation of emotional and social abilities is also crucial. This case report illustrates a compassion mapping paradigm that could potentially generalize to other emotions for brain areas where those emotions are implicated. Overall, stimulation during the viewing of video clips intended to elicit compassionate feelings showed no significant perturbation of the patient’s subjective experience of compassion when compared to matched video clips with no stimulation. Consistent with this preoperative compassion mapping result, postoperative testing of compassion and subcomponents of compassion (sadness and empathy) showed no significant changes.
The role of the insula in compassion and empathy raises valid concerns for patients with epilepsy who are considering the risks and benefits of surgical treatment. Because of its highly connected nature in the brain, the insula is a critical relay point to other high-level cortical regions collectively responsible for subjective feelings of compassion and empathy.17, 23–39 In combination with the surgical risks of resecting a deeply embedded structure such as the insula, careful presurgical testing and planning are warranted. Compassion and other emotion mapping paradigms can serve as useful tools to address concerns for changes in emotional temperament in patients undergoing surgical management of epilepsy.
In this report, preoperative compassion mapping showed a lack of compassion-related sites, which helped increase confidence for resection. Additionally, postoperative assessments showed no significant changes in compassion at 2 days, 2 weeks, and 7.5 months. However, it is important to note that this does not constitute proof that the mapping task is sufficient to avoid resecting necessary compassion-related cortex. It is possible that the mapping paradigm, as constructed, is not sufficient to reliably identify the compassion-related cortex, but in this patient, the nondominant insular cortex is not necessary for compassion, thus resulting in a good outcome. Future research is needed to assess the robustness of this mapping paradigm. However, this report does show the first structured approach to emotion mapping for preoperative assessment and has ultimately helped influence a clinical decision.
After anterior temporal lobectomy, new-onset psychiatric problems have been seen in up to 31% of patients shortly after resection (less than 6 months after surgery).40 These new-onset disorders are typically depression or anxiety and suggest that resection can lead to changes in emotion or mood. In addition to the neuroimaging literature,17, 23–39 data specifically arguing for changes in empathy or compassion after insular resection are lesion studies, including resections, that show changes in empathy10 or impaired subcomponents of these emotions, such as recognition of emotion in others.11, 12 While the loss of compassion and empathy are not reported in the epilepsy surgery literature, it is possible that empathy or compassion changes are present but remain undetected by physicians for several reasons. Two possible contributing factors are that 1) insular resections in epilepsy are less common relative to other procedures, such as anterior temporal lobectomies; and 2) compassion is a complex emotion that is difficult to measure or to even subjectively be assessed by the patient or the physician, making detection of changes more difficult. However, given the volume of literature implicating the right insula in compassion, this was a reasonable concern to address, and compassion mapping provided reassurance to the patient and the clinical team prior to resection.
Lessons
Although this is a single illustrative case, the ways that resections can affect emotional temperament and, consequently, quality of life are unknown and a concern for patients. Uncertainties regarding anticipated postoperative deficits are a barrier to elective resections. In the face of such uncertainties, a conservative approach is often favored, and resection is avoided to minimize the risk of harm to the patient. However, with more information and mapping paradigms such as the ones presented, these uncertainties can be reduced, thus allowing for better risk assessments to provide maximally effective surgeries and reassurance to the patient and the clinical team.
Acknowledgments
We want to thank the EEG technologists at the University of California, San Francisco, for their help in acquiring electrophysiological data.
Research reported in this publication was supported by National Institute of Mental Health (NIMH) of the National Institutes of Health under award number R01MH122431. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Chang, Hullett, Lin, Greicius, Shih, Sturm. Acquisition of data: Chang, Hullett, Lin, Greicius, Knowlton. Analysis and interpretation of data: Chang, Hullett, Lin, Rao, Sturm. Drafting the article: Hullett, Lin, Sturm. Critically revising the article: Chang, Hullett, Lin, Knowlton, Rao, Sturm. Reviewed submitted version of manuscript: Chang, Hullett, Lin, Greicius, Knowlton, Rao. Approved the final version of the manuscript on behalf of all authors: Chang. Statistical analysis: Hullett, Lin. Administrative/technical/material support: Lin, Greicius, Shih. Study supervision: Chang, Sturm.
Correspondence
Edward F. Chang: University of California, San Francisco, CA. edward.chang@ucsf.edu.
References
- 1.Jobst BC, Gonzalez-Martinez J, Isnard J, et al. The insula and its epilepsies. Epilepsy Curr. 2019;19(1):11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655-666. [DOI] [PubMed] [Google Scholar]
- 3.Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59-70. [DOI] [PubMed] [Google Scholar]
- 4.Brüne M, Lissek S, Fuchs N, et al. An fMRI study of theory of mind in schizophrenic patients with “passivity” symptoms. Neuropsychologia. 2008;46(7):1992-2001. [DOI] [PubMed] [Google Scholar]
- 5.Fan Y, Han S. Temporal dynamic of neural mechanisms involved in empathy for pain: an event-related brain potential study. Neuropsychologia. 2008;46(1):160-173. [DOI] [PubMed] [Google Scholar]
- 6.Gu X, Han S. Attention and reality constraints on the neural processes of empathy for pain. Neuroimage. 2007;36(1):256-267. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence EJ, Shaw P, Giampietro VP, Surguladze S, Brammer MJ, David AS. The role of “shared representations” in social perception and empathy: an fMRI study. Neuroimage. 2006;29(4):1173-1184. [DOI] [PubMed] [Google Scholar]
- 8.Moriguchi Y, Decety J, Ohnishi T, et al. Empathy and judging other’s pain: an fMRI study of alexithymia. Cereb Cortex. 2007;17(9):2223-2234. [DOI] [PubMed] [Google Scholar]
- 9.Sander K, Scheich H. Left auditory cortex and amygdala, but right insula dominance for human laughing and crying. J Cogn Neurosci. 2005;17(10):1519-1531. [DOI] [PubMed] [Google Scholar]
- 10.Leigh R, Oishi K, Hsu J, et al. Acute lesions that impair affective empathy. Brain. 2013;136(8):2539-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terasawa Y, Kurosaki Y, Ibata Y, Moriguchi Y, Umeda S. Attenuated sensitivity to the emotions of others by insular lesion. Front Psychol. 2015;6:1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klepzig K, Domin M, Wendt J, et al. Structural integrity of the insula and emotional facial recognition performance following stroke. Brain Commun. 2023;5(3):fcad144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goetz JL, Keltner D, Simon-Thomas E. Compassion: an evolutionary analysis and empirical review. Psychol Bull. 2010;136(3):351-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nat Neurosci. 2000;3(2):184-190. [DOI] [PubMed] [Google Scholar]
- 15.Brooks JCW, Nurmikko TJ, Bimson WE, Singh KD, Roberts N. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage. 2002;15(2):293-301. [DOI] [PubMed] [Google Scholar]
- 16.Kong J, White NS, Kwong KK, et al. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Hum Brain Mapp. 2006;27(9):715-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157-1162. [DOI] [PubMed] [Google Scholar]
- 18.Averill JR, Malmstrom EJ, Koriat A, Lazarus RS. Habituation to complex emotional stimuli. J Abnorm Psychol. 1972;80(1):20-28. [DOI] [PubMed] [Google Scholar]
- 19.LeDoux J. The Emotional Brain: the Mysterious Underpinnings of Emotional Life. Simon & Schuster; 1996. [Google Scholar]
- 20.Mauss IB, Robinson MD. Measures of emotion: a review. Cogn Emot. 2009;23(2):209-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. J Pers Soc Psychol. 1983;44(1):113-126. [Google Scholar]
- 22.Davis MH. Self report measures for love and compassion research: empathy Interpersonal Reactivity Index (IRI). JSAS Cat Sel Doc Psychol. 1980;10(85):3. [Google Scholar]
- 23.Preston SD, Bechara A, Damasio H, et al. The neural substrates of cognitive empathy. Soc Neurosci. 2007;2(3-4):254-275. [DOI] [PubMed] [Google Scholar]
- 24.De Gelder B, Snyder J, Greve D, Gerard G, Hadjikhani N. Fear fosters flight: a mechanism for fear contagion when perceiving emotion expressed by a whole body. Proc Natl Acad Sci U S A. 2004;101(47):16701-16706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blakemore SJ, Bristow D, Bird G, Frith C, Ward J. Somatosensory activations during the observation of touch and a case of vision-touch synaesthesia. Brain. 2005;128(7):1571-1583. [DOI] [PubMed] [Google Scholar]
- 26.Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci U S A. 2003;100(9):5497-5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakrabarti B, Bullmore E, Baron-Cohen S. Empathizing with basic emotions: common and discrete neural substrates. Soc Neurosci. 2006;1(3-4):364-384. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Y, Lin CP, Liu HL, et al. Expertise modulates the perception of pain in others. Curr Biol. 2007;17(19):1708-1713. [DOI] [PubMed] [Google Scholar]
- 29.Derntl B, Finkelmeyer A, Eickhoff S, et al. Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology. 2010;35(1):67-82. [DOI] [PubMed] [Google Scholar]
- 30.Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34(4):1744-1753. [DOI] [PubMed] [Google Scholar]
- 31.Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44(5):752-761. [DOI] [PubMed] [Google Scholar]
- 32.Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. J Cogn Neurosci. 2007;19(1):42-58. [DOI] [PubMed] [Google Scholar]
- 33.Lee TW, Dolan RJ, Critchley HD. Controlling emotional expression: behavioral and neural correlates of nonimitative emotional responses. Cereb Cortex. 2008;18(1):104-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris JS, Scott SK, Dolan RJ. Saying it with feeling: neural responses to emotional vocalizations. Neuropsychologia. 1999;37(10):1155-1163. [DOI] [PubMed] [Google Scholar]
- 35.Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439(7075):466-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Gaag C, Minderaa RB, Keysers C. Facial expressions: what the mirror neuron system can and cannot tell us. Soc Neurosci. 2007;2(3-4):179-222. [DOI] [PubMed] [Google Scholar]
- 37.Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655-664. [DOI] [PubMed] [Google Scholar]
- 38.Wiebking C, Bauer A, De Greck M, Duncan NW, Tempelmann C, Northoff G. Abnormal body perception and neural activity in the insula in depression: An fMRI study of the depressed “material me.” World J Biol Psychiatry. 2010;11(3):538-549. [DOI] [PubMed] [Google Scholar]
- 39.Saarela MV, Hlushchuk Y, Williams ACC, Schürmann M, Kalso E, Hari R. The compassionate brain: humans detect intensity of pain from another’s face. Cereb Cortex. 2007;17(1):230-237. [DOI] [PubMed] [Google Scholar]
- 40.Glosser G, Zwil AS, Glosser DS, O’Connor MJ, Sperling MR. Psychiatric aspects of temporal lobe epilepsy before and after anterior temporal lobectomy. J Neurol Neurosurg Psychiatry. 2000;68(1):53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]