Abstract
BACKGROUND
Endoscopic third ventriculostomy (ETV) is an effective procedure for the treatment of triventriculomegaly associated with aqueductal stenosis. However, some patients can develop severe and symptomatic intracranial pressure (ICP) elevations in the immediate postoperative period that can be monitored and treated with external ventricular drain (EVD) placement and controlled cerebrospinal fluid (CSF) diversion until the ICP normalizes and symptoms resolve.
OBSERVATIONS
The authors describe the case of a 39-year-old male who underwent ETV and intraoperative EVD placement for obstructive hydrocephalus associated with aqueductal stenosis. The patient was noted to have sustained ICP elevations in the immediate perioperative period but ultimately experienced a successful clinical outcome without requiring a ventriculoperitoneal shunt.
LESSONS
Significant sustained ICP elevations in the immediate postoperative period following ETV can occur and may indicate a prolonged adjustment period. These elevations can be tolerated if the patient’s symptoms and ICP are well controlled, with temporary external CSF diversion if the patient becomes symptomatic, as the ICP will likely normalize with a reassuring clinical outcome.
Keywords: endoscopic third ventriculostomy, high intracranial pressure, external ventricular drainage
ABBREVIATIONS: CSF = cerebrospinal fluid, ETV = endoscopic third ventriculostomy, EVD = external ventricular drain, ICP = intracranial pressure, MRI = magnetic resonance imaging.
Endoscopic third ventriculostomy (ETV) is a well-established procedure for the treatment of triventriculomegaly associated with aqueductal stenosis. ETV is preferred over ventriculoperitoneal shunt placement for aqueductal stenosis, as it avoids the shunt-associated risks of mechanical failure, shunt infection, and overdrainage.1 Lam et al. and Isaacs et al. have shown that ETV is effective in the adult population, demonstrating success rates of 74.7% and 87%, respectively.2, 3
In patients who have undergone ETV, elevated intracranial pressure (ICP) can occur postoperatively without clinical symptoms.3–13 Normal ICP is generally considered to range from approximately 0 to 20 mm Hg; however, in chronic hydrocephalus, some individuals can exhibit elevated ICP and remain asymptomatic.14 Physiologically, significantly elevated and sustained ICP can cause a decrease in the cerebral perfusion pressure, potentially resulting in cerebral hypoperfusion. This effect has been well documented in the acute setting of severe traumatic brain injury.15–17
Following ETV, some authors suggest intraoperative external ventricular drain (EVD) placement for postoperative ICP measurements. Normal ICP levels following ETV are reassuring and suggestive of a successful procedure. However, high sustained ICPs can also be seen during this period, and appropriate management remains unclear.5, 7, 8, 12, 13 Bellotti et al. have described an “adaptation period” following successful ETV, whereby an EVD placed following successful ETV with elevated ICP must be kept closed for the fenestration to mature.5 During this prolonged adjustment period, it may be important to allow sustained ICP elevations to ensure patency of the fenestration with controlled cerebrospinal fluid (CSF) drainage only if the patient becomes severely symptomatic. The time line for this period is not well established, but the current literature indicates that it can last several days to weeks.
We describe the case of a 39-year-old male who underwent ETV for obstructive hydrocephalus associated with aqueductal stenosis and who was noted to have elevated ICP in the immediate postoperative course but ultimately experienced a successful clinical outcome without requiring a ventriculoperitoneal shunt.
Illustrative Case
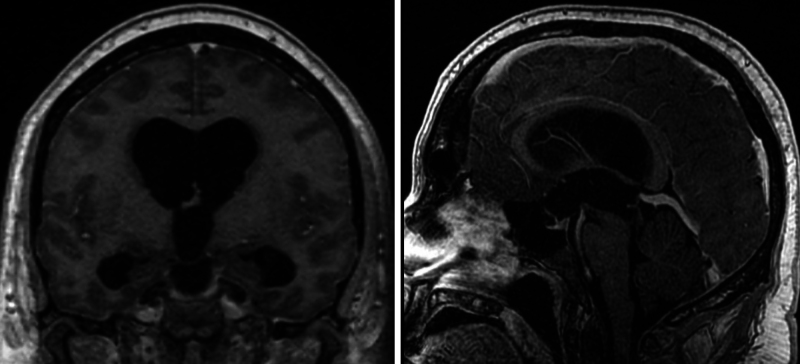
A 39-year-old male with no significant past medical history presented with an approximately 1-year history of progressive headaches accompanied by nausea and emesis. The patient exhibited an intact neurological examination, and head CT revealed triventriculomegaly with enlargement of the lateral and third ventricles, indicative of obstructive hydrocephalus. Brain magnetic resonance imaging (MRI) with and without contrast demonstrated aqueductal stenosis as the etiology of the triventriculomegaly (Fig. 1).
FIG. 1.
Preoperative coronal and sagittal postcontrast T1-weighted MRI sequences demonstrating triventriculomegaly (left) and aqueductal stenosis from a web (right).
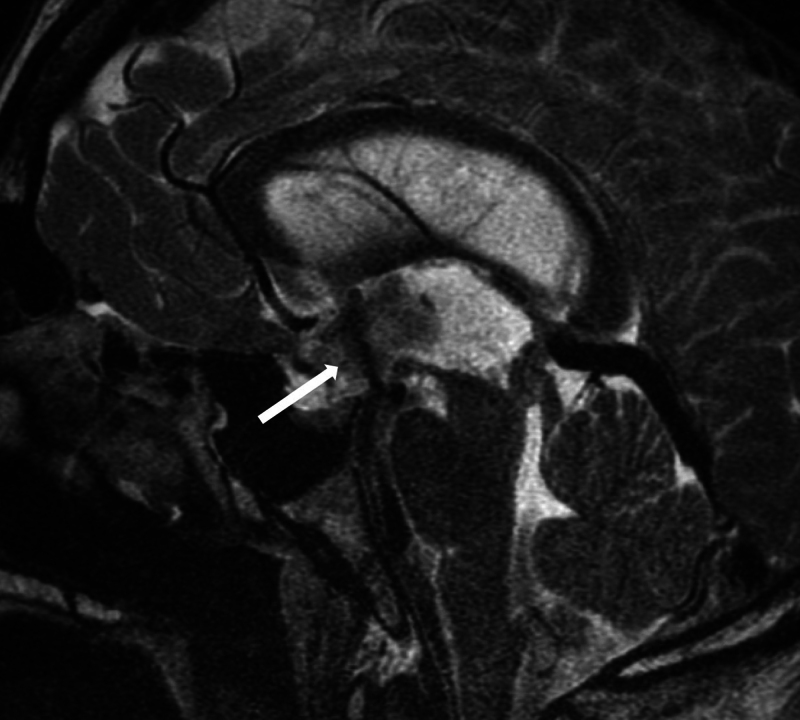
Given these clinical and radiographic findings, an ETV was recommended. The procedure was performed via a right frontal approach with a single burr hole, utilizing a neuroendoscope placed into the ventricular system under optical neuronavigation. The third ventriculostomy was initiated using a blunt probe, followed by serial dilation with a 0.2-mL Fogarty balloon. Pulsatile CSF flow was observed through the fenestration, and an EVD was placed under direct visualization into the lateral ventricles for postoperative ICP monitoring and possible CSF drainage (Fig. 2).
FIG. 2.
Immediately postoperative sagittal T2-weighted MRI sequence demonstrating a large flow void (arrow) through the floor of the third ventricle, indicating a patent fenestration with appropriate CSF flow.
On the first 2 postoperative days, the patient remained neurologically intact on examination but experienced periodic severe headaches accompanied by nausea and emesis. The ICPs during this period were predominantly in the 20– to40–mm Hg range, with transient and sustained spikes as high as 70 mm Hg. To alleviate the patient’s symptoms during this initial adjustment period, 5 mL of CSF was drained intermittently if the patient’s symptoms were accompanied by sustained and significantly elevated ICP spikes. On postoperative day 3, the EVD was opened at 30 cm H2O due to the patient’s symptoms, which were noted to improve with the resolution of nausea and emesis, as well as improvement in his headaches. A slow EVD weaning process was initiated on postoperative day 4, and the EVD was opened at 30 cm H2O every other hour for 24 hours followed by a gradual increase in the time of EVD closure (Table 1). On postoperative day 10, the EVD was opened once every 12 hours, and the patient’s only complaint was mild headaches. The EVD was then clamped, with monitoring of the patient’s symptoms and ICP. After the patient remained asymptomatic and ICPs remained within normal limits for 24 hours, the EVD was removed on postoperative day 11. Given the significant improvement in the patient’s symptoms, he was discharged from the hospital in stable condition on postoperative day 13.
TABLE 1.
Summary of EVD management strategy during the perioperative period
| Postop Day | Symptoms | EVD Plan |
|---|---|---|
| 1 | Severe headache/nausea/vomiting | Intermittent drainage (5 mL) |
| 2 | Severe headache/nausea/vomiting | Intermittent drainage (5 mL) |
| 3 | Moderate headache | Open (30 cm H2O) |
| 4 | Moderate headache | 1 hr closed & 1 hr open (30 cm H2O) |
| 5 | Moderate headache | 1 hr closed & 1 hr open (30 cm H2O) |
| 6 | Moderate headache | 2 hrs closed to 1 hr open (30 cm H2O) |
| 7 | Moderate headache | 3 hrs closed & 1 hr open (30 cm H2O) |
| 8 | Moderate headache | 6 hrs closed & 1 hr open (30 cm H2O) |
| 9 | Moderate headache | 12 hrs closed & 1 hr open (30 cm H2O) |
| 10 | Mild headache | Clamped |
| 11 | Mild headache | Removed |
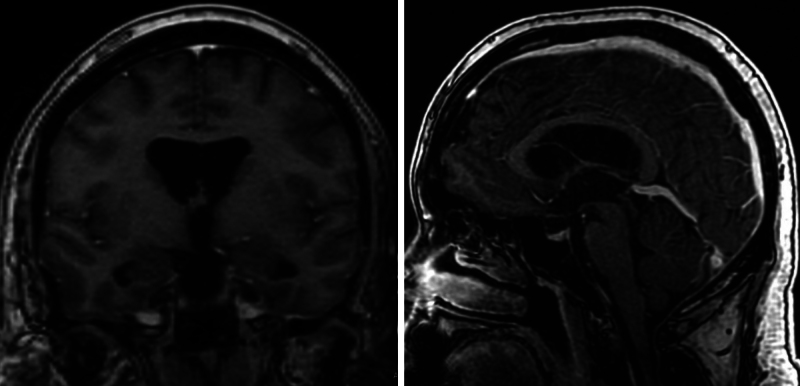
At the 1-month postoperative follow-up, the patient reported some mild intermittent headaches, with subsequent complete resolution of his headaches at 2 months. At the 2-year follow-up, the patient remained asymptomatic, and brain MRI showed resolution of the ventriculomegaly and a patent ventriculocisternostomy (Fig. 3).
FIG. 3.
Two-year postoperative coronal and sagittal postcontrast T1-weighted MRI sequences demonstrating significantly improved triventriculomegaly (left) and unchanged aqueductal stenosis from a web (right) in comparison to preoperative images in Fig. 1.
Informed Consent
The necessary informed consent was obtained in this study.
Discussion
Observations
There is evidence to suggest that an “adaptation period” following successful ETV, during which monitoring of ICP and controlled CSF drainage if patients have sustained ICP elevations and are significantly symptomatic, facilitates patient adjustment during the period of evolution of their CSF dynamics.5, 18 The time line for this period is not well established. We describe the case of a 39-year-old male who underwent ETV and was noted to have elevated ICPs in the immediate postoperative period but ultimately experienced a successful clinical outcome.
Several authors have discussed transient sustained ICP elevations following successful ETV, with some values exceeding 60 mm Hg in the postoperative period. It is evident that the absolute ICP measurements are not the sole factors dictating clinical management. Patients with chronic compensated obstructive hydrocephalus and long-standing ICP elevations can exhibit normal neurological examination findings with a low likelihood of acute decompensation, which is commonly observed in the perioperative adaptation period. A plausible hypothesis for this mechanism is that changes in the amount of interstitial fluid secreted by the brain will counteract high CSF pressures, which, combined with rapid CSF and interstitial fluid absorption, will allow cerebral blood flow to remain relatively normal.16
Management of extreme sustained ICP elevations following successful ETV remains controversial, with some authors advocating for either EVD or Rickham reservoir placement at the time of surgery, and others advocating for a lumbar puncture if patients become symptomatic. The precise mechanism of ICP elevations following successful ETV is not well understood. One plausible theory is that patients with long-standing CSF outflow obstruction exhibit delayed reopening of the subarachnoid spaces after alternative CSF outflow is established following long-standing obstruction.18 This transient ICP increase, generally most pronounced in the first few days after the procedure, is referred to as “transient intracranial hypertension,” as the ICP elevations are generally short-lived.18
Intraoperative EVD placement is commonly performed for patients undergoing ETV, as it enables clinicians to continuously monitor pressures and drain CSF if needed.6 Other authors have advocated for lumbar puncture, as it allows for rapid ICP normalization and symptom relief following significant elevations.7 However, authors arguing against EVD placement with continuous monitoring of ICP suggest that the approach provides no benefit in determining procedure success.12 If CSF drainage is utilized following an ETV, it must be performed carefully, as excessive drainage can lead to fenestration closure, ETV failure, and the need for ventriculoperitoneal shunt placement.1 Ultimately, the patient’s clinical presentation should dictate management in the immediate postoperative period, and procedural success cannot reliably be determined for a few weeks postprocedure, as it takes several days for the adaptation of CSF dynamics. This is often the case in patients with chronic compensated hydrocephalus; therefore, routine follow-up imaging to assess changes in ventricular caliber can potentially be delayed for 6–8 weeks.5, 18
The patient’s neurological examination and symptoms ultimately take precedence over ICP measurements in the postoperative period following successful ETV in the presence of a CSF drainage device when determining how much fluid to drain. Patients who become symptomatic with sustained ICP elevations during this adjustment period benefit from controlled CSF drainage and a prolonged EVD wean to assist with the maturation of the fenestration and the evolution of CSF dynamics.5, 7, 8, 12, 13
Lessons
Managing ICP elevations following successful ETV remains a controversial topic. In our study, we illustrate a case with extreme ICP elevations in the postoperative period, reaching as high as 72 mm Hg, despite a reassuring neurological examination and an excellent clinical outcome. Sustained ICP elevations in the immediate postoperative period with an EVD in place can be tolerated if the patient is clinically well, with temporary controlled CSF drainage performed if the patient becomes symptomatic. Ultimately, caution and patience should be exercised during this period, as ICP will likely normalize with a reassuring clinical outcome. Although this report details the findings of one patient, we believe it is strong proof of concept that the clinical picture, rather than ICP measurements alone, should drive patient management. Further studies are needed to understand the optimal management of elevated ICP after ETV.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Shepard, Sokol, Ziechmann. Acquisition of data: Sokol, Gupta, Ziechmann. Analysis and interpretation of data: Shepard, Sokol, Gupta. Drafting the article: all authors. Critically revising the article: Shepard, Sokol, Gupta. Reviewed submitted version of manuscript: Shepard, Sokol, Ziechmann. Approved the final version of the manuscript on behalf of all authors: Shepard. Statistical analysis: Sokol. Administrative/technical/material support: Gupta.
Correspondence
Scott R. Shepard: Temple University Hospital, Philadelphia, PA. scott.shepard@tuhs.temple.edu.
References
- 1.Schroeder HWS, Niendorf WR, Gaab MR. Complications of endoscopic third ventriculostomy. J Neurosurg. 2002;96(6):1032-1040. [DOI] [PubMed] [Google Scholar]
- 2.Lam S, Harris DA, Lin Y, Rocque BG, Ham S, Pan IW. Outcomes of endoscopic third ventriculostomy in adults. J Clin Neurosci. 2016;31:166-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isaacs AM, Bezchlibnyk YB, Yong H, et al. Endoscopic third ventriculostomy for treatment of adult hydrocephalus: long-term follow-up of 163 patients. Neurosurg Focus. 2016;41(3):E3. [DOI] [PubMed] [Google Scholar]
- 4.Kulkarni AV, Drake JM, Kestle JRW, et al. Predicting who will benefit from endoscopic third ventriculostomy compared with shunt insertion in childhood hydrocephalus using the ETV Success Score. J Neurosurg Pediatr. 2010;6(4):310-315. [DOI] [PubMed] [Google Scholar]
- 5.Bellotti A, Rapanà A, Iaccarino C, Schonauer M. Intracranial pressure monitoring after endoscopic third ventriculostomy: an effective method to manage the “adaptation period.” Clin Neurol Neurosurg. 2001;103(4):223-227. [DOI] [PubMed] [Google Scholar]
- 6.Brockmeyer D, Abtin K, Carey L, Walker ML. Endoscopic third ventriculostomy: an outcome analysis. Pediatr Neurosurg. 1998;28(5):236-240. [DOI] [PubMed] [Google Scholar]
- 7.Cinalli G, Spennato P, Ruggiero C, et al. Intracranial pressure monitoring and lumbar puncture after endoscopic third ventriculostomy in children. Neurosurgery. 2006;58(1):126-136. [DOI] [PubMed] [Google Scholar]
- 8.Elgamal EA. Continuous monitoring of intracranial pressure after endoscopic third ventriculostomy in the management of CSF shunt failure. Minim Invasive Neurosurg. 2010;53(2):49-54. [DOI] [PubMed] [Google Scholar]
- 9.Fukuhara T, Vorster SJ, Luciano MG. Risk factors for failure of endoscopic third ventriculostomy for obstructive hydrocephalus. Neurosurgery. 2000;46(5):1100-1109. [DOI] [PubMed] [Google Scholar]
- 10.Hopf NJ, Grunert P, Fries G, Resch KDM, Perneczky A. Endoscopic third ventriculostomy: outcome analysis of 100 consecutive procedures. Neurosurgery. 1999;44(4):795-804. [DOI] [PubMed] [Google Scholar]
- 11.Kulkarni AV, Drake JM, Armstrong DC, Dirks PB. Imaging correlates of successful endoscopic third ventriculostomy. J Neurosurg. 2000;92(6):915-919. [DOI] [PubMed] [Google Scholar]
- 12.Muroi A, Quezada JJ, McComb JG. Usefulness of postoperative ventriculography and intracranial pressure monitoring following endoscopic third ventriculostomy. Childs Nerv Syst. 2021;37(4):1151-1158. [DOI] [PubMed] [Google Scholar]
- 13.Roytowski D, Semple P, Padayachy L, Carara H. Intracranial pressure monitoring as an early predictor of third ventriculostomy outcome. World Neurosurg. 2013;80(5):605-611. [DOI] [PubMed] [Google Scholar]
- 14.Tefre S, Lilja-Cyron A, Arvidsson L, et al. Endoscopic third ventriculostomy for adults with hydrocephalus: creating a prognostic model for success: protocol for a retrospective multicentre study (Nordic ETV). BMJ Open. 2022;12(1):e055570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mokri B. The Monro-Kellie hypothesis: applications in CSF volume depletion. Neurology. 2001;56(12):1746-1748. [DOI] [PubMed] [Google Scholar]
- 16.Iencean SM, Ciurea AV. Intracranial hypertension: classification and patterns of evolution. J Med Life. 2008;1(2):101-107. [PMC free article] [PubMed] [Google Scholar]
- 17.Alali AS, Fowler RA, Mainprize TG, et al. Intracranial pressure monitoring in severe traumatic brain injury: results from the American College of Surgeons Trauma Quality Improvement Program. J Neurotrauma. 2013;30(20):1737-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amini A, Schmidt RH. Endoscopic third ventriculostomy in adult patients. Neurosurg Focus. 2005;19(6):E9. [PubMed] [Google Scholar]